Abstract
The cytokine/chemokine expression signature of a 60-year-old African American male with relapsing-remitting multiple sclerosis (RRMS) was analyzed using patient blood samples obtained from two separate visits to the clinic. Thirty-six different cytokines, chemokines, and growth factors were detected in the plasma of the RRMS patient using a multiplexed bead-based immunoassay. Results indicated that at least ten of these factors with a concentration of > 100 pg/mL are identified as pro-inflammatory. Calpain inhibition led to an anti-inflammatory effect, as indicated by a decrease in expression of pro-inflammatory cytokines/chemokines such as GM-CSF, IFNγ, and IL-17A, and a relative increase in two of the anti-inflammatory cytokines (IL-13 and IL-4) in the peripheral blood mononuclear cells activated with anti-CD3/CD28. Overall, these results suggest that the unique cytokine/chemokine pattern observed in the plasma of the RRMS patient can be used as a prognostic marker and calpain inhibition may be used as a novel therapeutic strategy for treating excessive inflammatory response specific to RRMS patients.
Keywords: Relapsing-remitting multiple sclerosis, Calpain, Cytokines, Chemokines, Plasma, Peripheral blood mononuclear cells
Introduction
Multiple sclerosis (MS) is a chronic autoimmune demyelinating disease associated with neurodegeneration affecting the central nervous system (CNS) leading to a gradual decrease in neurologic and cognitive function [1]. Globally, approximately 2.5 million individuals have been diagnosed with MS with more than 400,000 cases documented in the United States alone. Twice as many women as men develop MS with the majority diagnosed between the ages of 20 and 40 [2]. Although the exact pathogenesis is poorly understood, substantial evidence points to a combination of both environmental and genetic factors for influencing the development of MS [2].
MS has been classified into four major phenotypes: (a) clinically-isolated syndrome (CIS), (b) relapsing-remitting (RRMS), (c) secondary progressive (SPMS), and (d) primary progressive (PPMS) based on disease progression stage [3]. Of these, RRMS is the most widely prevalent phenotype with around 85% of the initial MS cases diagnosed [4], consisting, in general, of episodes of acute neurologic dysfunction (relapse) followed by partial or complete recovery (remission). After a period of time, the disease no longer goes into remission, leading to an aggressive MS phenotype known as SPMS [4]. Clinically-isolated syndrome usually refers to an initial incident of neurological dysfunction due to a single/multifocal CNS lesion which may or may not progress to active MS whereas the PPMS phenotype is characterized by the gradual decline in neurologic function from the onset of symptoms with an absence of early relapses or remissions. This particular case report focuses on a 60-year old male RRMS patient and the findings presented show changes in cytokine/chemokine expression associated with his clinical condition.
A major hallmark of MS pathology is the increased infiltration of myelin-specific T cells into the CNS. The Th1/Th17 cells, along with pro-inflammatory cytokines such as IFNγ, IL-1, IL-6, IL-12, IL-21, and IL-23 play prominent roles compared to the Th2/Treg cells in the active phase of MS. In contrast, the Th2/Treg cells and associated anti-inflammatory cytokines such as IL-4, IL-5, IL-10, and IL-13 tend to dominate the remission period in MS [5]. The onset of MS also involves an autoimmune inflammatory response directed specifically against myelin and is mediated by a range of cytokines and chemokines [1]. Hence, the main focus of MS therapies has been to target this inflammation with immunomodulatory strategies, and therefore the majority of drugs developed are immunomodulatory and do not ameliorate the disease progression [6–9]. This is further strengthened by a strong link between the neuroinflammation and neurodegeneration as well as different phenotypes in MS [10].
A number of animal and human studies have shown that calpain, a calcium-activated neutral protease, plays a critical role in MS pathophysiology, especially in myelin breakdown, cell death, and axonal degeneration [11–13]. Thus, neuron death with subsequent axonal degeneration promotes disability, and due to the complex pathology, development of an effective therapy to prevent both inflammation and neurodegeneration in MS patients has been difficult. One study also showed increased calpain expression and activity in a set of activated T cells localized in and around demyelinating lesions [6]. Calpeptin, a widely used calpain inhibitor has been shown to improve outcome in Experimental Autoimmune Encephalomyelitis (EAE), an animal model of MS [14], as well as modulate cytokine/chemokine expression profile in PBMCs of MS patients [15]. Therefore, RRMS patient PBMCs stimulated with anti-CD3/CD28 were treated with calpeptin, and the cytokine/chemokine profiles for both these supernatants and the patient’s plasma samples were analyzed. Results from the multiplex immunoassay revealed a unique cytokine and chemokine expression signature in this RRMS patient following calpain inhibition in PBMCs.
Materials and Methods
Patient Profile
The patient is a 61-year-old black male who was diagnosed with RRMS in 1999. His medication history includes the following (with time periods being approximate)—Avonex (September 2002 to February 2003 and April 2005 to June 2009), Amantadine (December 2005 to August 2006), Glatiramer (June 2010 to April 2017), and Dalfampridine (September 2012 to December 2013). The patient was started on Tysabri by October 2017.
Relevant medical history includes psoriasis, vitamin B12 deficiency, hypertension, and hypercholesterolemia. It also shows no admissions, visits for infectious symptoms or antibiotics prescribed during this time period as well as the several months preceding January 2015 and after January 2016. The two blood samples from this patient were taken July 10, 2015 and January 13, 2016; separated by a 6-month time gap and there was no clear relapse or remission between the two visits. Apparently, the patient was found to be stable at both the visits as he was under treatment with various MS-specific medications during the study.
Sample Collection
Whole blood was obtained from two separate clinic visits following informed consent according to our approved IRB protocol (Pro00028348). Samples were analyzed separately as described below. After collecting 10 mL of whole blood in heparinized tubes, 2 mL of the blood sample was used for plasma collection and the remaining 8 mL for isolation of peripheral blood mononuclear cells (PBMCs). Plasma was separated from whole blood by centrifugation at 7000 rpm for 2 min at 4 °C and stored in − 80 °C until used for further analysis.
PBMC Isolation, Stimulation and Calpeptin Treatment
PBMCs from whole blood were isolated using a laboratory standardized procedure, as described previously [16]. Ficoll separation was performed with centrifugation at 2000 rpm for 25 min with the brake off at room temperature, and the PBMC layer was collected. This PBMC layer was washed twice with HBSS, and then washed once with complete RPMI medium at a sample to media ratio of 1:5. For stimulation of PBMCs, 2 × 106 cells/well were treated in duplicate with 10 μg/mL of anti-CD3 and 5 μg/mL of anti-CD28 antibodies (Becton Dickinson, Franklin Lakes, NJ) in the presence or absence of 10 μM calpeptin dissolved in dimethyl sulfoxide (DMSO) for 48 h. The final DMSO concentration was less than 0.01%. Treated cells were centrifuged at 1200 rpm for 10 min at 4 °C, and supernatant was collected. The collected supernatants were stored at − 80 °C until further experimentation. Supernatant was also collected from the unstimulated PBMC fractions and were used as controls.
Cytokine and Chemokine Profiling
The plasma and the activated or unactivated PBMC supernatants were assayed using the Human Cytokine Array/Chemokine Array 42-Plex with IL-18 Discovery Assay (HD42, Eve Technologies, Calgary, Canada) with a Bio-Plex 200 system. This cytokine array (based on Millipore’s MILLIPLEX technology) allows the quantification of 42 cytokines, chemokines, and growth factors (EGF, Eotaxin-1, FGF-2, FLT-3L, Fractalkine, G-CSF, GM-CSF, GRO(pan), IFNα2, IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, MCP-1, MCP-3, MDC, MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, RANTES, sCD40L, TGFα, TNFα, TNFβ, VEGF-A). All samples were tested in duplicate, and cytokines with out-of-range (OOR) readings that were below their detection limits were recorded as 0 pg/mL for data analysis.
Statistical Analysis
Error bars were calculated based on mean ± SD using both the duplicate values (technical replicates) for each cytokine/chemokine. Significant differences between groups were determined by two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests using GraphPad Prism 7.03 software.
Results
For studying the cytokinome landscape of this particular RRMS patient, the levels of 42 well-known cytokines/chemokines in plasma and PBMC supernatant (both with and without calpain inhibition) were analyzed using a multiplex bead assay.
Cytokine and Chemokine Levels in RRMS Patient Plasma
Plasma cytokine/chemokine expression profiling indicated around 36 cytokines/chemokines at detectable limits in plasma of the RRMS patient for at least one of the visits (Table 1). Platelet-derived growth factor (PDGF-BB) (first visit, 2551.68 pg/mL; second visit, 1574.35 pg/mL), chemokine (C-C motif) ligand 5 or RANTES (first visit, 668.38 pg/mL; second visit, 582.83 pg/mL), GRO-α (first visit, 347.1 pg/mL; second visit, 97.18 pg/mL), IL-1α (first visit, 288.57 pg/mL; second visit, 8.94 pg/mL) and sCD40L (first visit, 181.4 pg/mL; second visit, 68.46 pg/mL) were the top five cytokines/chemokines detected in the plasma of the RRMS patient and all of these were decreased in samples from the second visit as compared to the first visit (Fig. 1).
Table 1.
List of cytokines and chemokines detected in plasma samples of a RRMS patient during the two separate visits
| Name of cytokine/chemokine | Concentration (pg/mL) | |
|---|---|---|
| First visit | Second visit | |
| PDGF-BB | 2551.68 | 1574.35 |
| RANTES | 668.38 | 582.83 |
| GRO-α | 347.1 | 97.18 |
| IL-1α | 288.57 | 8.94 |
| sCD40L | 181.4 | 68.46 |
| PDGF-AA | 177.01 | 61.66 |
| MDC | 137.91 | 360.81 |
| IP-10 | 125.17 | 369.87 |
| IFNα2 | 51.69 | 121.16 |
| FGF-2 | 44.1 | 112.43 |
| EGF | 34.75 | 18.62 |
| MCP-1 | 23.44 | 72.69 |
| Eotaxin-1 | 16.47 | 40.25 |
| IL-1β | 11.24 | 3.65 |
| Fractalkine | 10.81 | 13.85 |
| MIP-1β | 10.64 | 45.04 |
| TNF-α | 6.18 | 12.67 |
| G-CSF | 5.25 | 7.12 |
| IL-1RA | 2.7 | 2.23 |
| IFNγ | 2.16 | 14.15 |
| GM-CSF | 1.77 | 6.09 |
| IL-3 | 1.38 | 1 |
| IL-6 | 1.21 | 6.59 |
| IL-15 | 1.11 | 1.83 |
| IL-9 | 0.42 | 0.46 |
| IL-13 | 0.32 | 3.6 |
| IL-5 | 0.13 | 0.29 |
| IL-12p70 | 0.12 | 44.07 |
| TNF-β | 0.11 | 18.87 |
| IL-17A | 0.07 | 7.93 |
| IL-12p40 | OOR < | 6.72 |
| IL-8 | OOR < | 4.5 |
| MCP-3 | OOR < | 20.21 |
| IL-2 | OOR < | 1.06 |
| MIP-1α | OOR < | 17.91 |
| VEGF-A | OOR < | 120.59 |
Fig. 1.
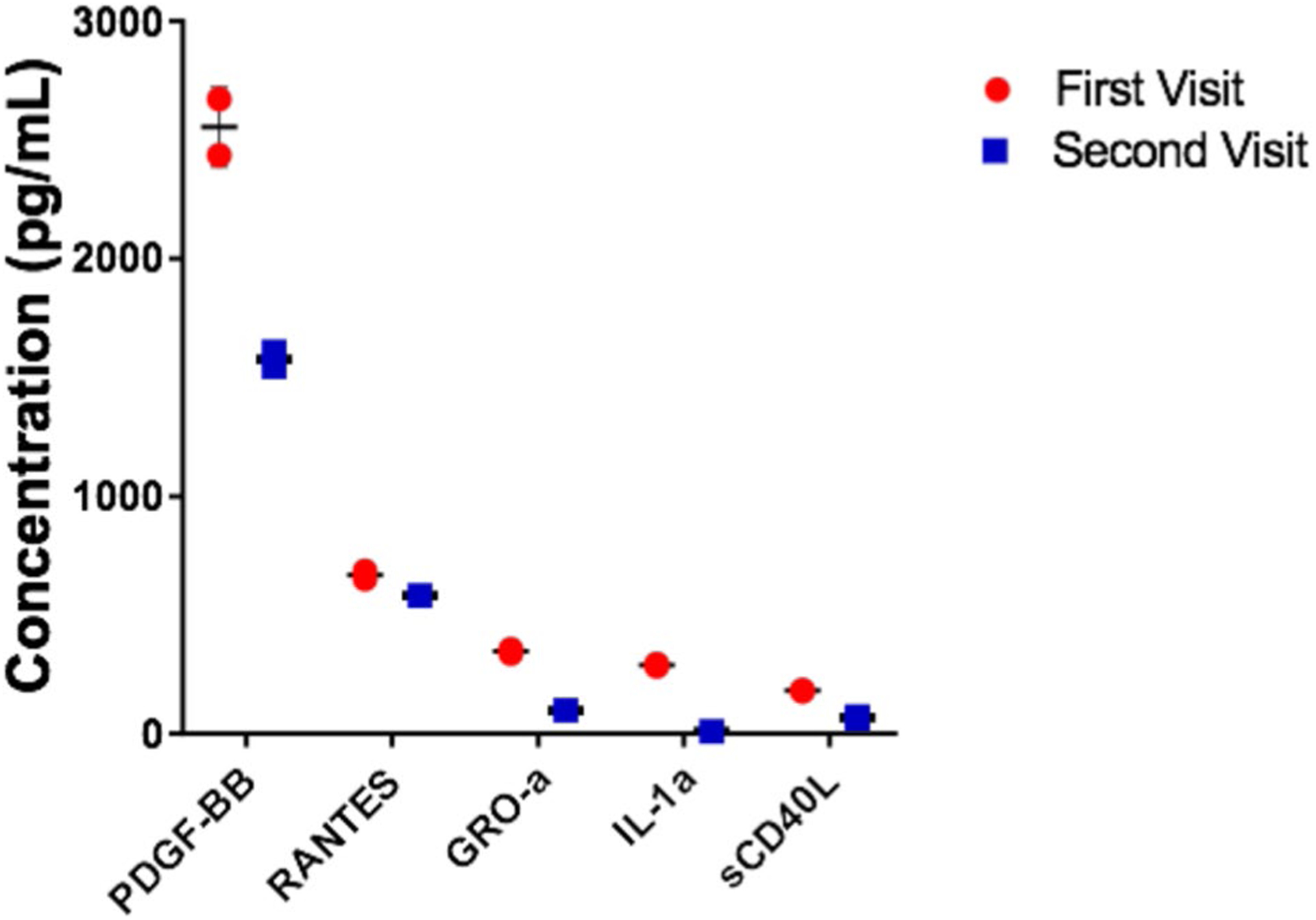
Cytokine and chemokine expression profile in plasma samples of a RRMS patient during both the visits. The top five cytokines/chemokines expressed in the RRMS patient plasma during both the visits. Values are mean ± SD (n = 2)
In spite of a small drop in their expression, some of the cytokines/chemokines such as PDGF-BB (first visit, 2551.68 pg/mL; second visit, 1574.35 pg/mL) and RANTES (first visit, 668.38 pg/mL; second visit, 582.83 pg/mL) were detected at consistent levels during both the visits. In contrast, few of the cytokines/chemokines in the expression profile were detected at higher levels in just one of the visits. Higher levels of GRO-α (first visit, 347.1 pg/mL; second visit, 97.18 pg/mL) and IL-1α (first visit, 288.57 pg/mL; second visit, 8.94 pg/mL) were also detected during the first visit. IP-10 (first visit, 125.17 pg/mL; second visit, 369.87 pg/mL) and IFNα2 (first visit, 51.69 pg/mL; second visit, 121.16 pg/mL) were found to be expressed in a higher concentration during the second visit as compared to the first visit. A group of cytokines/chemokines like IL-12(p40) (6.72 pg/mL), IL-8 (4.5 pg/mL) and VEGF-A (120.59 pg/mL) were also detected only in the second visit suggesting their possible correlation with the later phases of the RRMS time course.
Also, comparison of the expression of few of the cytokines/chemokines with a progressive MS patient showed some of the cytokines/chemokines to be increased in the RRMS patient (IL-1α, IFNα2, Eotaxin-1 and Fractalkine) and at least one of them in the progressive MS patient (MDC) in both the visits.
The increased expression of certain cytokines/chemokines and the difference in the expression profile in comparison with that of a progressive MS patient (Table 2) strongly suggest their specific involvement in maintenance of the RRMS disease phenotype. The dynamic nature of cytokine/chemokine expression differing among both the visits shows the temporal expression of these cytokines might be responsible for worsening and/or improvement of symptoms corresponding with the remission/relapse phases.
Table 2.
Comparison of few cytokines and chemokines detected in plasma samples of a RRMS patient and a progressive MS patient during the two separate visits
| Name of cytokine/chemokine | Concentration (pg/mL) | |||
|---|---|---|---|---|
| RRMS | Progressive MS | |||
| First visit | Second visit | First visit | second visit | |
| IL-1α | 288.57 | 8.94 | 22.02 | 19.15 |
| MDC | 137.91 | 360.81 | 638.32 | 669.02 |
| IFNα2 | 51.69 | 121.16 | OOR < | OOR < |
| Eotaxin-1 | 16.47 | 40.25 | 7.6 | 8.48 |
| Fractalkine | 10.81 | 13.85 | 3.2 | OOR < |
Effect of Calpeptin Treatment on Cytokine and Chemokine Release in Anti-CD3/CD28-Stimulated PBMC Supernatant
PBMC cytokine profile could be a better indicator of the disease-associated cytokine pattern compared to a plasma-based profile. Various studies have shown stimulated PBMCs to be a consistent model for studying disease-associated cytokine/chemokine expression. Many of the cytokines/chemokines that were detected in the anti-CD3/CD28 stimulated PBMC with or without calpeptin treatment were almost similar or relatively higher levels as in the control PBMC supernatant fractions (Table 3), thus making it difficult to pinpoint the effect of calpain inhibition on their individual expression in a single patient. However, calpain inhibition in anti-CD3/CD28 stimulated PBMCs clearly showed a marked alteration of a group of MS-associated cytokines such as GM-CSF, IFNγ, IL-13, IL-17A, IL-1α, IL-1β, IL-4 and MIP-1β.
Table 3.
List of cytokines and chemokines expressed in PBMCs of a RRMS patient during the two separate visits
| Name of cytokine/chemokine | Concentration (pg/mL) | |||||
|---|---|---|---|---|---|---|
| Control | Anti-CD3/CD28 | Anti-CD3/CD28 + CP | ||||
| First visit | Second visit | First visit | Second visit | First visit | Second visit | |
| EGF | 82.39 | 180.12 | 94.12 | 300.08 | 112.27 | 272.9 |
| FGF-2 | OOR < | 42.43 | OOR < | 70.62 | 129.81 | 68.25 |
| FLT3L | OOR < | 9.81 | OOR < | 67.01 | OOR < | 66.33 |
| Fractalkine | OOR < | 29.44 | 0.86 | 26.24 | 293.4 | 39.06 |
| G-CSF | 0.25 | 2.43 | 0.12 | 374.54 | 74.33 | 375.41 |
| GM-CSF | 5.14 | 2.28 | 141.13 | 1707.13 | 182.51 | 1413.79 |
| GRO-α | 665.59 | 3465.25 | 636.01 | 10175.14 | 760.06 | 8832.99 |
| IFNα2 | OOR < | 1.26 | 2.57 | 2.69 | 131.35 | 3.59 |
| IFNγ | OOR < | 2.06 | 82.59 | 12233.24 | 98.29 | 11802.46 |
| IL-10 | OOR < | 56.04 | 13 | 6229.1 | 13.36 | 5782.19 |
| IL-12p40 | OOR < | 5.64 | OOR < | 8.41 | 77.86 | 7.43 |
| IL-12p70 | OOR < | OOR < | 1.26 | 6.41 | 36.31 | 10.89 |
| IL-13 | OOR < | 0.03 | 0.70 | 2872.78 | 15.32 | 2937.46 |
| IL-15 | OOR < | 1.25 | OOR < | 2.58 | 19.39 | 3.62 |
| IL-17A | OOR < | 4.31 | 47.38 | 1481.38 | 70.37 | 1216.88 |
| IL-18 | OOR < | 23.71 | OOR < | 40.14 | OOR < | 40.85 |
| IL-1α | 9.85 | 1.79 | 29.9 | 1953.87 | 147.78 | 1199.5 |
| IL-1β | 3.95 | 5.12 | 10.4 | 2922.84 | 17.41 | 2546.77 |
| IL1RA | OOR < | 326.32 | OOR < | 2868.95 | 24.08 | 3024.76 |
| IL-2 | OOR < | 5.03 | 68.14 | 3.04 | 58.55 | 7.9 |
| IL-3 | 1.72 | OOR < | 3.5 | 46.13 | 13.51 | 33.42 |
| IL-4 | OOR < | 1.99 | 2.34 | 6.61 | 30.48 | 10.9 |
| IL-5 | OOR < | 0.42 | 14.91 | 1928.04 | 16.87 | 2037.28 |
| IL-6 | 18.86 | 7.48 | 30.93 | 1751.54 | 57.34 | 1803.72 |
| IL-7 | OOR < | 1.11 | OOR < | 4.88 | 3.38 | 4.32 |
| IL-8 | 3400.23 | 3705.31 | 2566.9 | 6951.18 | 3349.6 | 6212.4 |
| IL-9 | 0.21 | 0.32 | 3.97 | 387.37 | 10.26 | 355.93 |
| IP-10 | 5.89 | 334.69 | 131.42 | 11356.7 | 274.43 | 10994.14 |
| MCP-1 | 1759.08 | 2139.22 | 483.01 | 2345.07 | 778.83 | 2114.02 |
| MCP-3 | OOR < | 128.01 | OOR < | 12859.54 | OOR < | 11766.62 |
| MDC | 255.85 | 1196.56 | 161.47 | 6297.77 | 281.54 | 5652.95 |
| MIP-1α | 109.83 | 45.97 | 275.69 | 1692.36 | 324.32 | 1805.45 |
| MIP-1β | 42.13 | 12.61 | 360.49 | 10759.25 | 386.2 | 10168.86 |
| PDGF-AA | 255.38 | 890.95 | 359.73 | 1084.4 | 394.32 | 1105.17 |
| PDGF-BB | 1046.81 | 29230.55 | 483.98 | OOR > | 497.1 | 39065.12 |
| RANTES | OOR > | 2572.38 | OOR > | OOR > | OOR > | OOR > |
| sCD40L | 97.83 | 107.71 | 99.38 | 129.77 | 71.9 | 110.99 |
| TGF-α | OOR < | 3.75 | OOR < | 79.8 | 4.35 | 86.46 |
| TNF-α | 59.25 | 19.65 | 508.67 | 2075.17 | 652.95 | 2192.85 |
| TNF-β | OOR < | 1.64 | 17.68 | 940.8 | 97.96 | 1223.87 |
| VEGF-A | OOR < | 99.73 | OOR < | 7.82 | 158.29 | 9.35 |
A clear difference was also seen with almost all the pro-inflammatory cytokines, which decreased only during the second visit while the anti-inflammatory cytokines increased on both the visits following calpain inhibition. This is striking as calpain inhibition decreased the levels of several pro-inflammatory cytokines like GM-CSF (1707.13 pg/mL vs. 1413.79 pg/mL), IFNγ (12,233.24 pg/mL vs. 11,802.46 pg/mL), IL-17A (1481.38 pg/mL vs. 1216.88 pg/mL), IL-1α (1953.87 pg/mL vs. 1199.5 pg/mL), and IL-1β (2922.84 pg/mL vs. 2546.77 pg/mL). Similarly, the inflammatory chemokine MIP-1β was also decreased (10,759.25 pg/mL vs. 10,168.86 pg/mL) in PBMC supernatant of MS samples as compared to anti-CD3/CD28 stimulated PBMC control, but only during the second visit (Fig. 2). By contrast, calpain inhibition increased the levels of two anti-inflammatory cytokines IL-13 (first visit, 0.7 pg/mL vs. 15.32 pg/mL; second visit, 2872.78 pg/mL vs. 2937.46 pg/mL) and IL-4 (first visit, 2.34 pg/mL vs. 30.48 pg/mL; second visit, 6.61 pg/mL vs. 10.9 pg/mL) in both the visits (Fig. 2). In addition, all of the cytokines/chemokines except IL-1α which was highly expressed in plasma in both visits like PDGF-BB, RANTES, GRO-α and sCD40L were highly expressed in all the three different PBMC supernatant groups.
Fig. 2.
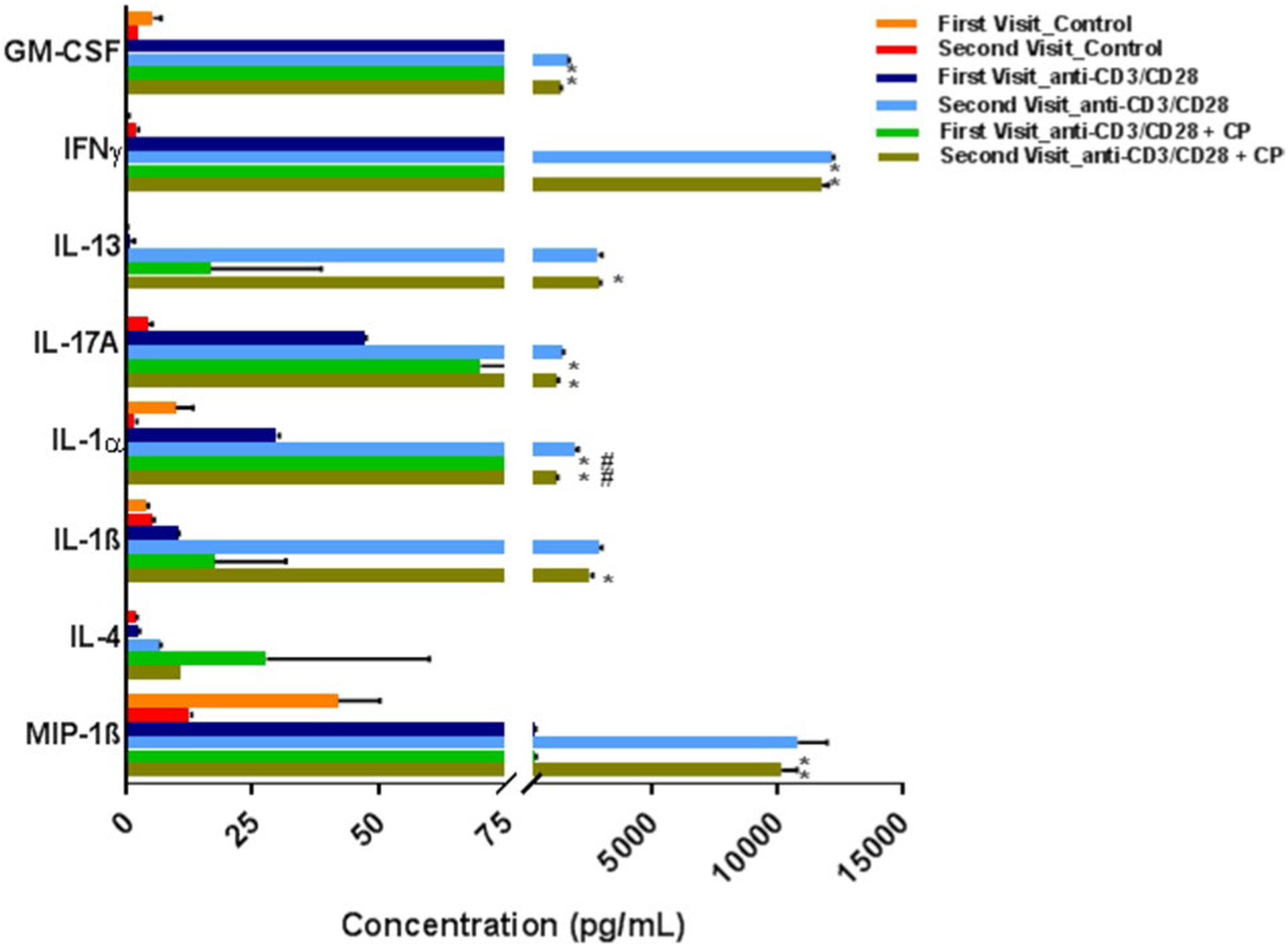
Cytokine and chemokine expression profile in stimulated PBMC supernatant of a RRMS patient during both the visits. The major cytokines/chemokines that were found to be significantly affected following calpain inhibition. Values are mean ± SD (n = 2). *p < 0.05 and #p < 0.05 denotes the anti-CD3/CD28 + CP group, compared with the PBMC control group and anti-CD3/CD28 control group respectively by two-way ANOVA followed by Tukey’s multiple-comparisons post-test. Few of the error bars are clipped due to axis limit
While calpain inhibition decreased inflammatory cytokines/chemokines, increases in anti-inflammatory cytokines in patient PBMCs were also found. For example, six pro-inflammatory cytokines/chemokines were decreased in this RRMS patient sample tested following calpain inhibition. Similarly, two of the anti-inflammatory cytokines were increased by calpeptin inhibition. However, many of the cytokines/chemokines were not altered following calpain inhibition, suggesting the complexity of the disease. Overall, this case study suggests that calpain inhibition may have an anti-inflammatory effect, leading to neuroprotection in RRMS patient.
Discussion
Cytokines and chemokines have been found to play a major role in the pathophysiology of MS [1]. Measurement of cytokine expression in stimulated PBMCs seems to be a more rational strategy in lieu of limitations in measuring them in blood components like plasma [17]. Anti-CD3/CD28 stimulation of T cells and the subsequent cytokine/chemokine release profile clearly represent the in vivo immune response to a particular disease [18]. In this study, the expression levels of 42 different cytokines, chemokines, and growth factors were analyzed using a multiplexed bead-based immunoassay in the plasma as well as in the supernatant of calpeptin-treated stimulated PBMCs from a single RRMS patient collected during two different visits to the clinic. Differential expression of 36 different cytokines, chemokines, and growth factors was shown in the plasma of the RRMS patient in at least one of the visits. Different expression profiles were also detected in terms of pro- and anti-inflammatory factors in both visits, indicating the time-dependent expression of the MS-associated cytokines/chemokines in regulating the disease. The cytokine/chemokine expression profile of anti-CD3/CD28 stimulated PBMCs from the RRMS patient showed an overall anti-inflammatory effect due to calpeptin treatment demonstrated by the decrease in the levels of six different pro-inflammatory and increase in two anti-inflammatory cytokines/chemokines. To our knowledge, this is one of the first studies to evaluate the effect of calpain inhibition directly on PBMCs isolated from an RRMS patient and to characterize the unique plasma cytokine/chemokine signature with a strong pro-inflammatory nature. Additionally, these results give insight into the use of plasma cytokinome/chemokinome evaluation using new-age multiplexed bead-based immunoassays for studying RRMS disease course as well as the possible use of calpain inhibition to treat the abnormal inflammatory response that is part of RRMS disease profile.
PDGF-BB, RANTES, GRO-α, IL-1α and sCD40L were detected as the top five highly expressed cytokines/chemokines in the plasma of the RRMS patient tested. PDGF is an important neurotrophic factor that is associated with improved clinical recovery after relapse in MS. Increased concentrations of both PDGFs (PDGF-BB and PDGF-AA) were detected in the plasma of the RRMS patient with PDGF-BB being the highest expressing immune factor in the panel. Higher levels of PDGF concentrations in the CSF of RRMS patients have been associated with improved neuronal plasticity, as demonstrated by an increase in the long-term potentiation in the brains [19]. The same study also found that a decrease in the PDGF levels may correlate with a relapse and in contrast stable RRMS patients may have higher levels of PDGF [19]. Another study showed that an increase in CSF PDGF does not influence radiological markers of disease activity, biochemical markers of neuronal damage like tau protein, and clinical parameters of MS disease progression measured mainly by worsening of the Expanded Disability Status Scale (EDSS) [20]. This strengthens the argument that PDGF acts in a beneficial manner by not modulating disease-associated parameters like inflammatory cytokine expression but rather leads to recovery by increasing long-term potentiation (LTP)-linked neuronal plasticity and other such parameters [20]. This situation, in which there are high levels of pro-inflammatory cytokines in spite of a spike in PDGF levels in these studies [20] as well as in this current patient, suggest that still therapeutic options might be needed to bring down the levels of other pro-inflammatory cytokines/chemokines so as to subvert their harmful effects on MS-linked inflammation.
Results also showed that RANTES was highly expressed in the patient’s plasma during the visits, and calpain inhibition did not show any decrease in their levels in stimulated PBMCs. RANTES has been associated with inflammation and synaptic excitability in MS brains [21]. The involvement of the other highly expressed cytokines/chemokines like GRO-α and sCD40L in MS pathophysiology has been shown in different studies [22–25]. The high levels of these cytokines/chemokines in plasma of this particular patient may indicate their possible functions in the maintenance of RRMS symptoms specific to this patient.
The observed changes in cytokine/chemokine expression can be either due to the action of the different medications or just the disease course. For example, Tysabri has been known to affect the entry of T cells into the CNS and thus may lead to an increase in the peripheral cytokine/chemokine expression. Pro-inflammatory T cells reactive to myelin basic protein can also exist in the blood stream of MS patients contributing to the peripheral cytokine/chemokine production, and could be reflective of the disease. Nonetheless, it remains unclear if Tysabri treatment can completely block the entry of T cells into the CNS. It is also equally possible that some of the cells (e.g., T cells) that did not cross the BBB might also be responsible for the spike in the levels of some of the cytokines/chemokines in plasma of MS patients.
Regardless, of medication history and other factors, it has been observed that the samples received from both the visits showed increased levels of certain inflammatory markers. Also, our main goal was to see if calpain inhibition altered the expression levels of these inflammatory markers in a 6-month period in this particular disease subtype irrespective of the location. A number of earlier studies from our laboratories have established the critical role that calpain plays in demyelination by demonstrating an increase in their expression and activity in animal models of demyelination [26–31]. These studies suggest that calpain inhibition by calpeptin treatment in an animal model of MS improves outcome following the onset of EAE [14]. The three previous studies have also shown that calpain inhibition attenuates inflammatory cytokines released from PBMCs of MS patients [15, 16, 32]. Thus, the PBMC cytokine/chemokine profile of an RRMS patient during two visits to the clinic was examined following calpain inhibition. Results obtained suggest that calpeptin treatment attenuates pro-inflammatory cytokines GM-CSF, IFNγ, IL-17A, IL-1α, IL-1β, and MIP-1β in RRMS patients. Data also suggest that calpeptin treatment increases the expression of anti-inflammatory cytokines IL-13 and IL-4 in anti-CD3/CD28 stimulated patient PBMCs. This outcome of an anti-inflammatory cytokine/chemokine profile following calpeptin treatment in stimulated PBMCs of this RRMS patient strengthens the argument of using calpain inhibition as a possible therapeutic strategy for treatment of MS and RRMS in particular. Interestingly, the decrease in inflammatory cytokine expression on calpain inhibition was observed only in the second visit, indicating a possible link between the time course of the disease and its responsiveness to the calpeptin action. Also, both the anti-inflammatory cytokines IL-13 and IL-4 were increased on both the visits in response to calpeptin treatment.
Increased calpain activation has been demonstrated in MS as well as animals with EAE [12, 27], and calpain has been implicated in cell death and degeneration of axons and myelin in EAE and animals models of Parkinson’s disease [14, 33–35]. Interestingly, calpain inhibitor treatment of these animals (EAE and PD) demonstrated amelioration of inflammation and neurodegeneration and improvement of function [14, 34, 36]. Likewise, such treatment showed reduction of inflammatory responses in MS plasma/serum, while any effect on neurodegenerative processes could not be assessed and may only be realized after these inhibitors are taken into the clinical setting. Nonetheless, studies on MS patients have clearly shown the heterogeneous nature of the cytokine expression in blood samples which can be a rich source for detection of MS-associated biomarkers. Further research on using calpeptin or other calpain inhibitors based on individual cytokine/chemokine profile of patients may help in designing personalized therapeutic strategies for treating RRMS and/or other MS phenotypes.
Acknowledgements
This work was supported in part by funding Veterans Administration (1I01BX002349-01); Department of Neurosurgery, MUSC, and MUSC-CTSA program; and the South Carolina State Spinal Cord Research Fund (SCIRF-2015P-01, SCIRF-2015P-04, SCIRF-2015-I-01, SCIRF-2016 I-03). Contents do not necessarily represent the policy of the SCIRF and do not imply endorsement by the funding agency.
Footnotes
Conflict of interest The authors have no financial conflicts of interest.
References
- 1.Amedei A, Prisco D, D’Elios MM (2012) Multiple sclerosis: the role of cytokines in pathogenesis and in therapies. Int J Mol Sci 13(10):13438–13460 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Tullman MJ (2013) Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Managed Care 19(2 Suppl):S15–S20 [PubMed] [Google Scholar]
- 3.Lublin FD (2014) New multiple sclerosis phenotypic classification. Eur Neurol 72(Suppl 1):1–5 [DOI] [PubMed] [Google Scholar]
- 4.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ et al. (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83(3):278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trager N, Butler JT, Haque A, Ray SK, Beeson C, Banik NL (2013) The involvement of calpain in CD4(+) T helper cell bias in multple sclerosis. J Clin Cell Immunol 4(4):1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dendrou CA, Fugger L (2017) Immunomodulation in multiple sclerosis: promises and pitfalls. Curr Opin Immunol 49:37–43 [DOI] [PubMed] [Google Scholar]
- 7.Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15(9):545–558 [DOI] [PubMed] [Google Scholar]
- 8.Dargahi N, Katsara M, Tselios T, Androutsou ME, de Courten M, Matsoukas J et al. (2017) Multiple sclerosis: immunopathology and treatment update. Brain Sci 7(7):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiendl H, Toyka KV, Rieckmann P, Gold R, Hartung HP, Hohlfeld R (2008) Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. J Neurol 255(10):1449–1463 [DOI] [PubMed] [Google Scholar]
- 10.Ellwardt E, Zipp F (2014) Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol 262(Pt A):8–17 [DOI] [PubMed] [Google Scholar]
- 11.Schaecher KE, Shields DC, Banik NL (2001) Mechanism of myelin breakdown in experimental demyelination: a putative role for calpain. Neurochem Res 26(6):731–737 [DOI] [PubMed] [Google Scholar]
- 12.Shields DC, Schaecher KE, Saido TC, Banik NL (1999) A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci USA 96(20):11486–11491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shields DC, Banik NL (1999) Pathophysiological role of calpain in experimental demyelination. J Neurosci Res 55(5):533–541 [DOI] [PubMed] [Google Scholar]
- 14.Guyton MK, Das A, Samantaray S, Wallace GC, Butler JT, Ray SK et al. (2010) Calpeptin attenuated inflammation, cell death, and axonal damage in animal model of multiple sclerosis. J Neurosci Res 88(11):2398–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podbielska M, Das A, Smith AW, Chauhan A, Ray SK, Inoue J et al. (2016) Neuron-microglia interaction induced bi-directional cytotoxicity associated with calpain activation. J Neurochem 139(3):440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AW, Doonan BP, Tyor WR, Abou-Fayssal N, Haque A, Banik NL (2011) Regulation of Th1/Th17 cytokines and IDO gene expression by inhibition of calpain in PBMCs from MS patients. J Neuroimmunol 232(1–2):179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan KE, Cutilli J, Piliero LM, Ghavimi-Alagha D, Starr SE, Campbell DE et al. (2000) Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol 7(6):920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhoef CM, Van Roon JA, Vianen ME, Glaudemans CA, Lafeber FP, Bijlsma JW (1999) Lymphocyte stimulation by CD3-CD28 enables detection of low T cell interferon-gamma and inter-leukin-4 production in rheumatoid arthritis. Scand J Immunol 50(4):427–432 [DOI] [PubMed] [Google Scholar]
- 19.Mori F, Rossi S, Piccinin S, Motta C, Mango D, Kusayanagi H et al. (2013) Synaptic plasticity and PDGF signaling defects underlie clinical progression in multiple sclerosis. J Neurosci 33(49):19112–19119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stampanoni Bassi M, Iezzi E, Marfia GA, Simonelli I, Musella A, Mandolesi G et al. (2018) Platelet-derived growth factor predicts prolonged relapse-free period in multiple sclerosis. J Neuroinflamm 15(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori F, Nistico R, Nicoletti CG, Zagaglia S, Mandolesi G, Piccinin S et al. (2016) RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis. Mult Scler (Basingstoke, England). 22(11):1405–1412 [DOI] [PubMed] [Google Scholar]
- 22.Filipovic R, Jakovcevski I, Zecevic N (2003) GRO-alpha and CXCR2 in the human fetal brain and multiple sclerosis lesions. Dev Neurosci 25(2–4):279–290 [DOI] [PubMed] [Google Scholar]
- 23.Glabinski AR, Tuohy VK, Ransohoff RM (1998) Expression of chemokines RANTES, MIP-1alpha and GRO-alpha correlates with inflammation in acute experimental autoimmune encephalomyelitis. Neuroimmunomodulation 5(3–4):166–171 [DOI] [PubMed] [Google Scholar]
- 24.Masuda H, Mori M, Uchida T, Uzawa A, Ohtani R, Kuwabara S (2017) Soluble CD40 ligand contributes to blood-brain barrier breakdown and central nervous system inflammation in multiple sclerosis and neuromyelitis optica spectrum disorder. J Neuroimmunol 305:102–107 [DOI] [PubMed] [Google Scholar]
- 25.Masuda H, Mori M, Umehara K, Furihata T, Uchida T, Uzawa A et al. (2018) Soluble CD40 ligand disrupts the blood-brain barrier and exacerbates inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol 316:117–120 [DOI] [PubMed] [Google Scholar]
- 26.Shields DC, Tyor WR, Deibler GE, Banik NL (1998) Increased calpain expression in experimental demyelinating optic neuritis: an immunocytochemical study. Brain Res 784(1–2):299–304 [DOI] [PubMed] [Google Scholar]
- 27.Shields DC, Tyor WR, Deibler GE, Hogan EL, Banik NL (1998) Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci USA 95(10):5768–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields DC, Banik NL (1998) Upregulation of calpain activity and expression in experimental allergic encephalomyelitis: a putative role for calpain in demyelination. Brain Res 794(1):68–74 [DOI] [PubMed] [Google Scholar]
- 29.Schaecher K, Rocchini A, Dinkins J, Matzelle DD, Banik NL (2002) Calpain expression and infiltration of activated T cells in experimental allergic encephalomyelitis over time: increased calpain activity begins with onset of disease. J Neuroimmunol 129(1–2):1–9 [DOI] [PubMed] [Google Scholar]
- 30.Shields DC, Schaecher KE, Goust JM, Banik NL (1999) Calpain activity and expression are increased in splenic inflammatory cells associated with experimental allergic encephalomyelitis. J Neuroimmunol 99(1):1–12 [DOI] [PubMed] [Google Scholar]
- 31.Das A, Guyton MK, Matzelle DD, Ray SK, Banik NL (2008) Time-dependent increases in protease activities for neuronal apoptosis in spinal cords of Lewis rats during development of acute experimental autoimmune encephalomyelitis. J Neurosci Res 86(13):2992–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imam SA, Guyton MK, Haque A, Vandenbark A, Tyor WR, Ray SK et al. (2007) Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol 190(1–2):139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trager N, Smith A, Wallace G IV, Azuma M, Inoue J, Beeson C et al. (2014) Effects of a novel orally administered calpain inhibitor SNJ-1945 on immunomodulation and neurodegeneration in a murine model of multiple sclerosis. J Neurochem 130(2):268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samantaray S, Knaryan VH, Shields DC, Cox AA, Haque A, Banik NL (2015) Inhibition of calpain activation protects MPTP-induced nigral and spinal cord neurodegeneration, reduces inflammation, and improves gait dynamics in mice. Mol Neurobiol 52(2):1054–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samantaray S, Knaryan VH, Le Gal C, Ray SK, Banik NL (2011) Calpain inhibition protected spinal cord motoneurons against 1-methyl-4-phenylpyridinium ion and rotenone. Neuroscience 192:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith AW, Rohrer B, Wheless L, Samantaray S, Ray SK, Inoue J et al. (2016) Calpain inhibition reduces structural and functional impairment of retinal ganglion cells in experimental optic neuritis. J Neurochem 139(2):270–284 [DOI] [PMC free article] [PubMed] [Google Scholar]


