Sun et al. show that proteins with marginally hydrophobic signal sequences pause at the Sec61 translocon on their way into the ER and require Sec63/BiP to overcome the pause and facilitate subsequent protein folding in the ER, which prevents protein aggregation during BiP deficiency.
Abstract
One-third of newly synthesized proteins in mammals are translocated into the endoplasmic reticulum (ER) through the Sec61 translocon. How protein translocation coordinates with chaperone availability in the ER to promote protein folding remains unclear. We find that marginally hydrophobic signal sequences and transmembrane domains cause transient retention at the Sec61 translocon and require the luminal BiP chaperone for efficient protein translocation. Using a substrate-trapping proteomic approach, we identify that nascent proteins bearing marginally hydrophobic signal sequences accumulate on the cytosolic side of the Sec61 translocon. Sec63 is co-translationally recruited to the translocation site and mediates BiP binding to incoming polypeptides. BiP binding not only releases translocationally paused nascent chains but also ensures protein folding in the ER. Increasing hydrophobicity of signal sequences bypasses Sec63/BiP-dependent translocation, but translocated proteins are prone to misfold and aggregate in the ER under limited BiP availability. Thus, the signal sequence–guided protein folding may explain why signal sequences are diverse and use multiple protein translocation pathways.
Introduction
About 30% of newly synthesized proteins from cytosolic ribosomes are delivered to the ER by the co-translational protein targeting pathway (Juszkiewicz and Hegde, 2018). These nascent polypeptides typically contain cleavable N-terminal signal sequences (SSs) or non-cleavable first transmembrane domains (TMDs). As SSs emerge from ribosomes, they are co-translationally recognized and captured by the signal recognition particle (SRP; Shan and Walter, 2005). The SRP-bound ribosome-associated nascent chains (RNCs) are then delivered to the ER membrane by interacting with the SRP receptor (Jiang et al., 2008). The SS is then transferred from SRP to the heterotrimeric Sec61 translocon complex (Rapoport, 2007). The SS is again recognized and bound, this time by the Sec61 translocon, allowing the translocation of the following nascent polypeptide across the ER membrane.
SSs are diverse and significantly differ in length, hydrophobicity, charge, and flanking sequences but contain a hydrophobic core (h-region) of at least seven non-hydrophilic amino acids (von Heijne, 1985). In mammals, SRP can efficiently recognize a broad range of hydrophobic SSs in RNCs and target them to the ER (Hegde and Kang, 2008; Jungnickel and Rapoport, 1995; Kim et al., 2002; Voorhees and Hegde, 2015). However, the interaction between the Sec61 translocon and SSs significantly varies. Strong SSs, such as the one from prolactin (Prl), efficiently engage the Sec61 translocon in a looped orientation in which the h-region of SS is intercalated into the lateral gate of the Sec61 translocon with its N-terminus facing the cytosol, and the following nascent chain is inserted into the aqueous pore of the Sec61 channel (Jungnickel and Rapoport, 1995; Li et al., 2016; Voorhees and Hegde, 2016). The looped engagement of the SS to the Sec61 translocon drives forward translocation of the nascent chain into the ER lumen upon further translation elongation. However, many SSs inefficiently engage the Sec61 translocon in a non-looped orientation, thus requiring accessory factors to promote protein translocation into the ER (Hegde and Kang, 2008). Earlier studies have shown that translocon-associated factors TRAM, TRAP, Sec62/Sec63, and the luminal chaperone BiP assist in the translocation of selected substrates (Fons et al., 2003; Lang et al., 2012; Nguyen et al., 2018; Schorr et al., 2020; Tyedmers et al., 2003; Voigt et al., 1996). It is poorly understood how these accessory factors are recruited to the Sec61 translocon to promote protein translocation. A previous study detected the selective assembly of the Sec61 translocon with Sec62/Sec63 during the co-translational translocation of prion protein (PrP), suggesting that substrate-driven changes in the Sec61 complexes promote protein translocation into the ER (Conti et al., 2015).
If the role of SSs is merely to mediate protein targeting and translocation into the ER, it is unclear why SSs are diverse and evolved to use various accessory factors for efficient translocation into the ER. An important function of SSs is that protein translocation into the ER can be selectively attenuated in an SS-dependent manner during ER stress to reduce the protein folding burden in the ER (Kang et al., 2006). In some cases, the SS can regulate the timing of signal peptide cleavage, thereby enhancing protein maturation (Rehm et al., 2001; Rutkowski et al., 2003; Snapp et al., 2017). However, the factors and mechanisms involved in SS-guided protein maturation are less understood.
Similar to SSs, TMDs of most integral membrane proteins are recognized by the Sec61 translocon, and they are partitioned into the lipid bilayer through the lateral gate of the translocon (Hegde and Keenan, 2022). In some instances, the insertion of the first TMD of polytopic membrane proteins is assisted by the ER membrane protein complex, and the following TMDs are inserted by the Sec61 translocon (Chitwood et al., 2018). Again, TMD hydrophobicity plays a crucial role in partitioning from the lateral gate of the translocon to the hydrophobic lipid bilayer. Yet, TMDs of membrane proteins greatly vary in their hydrophobicity. It is estimated that ∼25% of TMDs of polytopic membrane proteins are marginally hydrophobic (Elofsson and von Heijne, 2007; White and von Heijne, 2008). Recent studies have shown that marginally hydrophobic TMDs can transiently stall in the interior pore of the Sec61 translocon (Kida et al., 2016; Kida and Sakaguchi, 2018). It is unclear how and which factors assist in clearing these TMDs retained at the Sec61 translocon during the co-translational membrane protein insertion. In some cases, less hydrophobic TMDs are completely translocated into the ER lumen (Feige and Hendershot, 2013; Ojemalm et al., 2012; Skach et al., 1994; Sun and Mariappan, 2020). They can either be retrieved by interactions with the membrane-embedded TMDs or targeted for degradation by the ER-associated protein degradation pathways (Buck et al., 2017).
Upon translocation into the ER lumen, nascent chains are folded with the assistance of molecular chaperones and folding enzymes (Braakman and Hebert, 2013; Fewell et al., 2001; Gidalevitz et al., 2013; Helenius et al., 1992; Ma and Hendershot, 2004). Among the broad spectrum of chaperones in the ER, the member of the Hsp70 family chaperone BiP ATPase plays a central role in shielding exposed hydrophobic residues in nascent proteins, thereby preventing protein misfolding and aggregation. BiP also assists in protein folding by cycling between high-affinity and low-affinity substrate binding through ATP-dependent conformational changes that are regulated by co-chaperones and nucleotide exchange factors in the ER (Pobre et al., 2019). Considering the essential roles of BiP in preventing protein misfolding and promoting folding, it is less understood how the translocating nascent chains are accurately matched to the availability of BiP in the ER lumen. Studies of cytosolic proteins have shown that the ribosome-associated complex recruits Hsp70 to assist the folding of aggregation-prone nascent polypeptides (Döring et al., 2017; Willmund et al., 2013). It remains to be shown whether a similar mechanism exists in the ER since the ribosome is separated from the translocating nascent chain by the ER membrane bilayer.
We show that Sec63 activates the BiP ATPase to mediate the release of marginally hydrophobic TMDs and SSs retained at the Sec61 translocon. The action of BiP drives the translocation of the weak TMD into the ER lumen, presumably for subsequent assembly or disposal. In contrast, BiP drives the engagement of weak SSs at the translocon’s lateral gate, allowing translocation and folding of the mature portion of the polypeptide in the ER. Despite these different outcomes, Sec63-mediated translocation of weak TMDs and nascent chains bearing a weak SS operate similarly because both rely on the ATPase activity of BiP. The coupling of BiP-dependent protein translocation with the subsequent protein folding is crucial for preventing protein misfolding/aggregation inside the ER, particularly under the conditions of limited BiP availability. Thus, we propose that evolution has optimized SSs to coordinate chaperone-mediated protein translocation and folding in the ER.
Results
Sec63/BiP clear marginally hydrophobic TMDs retained at Sec61 translocons
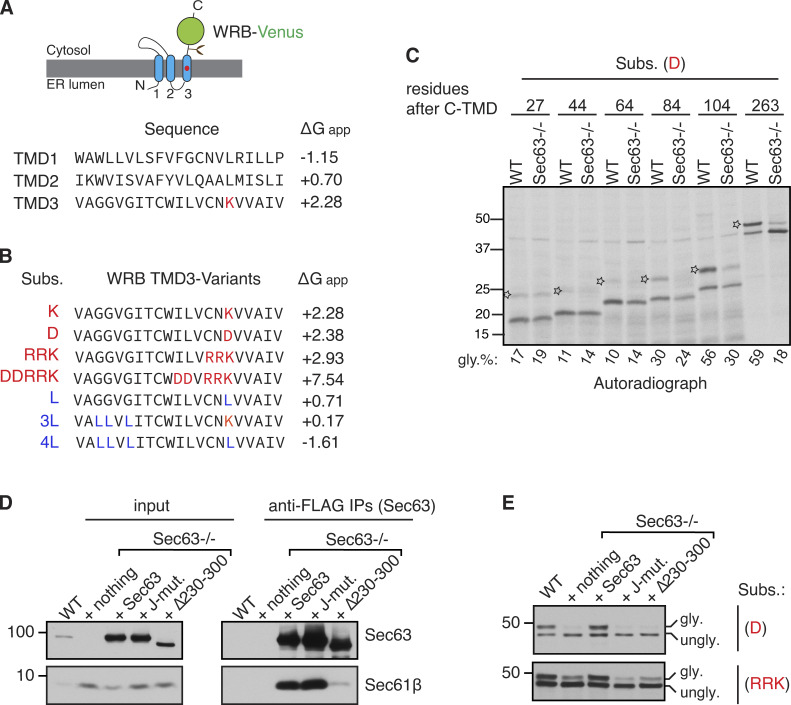
We first investigated how the Sec61 translocon deals with marginally hydrophobic (or weak) TMDs during membrane protein insertion into the ER membrane. Earlier studies have shown that such sequences are neither hydrophobic enough to be partitioned into the lipid bilayer through the lateral gate of the translocon nor sufficiently hydrophilic to be translocated into the ER lumen through the central pore of the translocon, leading to their transient retention at the Sec61 translocon (Kida et al., 2016; Kida and Sakaguchi, 2018). To investigate the mechanism by which the transiently retained weak TMD at the Sec61 translocon is cleared, we used our model C-terminally Venus-tagged substrates derived from WRB, a subunit of the tail-anchored membrane protein insertase (Fig. S1 A; Yamamoto and Sakisaka, 2012). We have previously shown that the unassembled C-terminal TMD (C-TMD) of WRB-Venus is translocated into the ER lumen because of a positively charged lysine residue (Fig. S1 A; Sun and Mariappan, 2020). The translocation of the C-TMD of WRB-Venus into the ER lumen can be further increased by incorporating more charged residues or reduced by adding more hydrophobic residues (Sun and Mariappan, 2020; Fig. S1 B). Consistent with our recent findings, substrates with marginally hydrophobic C-TMDs (K, D, RRK, and DDRRK) were poorly inserted into the ER membrane and were translocated into the ER lumen as evidenced by glycosylated bands (Fig. 1, A and B; and Fig. S1 B). By contrast, the substrate with sufficient hydrophobicity (L) was efficiently inserted into the ER membrane, as shown by the mostly unglycosylated form (Fig. 1, A and B).
Figure S1.
Characterization of Sec63-mediated clearance of translocon-occupied marginally hydrophobic TMDs. (A) A cartoon depicting the predicted topology of WRB fused with the C-terminus Venus tag. The red circle in the C-TMD (third TMD) indicates the charged lysine amino acid. The amino acid sequence and free energy prediction for all three TMDs of WRB are shown. The C-TMD has a high free energy (∆Gapp) value because it contains a positively charged lysine residue. (B) The C-TMD of WRB-Venus is mutated to either increase the free energy by introducing charged residues as specified in red color or reduce the free energy by introducing hydrophobic leucine residues as indicated in blue color (Sun and Mariappan, 2020). The free energy of all C-TMD variants was predicted as previously described (Hessa et al., 2007). (C) WT HEK293 or Sec63−/− cells were transfected with the indicated substrates with varying lengths of amino acids after their C-TMDs. Cells were metabolically labeled for 30 min and immunoprecipitated with anti-HA antibody beads. The immunoprecipitants were analyzed by autoradiography. The star symbol indicates the translocated/glycosylated form. (D) WT HEK293 or Sec63−/− cells stably expressing the indicated FLAG-tagged Sec63 constructs were lysed, immunoprecipitated with anti-FLAG beads, and analyzed by immunoblotting for indicated antigens. (E) The indicated cell lines were transfected with C-TMDs of substrates carrying the indicated charged amino acid(s). Transfected cells were directly analyzed by immunoblotting with anti-GFP antibodies for substrates. gly. indicates glycosylated forms. ungly. denotes non-translocated/unglycosylated forms with uncleaved SSs. Source data are available for this figure: SourceData FS1.
Figure 1.
Sec63/BiP are required for releasing marginally hydrophobic TMDs retained at the Sec61 translocon. (A) Diagram illustrating the translocon engaged with the marginally hydrophobic (or weak) C-TMD of WRB-Venus, which is then translocated into the ER lumen and is glycosylated. The C-TMD with sufficient hydrophobicity is correctly inserted into the ER membrane and, thereby, is not glycosylated. The red circle in the C-TMD indicates a charged residue. (B) Cells expressing C-TMDs of substrates bearing indicated charged residues (shown in red color) or hydrophobic residue (marked in blue color) were radiolabeled and analyzed by SDS-PAGE and autoradiography after IP of substrates with anti-GFP antibodies. The molecular weight measurements (kD) are listed beside the immunoblot and all other following immunoblots. gly. indicates glycosylated forms. ungly. denotes non-translocated/unglycosylated forms with uncleaved SSs. (C) The indicated substrates were expressed in chromosomally 2× Strep-tagged Sec61α cells, lysed, pulled down with Strep-Tactin beads, and analyzed by immunoblotting for the indicated antigens. The substrates were immunoblotted with anti-GFP antibodies. (D) Cells co-expressing the indicated versions of BiP and the substrate were analyzed by immunoblotting with anti-BiP or anti-GFP antibodies for substrates. The percent of glycosylation is shown below each panel. (E) WT HEK293, Sec62−/−, or Sec63−/− cells expressing the indicated substrates were radiolabeled and analyzed as in B. Immunoblots show the knockout of either Sec62 or Sec63. (F) WT HEK293 or Sec63−/− cells expressing the indicated substrates were immunoprecipitated with anti-GFP antibodies and analyzed by autoradiography. (G) Transcripts of the indicated substrates possessing or lacking a stop codon were in vitro translated in the RRL, including nothing or CRM. The asterisk symbol denotes tRNA-linked nascent chains. Transcripts with a stop codon produce free nascent chains that are likely ubiquitinated (indicated by black circles) in the cytosol lacking CRM. (H) The indicated transcripts lacking a stop codon were translated in the presence of microsomes derived from WT HEK293 or Sec63−/− cells. The reactions were treated with PK and analyzed by SDS-PAGE autoradiography. # denotes a background band. (I) Model showing Sec63/BiP-mediated release of a weak TMD retained at the Sec61 translocon. Source data are available for this figure: SourceData F1.
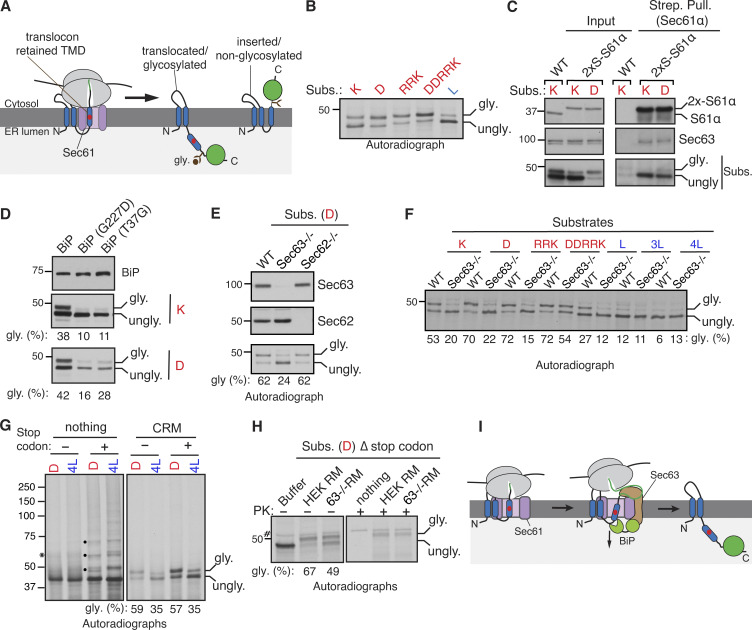
We hypothesized that weak TMD substrates might be transiently retained at the Sec61 translocon before translocation into the ER lumen. To test this, we precipitated the chromosomally 2xStrep-tagged Sec61α from cells expressing weak TMD substrates using Strep-Tactin beads. The unglycosylated forms were highly enriched with the Sec61 translocon (Fig. 1 C), whereas the glycosylated forms were less associated with the Sec61 translocon; presumably they had already translocated into the ER and moved away from the translocon. Next, we asked how the transiently retained weak TMD substrates are cleared from Sec61 translocons. We reasoned that the abundant luminal chaperone BiP ATPase might promote the translocation of substrates retained at the Sec61 translocon. Indeed, cells expressing BiP mutants (G227D and T37G), which are defective in ATP-induced release of substrates (Wei et al., 1995), inhibited the translocation of weak TMDs into the ER (Fig. 1 D).
The involvement of BiP in this process raises the question of how BiP is recruited to the Sec61 translocon. The translocon-associated membrane protein Sec63 is known to recruit and activate the BiP ATPase in order to facilitate co- and post-translational protein translocation into the ER (Brodsky et al., 1995; Deshaies et al., 1991; Matlack et al., 1999; Meyer et al., 2000; Schorr et al., 2020). To test whether Sec63 is involved in this process, we used knockout cells of either Sec63 or its interacting protein Sec62 generated by CRISPR/Cas9. Sec63 is required for the translocation of weak TMDs into the ER since the translocation was markedly reduced in Sec63−/− cells compared to WT cells. By contrast, the translocation of weak TMDs normally occurred in Sec62−/− cells (Fig. 1 E). To determine the biochemical features of the TMDs that depend on Sec63 activity, we tested the translocation of TMDs with varying hydrophobicity in Sec63−/− cells (Fig. 1 F and Fig. S1 B). The translocation of substrates with weak TMDs (K, D, and RRK) was significantly impaired, as demonstrated by reduced glycosylated forms in Sec63−/− cells relative to WT cells. By contrast, strong TMDs (L, 3L, and 4L) were efficiently inserted into the ER of WT and Sec63−/− cells, as evidenced by non-glycosylated forms (Fig. 1 F). Of note, the highly charged TMD (DDRRK) was only partially dependent on Sec63 (Fig. 1 F). These results suggest that the translocation of the Sec61 translocon occupied weak TMD into the ER relies on Sec63, whereas Sec63 is dispensable if the TMD is sufficiently hydrophobic or it is changed to a nearly hydrophilic sequence.
We next wanted to determine whether Sec63 could co-translationally, i.e., even when the nascent chain is still attached to the ribosome, mediate the translocation of the Sec61-occupied weak TMD into the ER. To test this, we in vitro translated mRNAs of substrates lacking a stop codon in the rabbit reticulocyte lysate (RRL), including 35S-methionine and canine rough microsomes (CRM). The lack of a stop codon leads to the formation of RNCs, where nascent polypeptides are covalently attached to ribosomes by a tRNA peptidyl linkage. As shown by the glycosylated form, the translocation of weak TMDs was similar for both nascent chains attached to ribosomes and nascent chains released from ribosomes when mRNAs possess a stop codon (Fig. 1 G). These data are consistent with the earlier report that showed a marginally hydrophobic TMD and its following C-terminus region could only be translocated into the ER co-translationally (Onishi et al., 2013). The co-translational translocation of a weak TMD was dependent on Sec63 since the translocation was reduced in microsomes derived from Sec63−/− cells compared to those prepared from WT cells (Fig. 1 H). Non-translocated nascent chains in the absence of Sec63 were entirely digested by proteinase K (PK), suggesting their localization to the cytosol (Fig. 1 H). Lastly, our truncation studies further revealed that weak TMD substrates containing a C-terminal tail shorter than 100 aa were poorly translocated independent of Sec63 activity (Fig. S1 C). By contrast, the substrates bearing a C-terminus longer than 100 aa were robustly translocated in a Sec63-dependent manner (Fig. S1 C). Altogether, these data indicate that Sec63 co-translationally mediates the translocation of weak TMDs transiently retained at Sec61 translocons by presumably recognizing their C-terminal region of more than 100 aa exposed to the cytosol (Fig. 1 I).
A substrate-trapping approach identifies endogenous substrates transiently retained at the Sec61 translocon
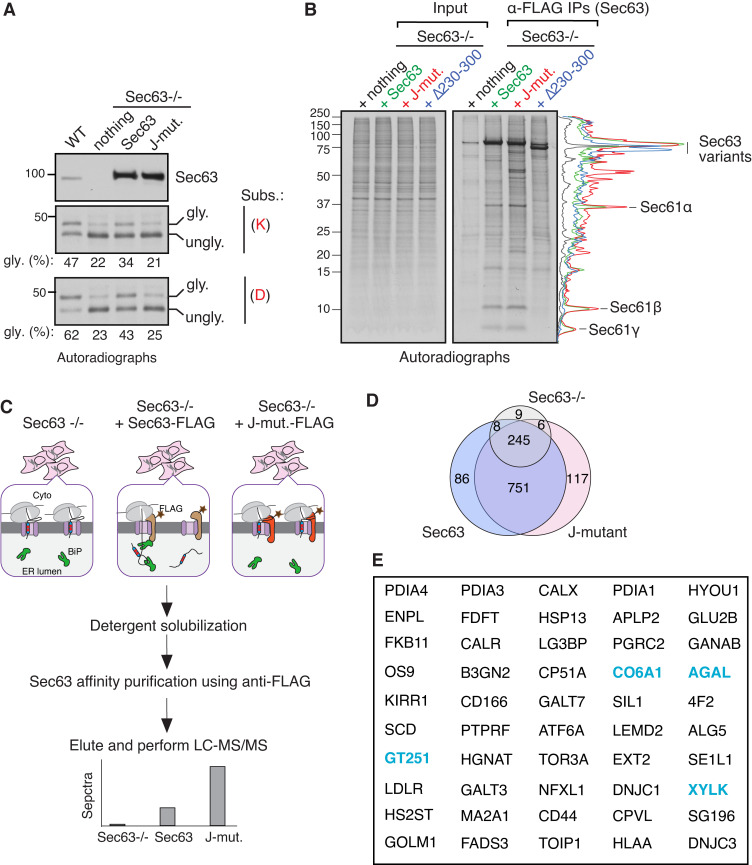
We next developed a substrate trapping strategy to determine the role of Sec63 in clearing endogenous substrates retained at the Sec61 translocon. We reasoned that a Sec63 J-domain mutant could trap nascent chains transiently retained at the Sec61 translocon due to its inability to activate the BiP ATPase. To examine this, we stably complemented either WT Sec63, Sec63 J-mutant (HPD/AAA), or the translocon interaction defective Sec63 mutant (Δ230–300) into Sec63−/− cells (Fig. S1 D). WT Sec63 complemented cells released weak TMDs retained at Sec61 translocons into the ER, as demonstrated by the glycosylated form of substrates (Fig. 2 A and Fig. S1 E). By contrast, Sec63 J-mutant and the Sec61 interaction-defective mutant (Δ230–300) were not able to clear the retained substrates, as shown by the primarily unglycosylated form of substrates (Fig. 2 A and Fig. S1 E). We next used these stable cell lines to perform metabolic labeling and immunoprecipitation (IP) to determine whether Sec63 J-mutant could trap endogenous substrates transiently retained at the Sec61 translocon and if the trapping depends on its interaction with the translocon. We found that all three subunits of the newly synthesized Sec61 translocon, α, β, and γ, were associated with both WT Sec63 and J-mutant but were not detected from Sec63−/− cells or the Sec61 interaction defective mutant (Δ230–300) expressing cells (Fig. 2 B). Quantification of bands further revealed that newly synthesized radiolabeled proteins were significantly enriched with Sec63 J-mutant relative to WT Sec63 or Sec63 (Δ230–300; Fig. 2 B). These findings support our conclusion that both Sec63 J-domain and its interaction with the translocon are required for clearing nascent polypeptides retained at Sec61 translocons in cells.
Figure 2.
Identification of endogenous Sec63 substrates retained at the Sec61 translocon. (A) WT HEK293 or Sec63−/− cells stably expressing the indicated Sec63 constructs were transfected with the C-TMD of WRB substrate carrying either lysine (K) or aspartic acid (D). The transfected cells were radiolabeled and analyzed by autoradiography after IP with anti-GFP antibodies for substrates. The immunoblot shows the expression of Sec63 from the indicated cell lines. (B) The indicated Sec63−/− complemented cell lines were radiolabeled and analyzed by autoradiography after IP with anti-FLAG beads. The traces are densitometry profiles of lanes and are shown on the right side of the autoradiograph, illustrating a selective enrichment of signals with the Sec63 J-mutant (HPD/AAA). (C) A scheme describing affinity purification and identification of translocon-occupied substrates by MS. (D) Venn diagram of protein distribution among the three groups of MS data from Table S1. Candidate proteins of 117 were at least twofold enriched in Sec63 J-mutant relative to WT Sec63 and fivefold increased than Sec63−/− cells. Candidate proteins of 86 were at least twofold enriched in WT Sec63 compared to Sec63 J-mutant and fivefold increased than Sec63−/− cells. (E) The top 50 secretory and membrane proteins from Table S2 that were enriched in Sec63 J-mutant. The blue color labeled proteins were previously identified in the study by Schorr et al. (2020). Source data are available for this figure: SourceData F2.
We next performed a large-scale affinity purification of Sec63 as depicted in Fig. 2 C and subjected immunoprecipitants to mass spectrometry (MS) to identify translocon-engaged endogenous clients. Our analysis showed many proteins associated with WT Sec63 and Sec63 J-mutant relative to control Sec63−/− cells (Fig. 2 D and Table S1). Consistent with our prediction, about 117 secretory and membrane proteins were at least twofold enriched in Sec63 J-mutant relative to WT Sec63 and fivefold increased than Sec63−/− cells (Fig. 2, D and E; and Table S2). Of the 117 proteins, about 65% of clients were non-resident ER proteins, indicating that these clients interacted with Sec63 J-mutant during their retention at the Sec61 translocon in the ER. Interestingly, about one-third of these candidate substrates have been previously implicated in human genetic disorders caused by mutations in corresponding genes (Table S2). Many of our Sec63 clients were also found in the independent proteomic study performed using Sec63-depleted cells (Fig. 2 E and Table S2). We found that about 86 secretory and membrane proteins were enriched with WT Sec63, but about 60% are ER-resident proteins, indicating that many are likely interacting proteins of Sec63 in the ER (Fig. 2 D and Table S3). Thus, our substrate-trapping proteomic approach identified potential endogenous secretory and membrane proteins that are transiently retained at the Sec61 translocon in cells.
Proteins containing a marginally hydrophobic SS depend on Sec63 for translocation
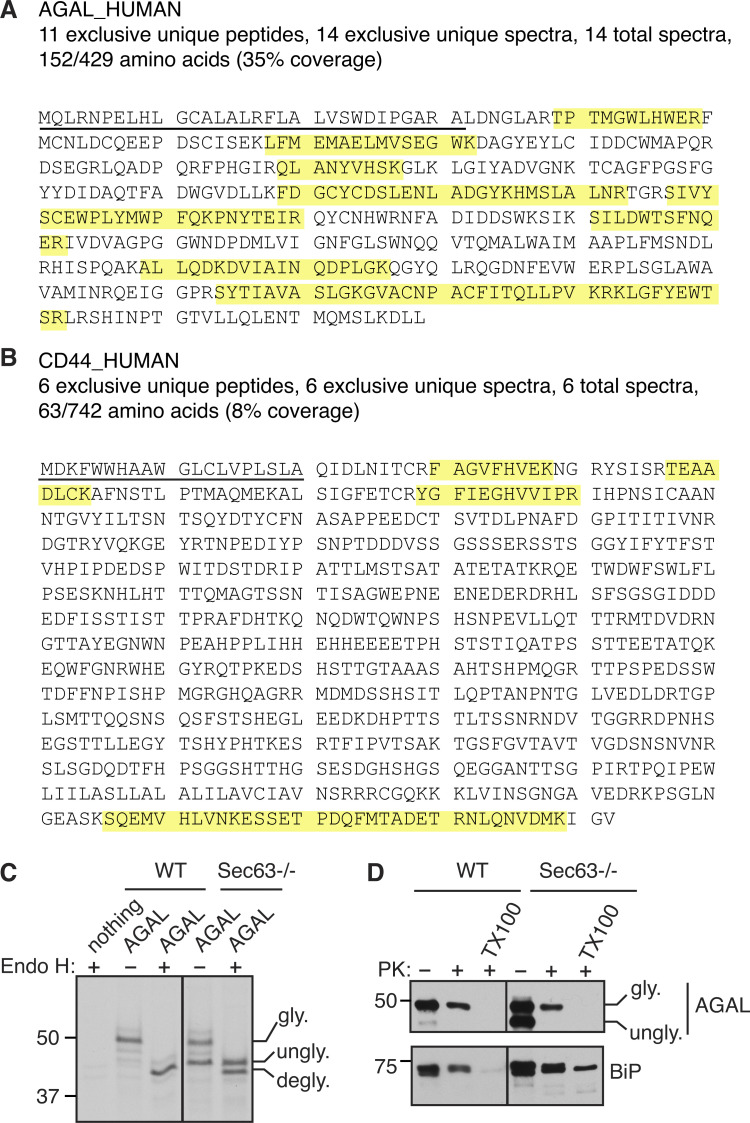
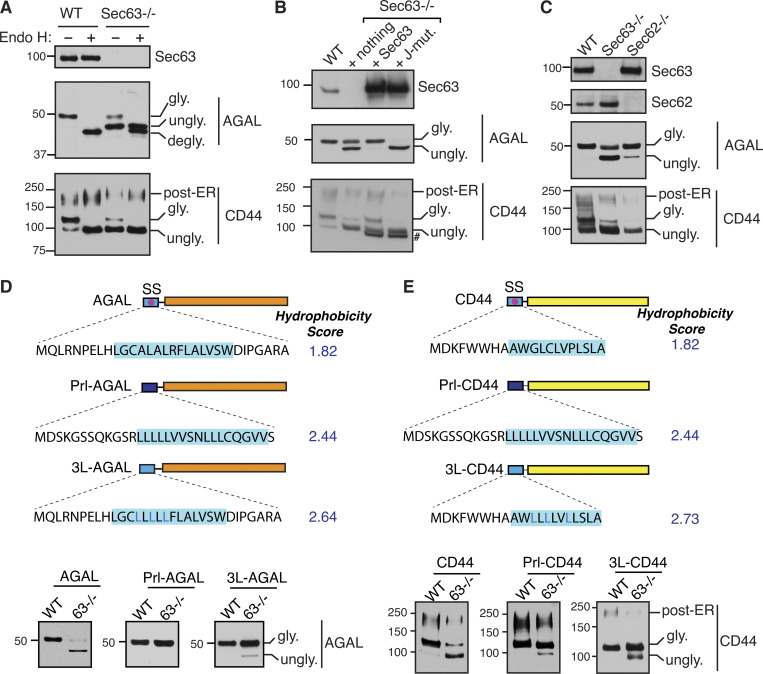
To validate our Sec63 substrates, we constructed C-terminally FLAG-tagged expression plasmids for two substrates, AGAL and CD44 (Fig. S2, A and B). The GLA gene encodes for a lysosomal enzyme called α-galactosidase A (AGAL), the activity of which is altered by genetic mutations in Fabry disease (Germain, 2010). The CD44 gene encodes a plasma membrane–localized single-spanning membrane protein with an N-terminal SS. Immunoblotting of AGAL from transiently expressing cells revealed that AGAL was fully glycosylated (Fig. 3 A). AGAL glycosylation was verified by digestion with Endo H, which removes high mannose N-linked glycans from glycoproteins. AGAL glycosylation was markedly reduced in Sec63−/− cells relative to WT cells (Fig. 3 A). Endo H digestion revealed that unglycosylated AGAL in Sec63−/− cells migrated slower than Endo H-deglycosylated AGAL (Fig. 3 A and Fig. S2 C), which suggests that unglycosylated AGAL in Sec63−/− cells still contains the uncleaved SS. The unglycosylated form of AGAL carrying the SS was mislocalized to the cytosol of Sec63−/− cells because it was digested by PK added to the semipermeabilized cells (Fig. S2 D). However, the translocated/glycosylated form was mostly protected from PK digestion. Expression of CD44 in WT cells showed two glycosylated bands. The faster migrating core glycosylated band was sensitive to Endo H. However, the slower migrating complex type of N-glycan was less susceptible to Endo H, indicating that it localizes to post-ER compartments (Fig. 3 A). Similar to AGAL, the translocation of CD44 was significantly reduced in Sec63−/− cells, as shown by the mostly unglycosylated form.
Figure S2.
AGAL mislocalizes to the cytosol in the absence of Sec63. (A and B) Amino acid sequences of AGAL and CD44 are annotated to indicate the peptides (yellow) identified by MS. The SSs were underlined. (C) AGAL-expressing cells were immunoprecipitated after radiolabeling for 30 min. The immunoprecipitants were either treated with or without Endo H and analyzed by SDS-PAGE autoradiography. degly. shows Endo H digested deglycosylated forms. (D) HEK293 or Sec63−/− cells expressing AGAL were treated with a low concentration of digitonin to selectively permeabilize the plasma membrane, followed by incubation with or without PK and analyzed by immunoblotting. Note that the non-translocated/unglycosylated AGAL with the SS is entirely digested by PK because it is localized to the cytosol. Source data are available for this figure: SourceData FS2.
Figure 3.
Translocation of proteins with a marginally hydrophobic SS depends on Sec63. (A) WT HEK293 or Sec63−/− cells were transfected with AGAL-FLAG or CD44-FLAG. Cell lysates were either untreated or treated with Endo H and analyzed by immunoblotting with an anti-FLAG antibody for substrates and anti-Sec63 antibodies. degly. means Endo H digested deglycosylated forms. Post-ER form of CD44 represents the Endo H resistant glycosylated form. (B) WT HEK293 or Sec63−/− cells stably expressing the indicated constructs were transfected with AGAL-FLAG or CD44-FLAG. Cell lysates were directly analyzed by immunoblotting as in A. # denotes FLAG-tagged Sec63 proteins. (C) WT HEK293, Sec63−/−, or Sec62−/− cells were transfected with plasmids expressing AGAL-FLAG or CD44-FLAG, and the cell lysates were directly analyzed as in A. (D) Top: Cartoons depicting the SS of AGAL are either replaced with the SS of Prl (Prl-AGAL) or mutated to generate 3L-AGAL. The blue shade denotes the SS’s h-region. Hydrophobicity scores of h-regions were analyzed by grand average hydropathy. Bottom: The indicated cell lines were transfected with the mentioned constructs and analyzed by immunoblotting with an anti-FLAG antibody. (E) Top: A cartoon depicting the SS of CD44 replaced with the SS of Prl (Prl-CD44) or mutated to generate 3L-CD44. Hydrophobicity scores were analyzed as in D. The indicated cell lines were transfected and analyzed as in D. Source data are available for this figure: SourceData F3.
We then asked if the translocation defects associated with AGAL and CD44 could be rescued by WT Sec63. Indeed, both AGAL and CD44 were translocated at similar levels to that of WT cells when Sec63−/− cells were complemented with WT Sec63 (Fig. 3 B). By contrast, Sec63 J-mutant complemented cells showed almost no translocation of AGAL and CD44 compared to WT Sec63 complemented and Sec63−/− cells (Fig. 3 B). This result suggests that the activation of BiP ATPase by the J-domain of Sec63 is critical for the translocation of these proteins. Since there is no glycosylated form of AGAL in the J-mutant lane, this result also indicates that Sec63 J-mutant completely inhibits Sec63-independent translocation in Sec63−/− cells (Fig. 3 B), presumably by blocking spontaneous BiP binding to translocating nascent chains. Since Sec63 functions along with Sec62 in protein translocation, we wondered whether Sec62 is also required for the translocation of AGAL and CD44. While the knockout of Sec62 had a significant effect on the translocation of CD44 as mirrored by less glycosylated forms, it had a subtle effect on the translocation of AGAL, suggesting that Sec62 plays a substrate-dependent role in promoting protein translocation (Fig. 3 C).
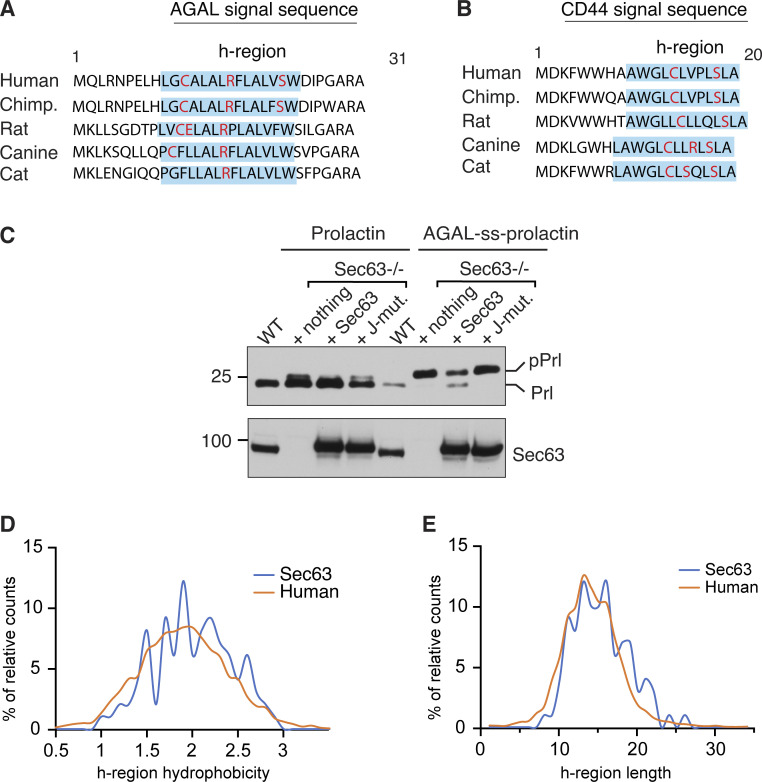
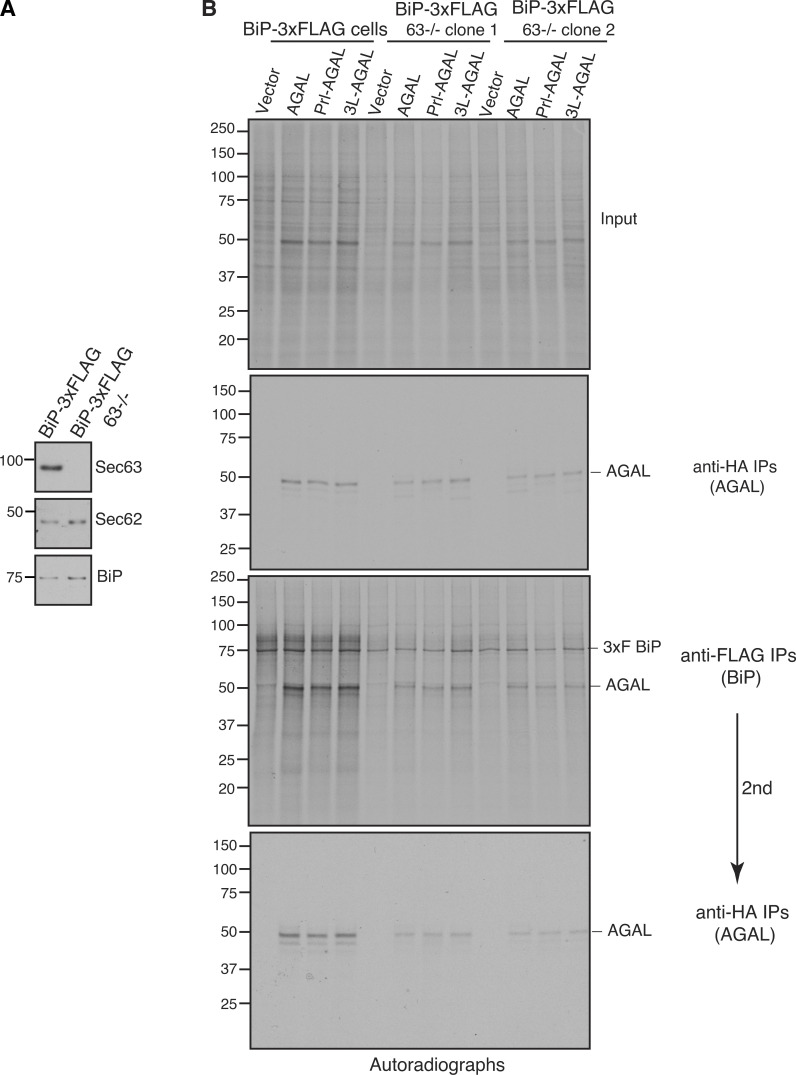
Since our data above showed that the translocation of weak TMDs relied on Sec63 activity, we asked if our new Sec63 substrates AGAL and CD44 also contain marginally hydrophobic (or weak) SSs. To address this, we swapped the SS of AGAL or CD44 with the strong SS from Prl and tested its Sec63 dependency by expressing it in Sec63−/− cells (Fig. 3 D). Prl-AGAL bypassed the Sec63 requirement as demonstrated by a fully glycosylated form of AGAL in WT and Sec63−/− cells, whereas AGAL with its endogenous SS was poorly glycosylated in Sec63−/− cells (Fig. 3 D). Interestingly, some of the hydrophilic residues within the h-region of AGAL SS are conserved in mammals, suggesting an evolutionary pressure to maintain Sec63 dependency (Fig. S3 A). Indeed, the hydrophobicity is the crucial determinant for Sec63 dependency since the translocation could occur independently of Sec63 activity for 3L-AGAL, which was generated by replacing less hydrophobic amino acids with hydrophobic leucine residues (Fig. 3 D). We also obtained a similar result for CD44, where Prl-CD44 and 3L-CD44 mostly translocated independent of Sec63 activity compared to WT CD44 (Fig. 3 E and Fig. S3 B). Furthermore, replacing Prl SS with less hydrophobic AGAL SS led to Sec63-dependent translocation of Prl into the ER, suggesting that the weak SS of AGAL is sufficient to render Sec63 dependency (Fig. S3 C). Analysis of SSs revealed that hydrophobicity and length of h-region significantly varied for both Sec63 clients and human secreted/membrane proteome (Fig. S3, D and E). However, there was no noticeable difference between the two datasets. This analysis suggests other features in precursor polypeptides, such as positively charged residues downstream of SSs (Schorr et al., 2020; Ziska et al., 2019), can also cause Sec63 dependency. Our results collectively show that less hydrophobic SSs, at least for AGAL and CD44, rely on Sec63/BiP to efficiently translocate their nascent chains into the ER.
Figure S3.
Conserved weak SSs determine Sec63 dependency. (A and B) Conservation of AGAL or CD44 SS in different mammalian species. Blue shade indicates the h-region of the SS defined by the Kyte-Doolittle scale. Charged and hydrophilic amino acids in the h-region are indicated in red color. (C) WT HEK293 cells, Sec63−/− cells, or Sec63−/− cells stably expressing WT Sec63 and or J-domain mutant (HPD/AAA) were transfected with plasmids expressing Prl or AGAL-ss-Prl. Cell lysates were analyzed by immunoblotting with an anti-FLAG antibody for substrates or Sec63 antibodies. Precursor (pPrl) and processed (Prl) forms are indicated. (D) H-regions in SSs of Sec63 clients (n = 99) and human secreted/membrane proteome (n = 5,294) were defined by custom scripts based on the Kyte-Doolittle algorithm described in Materials and methods. The calculated h-region hydrophobicity score frequency of the two datasets was compared and displayed. (E) The h-region length frequency of Sec63 clients (n = 99) and human secreted/membrane proteome (n = 5,294) was compared and displayed. Source data are available for this figure: SourceData FS3.
Sec63 co-translationally releases the translocation pause caused by a marginally hydrophobic SS
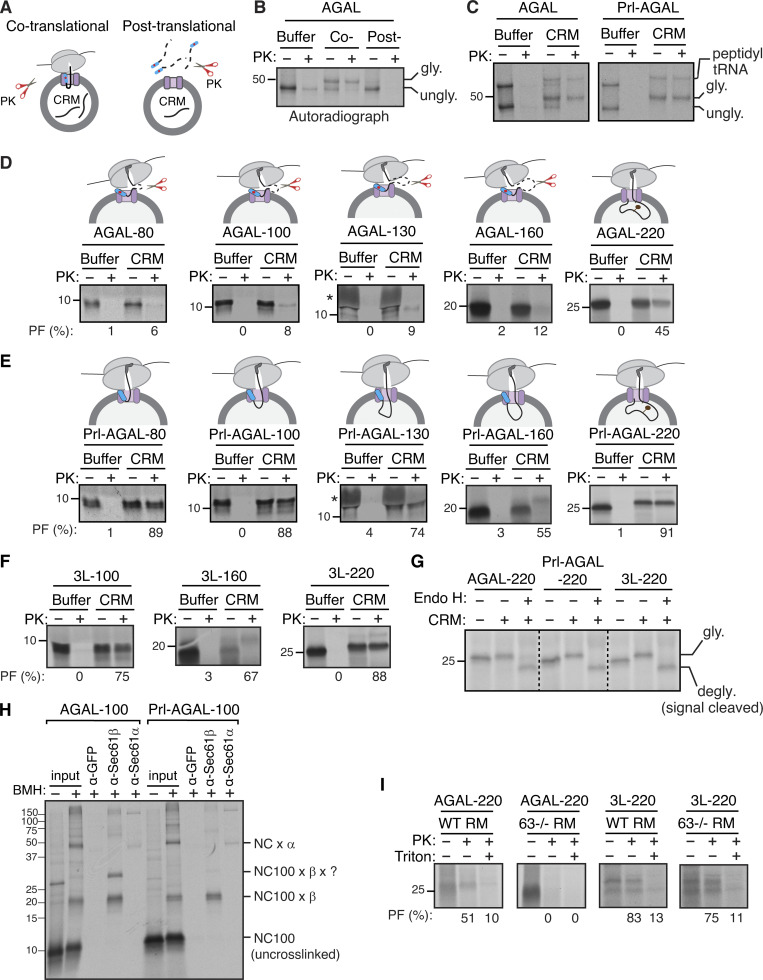
Earlier studies have established that Sec63 is predominantly involved in the post-translational translocation of substrates into the ER lumen by recruiting and activating BiP ATPase through its luminal J-domain (Brodsky et al., 1995; Corsi and Schekman, 1997; Matlack et al., 1999; Misselwitz et al., 1998). Studies have also shown that Sec63 plays a role in the co-translational protein translocation of selected substrates (Brodsky et al., 1995; Conti et al., 2015; Jung et al., 2014; Lang et al., 2012; Schorr et al., 2020; Young et al., 2001). The recent structural studies of the Sec61/Sec62/Sec63 complex from yeast suggest that both Sec63 and ribosome share the same binding site on the Sec61 translocon (Itskanov and Park, 2019; Wu et al., 2019; Weng et al., 2021). This means both the ribosome and Sec63 cannot bind to the Sec61 translocon simultaneously, questioning the role of Sec63 in the co-translational translocation process. To determine whether our new Sec63 substrates are co- or post-translationally translocated into the ER, we performed in vitro translation of AGAL transcripts using the RRL translation system, including 35S-methionine. AGAL was digested by PK when translated without microsomes (CRM), but it was translocated and glycosylated in the presence of microsomes and thereby insensitive to protease digestion (Fig. 4, A and B). In sharp contrast, AGAL was entirely digested by PK when microsomes were post-translationally incubated with the supernatant of the translation reaction, in which ribosomes were removed by centrifugation (Fig. 4, A and B). To directly detect the co-translational translocation of AGAL, we translated AGAL transcripts lacking a stop codon in vitro in the presence of microsomes. AGAL or Prl-AGAL was translocated into microsomes, as demonstrated by protease-resistant glycosylated bands, even when nascent chains were covalently attached to ribosomes through a peptidyl-tRNA bond (Fig. 4 C).
Figure 4.
Sec63 substrates are co-translationally translocated with an initial pause. (A) Scheme depicting the PK treatment protocol for the co- or post-translational protein translocation assay. (B) For the co-translational protein translocation, transcripts encoding AGAL-FLAG were in vitro translated in RRL, including either buffer or CRM. After translation, samples were digested with PK and analyzed by autoradiography after IP with an anti-FLAG antibody. For the post-translational translocation, transcripts encoding AGAL-FLAG were translated and centrifuged to remove ribosomes. The supernatant was incubated with CRM, digested with PK, and analyzed after IP with an anti-FLAG antibody. (C) AGAL or Prl-AGAL transcripts lacking a stop codon were in vitro translated in RRL, including either buffer or CRM. The reactions were treated with PK and analyzed as in B. (D) The indicated lengths of AGAL RNCs were produced in RRL, including either buffer or CRM, digested with PK, treated with RNase A to remove tRNA, and analyzed by autoradiography. The percent of protease-protected fragments (PF) is shown below each panel. The star symbol indicates the distortion of AGAL-130 nascent chain caused by the co-migration with hemoglobin from RRL. (E and F) Nascent chains of the indicated lengths of Prl-AGAL or 3L-AGAL were produced and analyzed as in D. (G) Nascent chains of AGAL-220, Prl-AGAL-220, or 3L-AGAL-220 were produced in the absence or presence of CRM. Translation reactions were denatured, treated with or without Endo H, and analyzed by autoradiography. (H) The membrane-targeted nascent chains of AGAL-100 or Prl-AGAL-100 were isolated by centrifugation and treated with bismaleimidohexane (BMH) crosslinker. An aliquot was directly analyzed (input), while the remainder was denatured and immunoprecipitated with the indicated antibodies. Anti-GFP antibodies were used as a control. The nascent chain (NC) crosslinked adducts are shown by “NC100 ×.” (I) Transcripts of AGAL-220 or 3L-AGAL-220 lacking a stop codon were translated in the presence of either rough microsomes (RM) derived from WT HEK293 cells or Sec63−/− cells and digested with PK before analyzing by autoradiography. Note that protease-protected fragments mostly disappeared in samples containing 1% Triton X-100, suggesting that they are translocated into the ER. Source data are available for this figure: SourceData F4.
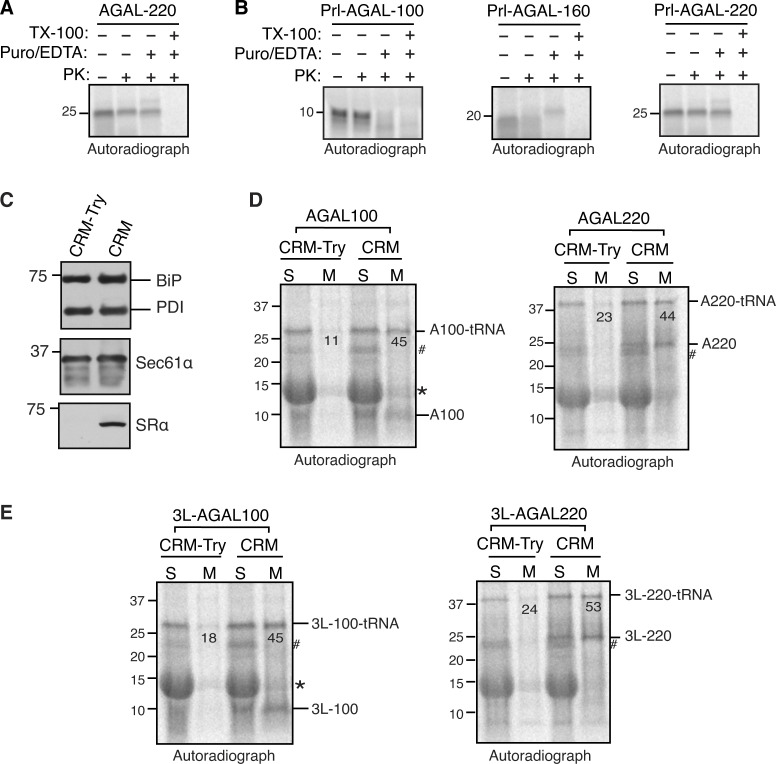
We next wanted to determine at which step Sec63 would assist the co-translational translocation of AGAL into the ER. To address this, we in vitro translated mRNAs lacking a stop codon encoding defined chain lengths of AGAL in the presence or absence of microsomes (Fig. 4 D). Translation of mRNAs without a stop codon yielded translationally arrested radiolabeled RNCs, representing physiological translocation intermediates. To our surprise, nascent chains of AGAL-80, -100, -130, and -160 aa were digested by PK even when translated in the presence of microsomes, as minimal protease-protected fragments were observed. (Fig. 4 D). However, nascent chains of AGAL-220 were protected from PK digestion. Puromycin treatment, which releases nascent chains from ribosomes, further confirmed that AGAL-220 nascent chains were protected from PK digestion by their successful translocation into the ER but not due to the protection by the ribosome–translocon complex (Fig. S4 A). However, the nascent chains were completely digested when treated with Triton X-100, which solubilizes the membrane. These data suggest that the AGAL nascent chain of ∼160 aa is translocationally paused and accumulated on the cytosolic side of the Sec61 translocon and thus accessible to PK digestion. The translocation pause was released when the nascent chain reached the length of 220 aa and hence became resistant to PK digestion. Conversely, Sec63-independent substrate Prl-AGAL showed protease-protected fragments for all lengths of nascent chains (Fig. 4 E). If the nascent chains were released from ribosomes by treatment with puromycin, Prl-AGAL-100 nascent chains were fully digested by PK (Fig. S4 B). This result suggests that Prl-AGAL-100 nascent chains are not yet translocated and are primarily shielded by the ribosome–translocon complex, whereas AGAL-100 nascent chains were poorly shielded even by the ribosome–translocon complex from PK digestion (Fig. 4 D). However, Prl-AGAL-160 and -220 nascent chains were translocated into the ER since they were protected from PK digestion when released from ribosomes by puromycin but digested upon treatment with Triton X-100 (Fig. S4 B).
Figure S4.
AGAL nascent chains are efficiently recruited to the ER and translocated across the membrane. (A and B) The indicated transcripts lacking a stop codon were in vitro translated in RRL in the presence of CRM. After translation, samples were either directly digested with PK or after the release of nascent chains with puromycin/EDTA. Note that protease-protected fragments seen in AGAL-220, Prl-AGAL-160, and Prl-AGAL-220 mostly disappeared in samples containing 1% Triton X-100, suggesting that they are translocated into the ER. (C) Untreated and trypsin (20 μg/ml) treated CRM were analyzed by immunoblotting for the indicated antigens. Note that mild trypsin digestion completely shaved off the α subunit of the SRP receptor, which is required to recruit the RNCs to the ER membrane. (D and E) RNCs at indicated lengths of AGAL or 3L-AGAL were produced in RRL in the presence of either trypsin-treated CRM (CRM-Try) or untreated CRM. The resulting reactions were layered on 1 M sucrose and centrifuged to collect supernatants (S) and membrane pellets (M), which were then analyzed by SDS-PAGE and autoradiography. The values indicate the percentage of membrane-associated tRNA-linked AGAL or 3L-AGAL. Note that tRNA moieties from AGAL nascent chains could be removed by digestion with RNase A (not shown). Star indicates the background signal from abundant hemoglobulin in RRL. # indicates a non-specific protein. Source data are available for this figure: SourceData FS4.
The protease protection experiment with varying lengths of 3L-AGAL nascent chains showed a similar result to that of Prl-AGAL (Fig. 4 F), demonstrating that the SS hydrophobicity determines the efficient translocation of nascent chains into the ER. Endo H digestion of nascent chains of 220 further revealed that SSs were cleaved, and nascent chains were glycosylated for both AGAL and its variants (Fig. 4 G). The translocation defects associated with AGAL nascent chains of <220 aa could be caused by their inability to either target to the ER membrane or interact with the Sec61 translocon. However, nascent chains of AGAL-100 and AGAL-220 were recruited to microsomes with comparable efficiency to nascent chains bearing a strong SS (Fig. S4, C−E). Also, AGAL-100 or Prl-AGAL-100 nascent chains crosslink similarly to α and β subunits of the translocon, except that AGAL100 formed a crosslinked adduct comprising β and an ∼10 kD unknown protein (Fig. 4 H). We next asked if Sec63 co-translationally assists the translocation of the AGAL nascent chain into the ER in vitro. Indeed, nascent chains of AGAL-220 were protease sensitive when incubated with microsomes derived from Sec63−/− cells compared to microsomes derived from WT cells (Fig. 4 I). In contrast, 3L-AGAL showed protease-protected fragments for both WT and Sec63−/− microsomes since its translocation into the ER was independent of Sec63. These data suggest that the weak SS of AGAL can target its nascent chain to the ER membrane and engages with the Sec61 translocon, but the nascent chain translocation is paused until it reaches the length of ∼220 aa.
Sec63 mediates BiP binding to translocating polypeptides in the ER
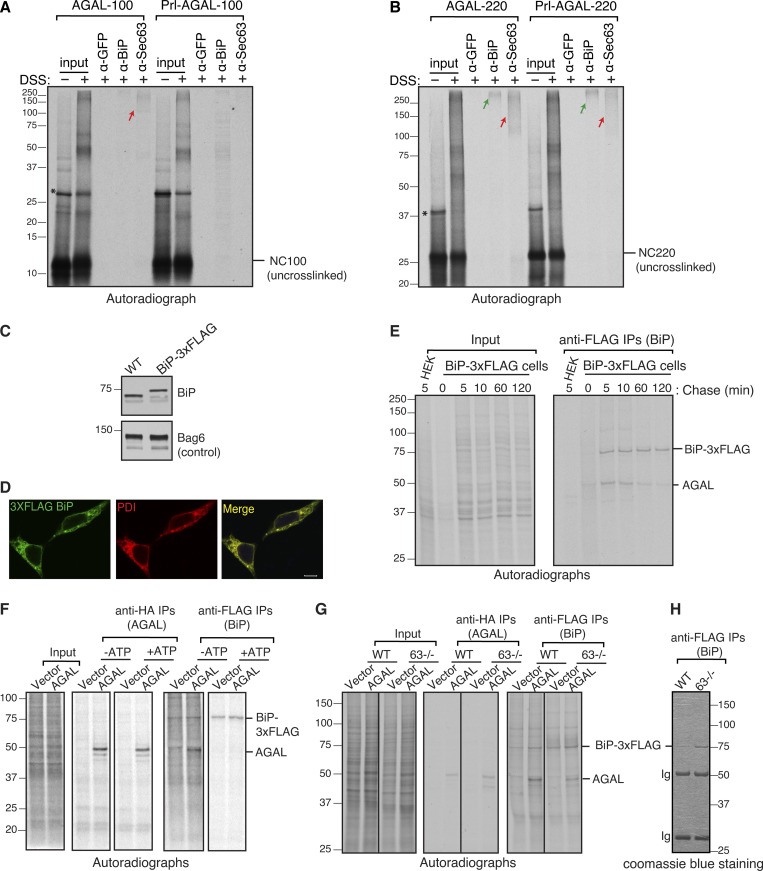
We hypothesized that SSs might direct the recruitment of luminal chaperones to bind onto incoming nascent chains through modulating the Sec61 translocon-associated factors. Accordingly, we predicted that AGAL nascent chains would preferentially bind to BiP chaperone since its translocation depends on the J-domain of Sec63. To investigate this hypothesis, we first examined if AGAL nascent chains could contact Sec63 at the ER membrane and bind to BiP upon entry into the ER lumen. To this end, we generated the ER membrane-targeted RNCs of 100 and 220 aa in length and treated them with a lysine reactive crosslinker to trap the interaction between nascent chains and Sec63 or BiP. AGAL100 nascent chains could interact with Sec63, as shown by a crosslinked adduct, which could be immunoprecipitated by antibodies against Sec63 but not by control antibodies against GFP (Fig. 5 A). AGAL crosslinking to Sec63 was further increased when nascent chains extended to 220 aa in length (Fig. 5 B). By contrast, no detectable crosslinking was observed between Sec63 and Prl-AGAL at the length of 100 aa, but the crosslinking was noticeable, albeit less than AGAL when the nascent chains reached 220 aa in length. Nascent chain crosslinked adducts appeared as a smeared band of 100–250 kD size on the gel, which suggests that they likely contain other translocon-associated factors in addition to Sec63. IP with anti-BiP antibodies revealed that both AGAL and Prl-AGAL nascent chains minimally crosslinked with BiP at the length of 100 aa. By contrast, a prominent crosslinking between BiP and AGAL or Prl-AGAL was observed when nascent chains entered the lumen at the length of 220 aa (Fig. 5 B; and Fig. S4, A and B). Thus, our in vitro data suggest that Sec63 is preferentially recruited to the Sec61 translocon harboring a weak SS by likely interacting with the nascent chain exposed on the cytosol. However, AGAL nascent chains bearing either weak or strong SS interact with BiP upon entry into the ER lumen.
Figure 5.
Sec63 is recruited to the translocation site to mediate BiP binding to polypeptides entering the ER. (A and B) The membrane-targeted nascent chains (NC) of indicated lengths were isolated by centrifugation and treated with disuccinimidyl suberate crosslinker. An aliquot was directly analyzed (input), while the remainder was denatured and immunoprecipitated with the indicated antibodies. Anti-GFP antibodies were used as a control. The red arrow shows crosslinked adducts containing Sec63. The green arrow indicates the crosslinked adducts containing BiP. The star symbol indicates tRNA-linked nascent chains. (C) WT HEK293 cells or HEK293 cells that contain a 3xFLAG tag at the C-terminus of endogenous BiP were analyzed by immunoblotting with an anti-BiP antibody. Bag6 serves as a loading control. (D) WT HEK293 and BiP-3xFLAG cell lines were immunostained with anti-FLAG for BiP and anti-PDI (protein disulfide isomerase) antibodies and imaged using a confocal microscope. Scale bars are 10 μm. (E) Cells expressing AGAL-HA were radiolabeled for 2 min and chased for the indicated time points. The immunoprecipitated cell lysates with anti-FLAG antibodies for BiP and analyzed by autoradiography. (F) BiP-3xFLAG HEK293 or BiP-3xFLAG HEK293 Sec63−/− cells were transfected with either empty vector or AGAL-HA. Cells were radiolabeled for 2 min, lysed with or without ATP, and immunoprecipitated with anti-FLAG beads before analyzing by autoradiography. (G) BiP-3xFLAG HEK293 or BiP-3xFLAG HEK293 Sec63−/− cells were transfected and radiolabeled for 2 min. Radiolabeled cells were lysed and immunoprecipitated with either anti-FLAG antibodies for BiP or anti-HA antibodies for AGAL and analyzed by autoradiography. (H) A coomassie stained gel showing immunoprecipitated 3xFLAG tagged endogenous BiP from BiP-3xFLAG HEK293 cells or BiP-3xFLAG HEK293 Sec63−/− cells. Source data are available for this figure: SourceData F5.
To examine if BiP binding to AGAL nascent chains depends on Sec63 activity in cells, we introduced a C-terminal 3xFLAG-tag on endogenous genes encoding BiP using CRISPR/Cas9 to efficiently pulldown endogenous BiP from HEK293. The C-terminal 3xFLAG introduced downstream of the KDEL retention signal of BiP did neither significantly change BiP expression levels nor its localization to the ER (Fig. 5, C and D). We first tested whether newly synthesized AGAL polypeptides bind to BiP in cells. BiP-3xFLAG cells expressing AGAL were radiolabeled with 35S-methionine/cysteine for 2 min, chased, and immunoprecipitated with an anti-FLAG antibody. Consistent with our prediction, the newly synthesized AGAL was co-immunoprecipitated with BiP. The interaction was reduced with the chase time because matured AGAL presumably left the ER (Fig. 5 E). BiP binding to AGAL and other endogenous substrates was sensitive to ATP since the incubation of cell lysates with ATP before IP completely abolished BiP binding to all newly synthesized polypeptides including AGAL (Fig. 5 F).
To next determine whether BiP binding to AGAL relies on Sec63, we generated Sec63 knockout in BiP-3xFLAG HEK293 cells using CRISPR/Cas9 (Fig. S5 A). Consistent with our model, BiP binding to AGAL markedly reduced in Sec63−/− cells, although about twofold more BiP was immunoprecipitated in Sec63−/− cells than WT cells due to BiP upregulation in Sec63−/− cells (Fig. 5, G and H). Lastly, we tested whether BiP binding to newly synthesized AGAL containing a strong SS depends on Sec63. Similar to AGAL, newly synthesized Prl-AGAL and 3L-AGAL also relied on Sec63 activity for binding to BiP, although they were translocated independent of Sec63 (Fig. S5 B). Taken together, our in vitro and cellular data suggest that AGAL depends on Sec63 activity for both its translocation and binding to BiP in the ER. However, AGAL bearing a strong SS translocate independent of Sec63 activity but still requires Sec63 for their BiP binding in the ER.
Figure S5.
Sec63 mediates BiP binding to newly synthesized polypeptides in the ER. (A) BiP-3xFLAG HEK293 or BiP-3xFLAG HEK293 Sec63−/− cells were analyzed by immunoblotting. (B) BiP-3xFLAG HEK293 or BiP-3xFLAG HEK293 Sec63−/− cells were transfected with either empty vector or AGAL constructs. Cells were radiolabeled for 2 min and immunoprecipitated with either anti-FLAG antibodies for BiP or anti-HA antibodies for AGAL and analyzed by autoradiography. Anti-FLAG immunoprecipitants were diluted and re-immunoprecipitated (second IPs) with anti-HA antibodies for AGAL. Source data are available for this figure: SourceData FS5.
BiP-dependent protein translocation is coupled with protein folding in the ER
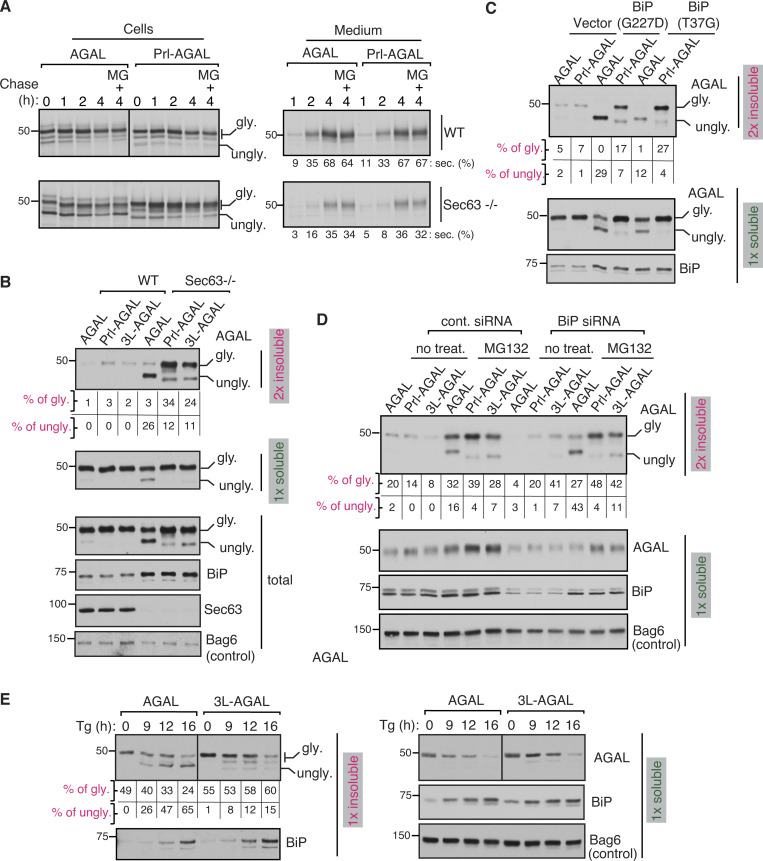
We hypothesized that BiP binding to AGAL nascent chains through Sec63 might not only facilitate their translocation into the ER but also promote their folding and maturation in the ER. To test this, we monitored the secretion of AGAL since properly folded AGAL is secreted into the extracellular medium upon overexpression (Ioannou et al., 1992). Pulse-chase analysis revealed that AGAL and Prl-AGAL were reduced in WT cells during the chase period with a concomitant increase in their secretion (Fig. 6 A). By contrast, the secretion of AGAL and Prl-AGAL was about twofold reduced in Sec63−/− cells (Fig. 6 A). The non-translocated/unglycosylated proteins in Sec63−/− cells were degraded by proteasomes, as they accumulated upon treatment with the proteasomal inhibitor MG132 (Fig. 6 A). Moreover, AGAL carrying either a weak or strong SS showed similar α-galactosidase activity in WT cells, but the activity was potently reduced for all AGAL variants in Sec63−/− cells (Fig. S6 A). Reduced activity and secretion in Sec63−/− cells could be attributed to the weak SS of AGAL that relies on Sec63 for efficient translocation into the ER. However, AGAL bearing a strong SS also showed these effects even though it is translocated into the ER independent of Sec63. This result suggested that AGAL with a strong SS is likely misfolded after translocation into the ER due to its inefficient binding to BiP in Sec63−/− cells (Fig. S5 B).
Figure 6.
Marginally hydrophobic SSs prevent protein misfolding and aggregation in the ER under limited BiP availability. (A) WT or Sec63−/− cells expressing AGAL or Prl-AGAL were radiolabeled for 30 min and chased in the presence or absence of 20 μM MG132 for the indicated time points. Both cells and medium were collected at each time point and analyzed by autoradiography after IP with an anti-HA antibody for AGAL. Secreted AGAL is given as the percentage of total signals from both secreted and intracellular bands. Images were taken from the same exposure and processed in parallel. (B) Cells expressing the indicated constructs were lysed in NP40 buffer, and the aliquots of total lysates and detergent-soluble fractions were analyzed by immunoblotting. The detergent-insoluble fractions were lysed in an SDS sample buffer and analyzed by immunoblotting. BiP, Sec63, and Bag6 were blotted from soluble fractions. The percent translocated/glycosylated protein values were calculated by insoluble glycosylated signal divided by the sum of glycosylated and unglycosylated signals from both soluble and insoluble fractions. The percent of non-translocated/unglycosylated form was calculated by insoluble unglycosylated signal divided by the sum of glycosylated and unglycosylated signals from both soluble and insoluble fractions. (C) HEK293 cells were co-transfected with the indicated AGAL constructs and vector, BiP (G227D) or BiP (T37G). Cells were treated with 10 μM MG132 for 6 h to detect non-translocated AGAL before harvesting and analyzing as in B. (D) Control or BiP siRNA treated HEK293 cells were transfected with the indicated AGAL construct and harvested after treating the cells with 10 μM MG132 for 6 h. Cells were lysed and analyzed as in B. (E) HeLa cells expressing AGAL constructs were treated with 5 μg/ml thapsigargin (Tg) along with 10 μM MG132 for the indicated time points and harvested in RIPA buffer. The soluble fractions were collected after centrifugation, and the insoluble fractions were solubilized by boiling in 1% SDS buffer. Both soluble and insoluble fractions were analyzed by immunoblotting for the indicated antigens. Source data are available for this figure: SourceData F6.
Figure S6.
Effect of SSs on protein translocation and protein folding. (A) WT or Sec63−/− cells expressing the indicated AGAL constructs were lysed in NP40 buffer, and the supernatant (soluble fraction) after centrifugation was assayed for α-galactosidase activity. Data are shown as a percentage of the activity of WT AGAL, and the error bar represents the SD (n = 3). (B) Sec63−/− cells expressing the indicated variants of AGAL were treated or not treated with Endo H and analyzed by immunoblotting. The unglycosylated AGAL migrates slower than the Endo H deglycosylated AGAL, suggesting that the unglycosylated form contains the uncleaved SS. However, unglycosylated forms of Prl-AGAL and 3L-AGAL carrying uncleaved SSs migrate only slightly slower than the Endo H digested deglycosylated bands. Presumably, this difference is caused by the presence of a positively charged arginine residue in the middle of the AGAL SS but not in the SS of Prl-AGAL or 3L-AGAL. (C) Sec63−/− cells expressing AGAL constructs along with vector, BiP (G227D), or BiP (T37G) were analyzed by immunoblotting. The unglycosylated band is the percentage of total signals from both glycosylated and unglycosylated bands. (D) CRMs were permeabilized with a low concentration of detergent to release the luminal content and were resealed by diluting with a detergent-free buffer and centrifugation. Transcripts encoding AGAL-220 and Prl-AGAL-220 were translated in the presence of indicated membranes or buffer followed by digestion with or without PK and analyzed by autoradiography. The percentage of protease-protected (PF) chains is shown below each autoradiograph. (E) Total lysate of BiP-depleted HEK293 cells shown in Fig. 6 D was treated or non-treated with Endo H and analyzed as in B. (F) Control or BiP siRNA-treated HeLa cells were transfected with the indicated AGAL construct and harvested in RIPA buffer after treating the cells with 10 μM MG132 for 6 h. The soluble fractions were collected after centrifugation, and the insoluble fractions were solubilized by boiling in 1% SDS buffer. Both soluble and insoluble fractions were analyzed by immunoblotting for the indicated antigens. Source data are available for this figure: SourceData FS6.
To determine whether AGAL carrying a strong SS is misfolded after translocation into the ER of Sec63−/− cells, we separated the detergent soluble fraction from the insoluble fraction, an indicator of misfolded/aggregated proteins. We found a subtle difference between AGAL variants in WT cells. By contrast, a significant fraction of translocated/glycosylated form of Prl-AGAL and 3L-AGAL was sedimented as detergent-insoluble fractions in Sec63−/− cells, supporting the notion that AGAL carrying a strong SS are misfolded and aggregated after translocation into the ER lumen (Fig. 6 B). To our surprise, most of the translocated/glycosylated form of AGAL moved to the soluble fraction. However, non-translocated/unglycosylated AGAL with the uncleaved SS sedimented as detergent-insoluble aggregates (Fig. 6 B and Fig. S6 B). This result suggested that the AGAL population translocated in the absence of Sec63 could be appropriately folded and matured in Sec63−/− cells. We reasoned that this is due to the upregulation of BiP in Sec63−/− cells, which could partly promote translocation and folding of AGAL nascent chains. Sec63-independent but BiP-dependent translocation of AGAL in Sec63−/− was supported by the observation that overexpression of BiP ATPase mutants significantly impaired translocation of AGAL in Sec63−/− cells with little effect on the translocation of AGAL with a strong SS (Fig. S6 C). Thus, these results indicate that strictly BiP-dependent translocation of AGAL in Sec63−/− promotes subsequent folding and maturation of AGAL in the ER, whereas BiP-independent translocation of AGAL carrying a strong SS is prone to misfolding after translocation into the ER of Sec63−/− cells.
Our data thus far suggest a model in which proteins with a weak SS translocate only upon binding to BiP molecules, which may also assist subsequent protein folding and maturation. However, in the absence of BiP binding, these proteins may be mislocalized in the cytosol and degraded by proteasomes. To test this model, we co-expressed AGAL variants with BiP mutants (G227D and T37G) that can bind substrates but fail to release them upon ATP binding (Wei et al., 1995). AGAL translocation was markedly reduced in cells expressing BiP mutants, and primarily, the non-translocated/unglycosylated AGAL was found in the insoluble fraction (Fig. 6 C). However, the population of AGAL that translocated into the ER was completely soluble, as shown by the presence of glycosylated form only in the soluble fraction. This result suggests that AGAL either translocates and folds properly by binding to active BiP molecules or does not translocate into the ER when it fails to bind active BiP molecules. Conversely, Prl-AGAL was efficiently translocated/glycosylated irrespective of the BiP functional status in the ER. Thus, a significant fraction of Prl-AGAL is misfolded after translocation into the ER and moved to the insoluble fraction (Fig. 6 C). Furthermore, our in vitro experiment demonstrated that the translocation of AGAL nascent chain was significantly inhibited in microsomes depleted of luminal proteins compared to that of AGAL containing a strong SS (Fig. S6 D).
To determine whether the weak SS of AGAL can respond to BiP availability in the ER by inhibiting the translocation of its mature domain, we depleted BiP in HEK293 cells using siRNA (Fig. 6 D). We briefly treated cells with or without MG132 to stabilize the non-translocated proteins in the cytosol. The translocation of AGAL, but not Prl-AGAL or 3L-AGAL, was impaired in BiP-depleted cells, which resembles the result seen in Sec63−/− cells (Fig. S6, B and E). In BiP-depleted cells, primarily the non-translocated/unglycosylated forms of AGAL moved to the insoluble fraction upon MG132 treatment. In contrast, a significant population of translocated/glycosylated form of Prl-AGAL and 3L-AGAL went to insoluble fractions upon MG132 treatment (Fig. 6 D). We also obtained a similar result of selective translocation attenuation of AGAL upon depletion of BiP in HeLa cells (Fig. S6 F). Lastly, we investigated whether the translocation of AGAL is reduced in response to ER stress, which likely depletes BiP availability due to the accumulation of misfolded proteins in the ER (Kang et al., 2006; Vitale et al., 2019). Upon ER stress induced by thapsigargin, a significant non-translocated/unglycosylated form of AGAL went to insoluble fractions in a time-dependent manner, which also correlated with a fraction of BiP moving to insoluble fractions, presumably its sequestration by aggregated proteins (Fig. 6 E). By contrast, mostly translocated/glycosylated form of AGAL with a strong SS moved to insoluble fractions during ER stress. Of note, we noticed that a significant fraction of AGAL and 3L-AGAL were aggregated even under normal conditions in HeLa cells (Fig. 6 E) compared to HEK293 cells (Fig. 6 C), indicating protein folding capacity of the ER varies between cell types. Collectively, these data suggest that proteins with weak SSs can respond to BiP deficiency by aborting translocation into the ER, where otherwise they would misfold and aggregate.
Discussion
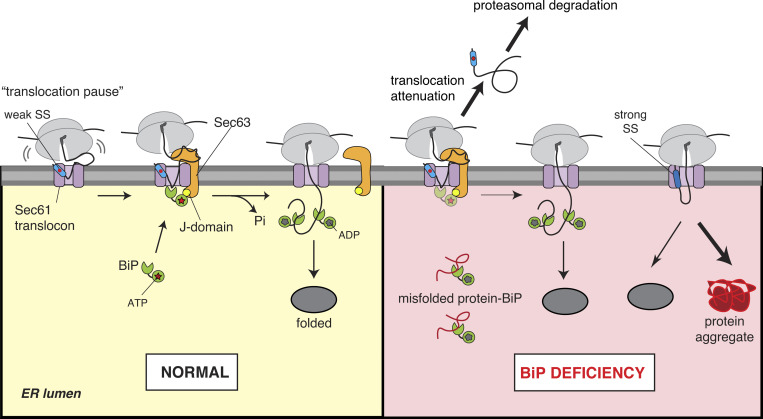
In addition to targeting roles (Blobel and Dobberstein, 1975), the findings in this study suggest SSs also provide protein folding information in the ER by coupling protein translocation with chaperone binding. We show that the RNC bearing a weak SS is efficiently recruited to the ER. However, the translocation is initially paused at the Sec61 translocon, thereby exposing the nascent chain to the cytosol (Fig. 7). Sec63 is co-translationally recruited to the translocation site and activates the BiP ATPase to bind the incoming nascent chain, thus driving forward translocation into the ER. This BiP-dependent translocation of the nascent chain promotes protein folding and prevents protein misfolding/aggregation under limited BiP availability in the ER. Thus, we suggest that evolution has exploited SSs diversity to recruit selective luminal chaperones for protein folding and maturation of newly synthesized proteins in the ER.
Figure 7.
Model for SS-guided protein folding in the ER. The RNC containing a marginally hydrophobic (or weak) SS is efficiently recruited to the ER membrane but loosely engages the Sec61 translocon. This can cause a pause in the initial translocation of ∼160 aa in length that accumulates on the cytosolic side of the membrane. Sec63 is recruited to the translocation site by interacting with the cytosolically accumulated nascent chains. The J-domain of Sec63 stimulates ATP hydrolysis by BiP to form a stable BiP–nascent chain complex, resulting in the forward translocation and subsequent protein folding in the ER. Under conditions of BiP deficiency, nascent chains with a weak SS still depend on BiP for translocation, and thus their BiP-assisted folding is ensured. However, if nascent chains fail to engage luminal BiP, their translocation is aborted, and they are mislocalized in the cytosol and eliminated by proteasomal degradation. Conversely, even under conditions of BiP deficiency, nascent chains with a strong SS are efficiently translocated in a Sec63/BiP-independent manner and thus are at a high risk of misfolding and aggregating after translocation into the ER.
The present studies were undertaken to understand how cells clear the marginally hydrophobic (or weak) TMD retained at the Sec61 translocon. The data suggest that Sec63 activates BiP to mediate the clearance of the Sec61 translocon occupied with a weak TMD. The “substrate-trapping” proteomic approach identified many endogenous secretory and membrane proteins transiently retained at the Sec61 translocon in the absence of Sec63 activity in cells. Mutagenesis experiments show that the hydrophobicity of SSs, at least for AGAL and CD44, determines Sec63 dependency. However, other features in SSs can also contribute to transient retention of nascent chains at the Sec61 translocon, thus necessitating Sec63 dependency. Our findings are consistent with earlier studies showing the involvement of Sec62/Sec63 and BiP in assisting the translocation of specific substrates, which often contain less hydrophobic SSs (Conti et al., 2015; Lang et al., 2012; Schorr et al., 2020; Tyedmers et al., 2003).
Our data raise the intriguing question of how Sec63 and ribosome can bind to the Sec61 translocon simultaneously because they share the same binding site (Itskanov and Park, 2019; Weng et al., 2021; Wu et al., 2019). The protease protection data suggest that the translocation of a nascent chain bearing a weak SS is initially paused at the Sec61 translocon, presumably due to a loose engagement of the weak SS to the lateral gate of the Sec61 translocon. Thus, the nascent chain may revert backward and be extruded to the cytosolic environment through the gap between the ribosome and translocon junction (Fig. 7). The nascent chain accumulation in the cytosol may further weaken the interaction between ribosome and Sec61 translocon. The chemical crosslinking data indicate that Sec63 is recruited to the Sec61 translocon by likely interacting with the nascent chain that accumulates in the cytosol. Once recruited, Sec63 might bind to the Sec61 translocon by temporarily displacing the ribosome that is weakly bound to the Sec61 translocon.
The Sec63 J-mutant data indicate that the J-domain activates ATP hydrolysis by BiP to stabilize BiP interaction with the translocationally paused nascent chain that may fluctuate between the Sec61 channel and the luminal space by random Brownian motion (Fig. 7). BiP binding could bias the nascent chain forward movement into the ER lumen. This mechanism is analogous to the post-translational translocation of proteins into the ER (Matlack et al., 1999), except that the ribosome is still associated with the nascent chain here. It is unclear why Sec63-mediated release of translocation pause requires the nascent chain length to be ∼220 aa. Perhaps, the accumulation of a longer nascent chain at the cytosolic vestibule of Sec61 may facilitate efficient interaction with the Sec63 cytosolic domain and other factors, which could exert a force on the nascent chain to enter into the ER lumen through the translocon. This may explain our observation that Sec63-dependent translocation of the weak TMD retained at the translocon also relies on the C-terminus length to be more than 100 aa. We predict that once the cytosolically accumulated nascent chain at the Sec61 translocon is ratcheted into the ER lumen by BiP, the translating ribosome may establish a tight complex with the Sec61 translocon by displacing Sec63 (Fig. 7).
How does Sec63/BiP act on two different classes of substrates (weak TMD and weak SS) that are differently engaged with the translocon (Fig. 1 I and Fig. 7)? As shown by earlier crosslinking studies (Kida and Sakaguchi, 2018), a weak TMD is stalled within the translocon due to its inability to diffuse into the hydrophobic lipid bilayer through the lateral gate, which would lead to the exposure of the downstream C-terminal segment into the cytosol. The recruitment of BiP through Sec63 would then drive the translocation of weak TMD along with the downstream segment into the ER lumen (Fig. 1 I), thus facilitating weak TMD disposal by ER-associated protein degradation (Feige and Hendershot, 2013) or assembly with partner TMDs (Skach, 2009). In contrast, a weak SS inefficiently engages the lateral gate of the translocon, causing a translocation pause and the exposure of the downstream polypeptide into the cytosol. Sec63/BiP would drive the weak SS to enter the lateral gate and enable translocation and folding of the downstream polypeptide (Fig. 7). In both cases, Sec63 is co-translationally recruited to the translocation site by presumably interacting with the cytosolically exposed nascent chain and mediates BiP binding to weak TMD or nascent chain of a weak SS that enters through the Sec61 translocon into the ER lumen.
Recent structural studies suggest that Sec63 acts as a “brace” holding Sec61α in a conformation with an open lateral gate (Itskanov et al., 2021; Itskanov and Park, 2019; Weng et al., 2021; Wu et al., 2019). This bracing function may promote the translocation of nascent chains bearing a weak SS. However, BiP binding to nascent chains appears to be strictly required for translocation of AGAL and CD44 because the Sec63 J-mutant is inactive for mediating protein translocation. The critical role of BiP is further supported by our observation that increased levels of BiP can partially compensate for the loss of Sec63 in mediating protein translocation. Besides binding to incoming nascent chains and assisting protein folding, BiP can also regulate the opening of the Sec61 channel, which may promote protein translocation (Alder et al., 2005; Haβdenteufel et al., 2018; Schäuble et al., 2012; Schorr et al., 2020).
Despite the requirement of additional factors for efficient translocation, weak SSs are commonly found across species. One reported function of weak SSs is that they can attenuate the translocation of their mature domains into the ER during ER stress conditions (Kang et al., 2006). Earlier studies suggest that the nature of the interaction between an SS and the Sec61 translocon can influence protein maturation by regulating the timing of SS cleavage and N-linked glycosylation (Lingappa et al., 2002; Rutkowski et al., 2001; Rutkowski et al., 2003). Our study now provides evidence that weak SSs contain information to recruit BiP using Sec63 for protein folding in the ER. We postulate that Sec61 translocon plays a central role in decoding information in SSs through its engagement with a SS, which could determine BiP-dependent or -independent protein translocation into the ER. The SS-mediated BiP recruitment may be crucial for the folding of AGAL since it contains the hydrophobic N-terminal region right after the SS that is buried inside the structure, which is also the hotspot for genetic mutations causing Fabry disease (Garman, 2007).
When functional BiP is scarce in the ER under conditions such as stress and starvation, the translocation of BiP-dependent substrates like AGAL is aborted, preventing protein misfolding and aggregation inside the ER. The mislocalized/non-translocated substrates in the cytosol are likely eliminated by the Bag6-mediated quality control pathway (Hessa et al., 2011). The results from BiP depletion and ATPase mutants support the model that BiP-dependent translocation of proteins bearing a weak SS is coupled with protein folding in the ER. Other experimental approaches, such as acute depletion of BiP combined with biophysical measurements of protein folding, are required to support further the SS-guided protein folding in live cells. Since we have previously shown that Sec63-recruited BiP also inhibits the activity of translocon-associated IRE1 (Li et al., 2020; Plumb et al., 2015), nascent chains containing weak SSs may locally activate IRE1 to match incoming proteins to BiP levels in the ER.
Our findings may explain why about 40% of the analyzed yeast secretome contains insufficiently hydrophobic SSs and use the Sec63/Sec62-mediated post-translational translocation pathway (Ast et al., 2013), which likely reflects their dependency on BiP for folding and maturation in the ER. We predict that the recruitment of BiP may occur at any time during translocation as long as nascent chains contain a translocon retention element, such as marginally hydrophobic sequences. Consistent with this notion, earlier studies have observed translocation pausing events in which the nascent chain is transiently exposed to the cytosol during the translocation of apolipoprotein B and immunoglobulin G (Hegde and Lingappa, 1996; Rutkowski et al., 2001). These translocation pausing events may promote the maturation of proteins by recruiting specific luminal chaperones to nascent chains.
Previous studies have shown that mutations in the Sec63 gene can cause autosomal dominant polycystic kidney diseases due to defects in protein maturation and cell surface localization of polycystin-1 and presumably other proteins (Besse et al., 2017; Davila et al., 2004). Around one-third of our Sec63 clients have been previously implicated in human genetic disorders, including many lysosomal storage disorders and polycystic kidney diseases. This finding suggests that these proteins are particularly vulnerable to misfolding and aggregation due to genetic mutations. In light of our findings, selective induction of BiP by small molecules, such as AA147 (Plate et al., 2016), might be a potential therapeutic approach for treating these diseases. Furthermore, our studies may guide the design of customized SSs to produce a growing number of therapeutic recombinant secretory proteins, including enzymes, growth factors, and antibodies.
Materials and methods
DNA constructs
Most of the cDNAs used in this study were cloned into pcDNA/FRT/TO vector (Invitrogen). pcDNA/FRT/TO containing WRB-HA-Venus substrates generated from inserting PCR-amplified fragments of WRB into pcDNA/FRT/TO-Venus (Sun and Mariappan, 2020). Mouse Sec63-FLAG (template mouse Sec63 plasmid; Fedeles et al., 2015) was a kind gift from Dr. Stefan Somlo (Yale School of Medicine, New Haven, CT). Sec63 HPD/AAA (H132A/P1133A/D134A) and Sec63 Δ230–330 were generated using phosphorylated primers and Phusion Site-Directed Mutagenesis procedure (Li et al., 2020). Prl-AGAL-FLAG and CD44-FLAG constructs were generated by replacing AGAL SS (1–31 aa) and CD44 SS (1–20 aa) with bovine Prl SS (1–31 aa) using the above procedure. Since the flanking threonine of Prl SS that remained after the SS cleavage inhibited AGAL activity, Prl-AGAL-HA used in Figs. 5 and 6 was created by replacing AGAL SS (1–31 aa) with bovine Prl SS (1–30 aa). 3L-AGAL and 3L-CD44 constructs were created using the Pfu polymerase (Agilent Technologies)–based site-directed mutagenesis method. AGAL-Prl-FLAG was cloned by replacing bovine Prl SS (1–30) with AGAL SS (1–31) using the above procedure. BiP mutants (G227D and T37G) were introduced into pcDNATM 3.1-BiP (rat) using the above site-directed mutagenesis method. The coding sequences of all constructs were verified by sequencing (Yale Keck DNA Sequencing).
Antibodies and reagents
Antibodies used for immunoblotting are as follows: Rat α-FLAG L5 (#637303; BioLegend), Rabbit α-HA, Rabbit α-GFP, Rabbit α-Sec62, Rabbit α-Sec63, Rabbit α-Sec61β, Rabbit α-BAG6, and Rabbit α-Sec61α (Chitwood et al., 2018; Mariappan et al., 2010; Snapp et al., 2004) are a gift from Dr. Ramanujan Hegde (MRC Laboratory of Molecular Biology, Cambridge, UK). Rabbit α-BiP (#11587-1-AP; Proteintech), Mouse α-HA (#901513; Biolegend), Mouse α-PDI (#MA3-018; Affinity Bioreagents), Goat α-Rat-HRP (#7077; Cell Signaling), Goat α-Mouse-HRP (#115-035-003; Jackson ImmunoResearch), Goat α-Rabbit-HRP (#111-035-003; Jackson ImmunoResearch), Goat anti-rat HRP (#7077S; Cell signaling), Goat α-RAT IgG-Cy2 (#112-225-167; Jackson ImmunoResearch), Goat α-Mouse IgG-Alexa657 (#A-21235; Invitrogen). Beads were purchased as follows: Strep-Tactin XT beads (#2-4010-010; IBA), Rat anti-FLAG L5 affinity gel (#651503; Biolegend), Protein A agarose (#CA-PRI-0100; Repligen), Mouse anti-HA magnetic beads (#88837; Pierce), Poly L-lysine (#OKK-3056; Peptides International). Rabbit Reticulocyte Lysate was purchased from Green Hectares (Ph:1-800-GHLYSAT). Detergents were purchased as follows: digitonin (EMD Millipore), Triton X-100 (Thermo Fisher Scientific), sodium deoxycholate (Sigma-Aldrich), SDS (Sigma-Aldrich), and Tween 20 (American Bioanalytical).
Cell culture and CRISPR/Cas9 genome editing
HEK293-Flp-In T-Rex cells (Invitrogen) and HeLa cells were cultured in high glucose DMEM containing 10% FBS at 5% CO2. The knockout cell lines of Sec63 or Sec62 and 2xStrep-Sec61α cells were previously described (Li et al., 2020; Sun and Mariappan, 2020). Briefly, the human Sec62 targeting sequence (5′-AGTATCTTCGATTCAACTG-3′) or Sec63 targeting sequence (5′-GCCAGAGGTAGTATGTCGC-3′) was cloned into the gRNA expression vector (Mali et al., 2013). HEK293-Flp-In T-Rex cells were transfected with the gRNA plasmid and pSpCas9(BB)-2A-Puro plasmid (Ran et al., 2013) using Lipofectamine 2000. Cells were treated with puromycin (2.5 μg/ml) for 48 h, after which cells were returned to non-selecting media for 72 h. Cells were then plated at 0.5 cell/well in 96-well plates and grown for 3 wk. Individual clones were analyzed for Sec62 or Sec63 by immunoblotting. For endogenous tagging of BiP, the BiP gRNA sequence (5′-CCTCTTCACCAGTTGGGGG-3′) was cloned into the gRNA expression vector. The single-strand DNA oligonucleotide homology-directed repair sequence (5′-CTGGAAGAAATTGTTCAACCAATTATCAGCAAACTCTATGGAAGTGCAGGCCCTCCCCCAGCCGACTACAAGGACCACGACGGCGATTATAAGGATCACGACATCGACTACAAAGACGACGATGACAAGGCAACTGGTGAAGAGGATACAGCAGAAAAAGATGAGTTGTAGACACTGATCTGCTAGTGCTGTAA-3′) was synthesized (IDT) for inserting 3xFLAG-tag into the C terminus of BiP encoding gene in HEK293 cells. 300 pmol of homology-directed repair oligonucleotide was electroporated into one million HEK293 Flp-In T-Rex cells along with 2.5 μg each of pSpCas9(BB)-2A-Puro and BiP gRNA plasmid (Amaxa kit R, program A-24; Lonza). Immediately after electroporation, cells were plated in a 6-well plate. After 24 h of electroporation, the expression of Cas9 was selected by puromycin treatment (2.5 μg/ml) for 48 h. Most cells died of electroporation and puromycin treatment, but about 5% of survived cells were grown for a week without puromycin. Cells were replated at 0.5 cell/well in 96-well plates and expanded for 3 wk. Individual clones were examined for the presence of 3xFLAG BiP by immunoblotting with anti-BiP antibodies. Positive clones containing 3xFLAG BiP exhibited a slower migrating band compared to BiP in control HEK293 cells.
Immunoprecipitation
To test the interaction between WRB-Venus substrates and the Sec61 translocon complex, the chromosomally Sec61α-2x Strep-tagged HEK293 cells were plated on a poly-lysine (0.15 mg/ml) coated 6-well plates at 0.6 million/well. The cells were transiently transfected with 2 μg WRB-Venus substrates using 5 μl of Lipofectamine 2000 (Invitrogen) and treated with 200 ng/ml doxycycline to induce protein expression. After 24 h of transfection, cells were harvested in 1× PBS and centrifuged for 2 min at 10,000 g. The cell pellet was lysed in 200 μl of lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MgAc, 2% digitonin, and 1× Roche EDTA-free protease inhibitor cocktail) by incubating on ice for 30 min and then diluted to 1 ml with the lysis buffer containing 0.1% digitonin. The 5% digitonin (EMD-Millipore) stock was boiled for 5 min just before adding it into the lysis buffer to avoid digitonin precipitation during IPs. The supernatants were collected by centrifugation at 15,000 g for 15 min at 4°C. For co-IP, the supernatant was rotated with StrepTactin-XT beads (IBA) for 2 h at 4°C. The beads were washed three times with 1 ml of wash buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MgAc, 0.1% digitonin). The bound material was eluted from the beads by directly boiling in 50 μl of 2× SDS sample buffer for 5 min and analyzed by immunoblotting. The interaction between Sec63-FLAG and Sec61 translocon shown in Fig. S1 D was tested by following the above digitonin IP procedure but using HEK293 Sec63−/− cells stably expressing variants of Sec63-FLAG. 20 ng/ml doxycycline was used to induce the protein expression for 24 h before harvesting for IPs.
Metabolic labeling and immunoprecipitationmmunoprecipitation
Indicated cells (0.6 million cells/well) were plated on poly-lysine (0.15 mg/ml) coated 6-well plates and transiently transfected with 2 μg indicated plasmids. The expression of indicated proteins was induced with doxycycline (200 ng/ml) for 24 h. The cells were starved with Met/Cys-free medium, including 10% dialyzed FBS and doxycycline for 30 min. Cells were then labeled with 80 μCi/ml Express-35S protein labeling Mix (NEG772007MC) for 30 min. The labeled cells were directly harvested in 1 ml of radioimmunoprecipitation assay (RIPA) buffer, including 1× EDTA-free protease inhibitor cocktail (Roche). The lysate was cleared by centrifugation at 20,000 g for 15 min. The supernatant was mixed with the unconjugated or antibodies conjugated to beads indicated in the figure legends for 1.5 h in the cold room. In the case of unconjugated anti-rabbit antibodies, 20 μl slurry of protein-A agarose was added to samples and incubated for another 1 h. The beads were washed three times with 1 ml of RIPA buffer, and proteins were eluted with 50 μl of 2× SDS sample buffer and run on 7.5% or 10% Tris-Tricine SDS-PAGE gels. The gels were dried and analyzed by autoradiography.
For isolating endogenous substrates transiently retained at the Sec61 translocon, Sec63−/− and Sec63−/− cells complemented with WT Sec63, Sec63 J-mutant (HPD/AAA), or the translocon interaction defective Sec63 mutant (Δ230–300) were labeled as described above. The labeled cells were washed and harvested with 1XPBS and centrifuged at 10,000 g for 2 min. The cell pellets were lysed in 200 μl of lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MgAc, 2% digitonin, and 1× Roche protease inhibitor cocktail) by incubating on ice for 30 min and then diluted to 1 ml with the lysis buffer containing 0.1% digitonin. The supernatant was collected by centrifugation at 15,000 g for 15 min and incubated with Rat anti-FLAG beads (BioLegend) for 2 h at 4°C. The beads were washed three times with 1 ml of wash buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM MgAc, 0.1% digitonin). The bound material was eluted from beads by directly boiling in 50 μl of 2× SDS sample buffer for 5 min and analyzed by SDS-PAGE and autoradiography.
For pulse-chase analysis, transfected cells were labeled as above for 30 min and chased in a complete DMEM medium supplemented with label-free 2 mM Methionine and 2 mM Cysteine for indicated time points. The cells were directly harvested in RIPA buffer, including 1× EDTA free protease inhibitor cocktail (Roche), and cleared by centrifugation at 15,000 g for 15 min. The supernatant was incubated with the indicated antibody conjugated to beads for 2 h. For checking the secretion of AGAL or Prl-AGAL, the medium was collected at each time point, adjusted to 50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100, and immunoprecipitated and analyzed as above.
Affinity purification of translocon-engaged endogenous substrates and MS
Three different HEK293 cell lines (Sec63−/−, Sec63−/− stably complemented with WT Sec63-FLAG, Sec63−/− complemented with Sec63-FLAG J-mutant [HPD/AAA]) were plated in 10-cm plates at 10 million cells/plate. Protein expression was induced by 20 ng/ml doxycycline. After 24 h of induction, the cells were transferred from one 10-cm plate to two 15-cm plates for each cell line, including 20 ng/ml doxycycline. After culturing for two d, cells at ∼100% confluence were treated with 100 μg/ml cycloheximide for 5 min. The cells were then collected using ice-cold 1XPBS and centrifuged at 1,500 g for 10 min to obtain the cell pellets. The cell pellets were resuspended in lysis buffer (50 mM Hepes, pH 7.4, 100 mM KAc, 50 mM NaCl, 10 mM MgAc, 1% digitonin, 1× Roche protease inhibitor cocktail, 1 mM DTT, 100 μg/ml cycloheximide). The lysates were further homogenized using a dounce by passing through manually 10 times. The lysates were then centrifuged at 35,000 g for 30 min at 4°C in an MLA80 rotor. The supernatant was incubated with 300 μl of pre-washed mouse anti-FLAG beads (Sigma-Aldrich) slurry for 2 h at 4°C. The beads were washed for six times with 5 ml of wash buffer (50 mM Hepes, pH 7.4, 100 mM KAC, 50 mM NaCl, 10 mM MgAC, 0.1% digitonin, 1 mM DTT). The samples were eluted with the elution buffer (0.1 M Glycine, pH 2.3, and 0.1% Triton X-100). The eluted materials were TCA precipitated and subjected to MS analysis.
The immunoprecipitants were digested with trypsin and analyzed by liquid chromatography with tandem MS (LC-MS/MS) using an LTQ Orbitrap Elite equipped with a Waters nanoACQUITY ultra-performance liquid chromatography system at the Mass Spectrometry & Proteomics Resource of the W.M. Keck Foundation Biotechnology Resource Laboratory, Yale University. Proteins were searched against the homo sapiens SwissProt protein database using the Mascot Search Engine (Matrix Science, LLC; version. 2.6.0). Scaffold program (version Scaffold_4.8.7; Proteome Software) was used to validate MS/MS-based peptide and protein identifications. Scaffold program was also used to generate normalized spectral counts for each sample. In Table S1, we listed protein hits from all three samples that have a protein threshold of 5.0% false discovery rate, at least two peptides, and a peptide threshold of 95%. In Table S2, we listed protein hits of secretory/membrane proteins from Sec63 J-mutant that are at least twofold enriched relative to WT Sec63 and fivefold enriched relative to negative control Sec63−/− cells. The information of SSs or the first TMD sequences, subcellular localization, and association with human diseases were extracted from http://uniprot.org. In Table S3, we listed secretory/membrane protein hits from WT Sec63 that are at least twofold enriched relative to Sec63 J-mutant and fivefold enriched relative to negative control Sec63−/− cells. The h-region of an SS shown in Fig. 3, D and E, was defined using the MacVector program by selecting the Kyte-Doolittle hydrophilicity option in windows of 3–19 aa. The defined h-region was analyzed by grand average hydropathy to obtain a hydrophobicity score. For Fig. S3, D and E, we first defined the h-region of the SS sets of the Sec63 clients (n = 99) and the human proteome from UniProt with the mitochondrial proteins deducted (n = 5,294). Similar to the methodology described in Schorr et al. (2020) using custom scripts, we calculated the window-averaged hydrophobicity score of each amino acid based on the Kyte-Doolittle method (the averaging of nince residues window for the central residue) and considered the continuous stretch of amino acids with a hydrophobicity score higher than 0.41 as the h-region. Based on the extracted h-region sequences, we then analyzed the properties, such as the hydrophobicity and length of the h-regions.
Endo H treatment
Indicated cell lines (0.15 million cells/well) were plated on poly-lysine (0.15 mg/ml) coated 24-well plates and transiently transfected with 400 ng indicated plasmids. Protein expression was induced with 200 ng/ml doxycycline. After 24 h of transfection, the cells were collected in 150 μl of 2× SDS sampled buffer and boiled for 10 min. 20 μl sample was mixed with 65 μl of water, 5 μl of 20% Triton X-100, 10 μl of 10× G5 buffer (NEB), and 1 μl of Endo H HF (NEB). The reactions were incubated for 4 h at 37°C and were terminated by adding 50 μl 5× SDS sample buffer and boiled for 5 min before analyzing by immunoblotting. The above procedure was used for Endo H digestion of immunoprecipitated samples that were already boiled in a 2× SDS sample.
In vitro transcription and translation
The open reading frames were PCR amplified from pcDNA/FRT/TO-based constructs using a forward primer bearing Sp6 promoter sequence and annealing sequence to the CMV promoter and a reverse primer that anneals to the poly-A region of the vector. For generating RNCs, PCR products were amplified using the above forward primer, but the reverse primer anneals to 3′ of the template cDNA without a stop codon. In vitro transcription reactions, including PCR product, SP6 polymerase (NEB), and RNasin (Promega), were performed at 37°C for 1.5 h as described previously (Sharma et al., 2010). The transcripts were translated in the RRL translation system, including 35S-methionine, and with or without rough microsomes. Rough microsomes were either prepared from the canine pancreas as described previously (Walter and Blobel, 1983) or cultured HEK293 or Sec63−/− cells as described previously (Zhang et al., 2013). Transcripts encoding WRB-Venus variants with and without a termination codon were translated in the presence or absence of HEK or Sec63−/− microsomes for 30 min at 32°C. The translated products (10 μl) were denatured with 5× volume of 2× SDS sample buffer and analyzed by SDS-PAGE and autoradiography. Alternatively, the translation products were treated or untreated with 0.5 mg/ml PK for 60 min on ice. PK digestion was terminated by adding 5 mM PMSF and incubating it on ice for 15 min. The resulting samples were transferred into the boiling 60 μl of 1.5× SDS sample buffer and incubated for an additional 5 min before analyzing by SDS-PAGE and autoradiography.
For the co-translational translocation assay, transcripts encoding AGAL-FLAG and Prl-AGAL-FLAG were translated in RRL in the presence or absence of microsomes for 30 min at 32°C. The translation products were treated or untreated with 0.5 mg/ml PK for 60 min on ice. PK digestion was terminated by adding 5 mM PMSF and incubating it on ice for 15 min. The resulting samples were transferred to the boiling SDS buffer (100 mM Tris, pH 8.0, and 1% SDS) and heated for an additional 5 min. The samples were diluted 10 times with Triton buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100). The diluted samples were incubated with anti-FLAG beads (Bio-legend) for 2 h at 4°C. The beads were washed three times with 1 ml of Triton buffer and eluted by directly boiling in 50 μl of 2× SDS sample buffer for 5 min and analyzed by SDS-PAGE and autoradiography. For the post-translational translocation assay, transcripts encoding AGAL-FLAG were translated in RRL for 30 min at 32°C. The translation reactions were centrifuged at 70,000 rpm in a TLA120.1 rotor for 30 min at 4°C. The post-ribosomal supernatant was incubated with or without microsomes for 30 min at 32°C. The resulting samples were treated with PK and analyzed as above.
RNCs of indicated lengths were produced from transcripts lacking a stop codon in the presence or absence of the indicated microsomes. The resulting translated products were split into 2 × 10 μl and incubated with or without 0.5 mg/ml PK for 60 min on ice. The PK digestion was terminated by adding 5 mM PMSF and incubating it on ice for 15 min. For Fig. S4, A and B, translated products were treated with 1 mM puromycin/10 mM EDTA for 15 min on ice followed by 15 min at 32°C and treated with PK. The PK treated or non-treated samples were transferred into boiling 60 μl of 1.5× SDS sample buffer and incubated for an additional 5 min. For RNCs of AGAL or Prl-ss-AGAL, the translated products were analyzed by SDS-PAGE autoradiography after denaturation and IP with anti-FLAG beads, as described above. In some cases, the denatured samples were treated with RNase A for 15 min at 37°C to remove tRNAs from nascent chains before analyzing by SDS-PAGE and autoradiography. For Endo H digestion in Fig. 4 G, the translated products denatured with an equal volume of 2% SDS for boiled for 5 min. 10 μl of denatured products were mixed with 1× G5 buffer from NEB, 1% Triton X-100, 1 μl of Endo H, and adjusted with water to 20 μl. After 4 h at 37°C, samples were mixed with an equal volume of 3× SDS sampled buffer and analyzed by SDS-PAGE autoradiography. Luminal proteins depleted CRM shown in Fig. S6 D was prepared as described previously (Kang et al., 2006), 100 μl CRM at a concentration of 1eq/μl was diluted with 400 μl buffer: 50 mM Hepes, pH 7.4, 250 mM sucrose, 2 mM MgCl2, 1 mM DTT, and 0.075% deoxy Big CHAP (DBC) for 10 min on ice. The detergent permeabilized microsomes were centrifuged at 70,000 rpm for 30 min in a TLA100.3 rotor. The resulting pellet was resuspended in 500 μl of buffer without DBC and centrifuged as above. The final pellet was resuspended in 50 μl of buffer without DBC and used for in vitro translation reactions as indicated above.
In vitro chemical crosslinking
Transcripts encoding versions of AGAL or Prl-AGAL lacking a termination codon were translated in the presence of CRM for 30 min at 32°C. The membrane-targeted RNCs were isolated by centrifugation through a 0.5 M sucrose cushion for 12 min at 70,000 rpm/TLA100.3, and the resulting pellet was resuspended in membrane buffer (MB: 50 mM Hepes, pH 7.4, 100 mM KAc, 5 mM MgAc, and 250 mM sucrose). For Fig. 4 H, crosslinking was performed with 400 μM bismaleimidohexane (Thermo Fisher Scientific) for 15 min at 25°C and quenched with 25 mM 2-mercaptoethanol. For Fig. 5, A and B, crosslinking was carried out with 200 μM disuccinimidyl suberate (Thermo Fisher Scientific) for 30 min at room temperature and quenched 20 mM Tris-HCl, pH 8.0, for 15 min. The cross-linked products were denatured with 1% SDS, 100 mM Tris-HCl, pH 8.0, for 30 min at 55°C, and diluted 10-fold in Triton buffer. The respective antibodies were added and incubated for 1.5 h at 4°C with end-to-end rotation, followed by incubation with protein-A agarose (Repligen) for 1.5 h at 4°C. The beads were washed three times with Triton buffer, eluted with SDS sample buffer, and analyzed after boiling for 5 min, but 55°C/30 min for the Sec61α sample. Samples were treated with RNase A (100 μg/ml) for 15 min at 37°C before analyzing by SDS-PAGE and autoradiography.
Membrane recruitment assay
We followed the previously published procedure (Yanagitani et al., 2011) with the following modifications. Transcripts encoding versions of AGAL lacking a stop codon were translated in RRL, including 35S-methionine in the presence of either trypsin (20 μg/ml) digested CRM (Plumb et al., 2015) or CRM for 20 min at 32°C. 25 μl translation reactions were layered on 100 μl of 1 M sucrose prepared in 50 mM Hepes, pH 7.5, 100 mM NaCl, 5 mM MgCl2. After sedimentation for 15 min at 20,000 g, 100 μl supernatants were carefully collected, and pellets were resuspended in 75 μl of 1 M sucrose. Aliquots of both supernatants and pellets were analyzed by SDS-PAGE and autoradiography.
Endogenous BiP-3xFLAG IP
BiP-3xFLAG HEK293 cells (0.9 million cells/well) were plated on poly-lysine coated 6-well plates and transiently transfected with 2 μg of indicated plasmids and induced with doxycycline (200 ng/ml) for 24 h. The cells were starved with Met/Cys-free medium, including 2.5% dialyzed FBS and doxycycline for 45 min. Cells were then labeled with 90 μCi/ml Express-35S protein labeling Mix (NEG772007MC) for 2 min at 37°C. The labeled cells were immediately placed on ice and harvested in 1 ml of ice-cold 1XPBS and centrifuged at 10,000 g for 2 min. Cell pellets were solubilized in 200 μl of lysis buffer (50 mM Tris, pH 7.5, 160 mM NaCl, 1% Digitonin, 1 mM CaCl2, Apyrase (10 U/ml)) for 30 min on ice. The lysate was diluted to 1 ml with lysis buffer containing 0.1% digitonin before centrifugation at 20,000 g for 15 min. While 800 μl of supernatant was incubated with 12 μl of mouse anti-FLAG beads (Sigma-Aldrich), the remaining 200 μl was diluted to 800 ul with NP40 buffer and incubated 15 μl mouse anti-HA magnetic beads (Thermo Fisher Scientific). After 90 min of incubation, anti-FLAG beads were washed 3× with 1 ml of lysis buffer containing 0.1% digitonin, but anti-HA magnetic beads were washed with 3× with 1 ml of NP40 buffer. The washed beads were boiled with 50 μl of 2× SDS samples buffer and analyzed by 7.5% Tris-Tricine SDS-PAGE and autoradiography. For the second IP, anti-FLAG immunoprecipitants (∼40 μl in 2× SDS sample buffer) were diluted to 1 ml with Triton buffer (50 mM Tris, pH 8, 150 mM NaCl, 1% Triton X-100) and incubated with 15 μl of anti-HA magnetic beads for 90 min. The beads were washed three times with 1 ml of Triton buffer and analyzed as above.
Isolation of detergent-soluble and -insoluble fractions
The indicated cells (0.3 × 106/well) were plated on a poly-lysine coated 12-well plate and transiently transfected with 0.8 μg of AGAL-HA or its variants. For Fig. 6 D and Fig. S6 F, cells (0.15 × 106/well) were plated and treated with control or BiP siRNA (5′-GGAGCGCAUUGAUACUAGA -3′) for 48 h and then transfected with variants of AGAL plasmids. Protein expression was induced with 200 ng/ml doxycycline for HEK293 cells. After 24 h of transfection, HEK293 cells were washed once with ice-cold 1XPBS and directly harvested in 400 μl of NP40 buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% sodium deoxycholate, 0.5% NP40, 1× complete EDTA protease inhibitor cocktail (Roche) by incubating 15 min at 4°C, while HeLa cells were harvested in 800 μl of RIPA buffer. The samples were centrifuged for 20 min at 15,000 rpm at 4°C. The detergent soluble supernatant was collected, and the detergent-insoluble pellet was directly boiled in 200 μl of 2× SDS buffer for 10 min, but RIPA pellets were reconstituted with 200 μl of 50 mM Tris-HCl, 150 mM NaCl, 2M Urea, and 1% SDS and boiled at 99°C for 15 min with shaking at 12,000 rpm. Both soluble and insoluble fractions were analyzed by immunoblotting.
Immunoblotting
Samples were typically run on 7.5% acrylamide Tris-Tricine SDS-PAGE gels at 100 V for 1.5 h. Proteins from the gels were transferred onto nitrocellulose membranes (Bio-Rad) using 100 V for 1 h. Membranes were blocked for 30 min at room temperature in 1XPBS/0.1% Tween 20 (PBST) containing 5% milk (American Bioanalytical). The primary antibodies were diluted in 5% BSA (Millipore Sigma), including 0.01% sodium azide (Millipore Sigma), and incubated with the membranes for 1 h 30 min at room temperature. Membranes were washed six times with PBST for 5 min per wash. The secondary antibodies conjugated with HRP were incubated for 1 h in 5% milk and washed as above. Membranes were blotted dry and incubated in ECL Western Blotting Substrate or SuperSignal West Pico (Thermo Fisher Scientific) for 2 min and finally exposed to HyBlot autoradiography films (Denville). The program ImageJ (National Institutes of Health) was used to quantify the intensity of bands from the scanned films.
Immunofluorescence
BiP-3xFLAG HEK293 cells (0.12 × 106) were plated on 12-mm round glass coverslips (Thermo Fisher Scientific) coated with 0.15 mg/ml poly-lysine in 24-well plates. The following day, cells were fixed and immunostained using a standard procedure (Sundaram et al., 2017). Rat anti-FLAG antibody (#637303; Biolegend) and mouse α-PDI (#MA3-018; Affinity Bioreagents) were used as primary antibodies. Goat α-RAT IgG-Cy2 (#112-225-167; Jackson ImmunoResearch) and Goat α-Mouse IgG-Alexa657 (#A-21235; Invitrogen) were used as secondary antibodies. Cells were imaged on Leica scanning confocal consisting of an inverted microscope (Leica SP6/SP8) and an HC PL APO 63×/1.4 (CS2 No: 11506350) oil objective lens (Leica) and was controlled by the Leica Application Suite X software. Sequential image scanning at 1× zoom, 100 Hz, 1,024 × 1,024 pixels, and with line averaging set at four was used to collect images for displayed images.
α-Galactosidase-A activity assay
Cells (0.35 × 106/well) were plated on poly-lysine (0.15 mg/ml) coated 12-well plates and transiently transfected with 800 ng AGAL-FLAG versions. After 24 h of transfection, the cells were washed once with 1XPBS and harvested in 1XPBS. After centrifugation at 10,000 g for 2 min, the cell pellet was resuspended in the 100 μl of NP40 buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% NP40, and 0.5% deoxycholate) and incubated on ice for 20 min. The lysate was centrifuged for 10 min at 14,000 g at 4°C. The assay was performed as described previously with the following modifications (Lukas et al., 2016). 50 μl of 2 mM artificial substrate 4-MU-α-D-galactopyranoside (Sigma-Aldrich) dissolved in 0.06 M phosphate citrate buffer (pH 4.7) was mixed well with 1 μl of the supernatant in a black 96-well plate (#655076; Greiner) for fluorescence reading, and incubated for 5 min at 37°C. The reaction was terminated by the addition of 200 μl 1 M glycine buffer (pH 10.5). The released 4-MU was measured by a fluorescence reader (TECAN) using a 360-nm excitation filter and a 465-nm emission filter. The readings were subtracted from empty vector-transfected cells, and AGAL variant activities were normalized to WT AGAL activity.
Quantification and statistical analysis
Quantification of bands in immunoblots and autoradiographs was performed using the ImageJ software (National Institutes of Health). The graphs were created by Prism7, and the error bars represent the SD from three independent experiments.
Online supplemental material
Fig. S1 shows the characterization of Sec63-mediated clearance of translocon-engaged marginally hydrophobic TMDs. Fig. S2 supports the mislocalization of AGAL in the cytosol of Sec63−/− cells. Fig. S3 shows that conserved weak SSs determine Sec63 dependency. Fig. S4 shows that AGAL nascent chains are efficiently recruited to the ER and translocated across the membrane. Fig. S5 suggests that Sec63 mediates BiP binding to newly synthesized polypeptides containing both weak and strong signal sequences. Fig. S6 demonstrates the effect of SSs on protein translocation and protein folding. Table S1 shows proteins identified from Sec63−/−, WT Sec63, or Sec63 J-domain mutant samples. Table S2 shows secretory and membrane proteins that are enriched in Sec63 J-mutant. Table S3 shows secretory and transmembrane proteins that are enriched in WT Sec63.
Supplementary Material
shows proteins identified from Sec63−/−, WT Sec63, or Sec63 J-domain mutant sample.
shows secretory and membrane proteins that are enriched in Sec63 J-mutant.
shows secretory and transmembrane proteins that are enriched in WT Sec63.
contains original blots for Fig. 1.
contains original blots for Fig. 2.
contains original blots for Fig. 3.
contains original blots for Fig. 4.
contains original blots for Fig. 5.
contains original blots for Fig. 6.
contains original blots for Fig. S1.
contains original blots for Fig. S2.
contains original blots for Fig. S3.
contains original blots for Fig. S4.
contains original blots for Fig. S5.
contains original blots for Fig. S6.
Acknowledgments
We thank Jean Kanyo and TuKiet Lam from the Yale Keck Proteomic facility for assisting with analyzing MS data. We are thankful to Rui Ma for helping analyze the SS hydrophobicity. We are grateful to Fred Gorelick, Karthikeyan Radhakrishnan, Jacob Culver, Sang-Wook Kang, and Zai-Rong Zhang for their comments on the manuscript. We greatly appreciate the reviewers’ constructive comments and suggestions.
This work is funded by National Institutes of Health grant R01GM117386 (M. Mariappan).
The authors declare no competing financial interests.
Author contributions: S. Sun designed and performed most of the experiments. X. Li performed imaging and BiP binding and dissociation experiments. M. Mariappan generated BiP-3xFLAG HEK293 cells, performed BiP interaction studies, soluble/insoluble assay, and supervised the project. S. Sun and M. Mariappan wrote the manuscript with inputs from X. Li.
References
- Alder, N.N., Shen Y., Brodsky J.L., Hendershot L.M., and Johnson A.E.. 2005. The molecular mechanisms underlying BiP-mediated gating of the Sec61 translocon of the endoplasmic reticulum. J. Cell Biol. 168:389–399. 10.1083/jcb.200409174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ast, T., Cohen G., and Schuldiner M.. 2013. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell. 152:1134–1145. 10.1016/j.cell.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Besse, W., Dong K., Choi J., Punia S., Fedeles S.V., Choi M., Gallagher A.R., Huang E.B., Gulati A., Knight J., et al. 2017. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J. Clin. Invest. 127:1772–1785. 10.1172/JCI90129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel, G., and Dobberstein B.. 1975. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67:835–851. 10.1083/jcb.67.3.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman, I., and Hebert D.N.. 2013. Protein folding in the endoplasmic reticulum. Csh Perspect. Biol. 5:a013201. 10.1101/cshperspect.a013201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky, J.L., Goeckeler J., and Schekman R.. 1995. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 92:9643–9646. 10.1073/pnas.92.21.9643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, T.M., Jordahl A.S., Yates M.E., Preston G.M., Cook E., Kleyman T.R., and Brodsky J.L.. 2017. Interactions between intersubunit transmembrane domains regulate the chaperone-dependent degradation of an oligomeric membrane protein. Biochem. J. 474:357–376. 10.1042/BCJ20160760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, P.J., Juszkiewicz S., Guna A., Shao S., and Hegde R.S.. 2018. EMC is required to initiate accurate membrane protein topogenesis. Cell. 175:1507–1519. 10.1016/j.cell.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, B.J., Devaraneni P.K., Yang Z., David L.L., and Skach W.R.. 2015. Cotranslational stabilization of Sec62/63 within the ER Sec61 translocon is controlled by distinct substrate-driven translocation events. Mol. Cell. 58:269–283. 10.1016/j.molcel.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi, A.K., and Schekman R.. 1997. The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. J. Cell Biol. 137:1483–1493. 10.1083/jcb.137.7.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila, S., Furu L., Gharavi A.G., Tian X., Onoe T., Qian Q., Li A., Cai Y., Kamath P.S., King B.F., et al. 2004. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat. Genet. 36:575–577. 10.1038/ng1357 [DOI] [PubMed] [Google Scholar]
- Deshaies, R.J., Sanders S.L., Feldheim D.A., and Schekman R.. 1991. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature. 349:806–808. 10.1038/349806a0 [DOI] [PubMed] [Google Scholar]
- Döring, K., Ahmed N., Riemer T., Suresh H.G., Vainshtein Y., Habich M., Riemer J., Mayer M.P., O’Brien E.P., Kramer G., and Bukau B.. 2017. Profiling ssb-nascent chain interactions reveals principles of hsp70-assisted folding. Cell. 170:298–311.e20. 10.1016/j.cell.2017.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elofsson, A., and von Heijne G.. 2007. Membrane protein structure: Prediction versus reality. Annu. Rev. Biochem. 76:125–140. 10.1146/annurev.biochem.76.052705.163539 [DOI] [PubMed] [Google Scholar]
- Fedeles, S.V., So J.S., Shrikhande A., Lee S.H., Gallagher A.R., Barkauskas C.E., Somlo S., and Lee A.H.. 2015. Sec63 and Xbp1 regulate IRE1α activity and polycystic disease severity. J. Clin. Invest. 125:1955–1967. 10.1172/JCI78863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige, M.J., and Hendershot L.M.. 2013. Quality control of integral membrane proteins by assembly-dependent membrane integration. Mol. Cell. 51:297–309. 10.1016/j.molcel.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell, S.W., Travers K.J., Weissman J.S., and Brodsky J.L.. 2001. The action of molecular chaperones in the early secretory pathway. Annu. Rev. Genet. 35:149–191. 10.1146/annurev.genet.35.102401.090313 [DOI] [PubMed] [Google Scholar]
- Fons, R.D., Bogert B.A., and Hegde R.S.. 2003. Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J. Cell Biol. 160:529–539. 10.1083/jcb.200210095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman, S.C. 2007. Structure-function relationships in alpha-galactosidase A. Acta Paediatr. 96:6–16. 10.1111/j.1651-2227.2007.00198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain, D.P. 2010. Fabry disease. Orphanet J. Rare Dis. 5:30. 10.1186/1750-1172-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz, T., Stevens F., and Argon Y.. 2013. Orchestration of secretory protein folding by ER chaperones. Biochim. Biophys. Acta 1833:2410–2424. 10.1016/j.bbamcr.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haßdenteufel, S., Johnson N., Paton A.W., Paton J.C., High S., and Zimmermann R.. 2018. Chaperone-mediated Sec61 channel gating during ER import of small precursor proteins overcomes Sec61 inhibitor-reinforced energy barrier. Cell Rep. 23:1373–1386. 10.1016/j.celrep.2018.03.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde, R.S., and Kang S.W.. 2008. The concept of translocational regulation. J. Cell Biol. 182:225–232. 10.1083/jcb.200804157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde, R.S., and Keenan R.J.. 2022. The mechanisms of integral membrane protein biogenesis. Nat. Rev. Mol. Cell Biol. 23:107–124. 10.1038/s41580-021-00413-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde, R.S., and Lingappa V.R.. 1996. Sequence-specific alteration of the ribosome-membrane junction exposes nascent secretory proteins to the cytosol. Cell. 85:217–228. 10.1016/S0092-8674(00)81098-3 [DOI] [PubMed] [Google Scholar]
- Helenius, A., Marquardt T., and Braakman I.. 1992. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 2:227–231. 10.1016/0962-8924(92)90309-B [DOI] [PubMed] [Google Scholar]
- Hessa, T., Meindl-Beinker N.M., Bernsel A., Kim H., Sato Y., Lerch-Bader M., Nilsson I., White S.H., and von Heijne G.. 2007. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature. 450(7172):1026–1030. 10.1038/nature06387 [DOI] [PubMed] [Google Scholar]
- Hessa, T., Sharma A., Mariappan M., Eshleman H.D., Gutierrez E., and Hegde R.S.. 2011. Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature. 475:394–397. 10.1038/nature10181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou, Y.A., Bishop D.F., and Desnick R.J.. 1992. Overexpression of human alpha-galactosidase A results in its intracellular aggregation, crystallization in lysosomes, and selective secretion. J. Cell Biol. 119:1137–1150. 10.1083/jcb.119.5.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskanov, S., Kuo K.M., Gumbart J.C., and Park E.. 2021. Stepwise gating of the Sec61 protein-conducting channel by Sec63 and Sec62. Nat. Struct. Mol. Biol. 28:162–172. 10.1038/s41594-020-00541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskanov, S., and Park E.. 2019. Structure of the posttranslational Sec protein-translocation channel complex from yeast. Science. 363:84–87. 10.1126/science.aav6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., Cheng Z., Mandon E.C., and Gilmore R.. 2008. An interaction between the SRP receptor and the translocon is critical during cotranslational protein translocation. J. Cell Biol. 180:1149–1161. 10.1083/jcb.200707196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, S.J., Kim J.E., Reithinger J.H., and Kim H.. 2014. The Sec62-Sec63 translocon facilitates translocation of the C-terminus of membrane proteins. J. Cell Sci. 127:4270–4278. 10.1242/jcs.153650 [DOI] [PubMed] [Google Scholar]
- Jungnickel, B., and Rapoport T.A.. 1995. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 82:261–270. 10.1016/0092-8674(95)90313-5 [DOI] [PubMed] [Google Scholar]
- Juszkiewicz, S., and Hegde R.S.. 2018. Quality control of orphaned proteins. Mol. Cell. 71:443–457. 10.1016/j.molcel.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S.W., Rane N.S., Kim S.J., Garrison J.L., Taunton J., and Hegde R.S.. 2006. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell. 127:999–1013. 10.1016/j.cell.2006.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida, Y., Ishihara Y., Fujita H., Onishi Y., and Sakaguchi M.. 2016. Stability and flexibility of marginally hydrophobic-segment stalling at the endoplasmic reticulum translocon. Mol. Biol. Cell. 27:930–940. 10.1091/mbc.E15-09-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida, Y., and Sakaguchi M.. 2018. Interaction mapping of the Sec61 translocon identifies two Sec61α regions interacting with hydrophobic segments in translocating chains. J. Biol. Chem. 293:17050–17060. 10.1074/jbc.RA118.003219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.J., Mitra D., Salerno J.R., and Hegde R.S.. 2002. Signal sequences control gating of the protein translocation channel in a substrate-specific manner. Dev. Cell. 2:207–217. 10.1016/S1534-5807(01)00120-4 [DOI] [PubMed] [Google Scholar]
- Lang, S., Benedix J., Fedeles S.V., Schorr S., Schirra C., Schäuble N., Jalal C., Greiner M., Hassdenteufel S., Tatzelt J., et al. 2012. Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J. Cell Sci. 125:1958–1969. 10.1242/jcs.096727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., Park E., Ling J., Ingram J., Ploegh H., and Rapoport T.A.. 2016. Crystal structure of a substrate-engaged SecY protein-translocation channel. Nature. 531:395–399. 10.1038/nature17163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Sun S., Appathurai S., Sundaram A., Plumb R., and Mariappan M.. 2020. A molecular mechanism for turning off IRE1α signaling during endoplasmic reticulum stress. Cell Rep. 33:108563. 10.1016/j.celrep.2020.108563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa, V.R., Rutkowski D.T., Hegde R.S., and Andersen O.S.. 2002. Conformational control through translocational regulation: A new view of secretory and membrane protein folding. BioEssays. 24:741–748. 10.1002/bies.10130 [DOI] [PubMed] [Google Scholar]
- Lukas, J., Scalia S., Eichler S., Pockrandt A.M., Dehn N., Cozma C., Giese A.K., and Rolfs A.. 2016. Functional and clinical consequences of novel α-galactosidase A mutations in Fabry disease. Hum. Mutat. 37:43–51. 10.1002/humu.22910 [DOI] [PubMed] [Google Scholar]
- Ma, Y., and Hendershot L.M.. 2004. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 28:51–65. 10.1016/j.jchemneu.2003.08.007 [DOI] [PubMed] [Google Scholar]
- Mali, P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., and Church G.M.. 2013. RNA-guided human genome engineering via Cas9. Science. 339:823–826. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan, M., Li X., Stefanovic S., Sharma A., Mateja A., Keenan R.J., and Hegde R.S.. 2010. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature. 466:1120–1124. 10.1038/nature09296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack, K.E., Misselwitz B., Plath K., and Rapoport T.A.. 1999. BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell. 97:553–564. 10.1016/S0092-8674(00)80767-9 [DOI] [PubMed] [Google Scholar]
- Meyer, H.A., Grau H., Kraft R., Kostka S., Prehn S., Kalies K.U., and Hartmann E.. 2000. Mammalian Sec61 is associated with Sec62 and Sec63. J. Biol. Chem. 275:14550–14557. 10.1074/jbc.275.19.14550 [DOI] [PubMed] [Google Scholar]
- Misselwitz, B., Staeck O., and Rapoport T.A.. 1998. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol. Cell. 2:593–603. 10.1016/S1097-2765(00)80158-6 [DOI] [PubMed] [Google Scholar]
- Nguyen, D., Stutz R., Schorr S., Lang S., Pfeffer S., Freeze H.H., Förster F., Helms V., Dudek J., and Zimmermann R.. 2018. Proteomics reveals signal peptide features determining the client specificity in human TRAP-dependent ER protein import. Nat. Commun. 9:3765. 10.1038/s41467-018-06188-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemalm, K., Halling K.K., Nilsson I., and von Heijne G.. 2012. Orientational preferences of neighboring helices can drive ER insertion of a marginally hydrophobic transmembrane helix. Mol. Cell. 45:529–540. 10.1016/j.molcel.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi, Y., Yamagishi M., Imai K., Fujita H., Kida Y., and Sakaguchi M.. 2013. Stop-and-move of a marginally hydrophobic segment translocating across the endoplasmic reticulum membrane. J. Mol. Biol. 425:3205–3216. 10.1016/j.jmb.2013.05.023 [DOI] [PubMed] [Google Scholar]
- Plate, L., Cooley C.B., Chen J.J., Paxman R.J., Gallagher C.M., Madoux F., Genereux J.C., Dobbs W., Garza D., Spicer T.P., et al. 2016. Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. Elife. 5:5. 10.7554/eLife.15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb, R., Zhang Z.R., Appathurai S., and Mariappan M.. 2015. A functional link between the co-translational protein translocation pathway and the UPR. Elife. 4:4. 10.7554/eLife.07426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobre, K.F.R., Poet G.J., and Hendershot L.M.. 2019. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 294:2098–2108. 10.1074/jbc.REV118.002804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran, F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., and Zhang F.. 2013. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8:2281–2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport, T.A. 2007. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 450:663–669. 10.1038/nature06384 [DOI] [PubMed] [Google Scholar]
- Rehm, A., Stern P., Ploegh H.L., and Tortorella D.. 2001. Signal peptide cleavage of a type I membrane protein, HCMV US11, is dependent on its membrane anchor. EMBO J. 20:1573–1582. 10.1093/emboj/20.7.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski, D.T., Lingappa V.R., and Hegde R.S.. 2001. Substrate-specific regulation of the ribosome- translocon junction by N-terminal signal sequences. Proc. Natl. Acad. Sci. USA. 98:7823–7828. 10.1073/pnas.141125098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski, D.T., Ott C.M., Polansky J.R., and Lingappa V.R.. 2003. Signal sequences initiate the pathway of maturation in the endoplasmic reticulum lumen. J. Biol. Chem. 278:30365–30372. 10.1074/jbc.M302117200 [DOI] [PubMed] [Google Scholar]
- Schäuble, N., Lang S., Jung M., Cappel S., Schorr S., Ulucan Ö., Linxweiler J., Dudek J., Blum R., Helms V., et al. 2012. BiP-mediated closing of the Sec61 channel limits Ca2+ leakage from the ER. EMBO J. 31:3282–3296. 10.1038/emboj.2012.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr, S., Nguyen D., Haßdenteufel S., Nagaraj N., Cavalié A., Greiner M., Weissgerber P., Loi M., Paton A.W., Paton J.C., et al. 2020. Identification of signal peptide features for substrate specificity in human Sec62/Sec63-dependent ER protein import. FEBS J. 287:4612–4640. 10.1111/febs.15274 [DOI] [PubMed] [Google Scholar]
- Shan, S.O., and Walter P.. 2005. Co-translational protein targeting by the signal recognition particle. FEBS Lett. 579:921–926. 10.1016/j.febslet.2004.11.049 [DOI] [PubMed] [Google Scholar]
- Sharma, A., Mariappan M., Appathurai S., and Hegde R.S.. 2010. In vitro dissection of protein translocation into the mammalian endoplasmic reticulum. Methods Mol. Biol. 619:339–363. 10.1007/978-1-60327-412-8_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skach, W.R. 2009. Cellular mechanisms of membrane protein folding. Nat. Struct. Mol. Biol. 16:606–612. 10.1038/nsmb.1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skach, W.R., Shi L.B., Calayag M.C., Frigeri A., Lingappa V.R., and Verkman A.S.. 1994. Biogenesis and transmembrane topology of the CHIP28 water channel at the endoplasmic reticulum. J. Cell Biol. 125:803–815. 10.1083/jcb.125.4.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp, E.L., McCaul N., Quandte M., Cabartova Z., Bontjer I., Källgren C., Nilsson I., Land A., von Heijne G., Sanders R.W., and Braakman I.. 2017. Structure and topology around the cleavage site regulate post-translational cleavage of the HIV-1 gp160 signal peptide. Elife. 6:e26067. 10.7554/eLife.26067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp, E.L., Reinhart G.A., Bogert B.A., Lippincott-Schwartz J., and Hegde R.S.. 2004. The organization of engaged and quiescent translocons in the endoplasmic reticulum of mammalian cells. J. Cell Biol. 164:997–1007. 10.1083/jcb.200312079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S., and Mariappan M.. 2020. C-terminal tail length guides insertion and assembly of membrane proteins. J. Biol. Chem. 295:15498–15510. 10.1074/jbc.RA120.012992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram, A., Plumb R., Appathurai S., and Mariappan M.. 2017. The Sec61 translocon limits IRE1α signaling during the unfolded protein response. ELife. 6:e27187. 10.7554/eLife.27187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers, J., Lerner M., Wiedmann M., Volkmer J., and Zimmermann R.. 2003. Polypeptide-binding proteins mediate completion of co-translational protein translocation into the mammalian endoplasmic reticulum. EMBO Rep. 4:505–510. 10.1038/sj.embor.embor826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, M., Bakunts A., Orsi A., Lari F., Tadè L., Danieli A., Rato C., Valetti C., Sitia R., Raimondi A., et al. 2019. Inadequate BiP availability defines endoplasmic reticulum stress. Elife. 8:e41168. 10.7554/eLife.41168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt, S., Jungnickel B., Hartmann E., and Rapoport T.A.. 1996. Signal sequence-dependent function of the TRAM protein during early phases of protein transport across the endoplasmic reticulum membrane. J. Cell Biol. 134:25–35. 10.1083/jcb.134.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne, G. 1985. Signal sequences. The limits of variation. J. Mol. Biol. 184:99–105. 10.1016/0022-2836(85)90046-4 [DOI] [PubMed] [Google Scholar]
- Voorhees, R.M., and Hegde R.S.. 2015. Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. Elife. 4:4. 10.7554/eLife.07975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees, R.M., and Hegde R.S.. 2016. Structure of the Sec61 channel opened by a signal sequence. Science. 351:88–91. 10.1126/science.aad4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, P., and Blobel G.. 1983. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 96:84–93. 10.1016/S0076-6879(83)96010-X [DOI] [PubMed] [Google Scholar]
- Wei, J., Gaut J.R., and Hendershot L.M.. 1995. In vitro dissociation of BiP-peptide complexes requires a conformational change in BiP after ATP binding but does not require ATP hydrolysis. J. Biol. Chem. 270:26677–26682. 10.1074/jbc.270.44.26677 [DOI] [PubMed] [Google Scholar]
- Weng, T.H., Steinchen W., Beatrix B., Berninghausen O., Becker T., Bange G., Cheng J., and Beckmann R.. 2021. Architecture of the active post-translational Sec translocon. EMBO J. 40:e105643. 10.15252/embj.2020105643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S.H., and von Heijne G.. 2008. How translocons select transmembrane helices. Annu. Rev. Biophys. 37:23–42. 10.1146/annurev.biophys.37.032807.125904 [DOI] [PubMed] [Google Scholar]
- Willmund, F., del Alamo M., Pechmann S., Chen T., Albanèse V., Dammer E.B., Peng J., and Frydman J.. 2013. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell. 152:196–209. 10.1016/j.cell.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Cabanos C., and Rapoport T.A.. 2019. Structure of the post-translational protein translocation machinery of the ER membrane. Nature. 566:136–139. 10.1038/s41586-018-0856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, Y., and Sakisaka T.. 2012. Molecular machinery for insertion of tail-anchored membrane proteins into the endoplasmic reticulum membrane in mammalian cells. Mol. Cell. 48:387–397. 10.1016/j.molcel.2012.08.028 [DOI] [PubMed] [Google Scholar]
- Yanagitani, K., Kimata Y., Kadokura H., and Kohno K.. 2011. Translational pausing ensures membrane targeting and cytoplasmic splicing of XBP1u mRNA. Science. 331:586–589. 10.1126/science.1197142 [DOI] [PubMed] [Google Scholar]
- Young, B.P., Craven R.A., Reid P.J., Willer M., and Stirling C.J.. 2001. Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J. 20:262–271. 10.1093/emboj/20.1.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.R., Bonifacino J.S., and Hegde R.S.. 2013. Deubiquitinases sharpen substrate discrimination during membrane protein degradation from the ER. Cell. 154:609–622. 10.1016/j.cell.2013.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska, A., Tatzelt J., Dudek J., Paton A.W., Paton J.C., Zimmermann R., and Haßdenteufel S.. 2019. The signal peptide plus a cluster of positive charges in prion protein dictate chaperone-mediated Sec61 channel gating. Biol. Open. 8:bio040691. 10.1242/bio.040691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
shows proteins identified from Sec63−/−, WT Sec63, or Sec63 J-domain mutant sample.
shows secretory and membrane proteins that are enriched in Sec63 J-mutant.
shows secretory and transmembrane proteins that are enriched in WT Sec63.
contains original blots for Fig. 1.
contains original blots for Fig. 2.
contains original blots for Fig. 3.
contains original blots for Fig. 4.
contains original blots for Fig. 5.
contains original blots for Fig. 6.
contains original blots for Fig. S1.
contains original blots for Fig. S2.
contains original blots for Fig. S3.
contains original blots for Fig. S4.
contains original blots for Fig. S5.
contains original blots for Fig. S6.