Abstract
In a search for new skin test reagents specific for tuberculosis, we found that the antigen encoded by gene Rv3874 of Mycobacterium tuberculosis elicited delayed-type hypersensitivity in M. tuberculosis-infected guinea pigs but not in control animals immunized with Mycobacterium bovis bacillus Calmette-Guérin (BCG) or Mycobacterium avium. The antigen, which was named MTSA-10 (for M. tuberculosis-specific antigen 10), is a prime candidate for a component of a new tuberculin that will allow discrimination by a skin test of latent M. tuberculosis infection from vaccination with BCG or from sensitization with environmental, nontuberculous mycobacteria.
The identification of persons infected with Mycobacterium tuberculosis—who account for one-third of the world's population—has a high priority in tuberculosis (TB) control programs, second only to the identification and treatment of infectious TB patients (1). To date, the only indicator of latent infection with M. tuberculosis is a positive tuberculin skin test, which measures delayed-type hypersensitivity (DTH) responses to the intradermal injection of the purified protein derivative (PPD) of tuberculin, an ammonium sulfate precipitate of filtrates of heat-inactivated, stationary-phase cultures (24). Unfortunately, the use of PPD imposes serious limitations on skin test accuracy. First, batches of PPD vary in protein composition and potency (13), making it exceedingly difficult to compare results obtained with different individuals, countries, or studies. Second, PPD is highly cross-reactive, for it shares many epitopes with antigens of other mycobacteria. Thus, the specificity of the PPD skin test (i.e., the ability of the test to correctly identify noninfected individuals) is very low for persons who have been vaccinated with Mycobacterium bovis bacillus Calmette-Guérin (BCG) and for persons living in areas that have a high environmental burden of nontuberculous mycobacteria (3, 4).
The premise of our work is that the accurate diagnosis of TB infection by skin testing requires a new tuberculin, one consisting of defined protein antigens that are unique to M. tuberculosis. A new tuberculin should contain many antigens, since cocktails of multiple antigens are needed to elicit strong DTH responses (16) and to cover the broad spectrum of antigen recognition by different individuals (9) typical of TB (23). We have shown that cocktails of M. tuberculosis complex-specific antigens elicit DTH responses that distinguish TB infection from sensitization with nontuberculous mycobacteria (16). The challenge now is to identify antigens unique to M. tuberculosis to develop multiantigen cocktails that allow discrimination between M. tuberculosis infection and vaccination with M. bovis BCG.
We have initiated a search for DTH-active antigens expressed by M. tuberculosis but not by M. bovis BCG. Prior to the present work, only one antigen, ESAT-6 (26), was known to have such properties (another antigen, MPT64 [29], is absent from only some BCG substrains [15, 17], and it evokes a vigorous DTH response to vaccination with some, but not all, substrains of BCG [11]). ESAT-6, which elicits strong, TB-specific DTH responses in guinea pigs (9), in cattle (21), and in humans (22, 27), is encoded by RD1, a DNA region present in the genome of M. tuberculosis and virulent M. bovis but missing from the DNA of all substrains of M. bovis BCG (17). In the present work we show that a second antigen encoded by the RD1 region of M. tuberculosis (gene Rv3874) elicits strong, M. tuberculosis-specific DTH responses in guinea pigs.
The Rv3874 gene product.
To identify DTH-eliciting antigens specific to M. tuberculosis, amino acid sequences, which were deduced from the nine putative genes in RD1 (7), were analyzed with a BLAST protein homology program to eliminate proteins having homologs encoded outside the RD1 region, since such proteins are likely to share epitopes with antigens of M. bovis BCG. The products of Rv3874 and Rv3879c shared no homology with proteins encoded elsewhere in the M. tuberculosis genome. Because of previous evidence that the Rv3874 gene is expressed and that the corresponding protein is found in the culture filtrate of M. tuberculosis (culture filtrate protein 10 [5]), we analyzed the Rv3874 product. The Rv3874 open reading frame was amplified from the chromosomal DNA of M. tuberculosis H37Rv by PCR and was cloned into the Escherichia coli expression vector pQE-30 (Qiagen) by following standard protocols (18, 19). Recombinant protein was expressed as a polyhistidine-tagged fusion protein and was purified to near homogeneity by using a three-step chromatography protocol (6). The purified protein had an apparent molecular mass of ∼10 kDa as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, in agreement with a calculated Mr of 10,794 (data not shown).
DTH responses in guinea pigs.
The ability of the Rv3874 gene product to elicit DTH was determined by guinea pig skin testing. We tested four groups of female outbred Hartley guinea pigs weighing approximately 300 g each. One group was infected with M. tuberculosis H37Rv by using an aerosol-generating device (Glas-Col, Middlebrook) calibrated to deliver about 100 tubercle bacilli into the lungs of each animal. Two additional, control groups were immunized with a suspension of 106 cells of M. bovis BCG or of M. avium (the most common cause of nontuberculous mycobacterioses in humans [10]) by intradermal injection in the shaved abdomen. A fourth group was mock immunized by intradermal injection of phosphate-buffered saline. This four-group design was utilized in two independent experiments that included two different substrains of M. bovis BCG (BCG Japan and BCG Pasteur).
Six to eight weeks after sensitization, animals were injected intradermally with 2 μg of purified antigen in 0.1 ml of phosphate-buffered saline. Each animal was also injected with 1 μg of PPD to control for sensitization. Single antigens of known DTH activity were also included as sensitization controls because, due to the complex composition of PPD, low levels of sensitization may still result in a sizable reaction to PPD with little or no reaction to single antigens (our unpublished observations). MPT64 was used as a sensitization control in the experiment that utilized BCG Japan, a substrain that produces the M. bovis homolog MPB64 (15, 17). In a second experiment that utilized BCG Pasteur, a substrain that does not produce MPB64 (15, 17), we used MPT32 as a sensitization control. The MPT32 antigen of M. tuberculosis (14, 20) elicits DTH reactions both in animals sensitized with mycobacteria of the M. tuberculosis complex and in those sensitized with nontuberculous mycobacteria (18). Skin reactions (diameter of erythema, in millimeters) were measured 24 h after antigen injection.
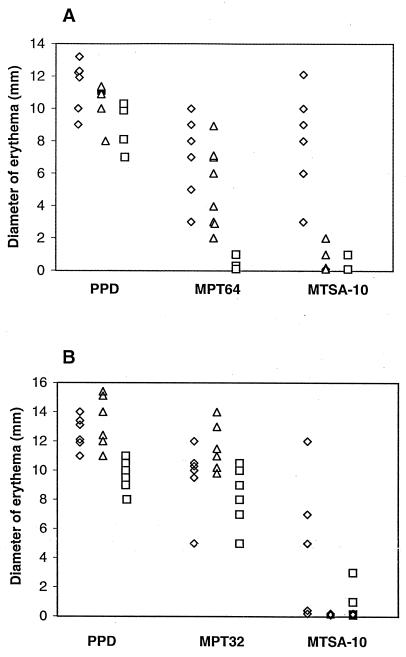
Measurement of skin reactions to PPD and to the control single antigens (MPT64 and MPT32) indicated that all animals were sensitized (Fig. 1). As expected, the control MPT64 antigen, which is specific for the M. tuberculosis complex (2), induced a strong response by the TB and BCG groups, but not by the M. avium group (Fig. 1A). In contrast, the control MPT32 antigen, which is cross-reactive (18), induced a strong response in the TB, BCG, and M. avium groups (Fig. 1B).
FIG. 1.
The DTH response to MTSA-10 distinguishes TB infection from immunization with BCG or with M. avium. For skin test experiments, M. tuberculosis H37Rv was grown to mid-log phase by shaking at 37°C in liquid glycerol-alanine-salts medium with 0.05% (vol/vol) Tween 80 in a biosafety level 3 laboratory. M. bovis BCG Japan and BCG Pasteur were grown in Sauton's medium with 0.025% (vol/vol) tyloxapol at 37°C. M. avium TMC 724 was grown by shaking at 37°C in Middlebrook 7H9 (Difco) liquid medium with 2% glycerol, 10% oleic acid-albumin-dextrose-catalase supplement, and 0.05% (vol/vol) Tween 80. PPD from M. tuberculosis was prepared at Colorado State University by using a standard protocol (25) and was applied to a Detoxi-gel column (Pierce, Rockford, Ill.) to remove the lipopolysaccharide. Purification of native MPT32 from M. tuberculosis H37Rv was performed by the method of Dobos et al. (8). Purification of recombinant MPT64 from E. coli was described previously (6). Guinea pigs were aerosol infected with M. tuberculosis (◊) or were immunized with BCG (▵) or with M. avium (□). Six to eight weeks later, animals were tested intradermally with 1 μg of PPD and 2 μg of purified recombinant antigens. Each point represents 1 animal. Results are expressed as diameter of erythema measured 24 h after antigen injection. (A) In a first experiment, the group sizes were six animals for M. tuberculosis, eight for M. bovis BCG Japan, four for M. avium, and four for saline. Antigens were PPD, MPT64 (BCG Japan produces the M. bovis homolog MPB64; see text), and MTSA-10. (B) In a second experiment, the group sizes were six animals for M. tuberculosis, six for M. bovis BCG Pasteur, six for M. avium, and five for saline. Antigens were PPD, MPT32 (BCG Pasteur does not produce MPB64; see text), and MTSA-10.
The Rv3874 gene product elicited DTH responses in the M. tuberculosis-infected animals, but not in the BCG- and M. avium-sensitized controls (Fig. 1), indicating that the DTH response to this antigen is TB specific. In the second experiment, three animals in the TB group showed no reactivity to the Rv3874 gene product (Fig. 1B). The biological basis of such animal-to-animal variability, which has been previously observed in the DTH response of guinea pigs to other antigens of M. tuberculosis (9), is presently unknown.
Since the Rv3874 gene product elicits DTH responses specific for TB, we call this protein MTSA-10 (for M. tuberculosis-specific antigen 10) and the corresponding gene mtsa-10.
Genome analysis of the mtsa-10 gene.
The distribution of the mtsa-10 gene in tuberculous and nontuberculous mycobacteria (listed in Table 1) was analyzed by Southern transfer hybridization. The presence of the mtsa-10 gene was visualized as a single hybridization signal of approximately 4 kb (data not shown), indicating that the mtsa-10 gene is present as a single copy in the mycobacterial chromosome. The mtsa-10 gene was found in M. tuberculosis (two laboratory strains and two clinical isolates), in virulent M. bovis, and in Mycobacterium africanum. As expected, the gene was absent from all BCG substrains (Table 2). The mtsa-10 gene was found in only 2 of 13 species of nontuberculous mycobacteria tested, suggesting a limited gene distribution. The mtsa-10 gene was notably absent from M. avium, the most common human pathogen among nontuberculous mycobacteria (10). Among other nontuberculous mycobacteria causing human disease with any significant frequency (10), the mtsa-10 gene was found in Mycobacterium kansasii but not in Mycobacterium fortuitum or Mycobacterium scrofulaceum (Table 2).
TABLE 1.
Mycobacterial strains used in this study
| Species | Straina |
|---|---|
| M. tuberculosis complex | |
| M. tuberculosis H37Rv | ATCC 25618 |
| M. tuberculosis H37Ra | ATCC 35836 |
| M. tuberculosis | CDC 1551 |
| M. tuberculosis W | PHRI TN3742 |
| M. bovis NADL | TMC 410 |
| M. bovis Ravenel | TMC 401 |
| M. bovis Branch | TMC 407 |
| M. bovis BCG Pasteur | ATCC 35734 |
| M. bovis BCG Japan | ATCC 35737 |
| M. bovis BCG Connaught | ATCC 35745 |
| M. bovis BCG Montreal | ATCC 35735 |
| M. bovis BCG Russia | ATCC 35736 |
| M. africanum | ATCC 5122 |
| Other mycobacteria | |
| M. asiaticum | TMC 803 |
| M. avium | TMC 724 |
| M. fortuitum | TMC 1530 |
| M. gastri | TMC 1456 |
| M. haemophilum | ATCC 29548 |
| M. kansasii | TMC 1201 |
| M. malmoense | TMC 802 |
| M. phlei | ATCC 11758 |
| M. scrofulaceum | ATCC 1302 |
| M. simiae | TMC 1226 |
| M. terrae | TMC 1450 |
| M. triviale | TMC 1453 |
| M. ulcerans | TMC 1615 |
ATCC, American Type Culture Collection; CDC, Centers for Disease Control and Prevention; PHRI, Public Health Research Institute; TMC, Trudeau Mycobacterial Collection.
TABLE 2.
Distribution of mtsa-10 and esat-6 among mycobacteria
| Species and strain | Presence ofa:
|
|
|---|---|---|
| mtsa-10 | esat-6 | |
| M. tuberculosis W | + | + |
| M. tuberculosis H37Ra | + | + |
| M. tuberculosis H37Rv | + | + |
| M. tuberculosis CDC 1551 | + | + |
| M. africanum | + | + |
| M. bovis NADL | + | + |
| M. bovis Ravenel | + | + |
| M. bovis Branch | + | + |
| BCG Pasteur | − | − |
| BCG Japan | − | − |
| BCG Connaught | − | − |
| BCG Montreal | − | − |
| BCG Russia | − | − |
| M. asiaticum | − | − |
| M. avium | − | − |
| M. fortuitum | − | − |
| M. gastri | + | + |
| M. haemophilum | − | − |
| M. kansasii | + | + |
| M. malmoense | − | − |
| M. phlei | − | − |
| M. scrofulaceum | − | − |
| M. simiae | − | − |
| M. terrae | − | − |
| M. triviale | − | − |
| M. ulcerans | − | − |
+, presence of hybridization signal; −, absence of hybridization signal. Mycobacterial strains were cultured at 36°C in an atmosphere containing 5% CO2 on Middlebrook 7H10 (Difco) agar plates containing 0.5% (vol/vol) glycerol and supplemented with 10% (vol/vol) albumin-dextrose-catalase. An exception was M. haemophilum, which was cultured on chocolate-agar plates at 30°C. The culture medium for M. africanum was supplemented with 0.5% (wt/vol) pyruvic acid. Cells were harvested after 4 days of culturing for fast-growing mycobacteria and 4 weeks of culturing for slow-growing mycobacteria. DNA isolation and Southern transfer hybridization were performed according to a standard protocol employed in DNA fingerprinting of M. tuberculosis (28).
Since mtsa-10 maps adjacent to esat-6 and since the two genes are cotranscribed (5), we compared the distribution of the two genes among mycobacteria. The distribution of mtsa-10 matched that of esat-6 (Table 2). The results obtained with esat-6 confirm and extend earlier studies with nontuberculous mycobacteria (12).
In conclusion, we show that the product of the mtsa-10 gene of M. tuberculosis elicits a strong DTH response in guinea pigs infected with M. tuberculosis but not in animals immunized with M. bovis BCG or M. avium. The characterization of MTSA-10 as an M. tuberculosis-specific skin test antigen advances our pursuit of a new tuberculin that will allow discrimination by skin testing of latent M. tuberculosis infection from vaccination with BCG or sensitization with environmental, nontuberculous mycobacteria.
Acknowledgments
We thank Patrick J. Brennan for guidance in establishing a collaborative project between PHRI and CSU, Alan Roberts for aerosol infection of guinea pigs, Elisa French and Julia Granowski for excellent animal care at the Painter Center at CSU, Barry Kreiswirth for advice on genome analyses, and Karl Drlica for comments on the manuscript.
This work was supported by NIH grant AI-36896 (M.L.G.) and by NIH/NIAID contract NO1-AI-75320, “Tuberculosis Research Materials and Vaccine Testing.” R.C. was the recipient of a fellowship on tuberculosis research from the Istituto Superiore di Sanità, Rome, Italy.
REFERENCES
- 1.Advisory Council for the Elimination of Tuberculosis. Screening for tuberculosis and tuberculosis infection in high-risk populations. Recommendations of the Advisory Council for the Elimination of Tuberculosis. Morbid Mortal Weekly Rep. 1995;44(RR-11):18–34. [PubMed] [Google Scholar]
- 2.Andersen Å B, Kjungqvist L, Hasløv K, Bentzon M W. MPT64 possesses “tuberculosis-complex”-specific B- and T-cell epitopes. Scand J Immunol. 1991;34:365–372. doi: 10.1111/j.1365-3083.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 3.Bass J B., Jr . The tuberculin test. In: Reichmann L B, Hershfield E S, editors. Tuberculosis. A comprehensive international approach. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 139–148. [Google Scholar]
- 4.Bates J H. The tuberculin skin test and preventive treatment for tuberculosis. In: Rom W N, Garay S, editors. Tuberculosis. Boston, Mass: Little, Brown and Co.; 1996. pp. 865–872. [Google Scholar]
- 5.Berthet F-X, Rasmussen P B, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 6.Colangeli R, Heijbel A, Williams A, Manca C, Chan J, Lyashchenko K, Gennaro M L. Three-step purification of lipopolysaccharide-free, polyhistidine-tagged recombinant antigens of Mycobacterium tuberculosis. J Chromatogr B. 1998;714:223–235. doi: 10.1016/s0378-4347(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 7.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Dobos K M, Swiderek K, Khoo K-H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elhay M J, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66:3454–3456. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grange J. Other mycobacterial diseases. In: Grange J, editor. Mycobacteria and human disease. London, England: Arnold; 1996. pp. 188–203. [Google Scholar]
- 11.Harboe M, Nagai S, Patarroyo M E, Torres M L, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landi S. Production and standardization of tuberculin. In: Kubica G P, Wayne L G, editors. The mycobacteria: a sourcebook, part A. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 505–535. [Google Scholar]
- 14.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Ulstrup J C, Jonassen T Ø, Melby K, Nagai S, Harboe M. Evidence for absence of the MPB64 gene in some substrains of Mycobacterium bovis BCG. Infect Immun. 1993;61:1730–1734. doi: 10.1128/iai.61.5.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyashchenko K, Manca C, Colangeli R, Heijbel A, Williams A, Gennaro M L. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect Immun. 1998;66:3606–3610. doi: 10.1128/iai.66.8.3606-3610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manca C, Lyashchenko K, Colangeli R, Gennaro M L. MTC28, a novel 28-kilodalton proline-rich secreted antigen specific for the Mycobacterium tuberculosis complex. Infect Immun. 1997;65:4951–4957. doi: 10.1128/iai.65.12.4951-4957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manca C, Lyashchenko K, Wiker H G, Usai D, Colangeli R, Gennaro M L. Molecular cloning, purification, and serological characterization of MPT63, a novel antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1997;65:16–23. doi: 10.1128/iai.65.1.16-23.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy H A, Mustafa A S, Jensen A K, Holm A, Rosenkrands I, Oftung F, Olobo J, von Reyn F, Andersen P. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 23.Schoel B, Gulle H, Kaufmann S H E. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts to Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect Immun. 1992;60:1717–1720. doi: 10.1128/iai.60.4.1717-1720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siebert F, Munday B. The chemical composition of the active principle of tuberculin. XV. A precipitated purified tuberculin protein suitable for the preparation of a standard tuberculin. Am Rev Tuberc. 1932;25:724–737. [Google Scholar]
- 25.Siebert F B, Glenn J T. Tuberculin purified protein derivative: preparation and analysis of a large quantity for standard. Am Rev Tuberc. 1941;44:9–25. [Google Scholar]
- 26.Sørensen A L, Nagai S, Houen G, Andersen P, Andersen Å B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S H. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. . (Erratum, 29:725, 1999.) [DOI] [PubMed] [Google Scholar]
- 28.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi R, Matsuo K, Yamazaki A, Abe C, Nagai S, Terasaka K, Yamada T. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG. Infect Immun. 1989;57:283–288. doi: 10.1128/iai.57.1.283-288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]