Abstract
Several group B streptococcal products have been previously found to stimulate human monocytes to produce tumor necrosis factor alpha. In order to identify the receptors involved in these responses, monocytes were stimulated with purified group- or type-specific carbohydrates or lipoteichoic acid in the presence of anti-receptor monoclonal antibodies, soluble CD14, or lipopolysaccharide-binding protein. Results indicate that CD14 plays an important role in tumor necrosis factor alpha responses to all of the stimuli tested. Moreover, both CD14 and complement receptor type 3 may be involved in responses to the group-antigen.
Group B streptococci (GBS) are a major cause of neonatal sepsis and meningitis (3). The course of these infections is often rapidly fatal, with shock and multiple-organ failure (2). Many of the manifestations of septic shock have been related to the exaggerated release of proinflammatory cytokines upon the interaction of host cells with microbial products. Previous studies have indicated that tumor necrosis factor alpha (TNF-α) plays an important pathophysiologic role in models of GBS sepsis (7, 19, 25). In fact, prophylaxis (25) or treatment (15) with anti-TNF-α antibodies was beneficial in neonatal rats infected with GBS.
Recent studies were aimed at identifying GBS components responsible for cytokine induction. A number of such components, including the type- and group-specific polysaccharides (CHOs), lipoteichoic acid (LTA), and cell walls, were shown to induce a significant release of TNF-α, interleukin 1, or interleukin 6 (20, 21, 23, 28–30). Little is known of monocyte receptors involved in cytokine responses to GBS components. Peptidoglycans and LTAs from other gram-positive bacteria were shown to stimulate cytokine release through the involvement of CD14, a glycosyl-phosphatidyl-inositol-anchored protein which has an important role in mediating cell activation by the lipopolysaccharide (LPS) component of gram-negative bacteria (6, 16, 31, 32). Binding of LPS to CD14 is greatly enhanced by LPS-binding protein (LBP), which may account for the ability of serum to enhance LPS responses (22). Moreover, soluble CD14 (sCD14), also a serum factor, can mediate LPS-induced activation phenomena in CD14-negative cells (13).
A recent study focused on receptors involved in cell activation by GBS cell walls (23). Blocking of either CD14 or CD18, the common β subunit of CR3 and CR4, decreased cell wall-induced TNF release. Purified cell walls of GBS contain large amounts of polysaccharides, which are covalently linked to the peptidoglycan moiety. Up to 37.4 and 22.1% of group- and type-specific CHOs, respectively, were estimated to be present in GBS cell walls (10). Therefore, it is unclear whether different cell wall components are separately responsible for the CD14- and CD18-dependent effects observed with the cell walls. The present study was undertaken to identify human monocyte receptors involved in TNF-α responses to purified surface GBS components, including the type- and group-specific carbohydrates and LTA.
Anti-receptor mouse monoclonal antibodies (MAbs) were purified by protein G affinity chromatography (GammaBind G Sepharose; Pharmacia Biotech, Milan, Italy) from the culture supernatants of the following hybridomas: TS1/18 (anti-CD18), LM-2/1.6 (anti-CD11b), HB 247 (60bca anti-CD14), and TIB 228 (3C10 anti-CD14). All of these hybridomas were purchased from the American Type Culture Collection (Manassas, Va.). MAb 6H8, which recognizes a widely distributed 180-kDa glycoprotein (T. Espevik and B. Naume, unpublished observation), or mouse immunoglobulin G1 (IgG1) (Sigma Chimica, Milan, Italy) were used as controls. Monocytes were obtained from the peripheral blood of healthy adult donors by centrifugation on Ficoll-Hypaque (Pharmacia) and adherence (8). Monolayers were incubated with plain medium (RPMI 1640; Life Technologies, Milan, Italy), control IgG1, or MAbs at the indicated concentrations for 30 min at 37°C before the addition of the stimuli. After a 4-h incubation with the stimuli, culture supernatants were collected and stored at −70°C until assayed for TNF-α production. TNF-α was detected by a cytotoxicity assay employing WEHI 164 clone 13 cells (12), as previously described (25). In some experiments, cytotoxicity results were confirmed with a commercial enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 5 pg/ml (Cytoscreen hTNF-α ELISA kit; BioSource International, distributed by Celbio, Milan, Italy). Results were converted into units with an rTNF-α standard with a specific activity of 7.6 × 107 U/mg.
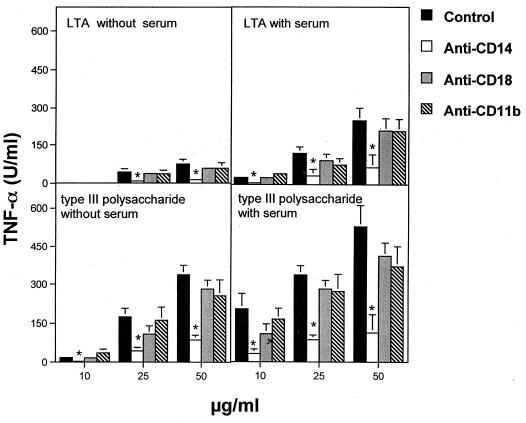
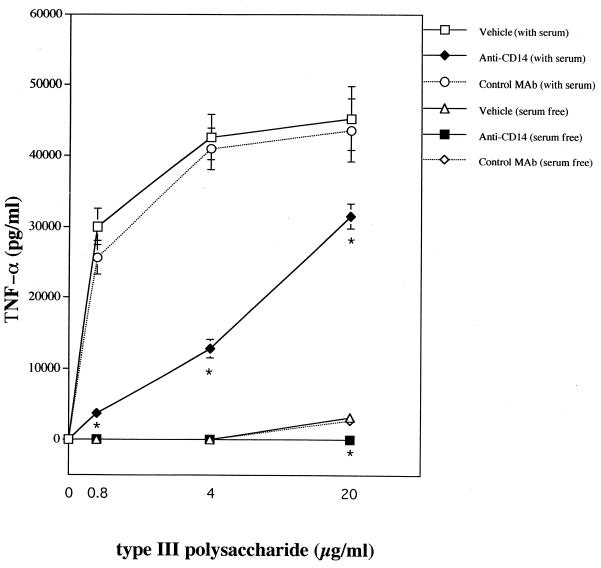
Since normal serum components are known to markedly affect LPS responses, monocytes were stimulated both in the presence and in the absence of heat-inactivated (56°C for 30 min) fetal calf serum (FCS; Life Technologies) with purified GBS products, obtained as previously described (21, 26). Significant endotoxin contamination in these preparations was excluded, based on observations that their TNF-α-inducing activities were not affected by the endotoxin-inactivating agent polymyxin B (20 μg/ml; Sigma) (not shown). Figure 1 shows the results obtained with the type III CHO and LTA. FCS was not an absolute requirement for TNF-α induction by the type CHO or LTA (Fig. 1). Its presence, however, significantly increased TNF-α release at all tested doses of these stimuli. Further experiments were performed with human serum obtained from healthy volunteers. These serum samples were devoid of antibodies against GBS and LTA as assessed by conventional ELISA and passive hemagglutination, respectively (20, 24, 30). These experiments showed that not only FCS but also human serum had marked costimulatory activities (not shown). Figure 1 shows that anti-CD14, but not anti-CD18 or anti-CD11b, significantly reduced TNF-α elevations produced by the type CHO or LTA, both in the presence and in the absence of serum. These effects were confirmed by showing that TNF-α induction by the type antigen could be inhibited by a different blocking anti-CD14 MAb (3C10), but not by the isotype-matched control MAb 6H8 (Fig. 2).
FIG. 1.
TNF-α production induced in human monocytes by GBS type III or LTA in the absence or presence of heat-inactivated FCS. Human monocytes were pretreated with MAbs (100 μg/ml) before stimulation with GBS components. The following MAbs were employed: TS1/18 (anti-CD18), LM-2/1.6 (anti-CD11b), and HB 247 (anti-CD14). Columns and bars represent means ± standard deviations of three independent observations. ∗, significantly (P < 0.05) different from controls by one-way analysis of variance and Student-Newman-Keuls test.
FIG. 2.
TNF-α production induced in human monocytes by GBS type III polysaccharide in the absence or in the presence of 25% A+ human serum. Human monocytes were pretreated with 100 μg of the blocking 3C10 anti-CD14 or the isotype-matched control 6H8 MAb per ml before stimulation with GBS type III CHO. Points and bars represent means ± standard deviations of three independent observations. ∗, significantly (P < 0.05) different from controls by one-way analysis of variance and Student-Newman-Keuls test.
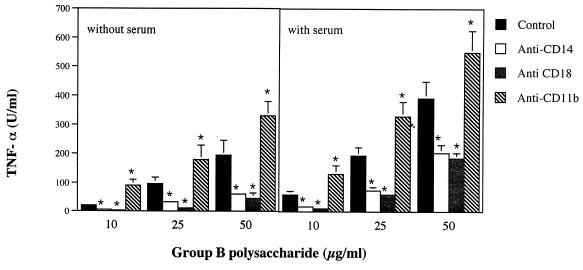
Figure 3 shows the effects of MAbs on group CHO-induced stimulation. As previously observed with the type CHO or LTA, heat-inactivated FCS enhanced, and anti-CD14 decreased, TNF-α responses (Fig. 3). Significant reduction by anti-CD14 was observed both in the presence and in the absence of FCS. However, anti-CR3 antibodies which, as shown above, had no effects on type CHO or LTA stimulation markedly influenced group B CHO-induced TNF-α release. In fact, while significant inhibition was produced by anti-CD18, anti-CD11b strongly enhanced TNF-α release (Fig. 3). Moreover, experiments using anti-CD11b controls concurrently with group CHO stimulation confirmed results shown in Fig. 3. Specifically, there was a marked enhancement of TNF-α release by combinations of group B antigen and anti-CD11b, relative to that of anti-CD11b controls (not shown). To rule out that the observed enhancing effects of anti-CD11b were due to endotoxin contamination, anti-CD11b was added to monocyte monolayers in the absence of bacterial stimuli. Anti-CD11b at levels of >10 μg/ml produced, in the absence of other stimuli, TNF-α elevations which never exceeded 6% of maximal, Salmonella enterica serovar Enteritidis LPS-induced stimulation (not shown). These slight TNF-α responses were not inhibited by polymyxin B, ruling out contamination of the antibody preparation.
FIG. 3.
TNF-α production induced in human monocytes by group B polysaccharide in the absence (A) or presence (B) of heat-inactivated FCS. Human monocytes were pretreated with MAbs (100 μg/ml) before stimulation with group B CHO. The following MAbs were employed: TS1/18 (anti-CD18); LM-2/1.6 (anti-CD11b); HB 247 (anti-CD14). Columns and bars represent means ± standard deviations of three independent observations. ∗, significantly (P < 0.05) different from controls by one-way analysis of variance and Student-Newman-Keuls test.
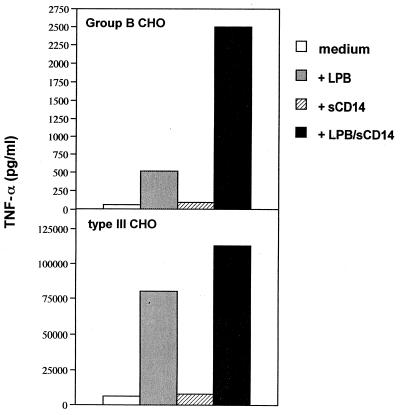
Further studies were conducted to identify the factors responsible for the observed serum-induced enhancement of TNF-α responses to GBS products. Specifically, we examined the effects of sCD14 and LBP, which are both normal serum components and can enhance LPS responses. Recombinant sCD14 and LBP were prepared as described previously (14). The addition of LBP, but not sCD14, increased TNF-α production when the type III CHO was used as stimulus (Fig. 4). However, the combination of LBP and sCD14 enhanced TNF-α release to higher levels than those observed with LBP alone. Similarly, LBP and sCD14, in combination, had marked synergistic effects in enhancing group CHO-induced TNF-α production (Fig. 4).
FIG. 4.
Effects of sCD14 and LBP on TNF-α production induced in human monocytes by GBS type III (lower panel) and group B polysaccharides (upper panel). Data are from one experiment, which was repeated three times with similar results.
Considerable amounts of extracellular products are released by GBS in body fluids during invasive infections, either spontaneously (4, 11) or by the effect of β-lactam antibiotics (1). In view of the potential pathogenetic role of TNF-α in GBS disease, the mechanisms leading to cytokine release upon stimulation with purified GBS products may be of interest. Our data indicate that the type III antigen, the group B CHO, and LTA can all induce TNF-α secretion through mechanisms that involve CD14, as suggested by the marked inhibitory activities of anti-CD14 MAbs. Our data do not exclude that, in addition to CD14, other receptors may be involved in such responses. In this respect, the role of Toll-like receptors should be further investigated, in view of the ability of Toll-like receptor 2 expression in CHO cells to confer responsiveness to whole staphylococci and pneumococci (34). Studies to address this point are underway.
Our data are in general agreement with the notion that CD14 is a broad-specificity receptor involved in cell activation phenomena by a large number of diverse microbial components, including LPS (9, 27, 33, 36), soluble peptidoglycan (16, 31, 32), LTAs (6, 17), mannuronic acids (18), and cryptococcal glycoproteins (5).
Binding of LPS to CD14 is greatly increased by serum LBP, which may account for the ability of serum to enhance LPS responses (22). Similarly, in the present study, the TNF-α-inducing activities of GBS products were enhanced by serum. These effects could, at least partially, be accounted for by combinations of LBP and sCD14, both normal serum components. In fact, the addition of LBP dramatically enhanced TNF-α secretion induced by the type and the group CHO, and this effect was further potentiated by sCD14. Collectively, our data demonstrate that GBS products can activate monocytes by mechanisms that at least partially resemble those involved in LPS responses, although their potency in these activities is considerably lower, relative to that of LPS. It is unlikely that endotoxin contamination of the GBS products used accounts for the similarity with LPS activation mechanisms. This is indicated by the inability of the endotoxin-inactivating agent polymyxin B to influence TNF-α release (not shown). Moreover, the endotoxin content of the type- and group-specific antigen preparations was <5 pg/ml.
A number of microbial products, including LPS, were previously shown to bind CR3, but whether such interactions can lead to TNF-α release has not been investigated. The present study indicates that, in addition to CD14, CR3 (CD18/CD11b) may be involved in responses to the group CHO, as evidenced by the ability of anti-CR3 to markedly affect stimulation by the group-antigen, but not by the type-antigen or LTA. This raises the possibility that the group CHO binds, either alone or in association with CD14 and LBP, to CR3 to induce activation phenomena. It may be interesting, in this context, that CR3 can bind to LBP and, in the presence of LPS, associate with surface CD14 on the plane of the membrane (35). Interestingly, while significant inhibition was produced by anti-CD18, anti-CD11b strongly enhanced the group CHO-induced TNF-α release. It is unlikely that the enhancing effects of anti-CD11b were due to endotoxin contamination since they were not abrogated by polymyxin B. We hypothesize that binding of the group CHO to CR3 may be insufficient, even in the presence of LBP or CD14, to produce maximal cross-linking of the receptor. This could be achieved experimentally with anti-CD11b antibodies, which could explain their ability to enhance group CHO-induced activation. Further studies are needed to prove this hypothesis. It is unlikely that the observed differences in the effects of anti-receptor antibodies using various GBS stimuli were due to differences in the kinetics of TNF-α responses. In fact, experiments performed by collecting supernatants at 24 h after addition of all of the GBS products produced similar results to those observed with supernatants collected at 4 h after the addition of the stimuli.
In conclusion, our data indicate that CD14 is involved in responses to the type III CHO and LTA of GBS while CR3, in addition to CD14, may be involved in responses to the group CHO. These data may be useful to devise alternative therapeutic strategies aimed at preventing mediator production during GBS sepsis.
Acknowledgments
This work was supported by grants from the CNR (Progetto Finalizzato Biotecnologie), MURST (Progetti di Rilevanza Nazionale ex 40%), and ISS (Progetto AIDS and Progetto Nazionale Tubercolosi) of Italy.
REFERENCES
- 1.Alkan M L, Beachey E. Excretion of lipoteichoic acid by group A streptococci. J Clin Investig. 1978;61:671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker C J. Group B streptococcal infections in newborns. N Engl J Med. 1986;314:1702–1708. doi: 10.1056/NEJM198606263142609. [DOI] [PubMed] [Google Scholar]
- 3.Bone R C. Gram positive organisms and sepsis. Arch Intern Med. 1994;154:26–34. [PubMed] [Google Scholar]
- 4.Carey R B, Eisenstein T K, Shockman G D, Greber T F, Swenson R M. Soluble group- and type-specific antigens from type III group B Streptococcus. Infect Immun. 1980;28:195–203. doi: 10.1128/iai.28.1.195-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaka W, Verheul A F M, Vaishnav V V, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman A I M. Induction of TNF-α in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J Immunol. 1997;159:2979–2985. [PubMed] [Google Scholar]
- 6.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparation of gram-positive bacteria induce interleukin-12 throughout a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cusumano V, Genovese F, Mancuso G, Carbone M, Fera M T, Teti G. Interleukin-10 protects neonatal mice from lethal group B streptococcal infection. Infect Immun. 1996;64:2850–2852. doi: 10.1128/iai.64.7.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delfino D, Cianci L, Lupis E, Celeste A, Petrelli M L, Currò F, Cusumano V, Teti G. Interleukin-6 production by human monocytes stimulated with Cryptococcus neoformans components. Infect Immun. 1997;65:2454–2456. doi: 10.1128/iai.65.6.2454-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delude R L, Savedra R, Zhao H, Thierienger R, Yamomoto S, Fenton M J, Golenbock D T. CD14 enhances cellular responses to endotoxin without imparting ligand-specific recognition. Proc Natl Acad Sci USA. 1995;92:9288–9292. doi: 10.1073/pnas.92.20.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doran T I, Mattingly S I. Association of type- and group-specific antigens with the cell wall of serotype III group B streptococcus. Infect Immun. 1982;36:1115–1122. doi: 10.1128/iai.36.3.1115-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards M S, Baker C J. Prospective diagnosis of early onset group B streptococcal infection by countercurrent immunoelectrophoresis. J Pediatr. 1979;94:286–288. doi: 10.1016/s0022-3476(79)80845-8. [DOI] [PubMed] [Google Scholar]
- 12.Espevik T, Nissen-Meyer J. A highly sensitive cell line WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 13.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzano-Santoro H, Parent J B, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon P J. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Givner L B, Gray L, O'Shea T M. Antibodies to tumor necrosis factor-α: use and adjunctive therapy in established group B streptococcal disease in newborn rats. Pediatr Res. 1995;38:551–554. doi: 10.1203/00006450-199510000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Gupta D, Kirkland T N, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23319. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 17.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14-dependent pathway. Biochem Biophys Res Commun. 1997;233:375–381. doi: 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 18.Jahr T G, Ryan L, Sundan A, Lichenstein H S, Skjak-Braek G, Espevik T. Induction of tumor necrosis factor production from monocytes stimulated with mannuronic acid polymers and involvement of lipopolysaccharide-binding protein, CD14, and bacterial/permeability-increasing factor. Infect Immun. 1997;65:89–94. doi: 10.1128/iai.65.1.89-94.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancuso G, Tomasello F, Migliardo M, Delfino D, Cochran J, Cook J A, Teti G. Beneficial effects of interleukin-6 in neonatal models of group B streptococcal disease. Infect Immun. 1994;62:4992–5002. doi: 10.1128/iai.62.11.4997-5002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancuso G, Tomasello F, Ofek I, Teti G. Anti-lipoteichoic acid antibodies enhance release of cytokines by monocytes sensitized with lipoteichoic acid. Infect Immun. 1994;62:1470–1473. doi: 10.1128/iai.62.4.1470-1473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancuso G, Tomasello F, von Hunolstein C, Orefici G, Teti G. Induction of tumor necrosis factor alpha by the group- and type-specific polysaccharides from type III group B streptococci. Infect Immun. 1994;62:2748–2753. doi: 10.1128/iai.62.7.2748-2753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathison J C, Tobias P S, Wolfson E, Ulevitch R J. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative bacteria. J Immunol. 1992;149:200–206. [PubMed] [Google Scholar]
- 23.Medvedev A E, Flo T, Ingalls R R, Golenbock D T, Teti G, Vogel S N, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- 24.Teti G, Calapai M, Calogero G, Tomasello F, Mancuso G, Galli A, Riggio G. Specificity and protective activity of murine monoclonal antibodies directed against the capsular polysaccharide of type III group B streptococci. Hybridoma. 1992;11:13–22. doi: 10.1089/hyb.1992.11.13. [DOI] [PubMed] [Google Scholar]
- 25.Teti G, Mancuso G, Tomasello F. Cytokine appearance and effects of anti-tumor necrosis factor antibodies in a neonatal rat model of group B streptococcal infection. Infect Immun. 1993;61:227–235. doi: 10.1128/iai.61.1.227-235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teti G, Tomasello F, Chiofalo M S, Orefici G, Mastroeni P. Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect Immun. 1987;55:3057–3064. doi: 10.1128/iai.55.12.3057-3064.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 28.Vallejo J G, Baker C L, Edwards M S. Interleukin-6 production by human neonatal monocytes stimulated by type III group B streptococci. J Infect Dis. 1996;174:332–337. doi: 10.1093/infdis/174.2.332. [DOI] [PubMed] [Google Scholar]
- 29.Vallejo J G, Baker C J, Edwards M S. Roles of the bacterial cell wall and capsule in induction of tumor necrosis factor alpha by type III group B streptococci. Infect Immun. 1996;64:5042–5046. doi: 10.1128/iai.64.12.5042-5046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Hunolstein C, Totolian A, Alfarone G, Mancuso G, Cusumano V, Teti G, Orefici G. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun. 1997;65:4017–4021. doi: 10.1128/iai.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flad H D, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidemann B, Schletter J, Dziarski R, Kusumoto S, Stelter F, Rietschel E T, Flad H D, Ulmer A J. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun. 1997;65:858–864. doi: 10.1128/iai.65.3.858-864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS-binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura A, Lien E, Tuomanen E, Dziarski R, Golenbock D. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 35.Zarewych D M, Kindzelskii A L, Todd III R F, Petty H R. LPS induces CD14 association with complement receptor type 3, which is reserved by neutrophil adhesion. J Immunol. 1996;156:430–433. [PubMed] [Google Scholar]
- 36.Ziegler-Heitbrock H W L, Ulevitch R J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]