Abstract
Background:
To define the location of the initial contralateral lymph node (LN) metastasis in patients with oropharynx cancer.
Methods:
The location of the LN centroids from patients with oropharynx cancer and a single radiographically positive contralateral LN was defined. A clinical target volume (CTV) inclusive of all LN centroids was created, and its impact on dose to organs at risk was assessed.
Results:
We identified 55 patients of which 49/55 had a single contralateral LN in level IIA, 4/55 in level III, 1/55 in level IIB and 1/55 in the retropharynx. Mean radiation dose to the contralateral parotid gland was 15.1 Gy and 21.0 Gy, (p < 0.001) using the modelled high-risk elective CTV and a consensus CTV, respectively.
Conclusions:
We present a systematic approach for identifying the contralateral nodal regions at highest risk of harboring subclinical disease in patients with oropharynx cancer that warrants prospective clinical study.
Keywords: oropharynx cancer, radiotherapy, elective, lymph nodes, toxicity
Introduction
Patients with human papillomavirus positive oropharyngeal squamous cell carcinomas (HPV+OPSCC) represent a favorable patient cohort with overall survival rates of 70–100% at 3-years depending on patient and disease characteristics 1–5. Comprehensive head and neck radiochemotherapy (RCT) remains standard of care for patients with locally advanced HPV+OPSCC 6. Despite modern RT techniques and improvement in supportive care measures, significant treatment related side-effects remain 4,5. Therefore, it is important to define management strategies that maintain excellent clinical outcomes while effectively reducing the risk of chronic toxicities.
Completed trials and studies in progress focus on several different thematic approaches to reduce the side-effects of RCT. These include replacing cisplatin with cetuximab 4,5, induction chemotherapy selection of patients with favorable responses followed by reduced RT doses 7, reduced-doses of RT and chemotherapy 3,8, accelerated RT without concurrent chemotherapy 9, minimally invasive surgery followed by de-intensified risk-adapted adjuvant therapy 10,11, and immunotherapy in the upfront concurrent setting 12.
The concept of RT volume reduction as a technique to reduce toxicity has been explored albeit to a lesser degree than the described approaches 13. One volume reduction approach is to strategically eliminate contralateral elective nodal treatment volumes in well-lateralized tonsil tumors with a minimal ipsilateral nodal burden 14. This approach is supported surgical series that report very low rates of contralateral failure in patients undergoing ipsilateral only neck dissection without adjuvant therapy 15. However, in patients undergoing definitive RCT, the majority receive elective contralateral nodal irradiation 5,9. Therefore, identifying approaches to reduce treatment volume in the contralateral elective neck has the potential to reduce toxicities in a significant percentage of patients with HPV+OPSCC.
We hypothesized that an empirically defined elective treatment volume would target the location at highest risk of containing occult contralateral nodal disease and reduce radiation dose to organs at risk. To this end, we used patients with OPSCC and a single radiographically positive contralateral LN to define a high-risk elective contralateral nodal volume. We compared the dose to organs at risk between the modeled CTV and a consensus guideline defined treatment volume 16.
Material and Methods
Patient selection
Retrospective review of patient data was approved by the University of Wisconsin-Madison and Cleveland Clinic Institutional Review Boards. Patients with histologically confirmed squamous cell carcinoma of the tonsil or base of tongue and a single radiographically positive contralateral LN were included in the analysis. Radiographically positive contralateral LNs were defined during Head and Neck tumor boards where the size, shape, contrast enhancement characteristics, lack or presence of a fatty hilum, and metabolic activity of the node were considered. Common characteristics used to define clinically involved LNs included a maximum standardized uptake value (SUVmax) > 3.0 and/or short-axis diameter > 1.5 cm for level II, > 1.0 cm for levels IB, III, IV, and V, > 0.8 cm for retropharyngeal lymph nodes and/or central necrosis and/or heterogeneous enhancement. LN drainage is similar between HPV/p16-negative and HPV/p16-positive cancers 17. Therefore HPV/p16 status was reported but not required. AJCC 7th edition was used for overall clinical, T- and N- staging of all patients.
Defining contralateral LN centroid location and high-risk CTV
For each patient, the single radiographically positive contralateral LN was contoured. The LN centroid (center of the LN) was defined using MIM software (MIM Inc., Cleveland, OH). The location of each LN centroid was classified as belonging to level IB, II, III, IV, V or lateral retropharyngeal as previously defined 16. Level II was subdivided into IIA and IIB. Level IIA was limited superiorly by the caudal edge of the C1 lateral process, inferiorly by the caudal edge of the hyoid bone, anteriorly by the posterior edge of the submandibular gland or posterior edge of the posterior belly of the digastric muscle, laterally by the medial surface of the sternocleidomastoid muscle or platysma, medially by the medial edge of the internal carotid artery, and posteriorly by the by posterior edge of the internal jugular vein. Level IIB included the same superior, inferior, lateral, and medial borders of IIA with an anterior border that was defined by the posterior edge of the internal jugular vein and a posterior border of the posterior edge of the sternocleidomastoid muscle 16,18–20.
Unique distances and angles between the anatomic structures of the neck between patients prohibited direct mapping of LN centroids onto a single representative CT scan. Therefore, the extreme superior, inferior, anterior, posterior, lateral, and medial location of the LN centroids within their respective LN station was defined. The extreme location of LN centroids within each LN station was used to contour the high-risk CTV on a single CT neck scan.
Defining factors associated with LN location
We evaluated the association of primary tumor and LN characteristics with the location of the radiographically positive contralateral LN. Primary tumor characteristics that were included in the analysis were tumor location (tonsil versus base of tongue), location of the primary tumor with regard to midline (reaches/crosses versus lateralized), and maximum dimension (< 4 cm). LN characteristic that were assessed included number of clinically involved ipsilateral LN (0–2 versus > 2), the presence of an ipsitlateral LN in level III, and size of the largest ipsilateral LN (> 2 cm). Extranodal extension was not included in the analysis because of limited radiographic positive predictive value 21.
Treatment planning
Ten patients with OPSCC (5 tonsil and 5 base of tongue) with N0–2b disease (AJCC 7th) who were previously treated with elective contralateral cervical nodal RT were re-planned using the modeled high-risk elective CTV and a consensus CTV 16. Previous primary tumor and involved ipsilateral neck treatment volumes were unchanged. We adjusted the elective contralateral nodal volume to included either the RTOG consensus cervical nodal volumes II-IV or the modelled elective contralateral volume as described. The primary tumor and involved nodes were contoured to create a high-risk gross tumor volume that received 70 Gy (GTV70). As per our institutional practice and supporting published literature, the GTV70 was expanded by 3mm to create a planning target volume (PTV70) without an intermediate CTV expansion 22–25. Intermediate- and low-risk elective CTV nodal volumes received 60 Gy and 52.5 Gy, respectively, in 33 fractions. All CTVs were expanded by 3 mm to create respective PTVs. Tomotherapy-based intensity modulated RT plans were generated using a commercially available planning software (Pinnacle, Philips Healthcare, Andover, MA). Planning parameters included a field width of 2.512 cm, dynamic jaw mode, a modulation factor of 3 and pitch of 0.267. Plans were optimized to ensure that 95% of each PTV was covered by 100% of the prescribed dose.
Normal tissue complication probability (NTCP) analysis
The NTCP for the contralateral parotid gland was compared between plans using the modelled CTV and a consensus CTV using NTCP parameters that have been previously described 26,27. Volumetric dose distributions for differing fractionation schemes were converted voxel-wise to iso-effective dose in 2 Gy fraction equivalent (EQD2) using the linear quadratic formula with α/β of 2.5. NTCP for the contralateral parotid gland was then calculated based on the volumetric mean EQD2 dose using the Lyman model with n of 1, m of 0.4, and TD50 of 39.9 Gy 28,29. The minimal clinically important difference in treatment-related toxicity is expected from a mean change in NTCP of > 5%, as previously described 27,30.
Statistical Analysis
Patient and disease characteristics were reported with descriptive statistics. Factors associated with the location of the radiographically positive contralateral LN were assessed by the Fisher exact test. Based on our institutional data, we assumed an average parotid dose of 22 Gy for the consensus CTV that would be reduced by 25% using the modelled CTV. Ten patients provided us 80% power with an alpha of 0.5 to detect this difference. The t-test was used to compare dosimetric data of dose to organs at risk using different elective contralateral CTVs. A p-value of > 0.05 was considered significant.
Results
Fifty-five patients with OPSCC and a single radiographically positive contralateral LN were identified for analysis. Patient and disease characteristics are shown in Table 1. Median age was 59 years (range, 35 – 76 years). Approximately two-thirds of patients had a smoking history with a reported median of 29 pack-years. Base of tongue was the most common primary tumor at 65% and cT2-T4 comprised 95% of the total patient cohort. For patients with known HPV/p16-status, 76% were positive.
Table 1.
Patient and disease characteristics
| UW n=25, (%) | CC n=30, (%) | Total n=55 | |
|---|---|---|---|
| Age | |||
| Median | 57 | 60 | 59 |
| Range | 43–76 | 35–76 | 35–76 |
| Sex | |||
| Male | 21 (84) | 30 (100) | 51 (93) |
| Race | |||
| White | 25 (100) | 26 (87) | 51 (93) |
| Black | 0 (0) | 4 (13) | 4 (7) |
| Smoking history | |||
| Never | 9 (36) | 9 (30) | 18 (33) |
| Previous/Current (median pack-years) | 25 | 39 | 29 |
| Tumor site | |||
| Tonsil | 9 (36) | 10 (33) | 19 (35) |
| Base of tongue | 16 (64) | 20 (67) | 36 (65) |
| Clinical T-stage | |||
| 1 | 1 (4) | 2 (7) | 3 (5) |
| 2 | 14 (56) | 11 (37) | 25 (45) |
| 3 | 3 (12) | 8 (27) | 11 (20) |
| 4 | 7 (28) | 9 (39) | 16 (29) |
| HPV/p16-status | |||
| Positive | 16 (64) | 26 (87) | 42 (76) |
| Negative | 4 (16) | 3 (10) | 7 (13) |
| Unknown | 5 (20) | 1 (3) | 6 (11) |
Contralateral LN mapping
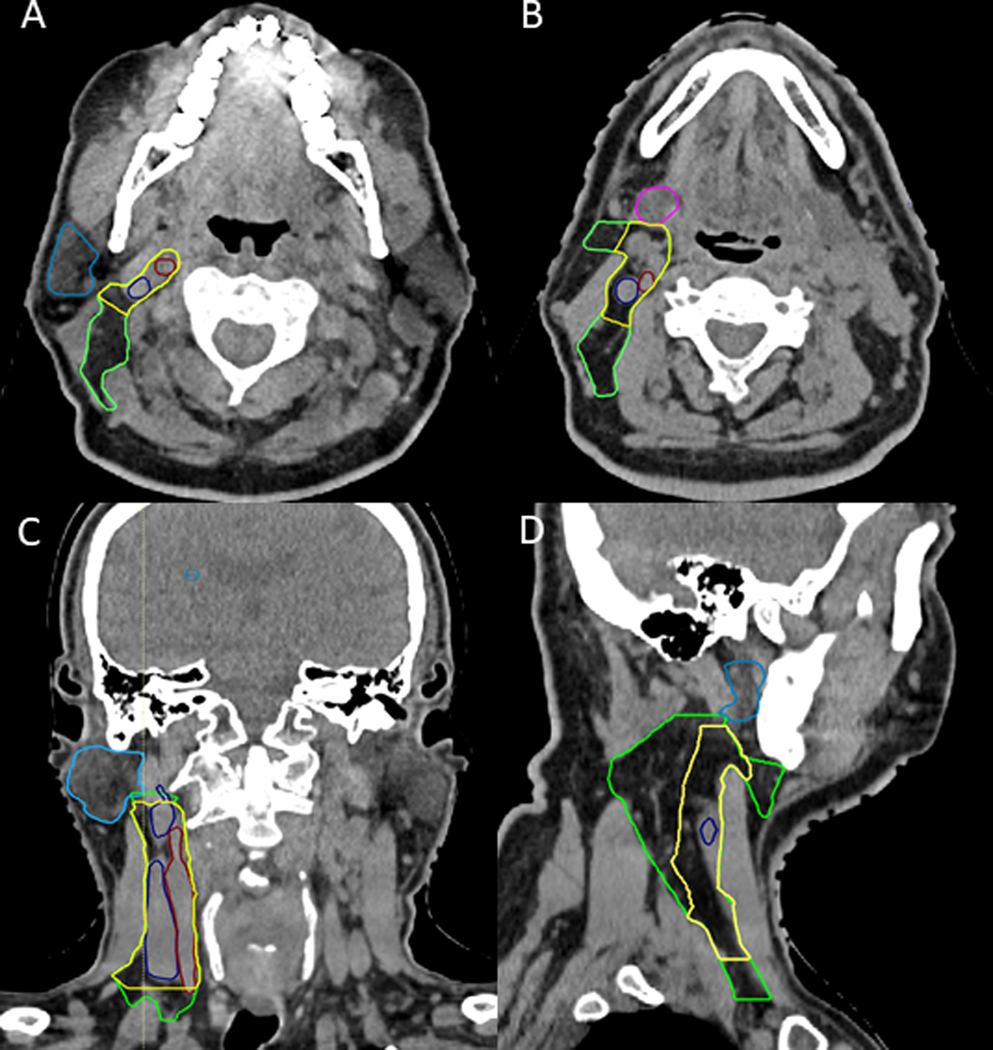
Level IIA was involved in 89% (49/55) of cases, 7% (4/55) of patients had a level III and 2% (1/55) of patients had either a level IIB or lateral retropharyngeal. The most superior LN centroid in level IIA was located 5 mm below the inferior surface of the C1 transverse process. There were no level IIA LNs that were lateral to the edge of the sternocleidomastoid muscle. There was a single LN medial to the internal carotid artery. This lateral retropharyngeal node was identified in a patient with a large primary tumor with extensive posterior pharyngeal wall involvement. The single level IIB LN centroid was within 5mm from the posterior edge of the internal jugular vein. There were no predictors for an initial contralateral level III LN. The modeled high-risk elective CTV that encompassed all contralateral LNs except for the lateral pharyngeal node was created and contoured on axial slices of neck CT with a consensus elective nodal CTV drawn for comparison (Figure 1A–D).
Figure 1.
CT neck slices in the axial (A and B), coronal (C), and sagittal (D) view demonstrating the differences between the modeled high-risk elective contralateral CTV (yellow) and the RTOG defined consensus elective neck contour (green) containing contours of the modeled CTV (green) and consensus CTV (yellow). The modeled high-risk CTV is defined by the extreme location of all LN centroids in each respective LN station. The internal jugular vein (dark blue), internal carotid artery (red), parotid gland (light blue), and submandibular gland (pink) are contoured for reference.
Dosimetric impact of the modeled high-risk elective CTV
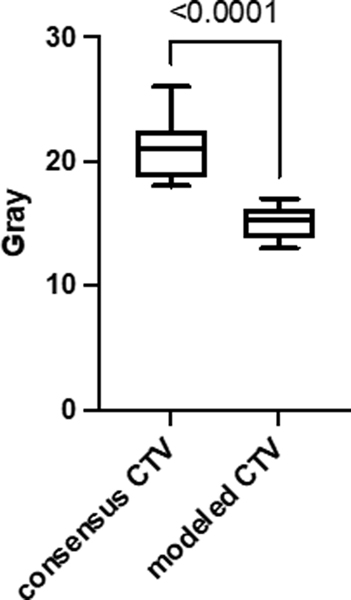
We examined the dosimetric impact of the modeled high-risk elective CTV on contralateral organs at risk from 10 patients, 5 base of tongue and 5 tonsil primaries, who previously received elective contralateral radiation. The mean dose and range for the contralateral parotid gland using the modeled high-risk CTV was 15 Gy (13 Gy – 17 Gy) and 22 Gy (18 Gy – 26 Gy) using a consensus CTV (p < 0.0001). A significant reduction was seen in NTCP for the contralateral parotid gland using the modeled CTV (p < 0.001). The NTCP mean for consensus CTV was 6.9% (range: 4.9% – 10.7%) and 3.6% (range: 2.6% – 4.3%) for the modeled high-risk CTV (Figure 3). This corresponded to a mean reduction of NTCP for the contralateral of 52%. Dose differences for the contralateral submandibular gland (p = 0.05), residual constrictors (p = 0.05) and esophagus (p = 0.06) approached but did not meet the predefined level of significance.
Discussion
Comprehensive head and neck radiation is associated with a spectrum of acute and chronic toxicities 4,31,32. We propose a modeled high-risk elective contralateral CTV (for the N0 neck) based on the location of known contralateral metastatic LNs in oropharynx cancer patients that resulted in an improvement in NTCP for the contralateral parotid gland. This minor modification in treatment technique for the N0 neck may favorably impact toxicity and long-term quality of life while successfully maintaining regional disease control.
The vast majority of local and regional failures in patients with HPV+OPSCC occur within high- and intermediate-dose treatment volumes while a minority occur in low-dose elective or untreated nodal stations 22. As such, radiotherapy field design and dose delivery should focus on gross disease and areas at highest risk of harboring subclinical disease. In patients with HPV+OPSCC warranting coverage of the contralateral N0 neck, historical standards have dictated coverage of the entire fat space encompassed by cervical nodal stations II-IV. The actual benefit of such broad coverage in patients with OPSCC at risk of contralateral dissemination is not clear. The hypothesis of this study is that the initial ipsilateral metastatic node location predicts the highest location likelihood for subclinical disease in the contralateral N0 neck. A further assumption in this study is that there are not multiple nodes containing microscopic disease in the clinically negative N0 neck. This is supported by a recent surgical series demonstrating that 75% of patients with HPV-positive base of tongue primaries found to have occult contralateral disease had a single node 33.
We used retrospective data to define the location of the initial radiographically positive contralateral LN in patients with oropharynx cancer. The resultant CTV established through the combination of these locations generated a volume that was 40% smaller than the corresponding consensus CTV. Contralateral level IIA was the most common location. We did not identify any contralateral LNs in levels IB, IV, or V. A single LN was identified in the contralateral retropharyngeal space, which is consistent with previous reports 34,35. Given this finding, caution in applying the modeled contralateral CTV in patients with extensive posterior pharyngeal wall involvement should be used. Dosimetric analysis of patients with either a tonsil or base of tongue primary planned using a consensus CTV compared to the modeled CTV demonstrated significant reduction in dose to organs at risk specifically the contralateral parotid gland, which exhibited a 32% reduction in mean dose and greater than 50% reduction in NTCP suggesting the potential to decrease the severity of treatment-related xerostomia 26. We recognized that the size and location of the primary tumor as well as the burden of ipsilateral nodal disease affects dose to contralateral organs at risk. Despite this limitation, these data support an elective volume reduction technique that may significantly improve the therapeutic index of comprehensive head and neck irradiation for patients with HPV+OPSCC.
Despite surgical data demonstrating limited contralateral failures in patients with early stage non-lateralized oropharynx cancer receiving ipsilateral only neck dissection and guidelines to support unilateral treatment of lateralized tonsil tumors with limited ipsilateral nodal disease burden 14,15, data from recent large, randomized trials suggests that most patients with HPV+OPSCC receive bilateral nodal irradiation. In De-Escalate, despite tonsil cancers accounting for 64% of the population, 80% received bilateral radiotherapy 5. Forty-four percent of patients enrolled in RTOG 1016 had base of tongue primaries while 50% had tonsil tumors suggesting that greater than half of all patients enrolled would have received bilateral neck radiotherapy 4. In the more recent cooperative group study HN002, 85.3% of patients received elective contralateral irradiation. Therefore, given the incidence of bilateral radiotherapy for highly curable HPV+OPSCC patients, reduction in contralateral parotid dose using a smaller contralateral elective target volume would represent a highly inclusive toxicity reducing technique for patients with HPV+OPSCC.
Therapy de-intensification for patients with HPV+OPSCC must be balanced with preserving the expected excellent clinical outcomes. To date, efficacy of therapy de-intensification has been mixed. In patients with low risk factors such as < T4 and a limited smoking history, reduction in both radiotherapy and chemotherapy is effective 3. However, clinical outcomes for patients with high-risk features such as advanced T and N stage and heavy smoking history resulted in decreased progression free survival when treated with therapy de-intensification 7. Consequently, a number of studies have excluded patients with high-risk factors 5,36. The proposed therapy de-intensification approach suggested would likely not be affected by tumor and nodal burden or smoking history and therefore could be considered for a broader cohort of patients. However, this assumption needs to be confirmed prospectively.
Elective nodal irradiation volume reduction is currently being developed as a harm-minimization approach for patients with oropharynx cancer. Sher et al., recently reported no elective regional failures in patients with non-well-lateralized primary oropharynx tumors receiving elective contralateral radiation to level II. This approach resulted in a reduction in the contralateral parotid gland dose to 16.9 Gy compared to 22.9 Gy to the ipsilateral superficial parotid gland 37. A similar approach is being evaluated in the Canadian Evader trial for patients with low-risk HPV-positive OPSCC (NCT03822897). These studies support our data. In our experience, parotid gland dose is affected more by level IIB than IIA. Prospective study will evaluate the impact of reducing level II coverage to level IIA and the anterior most portion of IIB.
This study has several limitations. First, it is retrospective in nature and defining the ultimate impact on regional control and toxicity will require prospective clinical evaluation. In addition, the total number of patients evaluated was limited. This is likely given the percentage of patients that present with contralateral nodal disease defined by a single positive node However, given that nearly 90% of patients had a single radiographically positive contralateral LN in level IIA, it is unlikely that additional patients would change the LN distribution. Second, the definition of a positive contralateral LN was based on radiologic findings rather than pathologic data. Lastly, although unlikely, it is unclear if prior resection of the primary tumor and dissection of the involved ipsilateral neck would influence contralateral drainage, which may limit this approach to patients undergoing non-surgical management.
Patients with HPV+OPSCC exhibit favorable clinical outcomes compared to those with HPV-negative disease. Patient selection and efforts to reduce toxicity while maintaining favorable outcomes are ongoing. We present a rational method to identify the contralateral N0 neck volume at highest risk for occult disease and a strategy to reduce elective neck volumes and contralateral parotid gland dose. Patterns of failure analyses and pathologic data suggest this approach would be effective in sterilizing occult contralateral regional disease. Prospective study is needed to confirm the impact of the modeled elective high-dose CTV on regional control and toxicity.
Figure 2.
Box and whisker plot of dose to the contralateral parotid gland from 10 oropharynx cancer patients planned with either a consensus CTV or modeled high-risk elective contralateral CTV.
Acknowledgments
Funding:
This work was supported in part by the NIH P50 DE026787- University of Wisconsin Head and Neck SPORE Grant.
Previous presentation:
This work was presented at the American Society of Radiation Oncology, 2018, San Antonio, TX, USA
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. [DOI] [PubMed] [Google Scholar]
- 3.Chera BS, Amdur RJ, Tepper JE, et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer. 2018;124(11):2347–2354. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colevas AD, Yom SS, Pfister DG, et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw. 2018;16(5):479–490. [DOI] [PubMed] [Google Scholar]
- 7.Marur S, Li S, Cmelak AJ, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35(5):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chera BS, Amdur RJ, Tepper J, et al. Phase 2 Trial of De-intensified Chemoradiation Therapy for Favorable-Risk Human Papillomavirus-Associated Oropharyngeal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2015;93(5):976–985. [DOI] [PubMed] [Google Scholar]
- 9.Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002). J Clin Oncol. 2021;39(9):956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma DJ, Price KA, Moore EJ, et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J Clin Oncol. 2019;37(22):1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swisher-McClure S, Lukens JN, Aggarwal C, et al. A Phase 2 Trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): Omission of the Resected Primary Tumor Bed After Transoral Robotic Surgery for Human Papilloma Virus-Related Squamous Cell Carcinoma of the Oropharynx. Int J Radiat Oncol Biol Phys. 2019. [DOI] [PubMed] [Google Scholar]
- 12.De-intensification Radiation Therapy With Chemotherapy (Cisplatin) or Immunotherapy (Nivolumab) in Treating Patients With Early-Stage, HPV-Positive, Non-Smoking Associated Oropharyngeal Cancer. [Google Scholar]
- 13.Villaflor VM, Melotek JM, Karrison TG, et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol. 2016;27(5):908–913. [DOI] [PubMed] [Google Scholar]
- 14.Huang SH, Waldron J, Bratman SV, et al. Re-evaluation of Ipsilateral Radiation for T1-T2N0-N2b Tonsil Carcinoma at the Princess Margaret Hospital in the Human Papillomavirus Era, 25 Years Later. Int J Radiat Oncol Biol Phys. 2017;98(1):159–169. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein GS, Quon H, O’Malley BW Jr., Kim GG, Cohen MA. Selective neck dissection and deintensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope. 2010;120(9):1749–1755. [DOI] [PubMed] [Google Scholar]
- 16.Gregoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–181. [DOI] [PubMed] [Google Scholar]
- 17.Bauwens L, Baltres A, Fiani DJ, et al. Prevalence and distribution of cervical lymph node metastases in HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma. Radiother Oncol. 2021;157:122–129. [DOI] [PubMed] [Google Scholar]
- 18.Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128(7):751–758. [DOI] [PubMed] [Google Scholar]
- 19.Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134(5):536–538. [DOI] [PubMed] [Google Scholar]
- 20.Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000;174(3):837–844. [DOI] [PubMed] [Google Scholar]
- 21.Patel MR, Hudgins PA, Beitler JJ, et al. Radiographic Imaging Does Not Reliably Predict Macroscopic Extranodal Extension in Human Papilloma Virus-Associated Oropharyngeal Cancer. ORL J Otorhinolaryngol Relat Spec. 2018;80(2):85–95. [DOI] [PubMed] [Google Scholar]
- 22.Burr AR, Harari PM, Ko HC, Bruce JY, Kimple RJ, Witek ME. Reducing radiotherapy target volume expansion for patients with HPV-associated oropharyngeal cancer. Oral Oncol. 2019;92:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burr AR, Harari PM, Haasl AM, et al. Clinical outcomes for larynx patients with cancer treated with refinement of high-dose radiation treatment volumes. Head Neck. 2020;42(8):1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caudell JJ, Meredith RF, Spencer SA, Keene KS, Dobelbower MC, Bonner JA. Margin on gross tumor volume and risk of local recurrence in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;76(1):164–168. [DOI] [PubMed] [Google Scholar]
- 25.Zukauskaite R, Hansen CR, Grau C, et al. Local recurrences after curative IMRT for HNSCC: Effect of different GTV to high-dose CTV margins. Radiother Oncol. 2018;126(1):48–55. [DOI] [PubMed] [Google Scholar]
- 26.Dijkema T, Raaijmakers CP, Ten Haken RK, et al. Parotid gland function after radiotherapy: the combined michigan and utrecht experience. Int J Radiat Oncol Biol Phys. 2010;78(2):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels SE, Eisbruch A, Vineberg K, et al. Methods for Reducing Normal Tissue Complication Probabilities in Oropharyngeal Cancer: Dose Reduction or Planning Target Volume Elimination. Int J Radiat Oncol Biol Phys. 2016;96(3):645–652. [DOI] [PubMed] [Google Scholar]
- 28.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16(6):1623–1630. [DOI] [PubMed] [Google Scholar]
- 29.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–19. [PubMed] [Google Scholar]
- 30.Binenbaum Y, Amit M, Billan S, Cohen JT, Gil Z. Minimal clinically important differences in quality of life scores of oral cavity and oropharynx cancer patients. Ann Surg Oncol. 2014;21(8):2773–2781. [DOI] [PubMed] [Google Scholar]
- 31.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110–1118. [DOI] [PubMed] [Google Scholar]
- 33.Last AS, Pipkorn P, Chen S, et al. Risk and Rate of Occult Contralateral Nodal Disease in Surgically Treated Patients With Human Papillomavirus-Related Squamous Cell Carcinoma of the Base of the Tongue. JAMA Otolaryngol Head Neck Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanguineti G, Pai S, Agbahiwe H, et al. HPV-related oropharyngeal carcinoma with Overt Level II and/or III metastases at presentation: The risk of subclinical disease in ipsilateral levels IB, IV and V. Acta Oncol. 2014;53(5):662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spencer CR, Gay HA, Haughey BH, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level II lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yom PT-S SS, Caudell JJ, Waldron JN, Gillison ML, Truong MT, Jordan R, Subramaniam R, Yao M, Chung C, Geiger JL, Chan J, O’Sullivan B, Blakaj DM, Mell LK, Thorstad WL, Jones CU, Banerjee RN, Lominska CE, Le QT NRG-HN002: A Randomized Phase II Trial for Patients with p16-Positive, Non-Smoking-Associated, Locoregionally Advanced Oropharyngeal Cancer. Int J Radiat Oncol Biol Phys. 2019;105(3):684–685. [Google Scholar]
- 37.Sher DJ, Pham NL, Shah JL, et al. Prospective Phase 2 Study of Radiation Therapy Dose and Volume De-escalation for Elective Neck Treatment of Oropharyngeal and Laryngeal Cancer. Int J Radiat Oncol Biol Phys. 2021;109(4):932–940. [DOI] [PubMed] [Google Scholar]