Abstract
Sulfate analog oxyanions that function as selective metabolic inhibitors of dissimilatory sulfate reducing microorganisms (SRM) are widely used in ecological studies and industrial applications. As such, it is important to understand the mode of action and mechanisms of tolerance or adaptation to these compounds. Different oxyanions vary widely in their inhibitory potency and mechanism of inhibition, but current evidence suggests that the sulfate adenylyl transferase/ATP sulfurylase (Sat) enzyme is an important target. We heterologously expressed and purified the Sat from the model SRM, Desulfovibrio alaskensis G20. With this enzyme we determined the turnover kinetics (kcat, KM) for alternative substrates (molybdate, selenate, arsenate, monofluorophosphate, and chromate) and inhibition constants (KI) for competitive inhibitors (perchlorate, chlorate, and nitrate). These measurements enable the first quantitative comparisons of these compounds as substrates or inhibitors of a purified Sat from a respiratory sulfate reducer. We compare predicted half-maximal inhibitory concentrations (IC50) based on Sat kinetics with measured IC50 values against D. alaskensis G20 growth and discuss our results in light of known mechanisms of sensitivity or resistance to oxyanions. This analysis helps with the interpretation of recent adaptive laboratory evolution studies and illustrates the value of interpreting gene–microbe–environment interactions through the lens of enzyme kinetics.
Subject terms: Biogeochemistry, Environmental microbiology
Selective inhibitors of dissimilatory sulfate reducing microorganisms (SRM) are both valuable tools for ecological studies and treatment strategies to abrogate unwanted sulfide production in industrial systems [1, 2]. The best studied selective SRM inhibitors are the inorganic oxyanion sulfate analogs including molybdate, tungstate, selenate, chromate, monofluorophosphate, arsenate, nitrate, perchlorate, and chlorate [3–6]. Remarkably, although direct interaction with the sulfate activating enzyme, sulfate adenylyl transferase (Sat) has been implicated as a primary mode of action for these compounds [3, 7], there is little kinetic data for these sulfate analogs as substrates or inhibitors of purified Sat enzymes from respiratory SRM. Other mechanisms of toxicity, tolerance and adaptation have been implicated for oxyanions against model SRM including competition for sulfate uptake [8, 9], ATP consuming futile cycles [10, 11] and detoxification via enzymatic reduction and efflux [5, 12].
We heterologously expressed, purified and kinetically characterized the Sat from the model SRM, Desulfovibrio alaskensis G20 as reported previously [13]. Briefly, the Sat was expressed in E. coli and purified using a combination of affinity chromatography and ion exchange chromatography. Substrate kinetic parameters were measured by monitoring pyrophosphate accumulation using a modified molybdenum blue assay. Alongside our previous measurements with molybdate [13], we report kinetic parameters for four other alternative substrates including selenate, arsenate, monofluorophosphate, and chromate [13–15] (Table 1A). Previously we found that perchlorate is a competitive inhibitor of the Sat [13], and we now report inhibition constants (KI) for the other competitive inhibitors nitrate and chlorate, both of which are competitive with the oxyanion substrate, molybdate, used in our assays but not competitive with the Sat co-substrate, ATP (Table 1B).
Table 1.
Kinetic parameters for D. alaskensis G20 sulfate adenylyl transferase (Sat).
| A. | ||||
|---|---|---|---|---|
| Substrate | kcat (s−1) | KM (mM) | kcat/KM (M−1 s−1) | Reference |
| MoO42− | 14.5 ± 1.2 | 3.26 ± 0.55 | 4.4 × 103 | 13 |
| SeO42− | 0.284 ± 0.032 | 1.77 ± 0.55 | 1.6 × 102 | This work |
| FPO42− | 11.0 ± 1.6 | 1.44 ± 0.56 | 7.6 × 103 | This work |
| AsO43− | 3.88 ± 0.32 | 0.0617 ± 0.026 | 6.3 × 104 | This work |
| CrO42− | 16.0 ± 0.79 | 0.793 ± 0.14 | 2.0 × 104 | This work |
| B. | ||||
|---|---|---|---|---|
| Inhibitor | KI—Varied MoO42− (mM) | KI—Varied ATP (mM) | Average KI (mM) | Reference |
| ClO3− | 0.316 ± 0.017 | 1.45 ± 0.076 | 0.833 | This work |
| NO3− | 3.60 ± 0.27 | 12.5 ± 0.98 | 8.05 | This work |
| ClO4− | 0.138 ± 0.014 | 0.915 ± 0.15 | 0.5265 | 13 |
While this set of sulfate analogs has previously been inferred to target Sat based on their selective influence on SRM metabolic activity and growth [4, 5, 7, 10], cell lysate enzyme assays [3], genetic screens [7, 16] and induction of the sulfate reduction regulon [7] our results with the purified enzyme enable the first quantitative ranking of these compounds as substrates or inhibitors of Sat. It is striking that the relative affinities (KM and KI) of these oxyanions vary by over an order of magnitude given their similar geometries and formal charges (Table 1). The highest affinity substrate is arsenate (KM = 0.0617 ± 0.026) while the lowest affinity substrate is molybdate (KM = 3.26 ± 0.55). The highest affinity inhibitor is perchlorate (KI = 0.138 ± 0.014) while the lowest affinity inhibitor is nitrate (KI = 3.60 ± 0.27).
The potency of a competitive substrate or inhibitor against cellular growth is determined by the affinity of the inhibitor for its primary target and detoxification reactions. For cytoplasmic enzymes, mechanisms of transport and efflux are important to consider. Thus, we can compare the measured affinities against Sat with known inhibitory potencies of these oxyanions against growth of D. alaskensis G20 [5] to infer the extent to which Sat is a primary target of these oxyanions and the magnitude of other processes that influence intracellular inhibitor concentration or other modes of toxicity. While inhibitor KI or KM is independent of the substrate concentration, enzyme IC50 increases at higher substrate concentrations for competitive substrates and inhibitors (Eq. 1) [17].
| 1 |
Thus, from measurements of sulfate KM and competitive inhibitor KI or competitive substrate KM, we can estimate the competitive substrate/inhibitor IC50 against Sat for a given concentration of sulfate (Fig. 1). The predicted IC50s can be compared against measured IC50s from previous replicate dose–response assays against the growth of G20 in liquid culture [5]. To model IC50s we use a sulfate KM of 2.93 ± 0.26 mM empirically determined for a homologous Desulfovibrio Sat [18]. The homologous Sat is 81% identical to the G20 Sat, and molybdate KM values only vary between 1 mM and 3 mM for different Desulfovibrio [19]. Thus, this is likely a reasonable approximation for the G20 Sat KM. Because we do not know the cytoplasmic concentrations of sulfate during batch growth in the presence of these inhibitors we calculated Sat IC50s at three concentrations of sulfate including: 15 mM sulfate (the mean extracellular concentration in G20 batch cultures [5, 9, 12, 13, 20]), 5 mM sulfate (the mean intracellular sulfate concentration in typical G20 batch cultures [21]), and 0.15 mM sulfate (which is close to the lowest intracellular concentration measured in G20 batch cultures [21] and an order of magnitude lower than the Sat sulfate KM).
Fig. 1. Measured and predicted IC50 values for D. alaskensis G20 Sat.
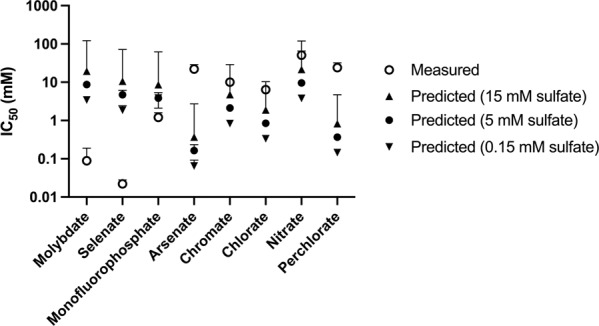
Measured IC50s are from Reference [5] and error bars represent the 95% confidence intervals of replicate dose–response fits. Predicted IC50s are based on measured kinetic parameters for for the G20 sulfate adenyl transferase (Table 1) using Eq. (1) and calculated using for intracellular sulfate concentrations of 15 mM (closed upright triangles) 5 mM (closed circles), and 0.15 mM (closed inverted triangles). Predicted IC50s error bars are upper and lower bounds calculated based on error in Sat kinetic measurements range (this work, References [13, 18]).
For the Sat substrates molybdate, selenate and monofluorophosphate, the predicted Sat IC50s are higher than the measured growth IC50s. As such, the measured IC50s must reflect an additional mode of growth inhibition apart from activity as competitive substrates of Sat or bioconcentration via uptake with minimal efflux of the inhibitor in the G20 cytoplasm. Molybdate and selenate form unstable APS analogs as products of Sat catalyzed reactions which rapidly decompose [22, 23]. This drives a non-productive, “futile” cycle which leads to rapid ATP hydrolysis and is thought to be a major mode of cellular toxicity of these compounds [10]. Selenate and molybdate are also competitive with sulfate uptake [8] and, at least in D. vulgaris Hildenborough, an additional protein aside from Sat may catalyze non-productive adenosine 5′-phosphomolybdate formation and ATP hydrolysis, but it is unknown if similar proteins are in D. alaskensis G20 [11]. Monofluorophosphate reacts with ATP at the Sat as a dead-end substrate to form the stable product adenosine 5′-(2- fluorodiphosphate), ADPβF [24], so ATP consumption through futile cycling is not a likely mechanism of cellular inhibition. Fluoride ion (F−) toxicity may contribute to the monofluorophosphate mechanism of action, but this is ameliorated by a fluoride efflux pump [5].
The other divalent oxyanions in our panel, arsenate and chromate, are less inhibitory to D. alaskensis G20 growth than predicted based on the Sat IC50. Arsenate is the highest affinity Sat substrate from our panel with a Sat KM 50–100-fold lower than molybdate or selenate (Table 1, Fig. 1A). The measured arsenate IC50 against G20 growth is ~10-fold higher than the predicted Sat arsenate IC50 and ~100-fold higher than the measured molybdate or selenate growth IC50s. However, arsenate is known to be catalytically reduced and effluxed by G20 and deletion of the arsenate reductase and efflux systems renders G20 ~10-fold more sensitive to arsenate [12]. Arsenate also reacts abiotically with sulfide [25]. These observations are consistent with the difference the measured arsenate IC50 being lower than predicted (Fig. 1). Chromate is the second highest affinity Sat substrate in our panel, and the predicted IC50 is slightly lower than the measured IC50. Chromate is catalytically reduced by G20 [26] and reacts abiotically with sulfide [27] which will increase the effective concentration required to inhibit Sat. Taken together, our results are consistent with Sat being an important target of both arsenate and chromate in G20, and this is consistent with the previous observation that both of these compounds are selective inhibitors of SRM in marine enrichment cultures [5].
We also compared predicted Sat IC50s with measured growth IC50s for the competitive inhibitors perchlorate, chlorate, and nitrate (Fig. 1). The mechanism of action of these compounds against SRM in complex natural systems is primarily due to bio-competitive exclusion via growth of nitrate or perchlorate respirers and the production of reactive nitrogen and potentially chlorine species [1, 28, 29]. However, at higher concentrations, these oxyanions are direct competitive inhibitors of the sulfate reduction pathway [7]. Understanding the targets and adaptation mechanisms to competitive inhibitors may aid in the development of next-generation small molecule inhibitors that are selective against SRM [30].
For nitrate and chlorate, predicted IC50s are less than 10-fold lower than the measured IC50s while the predicted perchlorate IC50 is nearly 50-fold lower than the measured IC50. Apart from competitive inhibition of Sat, cellular permeability, reactivity and efflux can influence the measured growth IC50s, cytoplasmic reactivity will make cells more sensitive to these compounds through the generation of reactive nitrogen and chlorine species (RNS/RCS). While perchlorate is kinetically very stable, chlorate and nitrate generate cytoplasmic RNS/RCS in G20 [7, 16]. No chlorate or nitrate efflux mechanisms in G20 are known and reduction or these compounds is minimal [7]. Thus, for these compounds it is most likely that measured growth IC50s are higher than are expected based on Sat kinetics because oxyanion transport keeps cytoplasmic concentrations of these compounds low.
Indeed, adaptive laboratory evolution and genetics indicate that changes in the permeability of cells to sulfate, nitrate and perchlorate influence sensitivity to these oxyanions. Mutants that increase the expression of sulfate transporters with low perchlorate affinity are implicated in perchlorate resistance [9], and loss of function mutants in putative thiosulfate transporters with high nitrate affinity are implicated in nitrate resistance [7, 16, 20]. Notably, point mutants that alter the activity of sulfate transporters were not observed suggesting that these compounds target a cytoplasmic enzyme, such as Sat, rather than sulfate uptake. In some perchlorate adapted cultures, point mutants in the Sat emerge that alter the KI, clearly indicating that Sat is under selection [9, 13].
Further characterization of transport kinetics, rates of reduction and efflux, and cytoplasmic concentrations will enable more quantitative predictions of the inhibitory potency and genetic targets of oxyanion inhibitors of sulfate respiration. Nevertheless, our results are consistent with Sat as an important target of these compounds. More generally, comparing the affinities of metabolic inhibitors for transport enzymes versus cytoplasmic metabolic enzymes will be essential for understanding the evolutionary landscapes that microorganisms navigate across environmental gradients of toxicants or nutrients [31–33]. The extent to which cell surface versus cytoplasmic enzymes are under selection depends on enzyme affinities and the relative concentrations of substrates and inhibitors. Thus, enzyme kinetics measurements are critical for understanding genotype–phenotype relationships in complex environments where a bacterial cell is confronted with physicochemically similar substrates and inhibitors such as metal ions, carbon sources or vitamins.
Acknowledgements
HKC acknowledges funding by ENIGMA, a Scientific Focus Area Program supported by the U. S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Genomics: GTL Foundational Science through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U. S. Department of Energy. JDC acknowledges funding from the UC Berkeley Energy and Biosciences Institute. The authors would like to thank Magdalena K. Stoeva and Morgan N. Price for critical feedback and suggestions.
Author contributions
HKC, MDY and JDC conceived of and designed the experiments. MDY, HKC, SAR, AJW conducted experiments. HKC wrote the manuscript. All authors contributed edits on the manuscript.
Competing interests
JDC declares that he holds IP on the application of (per)chlorate to control souring. The remaining authors declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu Y, Engelbrektson A, Stoeva M, Barnum T, Reyes-Umana V, Coates JD. Perchlorate and its application in the oil and gas industry. Oilfield microbiology. 2019. CRC Press, p. 109–28.
- 2.McGenity TJ. Microbial communities utilizing hydrocarbons and lipids: members, metagenomics and ecophysiology. 2019. Springer, Cham.
- 3.Peck HD. The ATP-dependent reduction of sulfate with hydrogen in extracts of Desulfovibrio desulfuricans. Proc Natl Acad Sci USA. 1959;45:701–8. doi: 10.1073/pnas.45.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postgate JR. Competitive and non-competitive inhibitors of bacterial sulphate reduction. Microbiology. 1952;6:128–42. doi: 10.1099/00221287-6-1-2-128. [DOI] [PubMed] [Google Scholar]
- 5.Carlson HK, Stoeva MK, Justice NB, Sczesnak A, Mullan MR, Mosqueda LA, et al. Monofluorophosphate is a selective inhibitor of respiratory sulfate-reducing microorganisms. Environ Sci Technol. 2015;49:3727–36. doi: 10.1021/es505843z. [DOI] [PubMed] [Google Scholar]
- 6.Williamson AJ, Engelbrektson AL, Liu Y, Huang LL, Kumar A, Menon AR, et al. Tungstate control of microbial sulfidogenesis and souring of the engineered environment. Environ Sci Technol. 2020;54:16119–27. [DOI] [PubMed]
- 7.Carlson HK, Kuehl JV, Hazra AB, Justice NB, Stoeva MK, Sczesnak A, et al. Mechanisms of direct inhibition of the respiratory sulfate-reduction pathway by (per)chlorate and nitrate. ISME J. 2015;9:1295–305. doi: 10.1038/ismej.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cypionka H. Characterization of sulfate transport in Desulfovibrio desulfuricans. Arch Microbiol. 1989;152:237–43. doi: 10.1007/BF00409657. [DOI] [PubMed] [Google Scholar]
- 9.Stoeva MK, Kuehl J, Kazakov AE, Wang O, Rushton-Green R, Coates JD. Anion transport as a target of adaption to perchlorate in sulfate-reducing communities. ISME J. 2020;14:450–62. doi: 10.1038/s41396-019-0540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor BF, Oremland RS. Specific depletion of cellular ATP in Desulfovibrio by molybdate and other Group VI anions. Ann Meet Amer Sot Microbial Abstract Q. 1977;41:268. [Google Scholar]
- 11.Zane GM, Wall JD, De Leon KB. Novel mode of molybdate inhibition of Desulfovibrio vulgaris Hildenborough. Front Microbiol. 2020;11:3162. doi: 10.3389/fmicb.2020.610455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Krumholz LR. Regulation of arsenate resistance in Desulfovibrio desulfuricans G20 by an arsRBCC operon and an arsC gene. J Bacteriol. 2007;189:3705–11. doi: 10.1128/JB.01913-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta-Kolte MG, Stoeva MK, Mehra A, Redford SA, Youngblut MD, Zane G, et al. Adaptation of Desulfovibrio alaskensis G20 to perchlorate, a specific inhibitor of sulfate reduction. Environ Microbiol. 2019;21:1395–406. doi: 10.1111/1462-2920.14570. [DOI] [PubMed] [Google Scholar]
- 14.Gavel OY, Bursakov SA, Calvete JJ, George GN, Moura JJ, Moura I. ATP sulfurylases from sulfate-reducing bacteria of the genus Desulfovibrio. A novel metalloprotein containing cobalt and zinc. Biochemistry. 1998;37:16225–32. doi: 10.1021/bi9816709. [DOI] [PubMed] [Google Scholar]
- 15.He Z, Honeycutt CW. A modified molybdenum blue method for orthophosphate determination suitable for investigating enzymatic hydrolysis of organic phosphates. Commun Soil Sci Plant Anal. 2005;36:1373–83. doi: 10.1081/CSS-200056954. [DOI] [Google Scholar]
- 16.Korte HL, Fels SR, Christensen GA, Price MN, Kuehl JV, Zane GM, et al. Genetic basis for nitrate resistance in Desulfovibrio strains. Front Microbiol. 2014;5:153. doi: 10.3389/fmicb.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 18.Kushkevych IV, Antonyak HL, Bartoš M. Kinetic properties of adenosine triphosphate sulfurylase of intestinal sulfate-reducing bacteria. Ukr Biochem J. 2014;86:129–38. doi: 10.15407/ubj86.06.129. [DOI] [PubMed] [Google Scholar]
- 19.Abdulina D, Kováč J, Iutynska G, Kushkevych I. ATP sulfurylase activity of sulfate-reducing bacteria from various ecotopes. 3 Biotech. 2020;10:55. doi: 10.1007/s13205-019-2041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu B, Liu F, Zhou A, Li J, Shu L, Kempher ML, et al. Experimental evolution reveals nitrate tolerance mechanisms in Desulfovibrio vulgaris. ISME J. 2020;14:2862–76. doi: 10.1038/s41396-020-00753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adkins JF, Orphan VJ, Sessions AL, Sim MS. Quantification and isotopic analysis of intracellular sulfur metabolites in the dissimilatory sulfate reduction pathway. 2016. p. B43D-05.
- 22.Hanna E, Ng KF, MacRae IJ, Bley CJ, Fisher AJ, Segel IH. Kinetic and stability properties of penicillium chrysogenum atp sulfurylase missing the C-terminal regulatory domain. J Biol Chem. 2004;279:4415–24. doi: 10.1074/jbc.M311317200. [DOI] [PubMed] [Google Scholar]
- 23.Renosto F, Patel HC, Martin RL, Thomassian C, Zimmerman G, Segel IH. ATP sulfurylase from higher plants: kinetic and structural characterization of the chloroplast and cytosol enzymes from spinach leaf. Arch Biochem Biophys. 1993;307:272–85. doi: 10.1006/abbi.1993.1590. [DOI] [PubMed] [Google Scholar]
- 24.Satishchandran C, Myers CB, Markham GD. Adenosine-5′-O-(2-fluorodiphosphate) (ADPβF), an analog of adenosine-5′-phosphosulfate. Bioorg Chem. 1992;20:107–14. doi: 10.1016/0045-2068(92)90031-W. [DOI] [Google Scholar]
- 25.Rochette EA, Bostick BC, Li G, Fendorf S. Kinetics of arsenate reduction by dissolved sulfide. Environ Sci Technol. 2000;34:4714–20. doi: 10.1021/es000963y. [DOI] [Google Scholar]
- 26.Li X, Krumholz LR. Thioredoxin is involved in U (VI) and Cr (VI) reduction in Desulfovibrio desulfuricans G20. J Bacteriol. 2009. [DOI] [PMC free article] [PubMed]
- 27.Kim C, Zhou Q, Deng B, Thornton EC, Xu H. Chromium (VI) reduction by hydrogen sulfide in aqueous media: stoichiometry and kinetics. Environ Sci Technol. 2001;35:2219–25. doi: 10.1021/es0017007. [DOI] [PubMed] [Google Scholar]
- 28.Engelbrektson A, Hubbard CG, Tom LM, Boussina A, Jin YT, Wong H, et al. Inhibition of microbial sulfate reduction in a flow-through column system by (per)chlorate treatment. Front Microbiol. 2014;5:315. doi: 10.3389/fmicb.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson HK, Hubert CRJ. Mechanisms and monitoring of oil reservoir souring control by nitrate or perchlorate injection. In: McGenity TJ (ed). Microbial communities utilizing hydrocarbons and lipids: members, metagenomics and ecophysiology. 2019. Springer International Publishing, Cham, p. 1–25.
- 30.Carlson HK, Mullan MR, Mosqueda LA, Chen S, Arkin MR, Coates JD. High-throughput screening to identify potent and specific inhibitors of microbial sulfate reduction. Environ Sci Technol. 2017;51:7278–85. doi: 10.1021/acs.est.7b00686. [DOI] [PubMed] [Google Scholar]
- 31.Carlson H, Deutschbauer A, Coates J. Microbial metal resistance and metabolism across dynamic landscapes: high-throughput environmental microbiology. F1000Res. 2017;6. [DOI] [PMC free article] [PubMed]
- 32.Chandrangsu P, Rensing C, Helmann JD. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol. 2017;15:338–50. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan TS, Gadd GM. Metal bioavailability and the soil microbiome. Adv Agron. 2019;155:79–120.


