Abstract
Tumor necrosis factor (TNF) antagonists have made significant progress in treating autoimmune diseases like inflammatory bowel disease. Adalimumab, a human anti-TNF monoclonal antibody, may be a treatment option for patients with moderate to severe Crohn’s disease for whom conventional treatments have not been effective. Central nervous system (CNS) and peripheral nervous system (PNS) demyelination may rarely develop during treatment. However, it is unclear whether CNS and PNS demyelination occurs as a coincidence or consequence. This report presents a 45-year-old male patient who developed multiple sclerosis-like demyelinating lesions at the seventh month of TNF alpha-blocker treatment. We discontinued anti-TNF therapy and initiated azathioprine. In the approximately eighteen-month follow-up, he did not show any neurological or radiological deterioration. When the autoimmune disease develops during anti-TNF blocker therapy, it would be a safe approach to choose a different group of biologic agents for disease control. The potential risk of developing neurological side effects requires closely follow-up of patients.
Keywords: TNF-alfa blocker, adalimumab, multiple sclerosis, demyelination, Crohn’s disease, inflammatory bowel disease
INTRODUCTION
Tumor necrosis factor (TNF)-alpha inhibitor drugs have been used in inflammatory diseases for over 20 years (1). Adalimumab, a TNF-alfa antagonist, may be a treatment option for patients with moderate to severe Crohn’s disease (CD) for whom conventional treatments have not been effective (2). Their increased use has brought along various side effects. For example, central nervous system (CNS) and peripheral nervous system (PNS) demyelination may rarely develop during treatment. The mechanism underlying this relationship has not yet been clarified (3). Here, we present a patient with CD who developed multiple sclerosis (MS)-like demyelinating lesions during adalimumab treatment.
CASE
In January 2020, a 45-year-old male patient was admitted to our neurology clinic with headaches and dizziness. Neurological examination revealed right-sided hypoesthesia on the face. He had no prior personal or family history of neurological diseases. In his medical history, he was diagnosed with enteropathic arthritis in 2018 and also with CD in February 2019. Four months after the last diagnosis, under 2000 mg/day sulfasalazine and 100 mg/day azathioprine treatment, he developed an attack presented with bloody diarrhea and abdominal pain. Therefore, adalimumab therapy was initiated in June 2019. At the seventh month of TNF alpha-blocker treatment, he experienced subacute onset headaches and dizziness. Initial brain and cervical magnetic resonance imaging (MRI) revealed pericallosal, juxtacortical, supratentorial, infratentorial, and spinal cord lesions suggesting MS (Figure 1). There was no contrast enhancement in these lesions. Cerebrospinal fluid (CSF) examination revealed mildly elevated CSF protein [43.9 mg/dL (standard range 15–40 mg/dL)], normal IgG index [0.74 (standard range 0.3–0.85)] and oligoclonal band negativity. Extensive laboratory workup for the vasculitic, infectious, and other rheumatological processes were normal. In this patient, the temporal relationship between adalimumab and CNS demyelination raised the question on the role of adalimumab. Thus, adalimumab treatment was switched to azathioprine 150 mg/day. At approximately eighteen months of follow-up, he did not experience any neurological symptoms, and besides, brain MRI did not show any new T2 or contrast-enhancing lesions
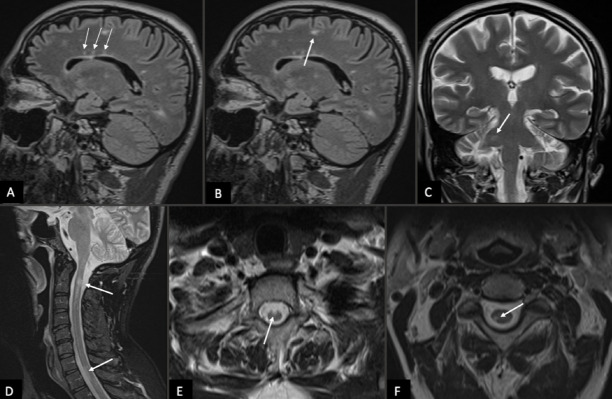
Figure 1.
a–f. The initial brain magnetic resonance imaging, sagittal fluid-attenuated inversion recovery (FLAIR) image shows a pericallosal (a, arrows) and juxtacortical (b, arrow) lesions suggesting of multiple sclerosis (c). The coronal T2-weighted (W) image discloses focal T2-hyperintense lesion in the right middle cerebellar peduncle (d, arrow). The sagittal short-tau inversion recovery sequence and axial T2W image of the spine demonstrate focal lesions (e and f, arrows) located at the C2 and T1 level indicating demyelinating lesion.
Highlights
CNS and PNS demyelination may develop during TNF-alfa antagonist therapy.
It is unclear whether the demyelination with anti-TNF is a coincidence or a result.
Patients under biological agents should be examined for neurological complications.
Neurological complications may require switching to a new class of biologic agents.
DISCUSSION
Inflammatory bowel disease (IBD) is a chronic process and requires long-term treatment. TNF-α blockers have a rapid onset of action and more effective control of disease progression when compared with traditional disease-modifying drugs (2). To date, five anti-TNF-α blockers have been approved for clinical use: Etanercept, Infliximab, Adalimumab, Golimumab, and Certolizumab (4). Adalimumab is a human anti-TNF monoclonal antibody approved for many indications involving inflammatory processes. Adalimumab can treat moderate to severe CD in patients who do not benefit from conventional treatments. In addition, available data suggest that adalimumab safety is not dose-dependent (2). Although these agents are thought to be well-tolerated, autoimmune-mediated adverse events such as CNS and PNS demyelination have been reported (3–5). Whether CNS and PNS demyelination may occur as a coincidence or a consequence during anti-TNF alpha-blocker therapy has not been clarified yet. Seror et al. studied 33 patients who developed the demyelinating disorder after TNF alpha-blocker therapy and found the CNS involvement ratio to PNS involvement of 2:1. In this cohort, CNS involvement included encephalic lesions, transverse myelitis, retrobulbar optic neuritis, and PNS involvement, including chronic or acute inflammatory demyelinating polyneuropathy (3).
A recent study reported the estimated frequency of bioagent-induced autoimmune disease as approximately 8 cases per 1000 patients. In addition CNS demyelinating diseases was found to be 0.03% in a review of cases exposure to different biological agents (5). Kemanetzoglou et al. studied 122 cases who developed CNS demyelination during treatment with TNF-α Blockers. Ten (8%) patients had CD, and in 19 (16%) cases, the responsible agent was Adalimumab. This study determined the time from initiation of anti-TNF-α to the development of neurological symptoms as approximately 5 months (range 1 week to 15 months). In this cohort, 7 (5.73%) patients were finally diagnosed with MS in the follow-up (mean follow-up 20.43 months) (4). In a recent study, researchers reported that patients with IBD had a 1.32 times greater risk of MS than healthy controls. In addition, this study found a 43% increase in the incidence of MS in patients with IBD exposed to anti-TNF-α treatments compared to patients with IBD who were not exposed (6).
Some patients suffering from this entity may have abnormalities in a CD4 + CD25 + regulatory T cell function that may play a significant role in controlling the development of the autoimmune process (5). It is unclear whether anti-TNF-related demyelination may origin from an environmental or genetic predisposition to MS (7). According to Williams et al., anti-TNF-associated demyelination does not have the same genetic background as MS. Furthermore, studies using anti-TNF agents for MS patients revealed that these drugs are ineffective and may increase immune activation and severity of the disease. The same researchers detected that three-quarters of patients with anti-TNF-induced demyelination continued to develop neurological problems despite stopping the drug (7).
An increase in the use of biological agents in various inflammatory processes will increase the frequency and variety of autoimmune diseases. In the management of this process, steroid therapy may give beneficial results (5). Of course, it is impossible to prove the relationship between Adalimumab and CNS demyelination in the patient presented herein. However, the obvious temporal connection with a clinic and radiological stabilization after discontinuation of Adalimumab despite not initiating typical MS therapy suggest this. As in our case, when the autoimmune disease process caused by anti-TNF agents regresses, to direct the treatment towards a new class of biologic agents may be a safe treatment approach to control the underlying active disease. Although a causal relationship cannot be fully proven, patients treated with biological agents should be followed closely to diagnose neurological complications that may require a change in therapy.
Footnotes
Peer-review: Externally peer-reviewed.
Informed Consent: Informed consent was obtained from the patient.
Author Contributions: Concept – OS, AT; Design – OS, AT, RG; Supervision – OS, AT, RG; Resource –OS, AT; Materials –OS, AT; Data Collection and/or Processing – OS, AT, RG; Analysis and/or Interpretation – OS, AT, RG; Literature Search –OS; Writing – OS, AT; Critical Reviews – OS, AT, RG.
Conflict of Interest: There are no conflicts of interest.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Willrich MAV, Murray DL, Snyder MR. Tumor necrosis factor inhibitors:clinical utility in autoimmune diseases. Transl Res. 2015;165:270–282. doi: 10.1016/j.trsl.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Colombel JF, Sandborn WJ, Reinisch W, Peyrin-Biroulet L, Panaccione R, Rutgeerts P, et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn's disease or ulcerative colitis. Aliment Pharmacol Ther. 2018;47:219–228. doi: 10.1111/apt.14420. [DOI] [PubMed] [Google Scholar]
- 3.Seror R, Richez C, Sordet C, Rist S, Gossec L, Direz G, et al. the Club Rhumatismes et Inflammation Section of the SFR. Pattern of demyelination occurring during anti-TNF-? therapy:a French national survey. Rheumatology (Oxford) 2013;52:868–874. doi: 10.1093/rheumatology/kes375. [DOI] [PubMed] [Google Scholar]
- 4.Kemanetzoglou E, Andreadou E. CNS Demyelination with TNF-? Blockers. Curr Neurol Neurosci Rep. 2017;17:36. doi: 10.1007/s11910-017-0742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-De-Lis M, Retamozo S, Flores-Chávez A, Kostov B, Perez-Alvarez R, Brito-Zerón P, et al. Autoimmune diseases induced by biological agents A review of 12, 731 cases (BIOGEAS Registry) Expert Opinion on Drug Safety. 2017;16:1255–1271. doi: 10.1080/14740338.2017.1372421. [DOI] [PubMed] [Google Scholar]
- 6.Avasarala J, Guduru Z, McLouth CJ, Wilburn A, Talbert J, Sutton P, et al. Use of anti-TNF-? therapy in Crohn's disease is associated with increased incidence of multiple sclerosis Mult Scler Relat Disord. 2021;51:102942. doi: 10.1016/j.msard.2021.102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams I, Uhlig HH. Demyelination After Anti-TNF Therapy:Who is at Risk? J Crohn Colitis. 2020;14:1651–1652. doi: 10.1093/ecco-jcc/jjaa144. [DOI] [PubMed] [Google Scholar]