Abstract
Linitis plastica is an intramural carcinoma that may occur in any hollow organ. Rectal linitis plastica (RLP) is a morphological variant cancer that may occur as a primary form of cancer or secondary as a metastasis of a primary malignancy. We report the case of a man in his 70s with RLP secondary to prostate carcinoma who was initially suspected to have an obstructing rectal adenocarcinoma. During colonoscopy a segment of cobblestone mucosa was seen in the distal rectum. Subsequent imaging showed enhancement of all wall-layers of the rectum and diffuse retroperitoneal fat infiltration with traction on both ureters. A prostate-specific membrane antigen scan confirmed RLP secondary to a prostate carcinoma mimicking the clinical and radiological signs of an obstructing rectal carcinoma with retroperitoneal fibrosis.
This case emphasises the possible pitfalls in the diagnosis of RLP and the importance of advanced imaging techniques, such as MRI, as well as appropriate histological samples. The patient underwent androgen deprivation therapy to which RLP responded well and neither systemic chemotherapy or surgery was necessary.
Keywords: Endoscopy, Prostate Cancer, Colon cancer
Background
Linitis plastica is an intramural carcinoma that is most often associated with a subtype of stomach cancer. However, linitis plastica is a condition that may occur in any hollow organ. The infiltrating cancer cells cause a desmoplastic reaction that causes a rigid (leather bottle) appearance of the organ wall while the mucosal layer is frequently spared.1 Linitis plastica of the rectum (RLP) is a rare entity which may occur as a primary colorectal carcinoma or a metastasis of another type of cancer (secondary RLP), most often gastric carcinoma. Cases of secondary RLP have also been described in bladder, breast, cervix, adnexa, skin (melanoma), colon and prostate cancer.2–6
In this case report we describe a patient with RLP secondary to prostate carcinoma. Secondary RLP has been found in increasing numbers because of the use of MRI in the staging of rectal cancer.3 6 To our knowledge, this report illustrates only the second case in which symptomatic RLP is the first manifestation of a prostate carcinoma and only the fourth case of RLP secondary to prostate carcinoma.3 6 7
Case presentation
A man in his 70s was referred to our gastroenterology department for colonoscopy with a 2-month history of change in bowel habit with non-bloody loose stool three times a day. The patient reported increasing episodes of generalised abdominal pain. His medical history revealed type II diabetes, chronic atrial fibrillation and mild chronic obstructive pulmonary disease. The patient had lost 4 kg of weight over the past 6 months and was unable to exercise because of increasing fatigue on exertion and abdominal pain. The patient did not experience rectal bleeding, nor was serum haematology suggestive of iron deficiency anaemia. The patient did not describe lower urinary tract symptoms. Because the patient was referred for colonoscopy, he had completed his bowel preparation which had vastly exacerbated his abdominal pain. Physical examination revealed a distended abdomen with tinkling bowel sounds on auscultation. The abdomen was diffusely tender without signs of peritonitis. After spontaneous defecation the patient’s symptoms were relieved considerably, thus, a diagnosis of partial bowel obstruction was made. On digital examination, at 2 cm from the anus, a circumferential solid rectal mass was felt. There was no blood on the examining finger. Examination of the prostate was not described. The patient was admitted with a working diagnosis of partial bowel obstruction secondary to an obstructing rectal carcinoma.
Investigations
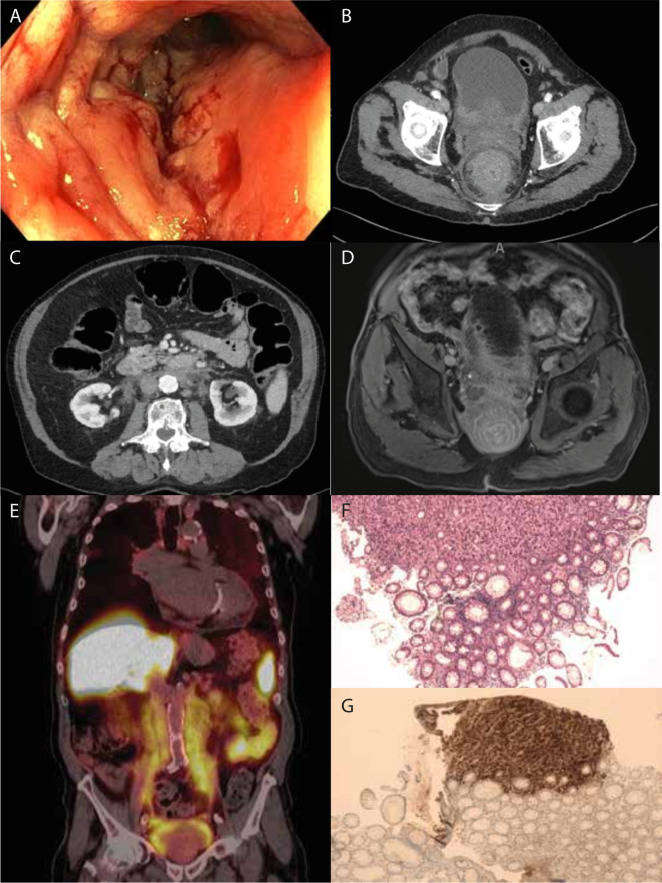
The partial colonoscopy that was subsequently performed showed an evident circular narrowing of the rectal lumen starting 2 cm proximal of the anal verge and extending proximally. The entire rectum was involved but the colon appeared normal. Notably, the rectal mucosa had a cobblestone appearance, evidently swollen and erythematous, on macroscopic mucosal inspection, while the colonic mucosa appeared normal. There were no typical endoscopic signs of colorectal carcinoma—no ulcerative or protruding lesions (figure 1A, video 1). Multiple biopsies were taken. Colonoscopy was not completed since the bowel was inadequately prepared.
Figure 1.
(A) Representative image from colonoscopy at approximately 10 cm from the linea dentata showing circumferential diffuse swelling, mild stenosis and a cobblestone appearance in the rectum from 15 to 2 cm from the anus. The colonic mucosa shows no signs of abnormalities. (B) Representative axial frame from contrast-enhanced abdominal CT scan showing the target sign in the rectum, as well as the primary prostate adenocarcinoma infiltrating into the bladder. (C) Representative axial frame from contrast-enhanced abdominal CT scan showing retroperitoneal involvement; that is, fat infiltration and traction of the ureters and renal arteries. A distended colon is also seen. (D) Representative axial frame from the gadolinium enhanced pelvic MRI showing a more defined target sign with individual epithelial layers as well as fat suppression. Further, there is suspicion of prostate adenocarcinoma with extension towards the rectal wall and the suggestion of bone metastases of the os ischium and os pubis on the right. (E) Prostate-specific membrane antigen (PSMA) scan (coronal) showing the full extent of involvement of the retroperitoneum. There is extensive PSMA positive prostate adenocarcinoma with extension to the rectum, bladder wall, para-iliac tissues and extension cranially into the duodenum with retroperitoneal fibrosis and traction on both ureters. Because of the extent of carcinoma lymph node, metastases cannot be separated from the carcinoma. There is a suspicion of bone metastases in the os ischium on the right side. (F) H&E staining of the rectal biopsy showing a layer of normal mucosa above while beneath there is cribriform disorganised growth of prostate adenocarcinoma. (G) Immunohistochemistry for prostate specific antigen (PSA) (brown) showing part of the biopsy containing malignancy to be strongly PSA positive, suggesting primary prostate adenocarcinoma.
Video 1. Video of colonoscopy showing the swollen mucosa and stenotic lumen over the full length of the rectum.
The patient subsequently underwent a thoracoabdominal contrast-enhanced CT scan. A circumferential thickening of the submucosa and muscular layers of the rectal wall was seen (target-sign appearance) (figure 1B) over a length of 15 cm. The CT scan also showed diffuse infiltration of the retroperitoneal fat, ascending to the level of the renal veins, with signs of traction on both ureters and extension caudally along the iliac vessels and surrounding the posterior part of the urinary bladder (figure 1C). Additionally, bilateral hilar and mediastinal lymphadenopathy was seen without evidence of pulmonary metastases. In retrospect, the lymphadenopathy, however, was also seen on a scan performed 5 years earlier, and appeared unchanged. At that time, cytological examination of the lymph nodes was performed and showed no abnormality—a definitive cause was not found. As clinical and colonoscopy findings did not fit with a diagnosis of rectal adenocarcinoma (which rarely obstructs and does not spread along the length of the rectum), our differential diagnosis included inflammatory diseases such as retroperitoneal fibrosis, sarcoidosis or amyloidosis, or malignancy—primary (lymphoma) or secondary (metastatic carcinoma).
MRI of the pelvis was performed which confirmed a diffuse infiltrative process in the rectal wall with oedema in the submucosal layer. There was markedly restricted diffusion in the muscular wall layers (MRI figure 1D). Postgadolinium series showed extensive enhancement of all wall-layers. An aberrant aspect of the peripheral zone of the prostate was seen with focal diffusion restriction. Diffuse and cell rich infiltration with diffusion restriction was seen around the prostate extending and infiltrating along the dorsolateral wall of the bladder with further extension towards the rectum (with some loss of the dorsal fat plane) and upward extension along the retroperitoneum, which also showed restricted diffusion.
Prostate-specific membrane antigen (PSMA) scan showed intense uptake of PSMA in a PSMA positive prostate carcinoma. This PSMA uptake was also seen in the thickened layers of the rectal wall. Further PSMA uptake was seen in the iliac region and around the ureters retroperitoneally and extended cranially as far as the duodenum. There was evidence of retroperitoneal fibrosis with traction on both ureters. The mediastinal lymph nodes we described earlier showed only mild uptake (figure 1E). Taken together these scans were highly suggestive of a primary prostate carcinoma. Current guidelines do not qualify these findings as a high-volume prostate carcinoma.8 Multidisciplinary review, however, deemed this an exceptionally extensive carcinoma on the basis of scan findings.
Histopathological examination of the biopsies taken during partial colonoscopy showed no significant abnormality of the rectal mucosa (figure 1F). However, beneath the rectal mucosa infiltrating atypical cells were found, showing irregular nuclei and only poorly formed glands suggestive of a poorly differentiated carcinoma. Immunohistochemistry of this material was negative for caudal type homeobox 2 (cdx2) which makes a colorectal origin more unlikely but positive for both cytokeratin 5.2 (Cam5.2) and prostate-specific antigen (PSA), suggesting either infiltrative growth or metastasis of a poorly differentiated primary prostate adenocarcinoma (figure 1G). Laboratory results showed a PSA level of 409.9 µg/L (reference <3 µg/L) and a CEA (carcinoembryonic antigen) level of 2 µg/L (reference <5 µg/L) further strengthening the diagnosis of a primary prostate adenocarcinoma. These investigations confirmed the diagnosis of RLP secondary to prostate adenocarcinoma.
Treatment
The patient was referred to both the urology and oncology departments for further treatment. In accordance with national guidelines, prostate adenocarcinoma was considered to have metastasised and thus curative treatment was no longer possible.8 9 Therefore, palliative hormonal treatment was started in the form of androgen deprivation therapy (ADT) (goserelin 10.8 mg every 12 weeks without abiraterone) in combination with 4 weeks of cyproterone tablets once per day 150 mg. To prevent the occurrence of osteoporosis subsequent to this therapy calcium carbonate/colecalciferol 500 mg/440IE once daily was started. The patient was informed of the survival benefits of add-on therapy to ADT (docetaxel or abiraterone).10 11 These survival benefits and possible negative implications for quality of life were evaluated (ie, starting these therapies would require frequent hospital visits as well as the possibility of toxicity resulting in neutropenia and neuropathy, for example). Because our patient wanted to spend as little time in the hospital as possible as he valued quality of life over length of survival and had a favourable response to ADT, he chose to continue goserelin injections every 12 weeks but decided against additional treatment. After 6 weeks of treatment PSA levels drastically improved to 87.26 µg/L (reference <3 µg/L), which is considered a good response to therapy.12 Had PSA levels risen, enzalutamide therapy once per day 160 mg as an add-on to goserelin therapy would have been added. Follow-up was planned every 3 months at the urology outpatient clinic, with regular intermittent check-ups by the specialised oncology nurse for guidance through this process as well as dietary support because of weight loss. At follow-up after 3 and 6 months, PSA levels continued to decline, 26.58 µg/L and 9.74 µg/L (reference <3 µg/L), respectively, indicating a persistent response to therapy.8 The patient did continue to experience mild faecal incontinence but there was no progressive bowel obstruction.
Outcome and follow-up
After treatment the patient’s condition remained stable for 8 months. Unfortunately, the patient developed cellulitis of the left leg from a non-healing ulcerating wound thought to be due to peripheral arterial disease. This was deemed unrelated to the prostate carcinoma. The patient eventually recovered and was admitted to a nursing home for further care. Unfortunately, the patient contracted COVID-19 and died approximately 10 months after the initial diagnosis of RLP.
Discussion
In the 17th century the first reports of ‘cirrhosis of the stomach’ described a thickened stiff wall of the stomach. In 1858, William Brinton defined this ‘leather bottle stomach’ as linitis plastica, because of the increase of irregular fibrotic connective tissue resembling linen cloth.13 14 Linitis plastica is most often associated with gastric adenocarcinoma, more specifically the diffuse type, but it may actually occur in any hollow organ. Secondary colorectal linitis plastica is more common than primary colorectal linitis plastica. This is more frequently diagnosed due to the standardised practice of pelvic MRI scan in the work up of rectal carcinoma. It most often occurs in association with metastatic gastric cancer, sometimes with metastatic urinary bladder or lobular breast cancer. RLP may extend throughout the entire gastrointestinal tract.4 5 15 16 To the best of our knowledge, there are only three previously reported cases of RLP secondary to prostate carcinoma.6 7 17 However, this is only the second reported case of RLP secondary to prostate carcinoma where partial obstruction due to RLP was the reason for presentation.6
Typical endoscopic findings of linitis plastica include swollen epithelium and reduced compliance (increased rigidity). There may be a cobblestone appearance as in this case. Most notably the mucosal pattern seems relatively undisturbed. This mucosal sparing, a clinical feature of this disease, makes it a difficult diagnosis. In this patient, the integrity of the superficial rectal mucosa was almost undisturbed. Only in the submucosa, infiltrative growth of atypical cubic cells (suspicious of malignancy) was found, rarely penetrating to the surface of the epithelium. These findings outline the importance of representative biopsies in this disease. As has been outlined before, endoscopic ultrasound guided fine needle aspiration might be needed to confirm the diagnosis.7 18 Immunohistochemical staining of the infiltrative growth showed no positivity for cdx2, which is an intestinal marker positive in a majority of adenocarcinomas of intestinal origin. Cam5.2, a marker for cells of epithelial origin, was, however, strongly positive. PSA, while not specific though highly suggestive for a prostate carcinoma, was very strongly positive in all atypical cells in the submucosa.
In the diagnosis of RLP, radiology plays an important role. There are several radiological features associated with this diagnosis that were also present in our patient.3 6 The trajectory of wall thickening is usually more than 5 cm; a target sign or double ring pattern was seen on both CT and MRI imaging. Of interest is that the carcinoma seemingly limits itself to the retroperitoneal space, essentially masquerading as retroperitoneal fibrosis. This has been described in gastric linitis plastica before.19 The intramural spread of poorly differentiated signet ring cells of the gastric carcinoma allows it to spread unnoticed in the retroperitoneal space where eventually only the traction caused by the increasing tumour mass can be seen. Prostate adenocarcinoma of our patient seems to have behaved in a remarkedly similar manner to gastric signet ring cell carcinomas, illustrating the clinical deceptiveness of linitis plastica, which may result in diagnostic delay.
Learning points.
Rectal linitis plastica may mimic different diseases in presentation: in this case the patient had symptoms of partial bowel obstruction.
The substantial and diffuse cell rich infiltration of the retroperitoneal space dominated the radiological findings, initially leading us in the direction of an inflammatory disease, such as retroperitoneal fibrosis or sarcoidosis. However, the combination of different imaging modalities, endoscopy and tissue biopsy lead to the definitive diagnosis.
Histology is crucial to diagnosis and submucosal findings are key.
Footnotes
Contributors: MCM and DAK wrote the manuscript. MCM developed the supporting images. JW and JvdL supervised and cowrote the manuscript. All authors critically reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Agnes A, Estrella JS, Badgwell B. The significance of a nineteenth century definition in the era of genomics: linitis plastica. World J Surg Oncol 2017;15:123. 10.1186/s12957-017-1187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.el Absi M, Elouannani M, Elmdarhri J, et al. [Primary linitis plastica of the rectum]. Rev Med Liege 2002;57:10–12. [PubMed] [Google Scholar]
- 3.Ha HK, Jee KR, Yu E, et al. CT features of metastatic linitis plastica to the rectum in patients with peritoneal carcinomatosis. AJR Am J Roentgenol 2000;174:463–6. 10.2214/ajr.174.2.1740463 [DOI] [PubMed] [Google Scholar]
- 4.Wei S-C, Su W-C, Chang M-C, et al. Incidence, endoscopic morphology and distribution of metastatic lesions in the gastrointestinal tract. J Gastroenterol Hepatol 2007;22:827–31. 10.1111/j.1440-1746.2006.04532.x [DOI] [PubMed] [Google Scholar]
- 5.Khor V, Khairul-Asri MG, Fahmy O, et al. Linitis plastica of the rectum secondary to metastatic prostate cancer: a case report of a rare presentation and literature review. Urol Ann 2021;13:442–5. 10.4103/UA.UA_188_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You JH, Song JS, Jang KY, et al. Computed tomography and magnetic resonance imaging findings of metastatic rectal linitis plastica from prostate cancer: a case report and review of literature. World J Clin Cases 2018;6:554–8. 10.12998/wjcc.v6.i12.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutani MS. EUS and EUS-guided fine-needle aspiration for the diagnosis of rectal linitis plastica secondary to prostate carcinoma. Gastrointest Endosc 1999;50:117–9. 10.1016/S0016-5107(99)70361-5 [DOI] [PubMed] [Google Scholar]
- 8.Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol 2021;79:263–82. 10.1016/j.eururo.2020.09.046 [DOI] [PubMed] [Google Scholar]
- 9.Richtlijn prostaatcarcinoom11 . Richtlijn prostaatcarcinoom richtlijnendatabase.nl2014 [updated 15-09-2016. Richtlijn prostaatcarcinoom], 2022. Available: https://richtlijnendatabase.nl/richtlijn/prostaatcarcinoom [Accessed 14 Feb 2022].
- 10.Gravis G, Boher J-M, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol 2016;70:256–62. 10.1016/j.eururo.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 11.James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338–51. 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm PD, Blasko JC, Sylvester JE, et al. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys 2001;51:31–40. 10.1016/S0360-3016(01)01601-7 [DOI] [PubMed] [Google Scholar]
- 13.Brinton W. The diseases of the stomach, with an introduction on its anatomy and physiology; being lectures delivered at St. Thomas's Hospital. London. J. Churchill 1859;406. [PMC free article] [PubMed] [Google Scholar]
- 14.Rijken A, Lurvink RJ, Luyer MDP, et al. The burden of peritoneal metastases from gastric cancer: a systematic review on the incidence, risk factors and survival. J Clin Med 2021;10. 10.3390/jcm10214882. [Epub ahead of print: 23 10 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernet P, Azar HA, Stout AP. Intramural (tubal) spread of LINITIS PLASTICA along the alimentary tract. Gastroenterology 1965;48:419–24. 10.1016/S0016-5085(65)80001-4 [DOI] [PubMed] [Google Scholar]
- 16.Mai KT, Isotalo PA, Guindi M, et al. Intestinal epithelial lesions associated with signet ring cell carcinoma of the colon and small intestine. Pathology 2002;34:51–6. 10.1080/00313020120105642 [DOI] [PubMed] [Google Scholar]
- 17.Dumontier I, Roseau G, Palazzo L, et al. Endoscopic ultrasonography in rectal linitis plastica. Gastrointest Endosc 1997;46:532–6. 10.1016/S0016-5107(97)70009-9 [DOI] [PubMed] [Google Scholar]
- 18.Yusuf TE, Levy MJ, Wiersema MJ. EUS features of recurrent transitional cell bladder cancer metastatic to the Gi tract. Gastrointest Endosc 2005;61:314–6. 10.1016/S0016-5107(04)02578-7 [DOI] [PubMed] [Google Scholar]
- 19.Karbasi A, Karbasi-Afshar R, Ahmadi J, et al. Retroperitoneal fibrosis as a result of signet ring cell gastric cancer: a case-based review. J Gastrointest Cancer 2013;44:94–7. 10.1007/s12029-012-9422-1 [DOI] [PubMed] [Google Scholar]