Abstract
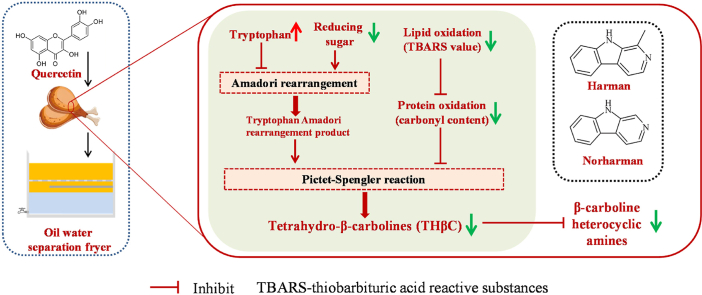
The effect of quercetin and oil water separation system on the formation of heterocyclic amines (HAs) was investigated during the frying process of braised chicken drumsticks. The results showed that two β-carboline HAs (β-CHAs) were detected in the chicken samples: 9H-pyrido [3,4-b] indole (Norharman) and1-methyl-9H-pyrido [3,4-b] indole (Harman). β-CHAs content in the chicken samples increased as the cycle times of the frying oil rose (P < 0.05). The addition of quercetin and use of oil water separation system significantly inhibited the formation of Harman (27.3% and 28.2%; P < 0.05) and Norharman (28.7% and 64.1%; P < 0.05), respectively, and the combined use of the two treatments had a better effect (47.0% and 80.2%; P < 0.05). It can be attributed to the lower consumption of β-CHAs precursors (tryptophan) and reduced generation of the intermediates (carbonyl compounds and 1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid). This provides a promising way for reducing β-CHAs during the frying process of braised chicken products.
Keywords: β-carboline heterocyclic amine, Quercetin, Oil-water separation frying, Braised chicken, Protein oxidation
Graphical abstract
Highlights
-
•
Two heterocyclic amines (HAs) were detected in the frying process of chicken.
-
•
The effect of oil water separation frying (OWSF) on HAs formation was analyzed.
-
•
The combined use of quercetin and OWSF reduced β-carboline HAs formation.
-
•
The protein and lipid oxidation of the chicken samples were inhibited.
-
•
The production of 1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid was suppressed.
1. Introduction
Heterocyclic amines (HAs), mainly composed of carbon, nitrogen, and hydrogen atom, are polycyclic aromatic compounds. These compounds are carcinogenic and mutagenic substances generated in protein-rich meat products during thermal processing (Yan et al., 2022). HAs can be divided into thermic HAs and pyrolytic HAs according to their formation pathway (Gibis, 2016; Bulanda and Janoszka, 2022). Thermic HAs are formed due to Maillard reaction, from α-amino acids, creatine and reducing sugars. Pyrolytic HAs are formed mainly from thermal decomposition products of single amino acids like tryptophan (Dong et al., 2020). Pyrolytic HAs mainly consist of carboline HAs and other pyrolytic HAs according to their chemical structures (Gibis, 2016). Among them, β-carboline HAs (β-CHAs) including Harman and Norharman were reported as two major pyrolytic HAs in meat products (Chang et al., 2018).
Deep-Frying is a typical dry cooking method immersing food in edible oils at high temperatures (Cheng et al., 2021). Short-time frying gives meat products attractive color, rich aroma and crispy texture, however, it also provides convenient conditions for the formation of consequent cooking-induced contaminants such as HAs. Braised chicken is a time-honored meat product in China, which undergoes a high-temperature frying process for a few minutes before braising at a low temperature for several hours (Cheng et al., 2019). Raw chicken is usually immersed with honey or maltose solution before frying to get a golden yellow color and an attractive taste of the product (Cheng et al., 2019). The frying oil tends to be used repeatedly to reduce the costs of the products (Xu et al., 2022), which may cause thermal decomposition and oxidation of fat (Wang et al., 2015). Under that circumstance, the content of HAs in the chicken increases dramatically as the frying time goes on (Yao et al., 2013). Consequently, it is important to restrict the production of HAs during the frying process of braised chicken.
Previous studies have reported that some plant extracts, spices and natural flavonoids can reduce the HAs formation and discussed the mechanism in chemical model systems (Keskekoglu and Uren, 2014; Tengilimoglu-Metin et al., 2017; Zeng et al., 2017, 2018). Quercetin is one of the most abundant flavonoids in many fruits and vegetables (Andres et al., 2018), which has been demonstrated to be a powerful antioxidant (Boots et al., 2008). It has been reported to be an effective inhibitor for the production of 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine in roasted beef (Zhu et al., 2016). As for pyrolytic HAs, other researchers also found that the addition of quercetin had an inhibitive effect on the synthesis of Harman and Norharman in roasted lamb meats (Ding et al., 2022).
Some publications also have described the phenomenon of alleviating HAs emergence in meat products by different cooking methods. The HAs level of electric grilled drumsticks was considerably lower compared with that of gas-grilled ones (Yao et al., 2020). The highest content of HAs was found in lamb patties cooked by stewing, followed by roasting, frying and pan-frying (Guo et al., 2014). For chicken meatballs, pan cooking led to lower levels of HAs compared with deep-fat frying (Keskekoglu and Uren, 2014). Oil water separation frying (OWSF) is a new technology, in which both water and oil are added in one frying tank and naturally stratified because of their incompatibility with indifferent densities (Zhang et al., 2016). The oil floats at the upper layer of the frying tank and the water sinks to the tank bottom. The OWSF machine only heats in the oil layer locally. During the process of OWSF, food residues in the oil-layer fall into the water-layer with the help of gravity, which can keep the residues from depositing in the oil and frying repeatedly (Ma et al., 2016). Therefore, compared with deep-fat frying, the OWSF process may significantly reduce the oxidation rate of the frying oil and alleviate its deterioration, especially when the oil is used repeatedly. Thus, it is a candidate method for preventing HAs development while food frying.
Thus, it has been found that quercetin was an effective inhibitor for HAs in meat products. Considering the antioxidant role of OWSF, it is necessary to evaluate the combined effect of quercetin and OWSF treatment on the formation of HAs in the frying process of braised chicken and clarify the mechanism. To our knowledge, there are few studies on this.
The study aimed to determine the combined effect of quercetin and OWSF on the formation of HAs during the frying process of braised chicken drumsticks. Moreover, some parameters related to HAs formation, such as tryptophan, reducing sugar, carbonyl content and thiobarbituric acid reactive substances (TBARS), were also monitored to reveal the mechanism.
2. Material and methods
2.1. Materials
2.1.1. Raw materials
Frozen chicken drumsticks and honey were purchased from a local market in Hefei, China. The chicken drumsticks were delivered to the laboratory within 0.5 h, and stored in a refrigerator at −20 °C. The frozen chicken drumsticks were thawed at 4 °C before use. Quercetin was obtained from Xi'an Shengqing Biotechnology Co. Ltd.
2.1.2. Chemicals
Heterocyclic amine standards were ordered from Toronto Research Chemicals (Downsview, Ontario, Canada): 1-methyl-9H-pyrido [3,4-b] indole (Harman, CAS no.: 486-84-0), 9H-pyrido [3,4-b] indole (Norharman, CAS no.: 244-63-3), 3-amino-1,4-dimethyl-5H-pyrido [4,3-b] indole (Trp-P-1, CAS no.: 68808-54-8), 3-amino-1-methyl-5H-pyrido [4,3-b] indole (Trp-P-2, CAS no.: 72254-58-1), 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PHIP, CAS no.: 105650-23-5), 2-amino-3,4-dimethylimidazo [4,5-f] quinoline (IQ, CAS no.: 76180-96-6), 2-amino-3,8- dimethylimidazo [4,5-f] quinoline (MeIQx, CAS no.: 77500-04-0), 2-amino-3,4- dimethylimidazo [4,5-f] quinoline (MeIQ, CAS no.: 77094-11-2), 2-amino-3,4,8-trimethylimidazoquinoxaline (4,8-DiMeIQx, CAS no: 95896-78-9) and 2-amino-3,7,8-trimethylimidazo [4,5-f] quinoxaline (7,8-DiMeIQx, CAS no: 92180-79-5). The internal standard for the analysis of HAs is 2-Amino-3,4,7,8-tetramethyl-3H-imidazo [4,5-f] quinoxaline (4,7,8-DiMeIQx, CAS no.: 132898-07-8). Other chemicals and solvents were of analytical or high-performance liquid chromatography (HPLC) grade.
2.2. Effect of cycle times of oil on the formation of HAs using different frying methods
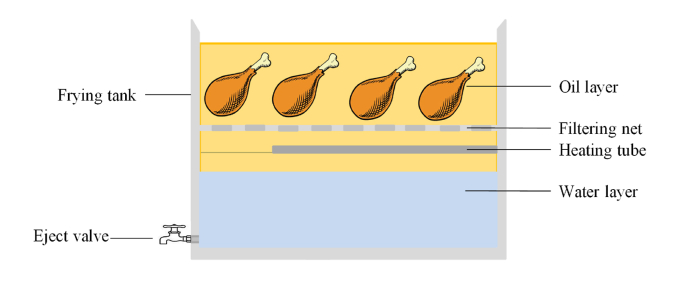
Four chicken drumsticks were chosen randomly and immersed in a 50% (w/w) honey solution. After drained for 1 min, the chicken drumsticks were fried in a thermostatic fryer with 4 L of oil (HH-WO-5L, Tanze Instrument Co., Ltd., Shanghai, China) or an oil water separation fryer with 8 L of oil (LN-100, Leiniao Instrument Ltd., Rui'an, China) at 180 °C for 2 min. The proportion of drumsticks and oil is equal in the two fryers. The schematic diagram of the oil water separation fryer is shown in Fig. 1. The oil was used to fry the drumsticks continuously for 10 times without any replenishment.
Fig. 1.
Schematic diagram of the oil water separation fryer.
The chicken drumsticks fried in the oil recycled for 8 and 10 times were selected. The skin was removed from the drumsticks and ground with a S1-M81 (D) blender (Joyoung Co., Ltd., Shandong, China). All the ground skin samples were stored at −80 °C before analysis of HAs.
2.3. Effect of quercetin and OWSF on the generation of β-CHAs during frying process of chicken drumsticks
Four groups of the chicken drumsticks were prepared. Two percent of quercetin (w/w) was dissolved into a 50% honey solution (w/v). The drumsticks were selected randomly in each group and immersed in the honey solution with or without adding quercetin. After drained for 1 min, the chicken drumsticks were fried using the thermostatic fryer or oil water separation fryer for 2 min when the oil reached 180 °C. Before frying, the oil in the both fryers has been recycled for 10 times. The fried chicken drumsticks were prepared in four groups as follows: (1) Control (deep-fat frying), (2) QC (treated with quercetin, deep-fat frying), (3) OW (OWSF), (4) QC-OW (treated with quercetin, OWSF). The ground skin samples were prepared as depicted in 2.2. to analysis for HAs, β-CHAs precursors (tryptophan and reducing sugar), carbonyl content and TBARS, 1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid (THCA).
2.4. Analysis of HAs
HAs content was determined using the method of Yan et al. (2022) with slight modifications. 2.0 g of the skin sample, 100 μL of the internal standard solution (200 μg/mL) and 3 mL of distilled water were added to a 50-mL centrifuge tube. The mixture was homogenized (9000 rpm, 15 s, 4 times) using a T18DS25 homogenizer (IKA Instrument Equipment Co., Ltd., Guangzhou, China). Later, the homogenizer's blade was rinsed with acetonitrile and n-hexane, and the rinsing solution was collected into the centrifuge tube containing the homogenized product. Then, the resultant mixture was vortexed for 1 min and followed by 30-min ultrasonic extraction steps. After centrifuged at 1320×g for 5 min, the acetonitrile layers of the mixture were removed into a new centrifuge tube. The extract procedures were repeated twice to collect the acetonitrile eluent for solid phase extraction (SPE).
The SPE procedures were carried out with MCX cartridges (60 mg, 3 mL) (Anpel Co., Ltd., shanghai, China). 3 mL of 0.1 mol/L HCl and 3 mL of methyl alcohol were used to eliminate impurities. The retained HAs were eluted by a 6-mL mixture of ammonium hydroxide and methyl alcohol (10:90, v/v), evaporated under nitrogen to dryness and redissolved in 1 mL 0.03 mol/L ammonium acetate - acetonitrile mixed solution (50:50, v/v) for HPLC-MS/MS analysis.
An AB Sciex UPLC–MS/MS system (API 5000, AB Sciex Pte., Ltd, Shanghai, China) was applied to analyze HAs. Separation process was carried out on a ZORBAX Eclipse Plus C18 column (100 mm × 2.1 mm, 1.8 μm) (Agilent Technologies Co., Ltd., California, USA) at 30 °C. 0.03 mol/L ammonium acetate (pH = 3.4) (A) and acetonitrile containing 1% acetic acid (B) were used as binary mobile phase. The flow rate was 0.25 mL/min. The elution program was 0–5 min, 95% A; 5–10 min, 95%–90% A; 10–15 min, 90-70% A; 15–22 min, 70-1% A; 22–25 min, 1–95% A; 25–30 min, 95% A. The HAs content was expressed as ng per g of the skin sample.
2.5. Analysis of tryptophan
Tryptophan was extracted and analyzed using the method described by Zhang et al. (2022) and Islam et al. (2016) with minor modifications. 4.0 g of the skin sample was mixed with 20 mL of distilled water and homogenized at 10,000 rpm for 30 s. The mixture was centrifuged at 2100×g for 15 min at 4 °C. Then, 4 mL of the supernatant was mixed with 2 mL n-hexane, vortexed for 15 s and filtered to vials through a 0.22-μm filter. The identification and quantification of tryptophan was carried out using a S6000 HPLC system (Acchrom-Tech Ltd., Beijing, China) based on a reversed-phase analytical column (TSKgel ODS-80 TM; 250 mm × 4.6 mm, 5 μm). Fluorescence was detected at the excitation wavelength and emission wavelength of 283 nm and 343 nm, respectively. The tryptophan content was expressed as microgram per gram of the skin sample.
2.6. Analysis of reducing sugar
Reducing sugar was measured according to Lou et al. (2018) using the 3, 5-dinitrosalicylic acid (DNS) method with minor modifications. 1.0 g of the ground skin sample and 9 mL distilled water were added to a 50-mL centrifuge tube and homogenized at 10,000 rpm for 30 s. The tube was placed in a water bath at 80 °C for 40 min and then cooled to room temperature. The samples were then centrifuged for 10 min at 8000×g. 1 mL of the supernatant was mixed with 9 mL of distilled water. 2 mL of DNS solution (10 mg/mL DNS, 300 mg/mL sodium potassium tartrate and 16 mg/mL NaOH in distilled water) was added to 1 mL of the diluent. The control consisted of 1 mL of distilled water and 2 mL of DNS solution. The samples and the control were kept in boiling water baths for 5 min and cooled on ice. The absorbance was determined at 540 nm with a microplate reader (H1, Boten Instrument Co., Ltd., Winooski, VT, USA). The contents of reducing sugars were calculated from the standard calibration curve prepared with glucose and expressed as milligram per gram of the sample.
2.7. Analysis of carbonyl content
Carbonyl contents in the skin samples were determined by 2,4- dinitrophenyl hydrazine (DNPH) method according to the method of Zhang et al. (2020) with slight modifications. 3.0 g of the skin sample was homogenized for 1 min at 10,000 rpm with 30 mL of phosphate buffer (20 mM, pH = 6.5, containing 0.6 M NaCl). The mixture was divided into four 0.2-mL aliquots and each was centrifuged with 1 mL of 10% cold trichloroacetic acid (TCA) at 8000×g for 10 min The protein pellet of two aliquots were added with 0.5 mL of 10 mmol/L DNPH (w/v) in 2.0 mol/L HCl and incubated for 1 h at 25 °C with continuous shaking, while the other two were treated with 0.5 mL of 2.0 mol/L HCl as blank. Then, the four aliquots were added with 0.5 mL of TCA (20%) before centrifugation at 8000×g for 10 min. The sediments were washed three times with 1 mL of ethanol and ethyl acetate solution (1:1, v/v) to remove excess DNPH. The precipitates were resuspended in 1 mL of phosphate buffer (20 mmol/L, pH = 6.5) with 6.0 mol/L guanidine hydrochloride. Finally, the solution absorbance of the protein mixture was measured at 280 nm and 370 nm. The carbonyl content (nmol/mg protein) was calculated using the following equation:
2.8. Analysis of TBARS
Lipid oxidation was determined according to the TBARS as described by Xiao et al. (2021) with minor modifications. 2.5 g of the skin sample from each group was homogenized for 1 min at 3000 rpm with 25 mL of cold trichloroacetic acid (TCA) reagent (7.5% TCA, 0.1% EDTA) using a homogenizer (T18DS25, IKA Instrument Equipment Co., Ltd., Guangzhou, China). Then, the mixture was filtered using filter paper and 2 mL of the filtrate was added with an equal volume of thiobarbituric acid (0.02 M). After incubated at 90 °C for 30 min and cooled to 25 °C, the absorbance of the mixture was determined at 532 nm using a microplate reader (H1, Boten Instrument Co., Ltd., Winooski, VT, USA). 1,1,3,3-Tetraethoxypropane was used to define the standard curve. The TBARS was expressed as mg of malonaldehyde (MDA) per kg of the skin sample.
2.9. Analysis of 1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (THCA)
The THCA content of the skin samples was measured according to the method of Herraiz (2000) and Wang et al. (2021) with slight modifications. 3.0 g of the sample was mixed with 15 mL of 0.6 mol/L HClO4 and then homogenized at 10,000 rpm for 30 s before solid-phase extraction. THCA analysis was performed on an Acchrom S6000 HPLC system with a TSKgel ODS-80 TM (250 mm × 4.6 mm, 5 μm) column. The mobile phase was 0.5 mol/L ammonium acetate-acetic acid buffer (pH = 3.4) (A)/acetonitrile (B), and the elution procedure was as followed: 0–8 min, 90%–68% A; 8–10 min, 68%–90% A; 10–15 min, 90% A. A fluorescence detector was employed, in which the excitation wavelength and emission wavelength were set as 270 nm and 343 nm, respectively. The THCA content was expressed as ng per g of the skin sample.
2.10. Statistical analysis
All the experiments were carried out in triplicate, with each being replicated three times. Values are presented as mean ± standard deviation. Statistical analysis was carried out using the SPSS software package (Version 26, SPSS Inc., Chicago, IL, USA). Independent two-sample t-test model was performed to identify the results among different oil cycle times. Tukey's multiple range test was used to compare the results of different treatments. The results were considered statistically significant at P < 0.05.
3. Results and discussion
3.1. Effect of cycle times of oil on the formation of HAs using different frying methods
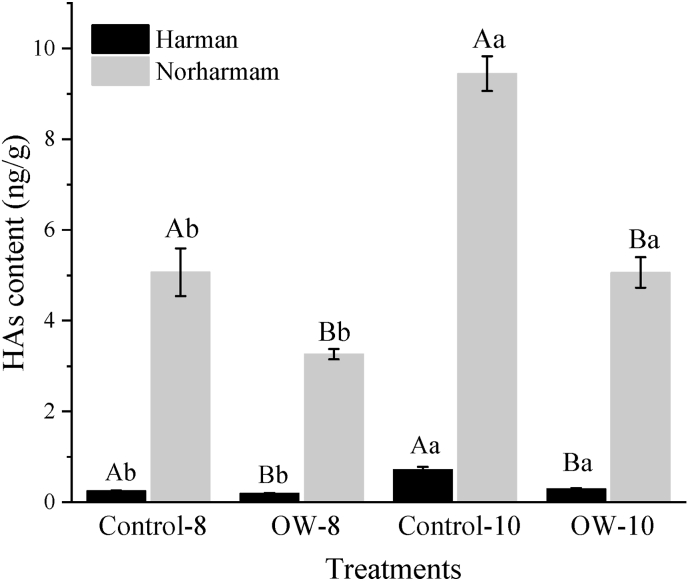
Norharman was the most abundant HA in the chicken drumsticks fried under different methods, followed by Harman, with a concentration below 1 ng/g (Fig. 2). The chicken drumsticks treated with OWSF showed considerably lower levels of HAs compared with the control (P < 0.05). Under different frying methods, the concentration of HAs increased rapidly as cycle times of the oil increased. When the cycle time of the oil increased from 8 to 10, the content of Harman and Norharman in the deep-fat fried chicken sample increased by 290.88% and 186.37%, respectively. For the samples treated with OWSF, an increase of 150.95% and 155.13% was observed for Harman and Norharman, respectively. Compared with the deep-fat frying group, the contents of Harman and Norharman detected in the OWSF group were obviously reduced when the oil was recycled for the same times (P < 0.05).
Fig. 2.
Effect of cycle times of oil on the formation of HAs in the chicken samples using deep-fat frying (Control) or OWSF (OW). Bars with different uppercase letters (A–B) indicate significant differences among different frying methods at the same cycle times of oil (P < 0.05) and means with different lowercase letters (a–b) indicate significant differences among different cycle times of oil using the same frying methods (P < 0.05). Comparisons were made within one heterocyclic amine.
The formation of HAs depends on the heating temperature, heating time and cooking method. When chicken drumsticks were grilled at 230 °C for 27 min, many HAs, including IQ, Harman, Norharman, PHIP, MeIQx and AαC were detected (Yao et al., 2020). However, only IQx was detected when drumsticks were deep-fat fried at 175 °C in sunflower oil for 8.5 min (Haskaraca et al., 2014). In this study, only Harman and Norharman were detected in the chicken skin samples which were fried for 2 min at 180 °C. Yao et al. (2013) also found that Harman and Norharman were detected in the chicken skin after fried at 160 °C for 2 min. Similar results were found in roasted pork and beef patties (Xu et al., 2021). Harman and Norharman are collectively called β-CHAs. They can be formed at a relatively low temperature (100 °C) in comparison to other HAs (> 150 °C) (Dong et al., 2020), which is responsible for the common occurrence of Harman and Norharman in thermally-processed meat products (Feng et al., 2022).
The preparation of traditional braised chicken involves a frying process for a few minutes before a relatively low temperature braising for several hours (Cheng et al., 2019). The main purpose of the short-time frying process is to generate a golden color and an attractive flavor, rather than make the product fully cooked (Yao et al., 2013). Thus, the heating conditions of the process are relatively mild and only two β-CHAs were detected. Moreover, previous research (Yao et al., 2013) and our preliminary experiments have demonstrated that HAs distributed mainly in the chicken skin after the short-time frying. Hence, the chicken skin was chosen to determine the contents of HAs.
Large quantities of oxides and abundant free radicals were formed in the oil when used repeatedly (Bulanda and Janoszka, 2022; Khan et al., 2022b), which promoted the formation of HAs in fried meat products. Previous research (Ma et al., 2016) demonstrated that the OWSF treatment imparted lower polymeric degradation and oxidation of the oil and compared with deep-fat frying. During the frying process, food residues exited the oil-layer and descended to the water-layer before carbonization (Zhang et al., 2016). Thus, the free radicals and oxides in the frying oil may keep at a relatively low content during the process. Based on these, the OWSF treatment is considered to be an effective way to reduce the content of HAs in chicken.
3.2. Effect of quercetin and OWSF on the formation of β-CHAs during frying process of chicken drumsticks
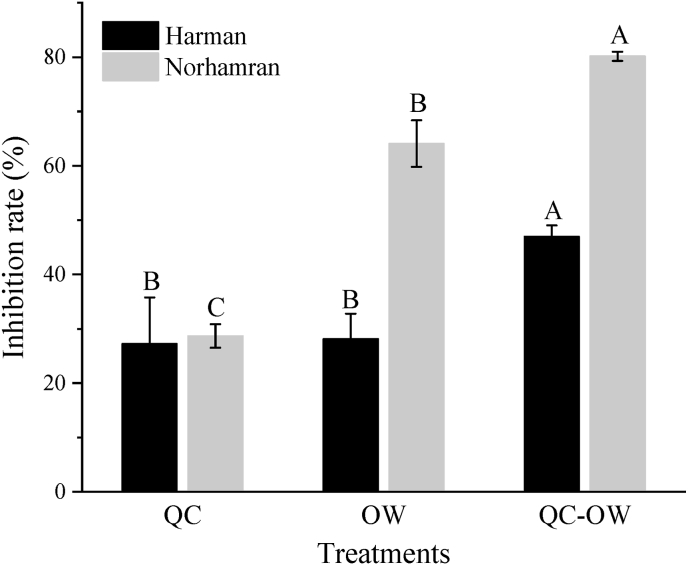
Both quercetin and OWSF were found to significantly inhibit the generation of β-CHAs (P < 0.05; Fig. 3). Norharman was significantly suppressed by the addition of quercetin (28.7%) and the use of oil water separation system (64.1%) (P < 0.05). The combination of the two treatments achieved a better inhibitory effect on Norharman (P < 0.05), with a higher inhibition rate of 80.2%. For Harman, when adding quercetin or using oil water separation system alone, the inhibition rate was 27.3% and 28.2%, respectively. When the two treatments were used in combination, the inhibition rate of Harman reached 47.0%.
Fig. 3.
Effect of quercetin (QC) and OWSF (OW) on the formation of β-CHAs during frying process of the chicken samples. Inhibition rate was defined as the ratio of β-CHAs content in the treatment to that in the control. Bars with different letters (A–C) indicate significant differences among different treatments (P < 0.05). Comparisons were made within one heterocyclic amine.
Quercetin, a member of flavonoids family, is one of the best in vitro antioxidants (Boots et al., 2008). Its antioxidative role is attributed to the existence of two antioxidant pharmacophores within the molecule that have the optimal configuration to scavenge free radical (Heijnen et al., 2009). Kikugawa (1999) found that antioxidants can scavenge the free radicals and inhibit the radical reactions involved in HAs formation, and thus reduce the HAs formation. Furthermore, it was also reported that the addition of quercetin had an inhibitory effect on the formation of Harman and Norharman in roasted lamb meats (Ding et al., 2022). The scavenge of free radicals by quercetin was considered to be involved in the blockade of the β-CHAs formation pathway.
In this study, quercetin was considered to block the formation pathway of β-CHAs in the chicken drumsticks by scavenging free radicals. Oil water separation system can also keep oxides and free radicals in the oil at a low level. Therefore, the combination of the two treatments can exhibit a better inhibitory effect on the formation of β-CHAs in the chicken drumsticks during the frying process.
3.3. Analysis of tryptophan and reducing sugar
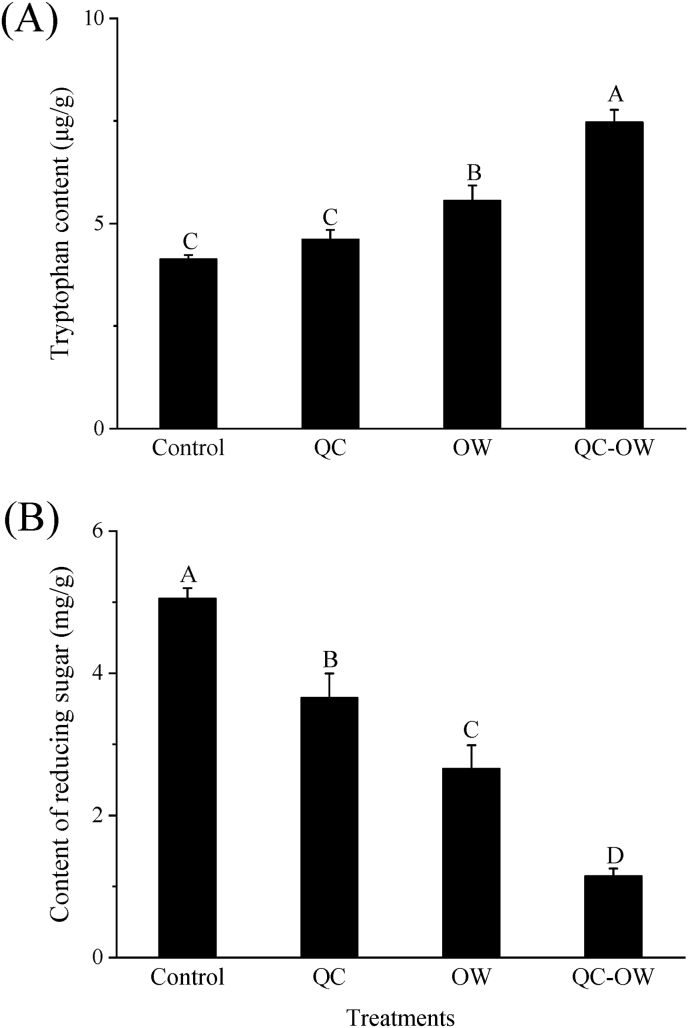
Tryptophan is considered as a precursor of β-CHAs formation and it can be initially converted to tryptophan Amadori rearrangement product with the presence of reducing sugar (Pfau and Skog, 2004; Rönner et al., 2000). The effects of quercetin and OWSF treatment on tryptophan content of the chicken skin samples were shown in Fig. 4A. The content of tryptophan of the OW and QC-OW groups was significantly higher than that of the control group (P < 0.05). Among them, the QC-OW group had the highest tryptophan content, which was 1.8 times higher than that of the control. The production of β-CHAs was related to the thermal-oxidative decomposition of tryptophan in food when treated at high temperatures (Pfau and Skog, 2004). The content of β-CHAs in the chicken samples treated with OWSF was substantially decreased compared with the control (Fig. 3), while the content of precursor tryptophan was significantly increased. Amadori rearrangement is an irreversible reaction (Lin et al., 2018). Therefore, it can be assumed that the OWSF treatment may inhibit the tryptophan Amadori rearrangement reaction and decrease the consumption of tryptophan in the frying process, thus reduce the production of β-CHAs in the chicken samples. The addition of quercetin alone did not significantly inhibit the consumption of tryptophan (Fig. 4A), but still reduced the generation of HAs in the chicken sample (Fig. 3), which suggested that quercetin may not inhibit HAs formation through block the Amadori rearrangement reaction.
Fig. 4.
Effect of quercetin (QC) and OWSF (OW) on the content of tryptophan (A) and reducing sugar (B) of the chicken samples. Bars with different letters (A–D) indicate significant differences among different treatments (P < 0.05).
Reducing sugar has been reported to promote the formation of β-CHAs (Gibis, 2016). Fig. 4B detailed the data on the reducing sugar content of the chicken samples treated with quercetin and OWSF. The content of reducing sugar was significantly decreased in all the treatment groups compared to the control (P < 0.05). In the QC-OW sample, the content of reducing sugar reached the lowest value (22.7% of the control). Based on the results of tryptophan (Fig. 4A), quercetin may not block the Amadori rearrangement reaction. However, the presence of the antioxidant can alter the reaction paths and products of reducing sugars involved in the Maillard reaction mainly by scavenging the free radicals, capturing the active carbonyl compounds and protecting the protein glycosylation sites (Jia et al., 2023). Thus, it was assumed that quercetin may promote reducing sugars to be involved in other pathways of Maillard reaction, which led to the reduction of β-CHAs formation. The OWSF treatment can also timely remove the free radicals and oxides produced during the frying process (Ma et al., 2016). These may cause the continuously positive shift of the chemical reaction equilibrium in which abundant reducing sugars are involved.
3.4. Analysis of carbonyl content and TBARS value
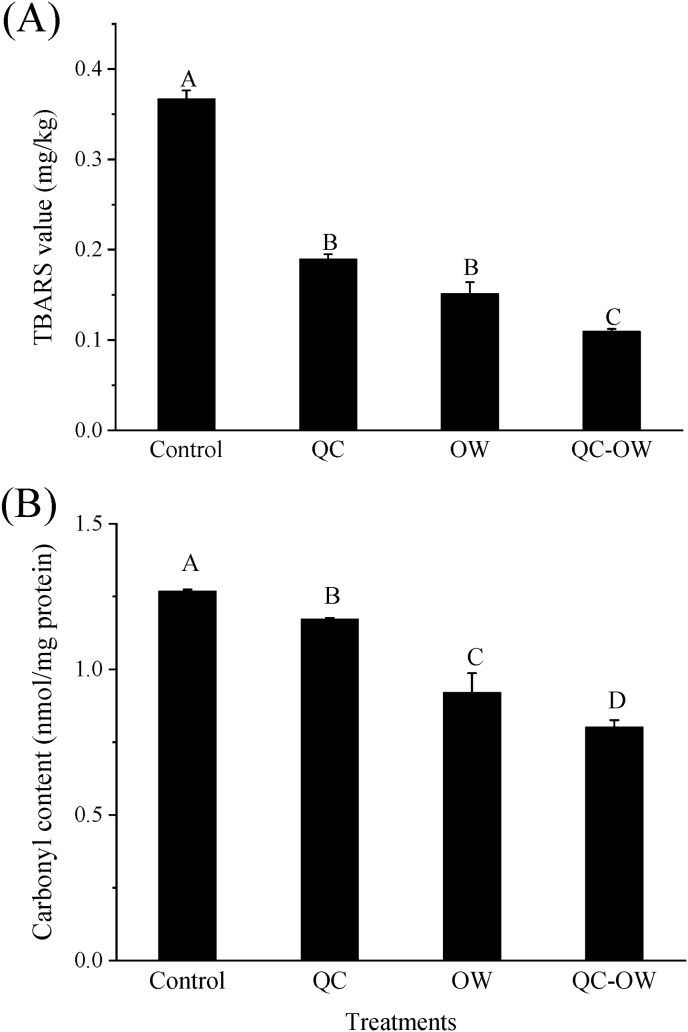
Carbonyl compounds such as acetaldehyde and α-ketonic acids are the intermediates in the β-CHAs formation (Totsuka et al., 1999). They can be involved in the Pictet-Spengler reaction with Amadori rearrangement product of tryptophan to generate tetrahydro-β-carbolines (THβC), the intermediates of β-CHAs (Rönner et al., 2000). Hence, the contents of carbonyl compounds in the chicken samples under different treatments were determined (Fig. 5A). The carbonyl contents of all the treatment groups were significantly reduced compared with that of the control (P < 0.05). The combined treatment of quercetin and OWSF led to the most remarkable decrease in the carbonyl content (0.80 mmol/mg protein).
Fig. 5.
Effect of quercetin (QC) and OWSF (OW) on carbonyl content (A) and TBARS value (B) in the chicken samples. Bars with different letters (A–D) indicate significant differences among different treatments (P < 0.05).
Previous studies have also shown that the HAs formation would be enhanced if lipid oxidation was accelerated (Zamora et al., 2012; Khan et al., 2022). The TBARS levels of all the treatment groups were lower as compared to that of the control (P < 0.05; Fig. 5B), and the QC-OW group had the lowest one (0.109 mg MDA/kg). The results of TBARS were consistent with those of the carbonyl compounds in all the groups.
Carbonyl compounds are active products of Maillard reaction, and may be produced from amino acids attacked by free radicals (Xue et al., 2020). Quercetin addition was found to reduce carbonyl contents of the chicken skin samples, which led to the decrease of β-CHAs formation (Fig. 3). These were in agreement with previous research using other antioxidant. Curcumin has been reported to decrease the contents of β-CHAs by inhibiting the production of carbonyl compounds (Wang et al., 2021). It has been widely accepted that antioxidants have excellent ability to remove free radicals (Meurillon and Engel, 2016), which may contribute to the inhibitory effects on the formation of carbonyl compounds.
The content of carbonyl compounds in the chicken samples treated with OWSF was found to be significantly lower than that of the QC group and Control. The key advantage of using OWSF was that the residues of the chicken drumsticks in the frying oil can be removed in time, which effectively alleviated the oxidation and polymerization deterioration in the oil (Ma et al., 2016). It has also been found that the carbonyl value of soybean oil using OWSF was lower than that using deep-fat frying (Ma et al., 2016). Meanwhile, during the frying process, food can absorb large amount of oil making oil become a component of the final product (Al-Khusaibi et al., 2012). Thus, OWSF is a promising way to retard the oxidation of fried meat products.
Lipid oxidation usually promotes protein oxidation (Berardo et al., 2016), and thus may enhance the formation of β-CHAs. This is because the free radicals generated in the lipid oxidation process are also the potential initiators of protein carbonylation (Estevez, 2011), which may lead to the β-CHAs production (Khan et al., 2022). Many studies have shown that the addition of antioxidant compounds to meat products was an effective way to scavenge the free radicals produced (Selani et al., 2011; Tengilimoglu-Metin et al., 2017; Wang et al., 2021), thus alleviated the lipid and protein oxidation. The OWSF treatment also had the ability to keep the free radical content in the oil at a low level by avoiding repeated heating of food residues (Ma et al., 2016), thus decreased the products of lipid and protein oxidation. Thus, the inhibition of lipid and protein oxidation caused by quercetin addition and OWSF treatment blocked the Pictet-Spengler reaction and subsequently led to the inhibition of β-CHAs generation in the chicken samples.
3.5. Analysis of THCA
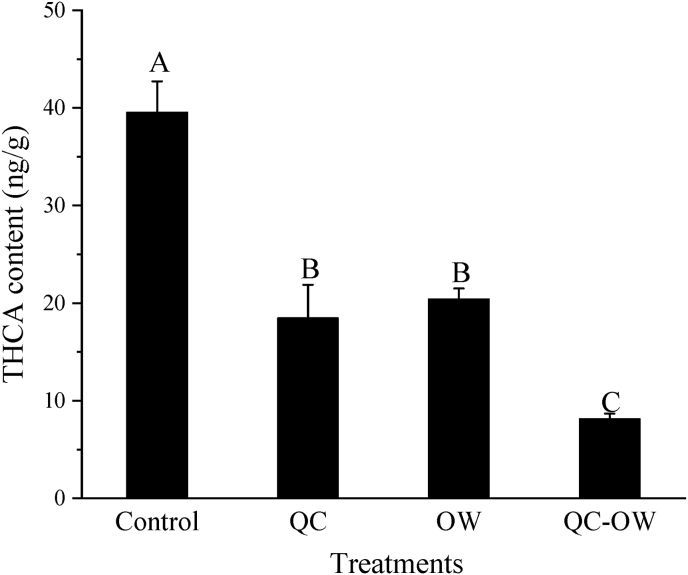
Tryptophan Amadori rearrangement products can react with carbonyl compounds such as acetaldehyde or α-ketonic acids to produce THCA and 1-methyl-1,2,3,4-tetrahydro-carboline-3-carboxylic acid via the Pictet-Spengler pathway, which are then oxidized and decarboxylated to generate Norharman and Harman, respectively (Herraiz, 2000). Norharman was the most abundant β-CHA in the frying process of the braised chicken drumsticks, and had stronger co-mutagenic activity than Harman (Totsuka et al., 1999). Therefore, the content of THCA was determined to investigate whether the quercetin and OWSF treatment can inhibit the Pictet-Spengler reaction. As shown in Fig. 6, both the quercetin addition and OWSF treatment had a strong inhibitory effect on THCA generation (46.7% and 51.6% of the control; P < 0.05). For the QC-OW sample, the content of THCA reached the lowest value (20.6% of the control; P < 0.05), indicating that the Pictet-Spengler reaction chain was blocked, which subsequently inhibited the formation of Norharman.
Fig. 6.
Effect of quercetin (QC) and OWSF (OW) on the content of THCA in the chicken samples. Bars with different letters (A–C) indicate significant differences among different treatments (P < 0.05).
Quercetin has been proven to be an excellent antioxidant (Boots et al., 2008), and has strong capacity to scavenge free radicals in food (Meurillon and Engel, 2016). In the present study, the addition of quercetin successfully inhibited the carbonyl compounds in the chicken samples (Fig. 5), led to the subsequent decrease of THβC generation (Fig. 6), therefore resulted in the reduced formation of β-CHAs (Fig. 3). For the OWSF treatment, it can not only keep free radicals at a relatively low content but also inhibit the consumption of tryptophan during the frying process of the chicken samples (Fig. 4A). Then, the formation of the carbonyl compounds and the tryptophan Amadori rearrangement products may be reduced. Therefore, the THβC content was significantly reduced in the chicken samples treated with OWSF (Fig. 6), which eventually led to the inhibition of β-CHAs generation (Fig. 3). Based on the different mechanisms for the β-CHAs inhibition of the quercetin and OWSF treatment, the combined use of them achieved a better effect.
4. Conclusion
Two β-CHAs (Harman and Norharman) were detected in the frying process of Chinese braised chicken. The quercetin addition and OWSF treatment significantly inhibited the formation of β-CHAs by inhibiting the consumption of β-CHAs precursors (tryptophan) and the formation of their intermediates (carbonyl compounds and THβC). The inhibition of the lipid oxidation helped to decrease the β-CHAs formation. The combined use of the two treatments had a better effect, with a reduction rate of 47.0% for Harman and 80.2% for Norharman, respectively. The study provides an effective and promising method to control β-CHAs formation in the frying process of braised chicken.
CRediT authorship contribution statement
Xuefei Li: Conceptualization, Methodology, Formal analysis, Data curation, Investigation, Writing – original draft. Zili Yang: Methodology, Formal analysis. Jieying Deng: Conceptualization, Validation, Resources, Writing – review & editing, Supervision. Conggui Chen: Resources. Baocai Xu: Resources. Peijun Li: Conceptualization, Validation, Resources, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Key R&D Program of China [grant no. 2019YFC1605902], the National Natural Science Foundation of China [grant no. 32172235] and the Major Science and Technology Program of Anhui, China [grant no. 2021d06050001].
Contributor Information
Jieying Deng, Email: dengjieying@hfut.edu.cn.
Peijun Li, Email: lipeijun@hfut.edu.cn.
Data availability
Data will be made available on request.
References
- Al-Khusaibi M., Gordon M.H., Lovegrove J.A., Niranjan K. Frying of potato chips in a blend of canola oil and palm olein: changes in levels of individual fatty acids and tocols. Int. J. Food Sci. Technol. 2012;47(8):1701–1709. doi: 10.1111/j.1365-2621.2012.03024.x. [DOI] [Google Scholar]
- Andres S., Pevny S., Ziegenhagen R., Bakhiya N., Schafer B., Hirsch-Ernst K.I., Lampen A. Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 2018;62(1) doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- Berardo A., De Maere H., Stavropoulou D.A., Rysman T., Leroy F., De Smet S. Effect of sodium ascorbate and sodium nitrite on protein and lipid oxidation in dry fermented sausages. Meat Sci. 2016;121:359–364. doi: 10.1016/j.meatsci.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Boots A.W., Haenen G.R., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585(2–3):325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Bulanda S., Janoszka B. Consumption of thermally processed meat containing carcinogenic compounds (polycyclic aromatic hydrocarbons and heterocyclic aromatic amines) versus a risk of some cancers in humans and the possibility of reducing their formation by natural food additives—a literature review. Int. J. Environ. Res. Publ. Health. 2022;19(8):4781. doi: 10.3390/ijerph19084781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Kao T., Zhang D., Wang Z., Inbaraj B.S., Hsu K.Y., Chen B.H. Application of quechers coupled with HPLC-DAD-ESI-MS/MS for determination of heterocyclic amines in commercial meat products. Food Anal. Methods. 2018;11(11):3243–3256. doi: 10.1007/s12161-018-1302-2. [DOI] [Google Scholar]
- Cheng Y., Yao M., Zhu Z., Dong X., Ali Khan I., Huang J., Zhou X., Huang M., Zhou G. Content, causes and analysis of heterocyclic amines in Chinese traditional braised chicken. Food Addit. Contam. 2019;36(7):1032–1041. doi: 10.1080/19440049.2019.1615136. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Yu Y., Wang C., Zhu Z., Huang M. Inhibitory effect of sugarcane (Saccharum officinarum L.) molasses extract on the formation of heterocyclic amines in deep-fried chicken wings. Food Control. 2021;119 doi: 10.1016/j.foodcont.2020.107490. [DOI] [Google Scholar]
- Ding X., Zhang D., Liu H., Wang Z., Hui T. Chlorogenic acid and epicatechin: an efficient inhibitor of heterocyclic amines in charcoal roasted lamb meats. Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130865. [DOI] [PubMed] [Google Scholar]
- Dong H., Xian Y., Li H., Bai W., Zeng X. Potential carcinogenic heterocyclic aromatic amines (HAAs) in foodstuffs: formation, extraction, analytical methods, and mitigation strategies. Compr. Rev. Food Sci. Food Saf. 2020;19(2):365–404. doi: 10.1111/1541-4337.12527. [DOI] [PubMed] [Google Scholar]
- Estevez M. Protein carbonyls in meat systems: a review. Meat Sci. 2011;89(3):259–279. doi: 10.1016/j.meatsci.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Feng Y., Xu Y., Li W., Chen S., Su Z., Xi L., Li G. Improved enrichment and analysis of heterocyclic aromatic amines in thermally processed foods by magnetic solid phase extraction combined with HPLC-MS/MS. Food Control. 2022;137 doi: 10.1016/j.foodcont.2022.108929. [DOI] [Google Scholar]
- Gibis M. Heterocyclic aromatic amines in cooked meat products: causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2016;15(2):269–302. doi: 10.1111/1541-4337.12186. [DOI] [PubMed] [Google Scholar]
- Guo H., Wang Z., Pan H., Li X., Chen L., Rao W., Gao Y., Zhang D. Effects of traditional Chinese cooking methods on formation of heterocyclic aromatic amines in lamb patties. Food Sci. Biotechnol. 2014;23(3):747–753. doi: 10.1007/s10068-014-0101-9. [DOI] [Google Scholar]
- Haskaraca G., Demirok E., Kolsarıcı N., Öz F., Özsaraç N. Effect of green tea extract and microwave pre-cooking on the formation of heterocyclic aromatic amines in fried chicken meat products. Food Res. Int. 2014;63:373–381. doi: 10.1016/j.foodres.2014.04.001. [DOI] [Google Scholar]
- Heijnen C.G.M., Haenen G.R.M.M., Minou Oostveen R., Stalpers E.M., Bast A. Protection of flavonoids against lipid peroxidation: the structure activity relationship revisited. Free Radic. Res. 2009;36(5):575–581. doi: 10.1080/10715760290025951. [DOI] [PubMed] [Google Scholar]
- Herraiz T. Tetrahydro-beta-carboline-3-carboxylic acid compounds in fish and meat: possible precursors of co-mutagenic beta-carbolines norharman and harman in cooked foods. Food Addit. Contam. 2000;17(10):859–866. doi: 10.1080/026520300420439. [DOI] [PubMed] [Google Scholar]
- Islam J., Shirakawa H., Nguyen T.K., Aso H., Komai M. Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Biosci. 2016;13:56–59. doi: 10.1016/j.fbio.2015.12.006. [DOI] [Google Scholar]
- Jia W., Guo A., Zhang R., Shi L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023;404 doi: 10.1016/j.foodchem.2022.134541. Forth coming. [DOI] [PubMed] [Google Scholar]
- Keskekoglu H., Uren A. Inhibitory effects of pomegranate seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs after cooking by four different methods. Meat Sci. 2014;96(4):1446–1451. doi: 10.1016/j.meatsci.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Khan A., Zou Y., Zongshuai Z., Xu W., Wang D., Huang M. Heterocyclic amines in cooked meat products, shortcomings during evaluation, factors influencing formation, risk assessment and mitigation strategies. Meat Sci. 2022;184 doi: 10.1016/j.meatsci.2021.108693. [DOI] [PubMed] [Google Scholar]
- Khan I.A., Luo J., Shi H., Zou Y., Khan A., Zhu Z., Xu W., Wang D., Huang M. Mitigation of heterocyclic amines by phenolic compounds in allspice and perilla frutescens seed extract: the correlation between antioxidant capacities and mitigating activities. Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130845. [DOI] [PubMed] [Google Scholar]
- Kikugawa K. Involvement of free radicals in the formation of heterocyclic amines and prevention by antioxidants. Cancer Lett. 1999;143(2):123–126. doi: 10.1016/S0304-3835(99)00140-8. [DOI] [PubMed] [Google Scholar]
- Lin Q., Han L., Liu G., Cheng W., Wang L. A preliminary study on the formation pathways of glycated phosphatidylethanolamine of food rich in phospholipid during the heat-processing. RSC Adv. 2018;8(21):11280–11288. doi: 10.1039/C8RA01072B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X.W., Zhang Y.B., Sun Y.Y., Wang Y., Pan D.D., Cao J.X. The change of volatile compounds of two kinds of vinasse-cured ducks during processing. Poultry Sci. 2018;97(7):2607–2617. doi: 10.3382/ps/pey109. [DOI] [PubMed] [Google Scholar]
- Ma R., Gao T., Song L., Zhang L., Jiang Y., Li J., Zhang X., Gao F., Zhou G. Effects of oil-water mixed frying and pure-oil frying on the quality characteristics of soybean oil and chicken chop. Food Sci. Technol. 2016;36(2):329–336. doi: 10.1590/1678-457X.0092. [DOI] [Google Scholar]
- Meurillon M., Engel E. Mitigation strategies to reduce the impact of heterocyclic aromatic amines in proteinaceous foods. Trends Food Sci. Technol. 2016;50:70–84. doi: 10.1016/j.tifs.2016.01.007. [DOI] [Google Scholar]
- Pfau W., Skog K. Exposure to beta-carbolines norharman and harman. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2004;802(1):115–126. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Rönner B., Lerche H., Bergmüller W., Freilinger C., Severin T., Pischetsrieder M. Formation of tetrahydro-β-carbolines and β-carbolines during the reaction of L-tryptophan with D-glucose. J. Agric. Food Chem. 2000;48(6):2111–2116. doi: 10.1021/jf991237l. [DOI] [PubMed] [Google Scholar]
- Selani M.M., Contreras-Castillo C.J., Shirahigue L.D., Gallo C.R., Plata-Oviedo M., Montes-Villanueva N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011;88(3):397–403. doi: 10.1016/j.meatsci.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Tengilimoglu-Metin M.M., Hamzalioglu A., Gokmen V., Kizil M. Inhibitory effect of hawthorn extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Food Res. Int. 2017;99(Pt 1):586–595. doi: 10.1016/j.foodres.2017.06.044. [DOI] [PubMed] [Google Scholar]
- Totsuka Y., Ushiyama H., Ishihara J., Sinha R., Goto S., Sugimura T., Wakabayashi K. Quantification of the co-mutagenic β-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143(2):139–143. doi: 10.1016/S0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li J., Li K., Li C. Effects of turmeric on reducing heterocyclic aromatic amines in Chinese tradition braised meat products and the underlying mechanism. Food Sci. Nutr. 2021;9(10):5575–5582. doi: 10.1002/fsn3.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hui T., Zhang Y.W., Liu B., Wang F.L., Li J.K., Cui B.W., Guo X.Y., Peng Z.Q. Effects of frying conditions on the formation of heterocyclic amines and trans fatty acids in grass carp (Ctenopharyngodon idellus) Food Chem. 2015;167:251–257. doi: 10.1016/j.foodchem.2014.06.109. [DOI] [PubMed] [Google Scholar]
- Xiao Q., Xu M., Xu B., Chen C., Deng J., Li P. Combined effect of high-pressure processing with spice extracts on quality of low-salt sausage during refrigerated storage. Foods. 2021;10(11):2610. doi: 10.3390/foods10112610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Thompson A., Chen B. Dynamic changes of 3-MCPD esters and glycidyl esters contents as well as oil quality during repeated deep-frying. Lebensm. Wiss. Technol. 2022;153 doi: 10.1016/j.lwt.2021.112568. [DOI] [Google Scholar]
- Xu Y., Li H., Liang J., Ma J., Yang J., Zhao X., Zhao W., Bai W., Zeng X., Dong H. High-throughput quantification of eighteen heterocyclic aromatic amines in roasted and pan-fried meat on the basis of high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry. Food Chem. 2021;361 doi: 10.1016/j.foodchem.2021.130147. [DOI] [PubMed] [Google Scholar]
- Xue C., Chen Q., He Z., Wang Z., Qin F., Yang T., Chen J., Zeng M. Non-precursors amino acids can inhibit beta-carbolines through free radical scavenging pathways and competitive inhibition in roast beef patties and model food systems. Meat Sci. 2020;169 doi: 10.1016/j.meatsci.2020.108203. [DOI] [PubMed] [Google Scholar]
- Yan Y., Zhou Y.Q., Huang J.J., Wan X., Zeng M.M., Chen J., Li W.W., Jiang J. Influence of soybean isolate on the formation of heterocyclic aromatic amines in roasted pork and its possible mechanism. Food Chem. 2022;369 doi: 10.1016/j.foodchem.2021.130978. [DOI] [PubMed] [Google Scholar]
- Yao M., Khan I.A., Cheng Y., Ang Y., Zhou X., Huang M. Effects of cooking methods and tea marinades on the formation of heterocyclic amines and benzo[a]pyrene in grilled drumsticks. J. Food Protect. 2020;83(2):365–376. doi: 10.4315/0362-028X.JFP-19-084. [DOI] [PubMed] [Google Scholar]
- Yao Y., Peng Z.Q., Shao B., Wan K.H., Wang F.L., Zhang Y.W., Li J.K., Hui T. Effects of frying and boiling on the formation of heterocyclic amines in braised chicken. Poultry Sci. 2013;92(11):3017–3025. doi: 10.3382/ps.2013-03216. [DOI] [PubMed] [Google Scholar]
- Zamora R., Alcon E., Hidalgo F.J. Effect of lipid oxidation products on the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) in model systems. Food Chem. 2012;135(4):2569–2574. doi: 10.1016/j.foodchem.2012.06.062. [DOI] [PubMed] [Google Scholar]
- Zeng M., Wang J., Zhang M., Chen J., He Z., Qin F., Xu Z., Cao D., Chen J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018;239:111–118. doi: 10.1016/j.foodchem.2017.06.097. [DOI] [PubMed] [Google Scholar]
- Zeng M., Zhang M., He Z., Qin F., Tao G., Zhang S., Gao Y., Chen J. Inhibitory profiles of chilli pepper and capsaicin on heterocyclic amine formation in roast beef patties. Food Chem. 2017;221:404–411. doi: 10.1016/j.foodchem.2016.10.061. [DOI] [PubMed] [Google Scholar]
- Zhang L., Du H., Zhang P., Kong B., Liu Q. Heterocyclic aromatic amine concentrations and quality characteristics of traditional smoked and roasted poultry products on the northern Chinese market. Food Chem. Toxicol. 2020;135 doi: 10.1016/j.fct.2019.110931. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang Q., Wang Z., Chen Q., Sun F., Xu M., Kong B. Influence of different ratios of sucrose and green tea leaves on heterocyclic aromatic amine formation and quality characteristics of smoked chicken drumsticks. Food Control. 2022;133 doi: 10.1016/j.foodcont.2021.108613. [DOI] [Google Scholar]
- Zhang X., Yu Z., Yousaf K., Chen K. Experiment and amount optimization analysis of oil-water mixture frying machine based on FLUENT. Adv Comput. Sci. Res. 2016;58:257–261. doi: 10.2991/msota-16.2016.56. [DOI] [Google Scholar]
- Zhu Q., Zhang S., Wang M., Chen J., Zheng Z.P. Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) in roast beef patties and in chemical models. Food Funct. 2016;7(2):1057–1066. doi: 10.1039/c5fo01055a. (c) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.