Abstract
The calcineurin gene was cloned and disrupted in serotype D strains of Cryptococcus neoformans. Serotype A and serotype D calcineurin mutants were inviable at 37°C and avirulent in mice, whereas only serotype A mutants were cation stress sensitive. Thus, calcineurin plays conserved and divergent roles in serotype A and serotype D strains.
Cryptococcus neoformans is an encapsulated basidiomycete that is the most common cause of systemic mycosis in AIDS patients. C. neoformans strains are classified into five serotypes (A, B, C, D, and AD) and two varieties: C. neoformans var. neoformans (serotypes A, D, and AD) and C. neoformans var. gattii (serotypes B and C). Serotype A and serotype D strains exhibit significant variation and may represent distinct varieties that have diverged in ∼18 million years of evolution (8, 9, 16, 19, 25). C. neoformans virulence factors include the capsule (3–5), melanin (22), prototrophy (17), and growth at 37°C (14). The protein phosphatase calcineurin is required for C. neoformans growth at 37°C and virulence (14).
Calcineurin is a Ca2+-calmodulin-activated phosphatase with catalytic and regulatory subunits (10). Calcineurin is the target of the T-cell-specific immunosuppressants cyclosporine (CsA) and tacrolimus (FK506). CsA and FK506 suppress the immune system by inhibiting calcineurin and preventing gene expression during T-cell activation. The antifungal activities of CsA and FK506 are mediated by a similar mechanism involving fungal homologs of calcineurin and the drug-binding proteins cyclophilin A and FKBP12 (1, 2, 6, 7, 14, 15).
Calcineurin has been identified from several fungi and regulates cell cycle progression in Aspergillus nidulans (21), hyphal elongation and growth in Neurospora crassa (11, 20), and mating and cytokinesis in Schizosaccharomyces pombe (18, 28). In Saccharomyces cerevisiae, calcineurin is required for recovery from pheromone arrest (12, 27) and regulates cation homeostasis and cell wall biosynthesis via the transcription factor Crz1 (1, 13, 23, 24).
The calcineurin gene has been identified, sequenced, and disrupted by homologous recombination in C. neoformans serotype A strain H99 (14). Calcineurin is essential for growth at 37°C, virulence in a rabbit model of cryptococcal meningitis, and cation homeostasis (14). Here, we isolated and disrupted the gene encoding the calcineurin A catalytic subunit (CNA1) from the congenic serotype D strains of C. neoformans and compared the functions of calcineurin in serotype A and serotype D strains.
Identification, sequence, and disruption of the serotype D calcineurin A CNA1 gene.
The CNA1 gene encoding calcineurin A was cloned from the serotype D strain JEC21 by PCR amplification with primers to conserved sequences in the serotype A CNA1 gene. A 1.8-kb CNA1 gene fragment was sequenced, revealing identity to calcineurin genes, and used in Southern blot analysis to show that the CNA1 gene is contained on an 8-kb EagI fragment. The gene was recovered from a size-selected EagI genomic library and sequenced. There were seven amino acid differences between the calcineurin A protein in serotype A and serotype D.
To disrupt the serotype D CNA1 gene, the ADE2 gene was inserted at an HpaI site in the CNA1 gene. The cna1::ADE2 allele was transformed with a biolistic apparatus into serotype D MATα ade2 strain JEC50 and MATa ade2 ura5 strain JEC156. A total of 3 of 200 Ade+ transformants (1.5%) were viable at 24°C, grew poorly at 30°C, and were inviable at 37°C. The cna1 mutation confers a more severe growth defect at 30°C in serotype D than in serotype A strains, consistent with findings that FK506 and CsA are more toxic at 30°C to serotype D than to serotype A strains (14). Southern blotting confirmed that the CNA1 gene was replaced by the cna1::ADE2 allele without ectopic integration in all three isolates (data not shown). In one isolate, the wild-type CNA1 gene was precisely replaced by the cna1::ADE2 allele by a double crossover. In two other isolates, tandem integrations had occurred at the CNA1 locus. By an overlay blot with 125I-calmodulin, calcineurin was expressed in CNA1 wild-type strains but not in the cna1::ADE2 mutant strains (data not shown). Genetic crosses and analysis of basidiospores by micromanipulation revealed that the ADE2 gene was integrated into a single genomic locus. All Ade+ meiotic segregants exhibited the cna1 temperature-sensitive growth defect, and all Ade− segregants grew at 37°C, indicating that the cna1 mutation is linked to the temperature-sensitive defect and the ADE2 gene.
Comparison of calcineurin cna1 mutant phenotypes in serotype A and serotype D strains.
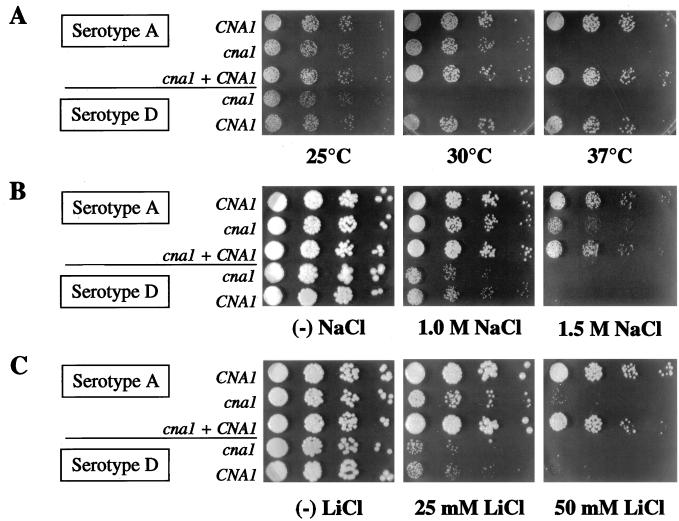
The growth of serotype A and serotype D wild-type and cna1 mutant strains was compared using a quantitative dilution assay for viability at 37°C and sensitivity to Na+ and Li+ (Fig. 1). At 25°C, growth of serotype A and serotype D cna1 mutants was comparable to that of wild-type strains. At 30°C, viability of serotype D cna1 mutants was severely reduced compared to the wild-type strain; growth of wild-type and cna1 mutant serotype A strains did not differ at 30°C. At 37°C, both serotype A and serotype D cna1 mutant strains were inviable. Reintroduction of the CNA1 gene restored growth at 37°C; thus, calcineurin is required for growth at 37°C for both serotype A and serotype D strains.
FIG. 1.
Growth properties of serotype A and serotype D calcineurin mutants of C. neoformans. Isogenic serotype A CNA1 wild-type (H99), calcineurin A cna1 mutant (AO4), and cna1 plus CNA1 reconstituted (AO10) strains and isogenic serotype D CNA1 wild-type (JEC21) and cna1 mutant (MCC3) strains were grown in YPD liquid medium and spotted onto YPD medium to compare growth at different temperatures (25, 30, and 37°C) (A), on medium containing 0 (−), 1, or 1.5 M NaCl (B) and 0 (−), 25, or 50 mM LiCl (C). Each dilution contained (from left to right) 1,250, 250, 50, and 10 cells of each strain. Cells shown in panels B and C were incubated for 72 h at 25°C.
Growth of serotype A cna1 mutants was impaired compared to that of the CNA1 wild-type or cna1 plus CNA1 strains on 1.5 M NaCl (Fig. 1B). Serotype D cna1 mutants showed only a slight reduction in the number of CFU on 1 M NaCl. The growth of wild-type serotype D strains was completely inhibited on 1.5 M NaCl, whereas serotype A wild-type strain H99 was viable (Fig. 1B). Thus, the congenic serotype D strain are more sensitive to cation stress than serotype A strain H99, and the cna1 mutation has little effect on cation resistance in serotype D.
Growth of serotype A cna1 mutants was compromised on yeast extract-peptone-dextrose (YPD) medium with 50 mM LiCl compared to the wild-type or cna1 plus CNA1 reconstituted strains (Fig. 1C). Growth of serotype D cna1 mutants was not reduced on 25 mM LiCl compared to wild-type strains. Both the wild-type and the cna1 mutant serotype D strains were inviable on 50 mM LiCl, in contrast to serotype A strain H99 (Fig. 1C).
Calcineurin is required for virulence of serotype A and serotype D strains in mice.
We tested if calcineurin is required for virulence of serotype D strains of C. neoformans in the murine model of cryptococcosis. Each animal was infected with 107 C. neoformans cells by lateral tail vein injection. Ten animals were analyzed for each strain, and survival was monitored as the endpoint.
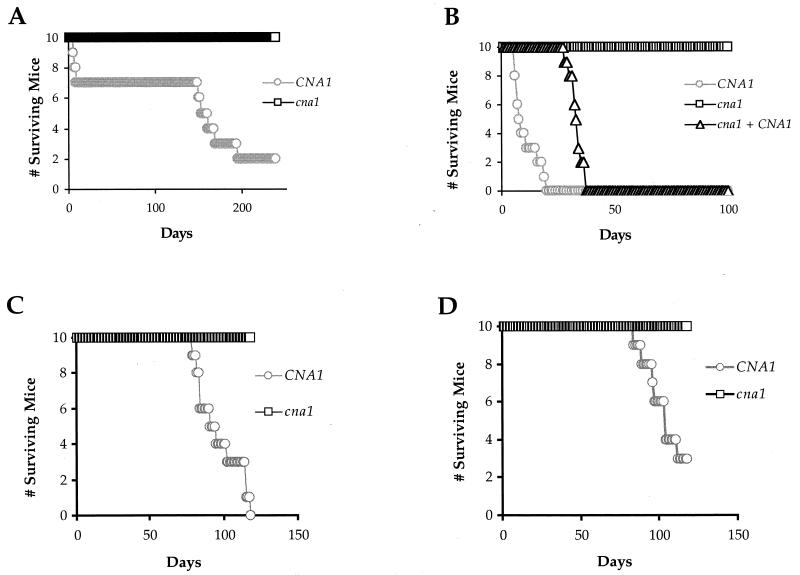
Infection of BALB/c mice with the serotype D CNA1 wild-type strain JEC21 resulted in the death of 50% of infected animals by day 153 and 80% by day 195. In comparison, all 10 mice infected with the isogenic cna1 mutant strain MCC2 survived to day 238 (Fig. 2A). No viable fungal cells could be cultured from the brains or lungs of mice infected with the serotype D cna1 mutant strain, indicating that the mutant had been eradicated (results not shown).
FIG. 2.
Calcineurin is required for virulence of C. neoformans serotype D and serotype A strains in mice. BALB/c mice (10 for each strain) received injections in the lateral tail vein of 107 cells of the prototrophic CNA1 wild-type MATα serotype D strain (JEC21) or the congenic MATα cna1 mutant strain (MCC2) lacking calcineurin A (A) or the isogenic serotype A CNA1 wild-type (H99), cna1 mutant (AO4), and cna1 plus CNA1 reconstituted (AO10) strains (B). C5-deficient mice (10 each) received injections in the lateral tail vein of 107 cells of prototrophic CNA1 wild-type MATa serotype D strain (JEC20) or the isogenic MATa cna1 mutant strain (MCC10) lacking calcineurin (C) or the prototrophic CNA1 wild-type MATα serotype D strain (JEC21) or the congenic MATα cna1 mutant (MCC5) lacking calcineurin (D). Mice were observed twice daily and the number of mice surviving versus time were plotted.
Comparable studies were conducted with the serotype A CNA1 wild-type, cna1 mutant, and cna1 plus CNA1 reconstituted strains (Fig. 1B). Infection with CNA1 wild-type strain H99 resulted in 50% mortality by day 8 and 100% mortality by day 20. The cna1 mutation severely attenuated virulence, and all 10 mice infected with the cna1 mutant survived to day 100. Reintroduction of the wild-type CNA1 gene restored the virulence of the cna1 mutant, albeit not to the wild-type level, resulting in 100% mortality by day 38. Thus, calcineurin is required for virulence of serotype A strain H99 in two different animal models.
Studies conducted with C5-deficient mice resulted in 100 and 70% mortality at day 120 following infection with wild-type strains JEC20 or JEC21, whereas 100% of mice infected with serotype D MATa or MATα cna1 mutant strains survived to day 120 (Fig. 2C and D). Thus, calcineurin is required for the virulence of both MATa and MATα serotype D strains in both immunocompetent and immunodeficient mice.
Summary and conclusions.
Serotype A calcineurin A mutants are hypersensitive to Na+ and Li+ compared to the isogenic wild-type strain (14). These findings suggest a role for calcineurin analogous to that in S. cerevisiae, where calcineurin controls expression of the Pmr2 ion pump that effluxes Na+ and Li+ (26). In contrast, in the C. neoformans serotype D strain, calcineurin does not appear to regulate cation homeostasis because calcineurin mutants were as sensitive to cations as were isogenic wild-type strains. In fact, the wild-type congenic serotype D strains were inherently more sensitive to cation stress than serotype A strain H99, indicating significant physiological differences between serotype A and serotype D strains under stress growth conditions. Our findings provide further support for the classification of serotype A and serotype D strains into distinct varieties of C. neoformans: C. neoformans var. grubii (serotype A) and C. neoformans var. neoformans (serotype D) (9).
Calcineurin is required for virulence of serotype D and serotype A strains in both rabbits and mice. Calcineurin is the target for the immunosuppressive antifungal agents CsA and FK506 and thus is an attractive target for antifungal agents (6, 7, 14, 15). The role of calcineurin in growth at 37°C and virulence in C. neoformans could not have been predicted from model organisms, because calcineurin is not required for growth at 37°C in S. cerevisiae and is only required for normal growth at 22°C but not at 37°C in S. pombe (28). Thus, studies of pathogens are necessary to establish the functions of specific genes in virulence.
Nucleotide sequence accession number.
The nucleotide sequence of the serotype D calcineurin A CNA1 gene has been deposited in the GenBank database under accession no. AF1559511.
Acknowledgments
We thank Tony Means and Elizabeth McDougal for generously providing 125I-calmodulin.
This work was supported by grants RO1 AI39115 and AI42159 from NIAID (to J.H.), supplement AI41937-S1 from the NIAID (to M.C.C. and J.H.), and PO1 grant AI44975 to the Duke University Mycology Research Unit. Joseph Heitman is an associate investigator of the Howard Hughes Medical Institute and a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology.
M.C.C. and R.A.L.S. contributed equally to this work.
REFERENCES
- 1.Breuder T, Hemenway C S, Movva N R, Cardenas M E, Heitman J. Calcineurin is essential in cyclosporin A- and FK506-sensitive yeast strains. Proc Natl Acad Sci USA. 1994;91:5372–5376. doi: 10.1073/pnas.91.12.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardenas M E, Muir R S, Breuder T, Heitman J. Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin A. EMBO J. 1995;14:2772–2783. doi: 10.1002/j.1460-2075.1995.tb07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang Y C, Kwon-Chung K J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y C, Kwon-Chung K J. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect Immun. 1998;66:2230–2236. doi: 10.1128/iai.66.5.2230-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y C, Penoyer L A, Kwon-Chung K J. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun. 1996;64:1977–1983. doi: 10.1128/iai.64.6.1977-1983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz M C, Cavallo L M, Görlach J M, Cox G, Perfect J R, Cardenas M E, Heitman J. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol Cell Biol. 1999;19:4101–4112. doi: 10.1128/mcb.19.6.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz M C, Poeta M D, Wang P, Wenger R, Zenke G, Quesniaux V F J, Movva N R, Perfect J R, Cardenas M E, Heitman J. Immunosuppressive and nonimmunosuppressive cyclosporin analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob Agents Chemother. 2000;44:143–149. doi: 10.1128/aac.44.1.143-149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzot S P, Fries B C, Cleare W, Casadevall A. Genetic relationship between Cryptococcus neoformans var. neoformans strains of serotypes A and D. J Clin Microbiol. 1998;36:2200–2204. doi: 10.1128/jcm.36.8.2200-2204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franzot S P, Salkin I F, Casadevall A. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. J Clin Microbiol. 1999;37:838–840. doi: 10.1128/jcm.37.3.838-840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemenway C S, Heitman J. Calcineurin: structure, function, and inhibition. Cell Biochem Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- 11.Kothe G O, Free S J. Calcineurin subunit B is required for normal vegetative growth in Neurospora crassa. Fungal Genet Biol. 1998;23:248–258. doi: 10.1006/fgbi.1998.1037. [DOI] [PubMed] [Google Scholar]
- 12.Moser M J, Geiser J R, Davis T N. Ca2+-calmodulin promotes survival of pheromone-induced growth arrest by activation of calcineurin and Ca2+-calmodulin-dependent protein kinase. Mol Cell Biol. 1996;16:4824–4831. doi: 10.1128/mcb.16.9.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T, Liu Y, Hirata D, Namba H, Harada S-I, Hirokawa T, Miyakawa T. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 1993;12:4063–4071. doi: 10.1002/j.1460-2075.1993.tb06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odom A, Muir S, Lim E, Toffaletti D L, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odom A, Poeta M D, Perfect J, Heitman J. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother. 1997;41:156–161. doi: 10.1128/aac.41.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perfect J R, Magee B B, Magee P T. Separation of chromosomes of Cryptococcus neoformans by pulsed field gel electrophoresis. Infect Immun. 1989;57:2624–2627. doi: 10.1128/iai.57.9.2624-2627.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perfect J R, Toffaletti D L, Rude T H. The gene encoding phosphoribosylaminoimidazole carboxylase (ADE2) is essential for growth of Cryptococcus neoformans in cerebrospinal fluid. Infect Immun. 1993;61:4446–4451. doi: 10.1128/iai.61.10.4446-4451.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plochocka-Zulinska D, Rasmussen G, Rasmussen C. Regulation of calcineurin gene expression in Schizosaccharomyces pombe. J Biol Chem. 1995;270:24794–24799. doi: 10.1074/jbc.270.42.24794. [DOI] [PubMed] [Google Scholar]
- 19.Polacheck I, Lebens G A. Electrophoretic karyotype of the pathogenic yeast Cryptococcus neoformans. J Gen Microbiol. 1989;135:65–71. doi: 10.1099/00221287-135-1-65. [DOI] [PubMed] [Google Scholar]
- 20.Prokisch H, Yarden O, Dieminger M, Tropschug M, Barthelmess I B. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol Gen Genet. 1997;256:104–114. doi: 10.1007/s004380050551. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen C, Garen C, Brining S, Kincaid R L, Means R L, Means A R. The calmodulin-dependent protein phosphatase catalytic subunit (calcineurin A) is an essential gene in Aspergillus nidulans. EMBO J. 1994;13:2545–2552. doi: 10.1002/j.1460-2075.1994.tb06544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salas S D, Bennett J E, Kwon-Chung K J, Perfect J R, Williamson P R. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stathopoulos A M, Cyert M S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stathopoulos-Gerontides A, Guo J J, Cyert M S. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 1999;13:798–803. doi: 10.1101/gad.13.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudarshan S, Davidson R C, Heitman J, Alspaugh J A. Molecular analysis of the Cryptococcus neoformans ADE2 gene, a selectable marker for transformation and gene disruption. Fungal Genet Biol. 1999;27:36–48. doi: 10.1006/fgbi.1999.1126. [DOI] [PubMed] [Google Scholar]
- 26.Wieland J, Nitsche A M, Strayle J, Steiner H, Rudolph H K. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 1995;14:3870–3882. doi: 10.1002/j.1460-2075.1995.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Withee J L, Mulholland J, Jeng R, Cyert M S. An essential role of the yeast pheromone-induced Ca2+ signal is to activate calcineurin. Mol Biol Cell. 1997;8:263–277. doi: 10.1091/mbc.8.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida T, Toda T, Yanagida M. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J Cell Sci. 1994;107:1725–1735. doi: 10.1242/jcs.107.7.1725. [DOI] [PubMed] [Google Scholar]