Abstract
Objectives
We aim to establish daily risk estimates of the relationships between grass, tree and weed pollen and asthma health outcomes.
Design
Time series regression analysis of exposure and health outcomes using interaction by month to determine risk estimates all year round.
Setting
Metropolitan Adelaide, South Australia.
Participants
Health outcomes for asthma are based on 15 years of hospital admissions, 13 years emergency presentations and ambulance callouts. In adults (≥18 years), there were 10 381 hospitalisations, 26 098 emergency department (ED) presentations and 11 799 ambulance callouts and in children (0–17 years), 22 114, 39 813 and 3774, respectively.
Outcome measures
The cumulative effect of 7 day lags was calculated as the sum of the coefficients and reported as incidence rate ratio (IRR) related to an increase in 10 grains of pollen/m3.
Results
In relation to grass pollen, children and adults were disparate in their timing of health effects. Asthma outcomes in children were positively related to grass pollen in May, and for adults in October. Positive associations with weed pollen in children was seen from February to May across all health outcomes. For adults, weed pollen-related health outcomes were restricted to February. Adults were not affected by tree pollen, while children’s asthma morbidity was associated with tree pollen in August and September. In children, IRRs ranged from 1.14 (95% CI 1.06 to 1.21) for ED presentations for tree pollen in August to 1.98 (95% CI 1.06 to 3.72) for weed pollen in February. In adults, IRRs ranged from 1.28 (95% CI 1.01 to 1.62) for weed pollen in February to 1.31 (95% CI 1.08 to 1.57) for grass pollen in October.
Conclusion
Monthly risk assessment indicated that most pollen-related asthma health outcomes in children occur in the colder part of the year, while adults are affected in the warm season. The findings indicate a need for year-round pollen monitoring and related health campaigns to provide effective public health prevention.
Keywords: Asthma, PUBLIC HEALTH, EPIDEMIOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study is based on 15 years of grass, tree and weed pollen exposure data, ensuring high statistical power.
Instead of the usual paradigm of spring-time risk assessment, this study provides risk estimates of the relationship between pollen and health outcomes all year round.
Misclassification of pollen exposure due to only one measurement site may move the results towards the null hypothesis.
The time series design cannot consider individual characteristics that may cause the health outcomes in question.
Introduction
Detrimental effects of airborne pollen on people with asthma are a topic of current research in Australia and overseas. Two recent systematic reviews of population-based studies have shown that short-term exposure to pollen can trigger emergency department (ED) presentations and hospitalisations for asthma in children and adolescents.1 2 Another review focusing on individuals with asthma or with allergic status demonstrated an association between pollen exposure and asthma and lower respiratory symptoms in adults and children.3 Population studies including adults have also indicated positive relationships between pollen and asthma-related ED presentations and hospitalisation, although not all looked at differential effects by age group.4–6
While specific pollen species were assessed in some studies,6 7 others generalised to categories of weed, tree and grass pollens with different results depending on the geographical context. Some studies reported strong asthma-related morbidity related to grasses,5 8 while other studies found that tree pollen7 9 or both tree and weed pollen had a stronger relationship with asthma.10
The study findings are underpinned by a plausible biological mechanism whereby pollen grains rupture, causing release of allergenic particles of respirable size. Inhaled pollen particles are small enough to enter the small airways, triggering asthma in susceptible individuals.11 12 The pollen rupture is thought to be assisted by rainy and windy conditions and possibly other weather conditions.13
Asthma is a relevant chronic disease in Australia and exposure to pollen is an important asthma trigger in susceptible individuals. Asthma is associated with substantial personal burden, high health costs and substantial time off work and school.14 South Australia (SA) had the highest asthma and respiratory hospital admission rate for people aged 3–19 years compared with other states from 2010–2011 to 2012–2013.15 In 2018, the asthma prevalence in SA based on self-reported diagnosis was 14.8% for adults 18 years and over and 15% for children (2–17 years).16 17 According to numbers from the Australian National Health Survey, national asthma prevalence has increased from 10.8% in 2014–2015 to 11.2% in 2017–2018.18 In SA, the increase was particularly steep from 10.6% to 13% in the same time frame.
Australian studies of pollen and asthma have thus far focused on grass pollen.8 19 A previous Adelaide study using asthma hospitalisations and pollen data had intriguing results.20 Daily total pollen count, including combined grass, weed and tree pollen, adjusted by daily air pollution showed a significant 5-day cumulative effect of 4.2% increase in daily hospitalisations per 10 grains/m3 increase in pollen in children (0–17 years) in the cold season only (April– September), but not in adults. While this finding has indicated that there are short-term effects of total pollen on children’s asthma, it left the quandary about the timing of when this risk phase is occurring in terms of providing health prevention in SA. Traditionally, such initiatives have commenced in late September to coincide with increasing grass pollen counts during spring, however, alerting potential pollen allergic asthmatics and hay fever sufferers may also be necessary at other times of the year. Furthermore, the study considered only the effect of total pollen count on asthma, without differentiating between pollen types.20 Studies in Australia and overseas have indicated that pollen other than grass pollen can be important triggers of asthma.7 10 21 22 This adds another layer to the pollen quandary and the need to ask further questions. While a recent review confirmed the significance of short-term exposure to pollen to the respiratory health burden, it also noted that large time series studies are still necessary to detect area specific associations and possible synergistic effects.23
This study was conducted to bring about evidence of timing of the asthma and pollen relationship in Adelaide, and to find out how this relates to pollen of grasses, weeds and trees in children and adults and their asthma exacerbation. Hence, this study sets out on a risk assessment procedure delivering risk association measures by months for grass, weed and tree pollen for children and adults, to guide the appropriate content and timing of pollen-related public health initiatives.
Methods
The study took place in Adelaide, SA. The relationships between daily pollen counts for grasses, weeds and trees and daily asthma morbidity were examined using a time series analysis involving the population (approximately 1.3 million people) of the Adelaide metropolitan area.
Lack of pollen data precluded inclusion of regional SA into the study. The study’s focus was on daily risk estimates with an interaction by months for the relationship between pollen and asthma based on our preliminary findings that pollen is present all year round (see results), allowing for time lags between pollen exposure and health outcomes.
Asthma data
Health data for asthma were sourced from SA Health for hospital admissions and ED presentations using the International Classification of Disease 10th version codes (J45 and J46). SA Ambulance Service codes for asthma callouts were obtained from the provisional medical diagnosis field in ambulance records. The asthma data for hospital admissions were sourced from 1 January 2003 to 31 August 2017 (15 years) and for ED presentations and ambulance callouts from 1 January 2004 to 31 December 2017 (14 years). Daily asthma health outcomes in relation to pollen exposure were analysed by children (0–17 years) and adults (18 years and over).
Exposure assessment
Pollen
Daily pollen grain data were obtained from the Adelaide Aerobiology Laboratory for 1 January 2003 to 31 December 2017. Daily pollen was categorised as grasses, trees and weeds. Pollen was collected with a Hirst automatic volumetric spore trap and calculated as number of grains per cubic metre.24
Specifically, air was drawn into the Hirst trap at 10 L/min, the rate of breathing. Glass slides were exposed for 24 hours and examined at 9:00 hours. Pollen was caught on the Vaseline coated glass surface. The volume of air contacting the glass was 0.077 m3, therefore, one pollen particle counted was equivalent to 13 particles/m3, which was used as the correction factor.
Air pollution
Daily air pollutants used as possible confounders in the analysis were daily mean PM10 and PM2.5 in µg/m3, and daily maximum 1-hour average concentrations of NO2 and O3 in ppb. The data were provided by the SA Environment Protection Authority from a collection site with high accuracy located 5 km west of the Adelaide central business district (CBD).
Meteorological data
Adelaide’s seasons are opposite to the northern hemisphere, with mild winters (June–August), and warm, dry summers (December–February). Daily weather data were obtained from the Australian Bureau of Meteorology (BOM) Kent Town station (BOM code 23090), approximately 1 km from the Adelaide CBD. Variables included were maximum and minimum temperature (MaxT and MinT), rainfall in millimetres and daily average relative humidity.
Statistical analysis
Time series regression analysis has been widely used to explore short-term relationships between environmental exposures and health outcomes over a long period of time. We followed the methods by Bhaskaran et al to control for seasonality and long-term trends25 in the asthma data using Poisson regression in Stata V.17.26 The ‘splinegen’ command was used to create cubic splines of time with 104 knots based on 15 years of data and 7 knots per calendar year as suggested by Bhaskaran et al.
Missing covariate data were imputed by linear regression, using Stata’s ‘impute’ command. The percent of data imputed ranged from less than 0.1% for MaxT and MinT to 3.8% for PM10 and PM2.5. Imputed pollen data were 2.5%. Pollen counts were log transformed using logpollen=log2(1+pollen/10) to work with the positive skew of the pollen data. This transformation maps zero pollen to 0 and 10 onto one so that the model coefficients measure the incidence rate ratio (IRR) between no pollen and 10 grains/m3. In addition, an interaction term between month (January–December) and pollen concentration assessed the IRR by months. Correlations between the three pollen taxa within each month (36 correlations) were all positive and ranged from 0.03 between weeds and trees in June to 0.8 between grasses and weeds in January. Only three correlations exceeded 0.6. While pollen correlations were generally not large, pollen data were not considered independent, requiring inclusion of all three pollen taxa into the same regression analysis for mutual adjustment.
Environmental confounders that change from day to day and are likely to be related to pollen such as air pollutants and weather variables were included in the regression model. MaxT, MinT and relative humidity were included in the form of cubic splines with 2 df. Rainfall in mm was log transformed for days where rainfall was present, likewise with a cubic spline with 2 df, and a binary indicator included for dry days. Additional confounders were day of the week and public holidays which were included as categorical variables.
The shape of the exposure response relationship was assessed by fitting 2 df spline curves relating IRRs to log pollen counts. Visual inspection of these 216 curves (3 outcomes, 2 age groups, 3 pollen types, 12 months), together with their 95% CIs, showed little evidence of departure from log-linearity.
Short-term autocorrelation of asthma outcomes was assessed by viewing the correlograms. Presence of correlation was adjusted by inclusion of the lagged Pearson residual (r). The Pearson residual is calculated by dividing the residuals of the health outcomes, r=Y fv, where Y stands for health outcomes and fv for fitted values, by their SD, which is estimated by the square root of the fitted values (r = (Y-fv)/√fv).27 The Pearson residual is thus standardised to have constant variance, and the inclusion of lagged residuals in the model corrects for autocorrelation. Hospitalisation and ED asthma outcomes for both children and adults were adjusted by inclusion of a 3-day lagged Pearson residual to control for autocorrelation, but no adjustment was necessary for ambulance callouts.
Seven-day lags between exposure and health outcomes were used to assess the effects of pollen on asthma on day 0 and the next 7 days to allow for delayed associations. The maximum lag of 7 days was chosen by comparing models using the Akaike information criterion (AIC). Covariates were included in their lagged form if including lags significantly improved the model by the likelihood ratio test.
The unconstrained distributed lag models (all lag terms modelled together) provided eight coefficients per health outcome for each pollen type, by months for children and adults. The cumulative effects over lags were calculated as the sum of the coefficients and tested using the ‘lincom’ command in Stata.26 28 For the result in the form of an incidence rate ratio (IRR), the sum of the coefficients was exponentiated and the standard errors from the linear combinations of the estimators were used to calculate the lower and upper 95% CI for the risk ratio.
Patient and public involvement
None.
Results
Figure 1 depicts the annual cycles of averaged grass, weed and tree pollen from 2003 to 2017. They provide an overview of the distribution of pollen by month, showing low counts for grass pollen in June, July and August and for weed pollen in July, and the constant presence of tree pollen throughout the year. These results informed the data analysis between daily pollen and health outcomes by including an interaction by month across the entire year, rather than restricting the analysis to a predefined pollen season.
Figure 1.
Box plots of daily grass, weed and tree pollen by month. Data are from 5478 days of observation over 15 years.
Trend analyses for all three pollen types over 15 years were modelled using 5 df for the spline; this minimised the AIC based on a log-linear regression of daily pollen over the years in relation to weather and time. The models indicated highly significant variations for all three pollen types over the years, as shown in figure 2.
Figure 2.
Trend of log pollen from 2003 to 2017 using a 5 df spline with 95% CIs (left axis) and annual mean pollen counts (right axis).
Descriptive statistics indicating the distribution (mean, SD, minimum, maximum, quartiles and 95th percentile) of the data for daily health outcomes, pollen species and environmental data are presented in table 1. The total incidence of hospitalisation for asthma over the 15 years for adults was 10 381, for ED presentations 26 098 and for ambulance callouts 11 799 cases. In children, there were 22 114 hospitalisations, 39 813 ED presentations and 3774 ambulance callouts. There were 3614 days (66%) without rain and 1865 days (34%) with rain.
Table 1.
Descriptive data on health outcomes, pollen and environmental confounders
| Variables | Mean | SD | Min | Max | p25 | p50 | p75 | p95 |
| Adults | ||||||||
| Asthma hospital admissions | 1.9 | 1.5 | 0 | 12 | 1 | 2 | 3 | 5 |
| Asthma emergency presentations | 5.1 | 2.6 | 0 | 20 | 3 | 5 | 7 | 10 |
| Asthma ambulance callouts | 2.4 | 1.6 | 0 | 10 | 1 | 2 | 3 | 5 |
| Children | ||||||||
| Asthma hospital admissions | 4.0 | 2.6 | 0 | 19 | 2 | 4 | 6 | 9 |
| Asthma emergency presentations | 7.8 | 4.1 | 0 | 29 | 5 | 7 | 10 | 15 |
| Asthma ambulance callouts | 0.8 | 0.9 | 0 | 6 | 0 | 1 | 1 | 3 |
| Pollen | ||||||||
| Grasses (grains/m3) | 17.0 | 17.3 | 0 | 104.0 | 0 | 13.0 | 26.0 | 52.0 |
| Weeds (grains/m3) | 23.6 | 21.9 | 0 | 127.0 | 0 | 20.0 | 39.0 | 62.4 |
| Trees (grains/m3) | 40.6 | 27.0 | 0 | 273.0 | 23.0 | 36.0 | 53.0 | 88.0 |
| Environmental confounders | ||||||||
| PM10 (µg/m3) | 17.7 | 9.2 | 1.0 | 125.9 | 12.1 | 15.9 | 20.8 | 32.4 |
| PM2.5 (µg/m3) | 7.9 | 2.8 | 1.6 | 61.2 | 6.0 | 7.4 | 9.2 | 12.7 |
| Max daily O3 (ppb) | 29.0 | 7.8 | 2.0 | 105.0 | 24.0 | 28.0 | 32.0 | 44.0 |
| Max daily NO2 (ppb) | 19.0 | 9.5 | 1.0 | 103.0 | 11.0 | 20.0 | 26.0 | 33.0 |
| Maximum daily temperature (°C) | 23.0 | 7.0 | 9.9 | 45.7 | 17.3 | 21.8 | 27.6 | 36.3 |
| Minimum daily temperature (°C) | 12.6 | 5.0 | 0.2 | 33.9 | 9.0 | 12.1 | 15.5 | 21.8 |
| Mean daily temperature (°C) | 14.5 | 5.6 | 1.3 | 35.5 | 10.6 | 13.8 | 17.5 | 25.4 |
| Daily rainfall (mm) | 1.5 | 4.3 | 0 | 75.2 | 0 | 0 | 0.8 | 8.6 |
| Daily mean humidity (%) | 69.1 | 20.4 | 5.0 | 100 | 58.0 | 72.0 | 86.0 | 95.0 |
Number of days of observation for hospitalisation n=5478; ED=5113; ambulance n=4935; for pollen and environmental confounder n=5479.
The short-term (daily) risk estimates as IRRs, related to an increase in 10 grains/m3, for children and adults, by months, by health outcomes and pollen species are shown graphically in figures 3 and 4. The monthly IRRs show distinct patterns of risk which do not necessarily coincide in the population of children compared with adults. As no consistent individual lag effect patterns for days 0–7 were found (data not shown), cumulative 7-day lag effects in the form of IRRs are presented.
Figure 3.
Short-term incidence rate ratios (IRRs) in children. IRRs significantly different from 1 are highlighted using solid dots. IRRs refer to asthma-related hospital admissions (H), emergency department presentations (E) and ambulance callouts (A) and pollen (grasses, weeds, trees) by month. The estimated IRRs and associated 95% CIs are based on an increase in 10 grains/m3 compared with zero grains/m3. Furthermore, the IRRs are based on the sum of the coefficients of the 0–7 day lags between the exposure on day 0 and the daily health outcomes from day 0 to day 7. Adjustment factors included in the model were PM10, PM2.5, NO2, O3, MaxT, MinT, rainy day, rainfall, humidity, public holidays and day of the week.
Figure 4.
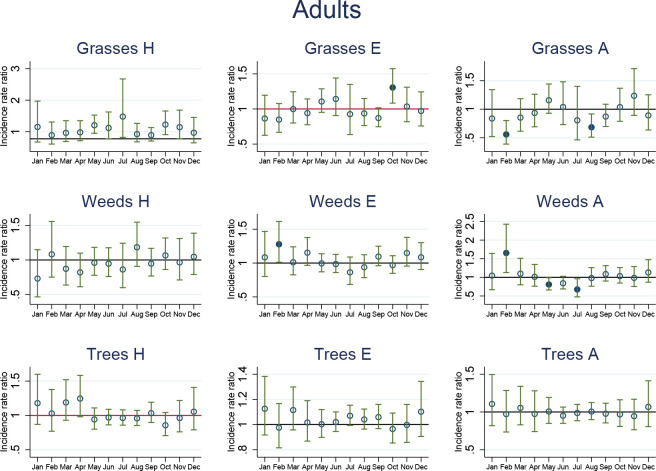
Short-term incidence rate ratios (IRRs) in adults. IRRs significantly different from 1 are highlighted using solid dots. IRRs refer to asthma-related hospital admissions (H), emergency department presentations (E) and ambulance callouts (A) and pollen (grasses, weeds, trees) by month. The estimated IRRs and associated 95% CIs are based on an increase in 10 grains/m3 compared with zero grains/m3. Furthermore, the IRRs are based on the sum of the coefficients of the 0–7 day lags between the exposure on day 0 and the daily health outcomes from day 0 to day 7. Adjustment factors included in the model were PM10, PM2.5, NO2, O3, MaxT, MinT, rainy day, rainfall, humidity, public holidays and day of the week.
Children and weed pollen
When examining the trajectory of statistically significant increases in IRRs in children across the year, a pattern of significant positive associations with weed pollen can be seen from February to May across the three health outcomes. For February, the IRR was significantly increased for asthma-related ambulance call-outs (IRR 1.98; 95% CI 1.06 to 3.72, p=0.03), emergency presentations (IRR 1.36; 95% CI 1.15 to 1.61, p<0.001) and with borderline significance for hospitalisations (IRR 1.22; 95% CI 1.00 to 1.48, p=0.05). For March, the significant increases continued for emergency presentations (IRR 1,22; 95% CI 1.04 to 1.43, p=0.01) and hospitalisations (IRR 1.27; 95% CI 1.05 to 1.54, p=0.02), while for April and May, significant IRR increases were only observed for hospitalisations with an IRR of 1.26 (95% CI 1.03 to 1.54, p=0.03) and IRR 1.16 (95% CI 1.01 to 1.33, p=0.04), respectively. Another month of positive associations between weed pollen and asthma in children occurred in August, but only for ambulance callouts (IRR 1.65; 95% CI 1.04 to 2.60, p=0.03).
Children and tree pollen
Another cluster of significant health outcomes was observed for August and September in association with tree pollen. The IRR for emergency presentations for children in August was 1.14 (95% CI 1.06 to 1.21, p≤0.001) and for hospitalisations 1.17 (95% CI 1.08 to 1.28, p≤0.001). In September, all three health outcomes showed a significant IRR rise with 1.31 for ambulance callouts (95% CI 1.02 to 1.69, p=0.03), IRR 1.16 (95% CI 1.07 to 1.25, p<0.001) for emergency presentations and IRR 1.23 (95% CI 1.11 to 1.37, p<0.001) for hospitalisations.
Children and grass pollen
A positive relationship with grass pollen in children was found only in May and was confined to emergency presentations (IRR 1.14; 95% CI 1.02 to 1.28, p=0.02).
Adults and weed pollen
As for children, IRR increases were observed in relation to weed pollen for adults in February, when there was a positive relationship with ambulance callouts (IRR 1.65; 95% CI 1.13 to 2.43, p=0.01) and ED presentations (IRR 1.28 95% CI 1.01 to 1.62, p=0.04).
Adults and tree pollen
Results indicated no positive relationships between tree pollen and asthma in adults.
Adults and grass pollen
The only other month with a significant relationship for adults was October, where the IRR in relation to grass pollen was 1.31 (95% CI 1.08 to 1.57, p=0.01) for ED presentations.
Several significant (p<0.05) reduced IRRs were observed in the relationship between pollen and health outcomes in children and adults.
The graphs of estimated monthly IRRs depicted in figures 3 and 4 are accompanied by tables of IRRs, their 95% CIs and associated p values for adults and children in online supplemental tables 1; 2.
bmjopen-2022-066851supp001.pdf (56KB, pdf)
bmjopen-2022-066851supp002.pdf (57.3KB, pdf)
Discussion
The findings for three pollen taxa in relation to asthma health outcomes using an extended dataset delivers an in-depth overview of pollen-related health effects across the seasons for metropolitan Adelaide. The methodology may be useful for studies in similar geographical areas and for pollen and health research in general. The use of year-round pollen data avoids a restriction to a single season, which has been used in most studies.6 8 29 The restrictive approach is valid for regions with a continental climate, characterised by minimal growth during late autumn and winter and a distinctive pollen season during spring and summer. However, in Australia’s south-east and south-west with their temperate climate, pollen can have several peaks. This has been shown in a recent study in Sydney, where two distinct pollen peaks, one from January to April and a second peak in July to October were reported.21 For Adelaide, the course of pollen distribution is shown in figure 1, and the relationship between pollen and health outcomes points to two periods when health effects occur, including late summer to autumn and in late winter extending into spring.
This study is built on a previous study of asthma and pollen in Adelaide by Chen et al, but with a longer study period (2003–2017, compared with 2003–2013).20 Further extensions include analysis by grass, weed and tree pollen instead of the total pollen count, and adding emergency presentations and ambulance callouts to the health outcomes. The results of the previous study were interesting in that significant pollen relationships were found only in children (aged 0–17 years) and were limited to the cool season (April to September). The results of this study show that episodes of positive associations between weed, and tree pollen count and asthma occurred in children predominantly in the cooler months. In contrast, risk episodes in adults occurred exclusively in the warm season, specifically for weed pollen in February and for grass pollen in October.
In late summer and autumn (February to May), weed pollen showed a consistent positive relationship with all three health outcomes in children. Adults were also affected by weed pollen in February. A second period of asthma risk occurs in children in late winter-early spring in relation to tree pollen, with consistency sustained across the three asthma health outcomes. Adults, on the other hand, are not affected by tree pollen, but by grass pollen in October, and only for ED presentations. Grass pollen-related asthma morbidity was not observed in children during spring, but instead was demonstrated as increased ED presentations in May.
August is a very likely month for tree pollen to be relevant in SA. Trees start to flower, and pollen is wind-distributed from a range of sources including cypresses, birch, Myrtaceae-related bushes and trees, pines, olive trees and casuarina. The positive relationship between children and tree pollen has been noted in other studies.21 More specifically, a study in New York showed strong relationships between tree pollen, ED visits for asthma and over the counter medication for allergy, particularly in children.7 Similarly, ED presentations among children were strongly related to tree and weed pollen in a New Jersey study, while grass pollen was only marginally related to the 5-day average (0–4 lag) risk ratio.10 All three pollen categories were studied in relation to all-age ED visits in Wake County, North Carolina, with tree pollen showing a strong relationship with ED visits, while weed and grass pollen were only weakly associated.9 The most recent study exploring pollen and asthma exacerbations in children in Philadelphia showed increased risks for total tree pollen and most individual tree species, as well as for most weeds. Only grass pollen counts above the 99th percentile were associated with health effects which occurred over only 2 days of the study period.30 A recent birth cohort study showed evidence of significant clinical outcomes among children at high risk of allergic asthma in relation to tree pollen.22 Lung function reduction was significantly related to Cypress, Casuarina and Pinus-related tree pollen, but not to grasses and weeds at age 8 of the follow-up. The recent evidence of tree pollen effects and asthma in children instigated cautionary voices around planting urban forests, suggesting that improved knowledge will be necessary to avoid health effects in exposed populations.31
Based on a recent meta-analysis and evidence gathered from studies in Victoria, grass pollen is an important asthma trigger for children in various places worldwide and in Australia.1 5 19 29 The Melbourne thunderstorm asthma event in 2016 and other smaller events in New South Wales have firmly determined a relationship between high concentrations of grass pollen, weather events, asthma and hay fever in both children and adults.32 On a smaller scale, even low concentrations of grass pollen have been associated with hospital admissions and ED presentations for asthma among children in Melbourne.19
In our study, for adults, grass pollen was a trigger for asthma morbidity in October, which aligns with the Australian paradigm of an asthma and hay fever season in spring. For children, this paradigm did not hold. Children were affected by grass pollen in autumn (May), not in spring. Overseas, grass pollen has also been associated with asthma outcomes in adults. A study in Brussels reported increased asthma hospitalisation in relation to grass pollen in the 15–59 years age group, but not in children.4 Moreover, a London-based study of adults reported significantly increased asthma hospitalisations in association with grass pollen during their yearly pollen monitoring period (approximately March–September).5
Looking at the results of this study, there is a discrepancy in tree and grass pollen-related asthma morbidity between children and adults and compared with evidence from other states in Australia and overseas. We can offer some possible explanations for these surprising results. Research has shown that there is an age-related decrease in allergic asthma in terms of symptoms over time.33 This could explain why adults do not react to tree pollen as children do. To explain why children do not have asthma exacerbations in October in relation to grass pollen in SA, but adults do, we speculate that this could be related to an age-related differential uptake of the annual asthma campaign which commences in mid-September and encourages compliance with asthma preventer medications. As children are more likely to be diagnosed with allergic asthma than adults, they are more likely to be encouraged to take asthma medication during this campaign period, which also highlights the need for adherence with asthma action plans.33 If this is the case, then children with asthma would be protected in October, but not in August and September (due to the timing of the campaign). Based on the results of our study, the asthma awareness campaign in Adelaide should start in August and continue until November, to help children and parents to be equally prepared for pollen exposure. However, considering the paucity of knowledge about plant allergens, their relationship to diseases and spatiotemporal patterns in Australia, it is premature to hypothesise about differences in tree and grass pollen allergenicity in children and adults.34 It can only be speculated that there may be geographical differences in the type of tree and grass pollen across south-east Australia, which may influence the distribution of the public health burden related to pollen. While there have been preliminary investigations into airborne pollen in Australia in the past, SA had never been part of this Australian research effort. General evidence suggests that pollen exposure is modified by variables related to geography, meteorology, climate change and land use.35 36
Likewise, there is limited knowledge of weed pollen which, according to this study, affects children and adults in late summer to autumn in Adelaide. The only other Australian study which measured weed pollen in relation to asthma hospitalisations in children took place in Sydney and found that weed, unidentified pollen and grass pollen contributed to asthma morbidity.21 Overseas, weed pollen also seems to affect asthma in children.10 30 In studies where weed pollen was positively related to asthma, the timing of the allergic effect over seasons was not clear. This paucity of data makes it hard to compare and verify our autumn findings for Adelaide. Furthermore, not much is known about the allergenicity of weed pollen in SA nor of the prevalence of allergic sensitivity to weed pollen, which add to the complexity in interpreting the results.
Considering the at times sharp upward trend in pollen concentrations over the study period, it will be necessary to observe pollen in concurrence with ongoing climate change, specifically since the pollen season, as shown in this study, is not confined to spring. Rising CO2 levels, warming and changing rainfall patterns may increase and shift the flowering seasons in conjunction with increased pollen load, possible changing allergenicity of pollen and potentially increasing the risk to public health year-round.34 37 38 Improved understanding of the spatiotemporal distribution of pollen and its allergenicity is required, with a necessary aim being the establishment of an extensive, standardised aerobiology monitoring programme. This will improve knowledge of pollen taxa and subspecies across Australia and aid understanding of associated health outcomes, the importance of which has been voiced by other researchers in Australia.34 35 38
Several monthly IRRs indicated significantly decreased effects of pollen exposure on asthma, mostly in children, but also in adults. This may have two reasons. First, the pollen may be directly protective. Second, and more plausibly, pollen counts may be a proxy for confounders which we do not know and therefore could not adjust for.
The strength of the study lies with the extensive time series data set of 15 years, which allowed enough statistical power for the exploration of the parameters in question. Furthermore, daily data were not restricted to one season, but all seasons by means of monthly risk estimates. The time series model has been used extensively in environmental epidemiology for short-term outcomes. Using the time series approach, it was possible to adjust for long-term and seasonal trends and focus on short-term exposures and asthma events, as well as the inclusion of all possible confounders, with or without lags, depending on the model assessment.25
While the results are not directly generalisable to other locations and populations due to differences such as climate, geography, socioeconomic determinants and local plant species, the methodology may be useful for studies in similar geographical areas with continuous plant growth throughout the year.
The study has several limitations. Pollen was only measured at one location, as were air pollutants, and misclassification is therefore possible as no spatial variability was available. This may bias the results towards the null hypothesis, though high correlation of grass pollen from nearby sites has been shown in a study in Melbourne.19 Furthermore, it has been reported that pollen counts do not always represent the allergen content.37 This can lead to erroneous interpretations of the relationship between pollen count and health outcomes. Allergen content analysis is a new research field and practical evidence is scarce.
Lack of influenza data for most of the study period prevented an assessment of correlation with clinical asthma exacerbations, and it is possible that the strong winter and early spring relationships between tree pollen and asthma in children may be partly affected by confounding by influenza and/or other respiratory tract infections.
Furthermore, the study also cannot offer any conclusions about asthma and pollen in regional SA because pollen exposure data were limited to metropolitan Adelaide. Moreover, the time series approach does not allow for inclusion of characteristics of the population that may affect asthma health outcomes, such as allergic sensitisation status or presence of indoor asthma triggers. To take account of those factors, other epidemiological study designs would have to be employed.
Finally, the most important aspect of the findings is their relevance for public health intervention and policy. This study provides pollen information for grasses, weeds and tress in association with three types of health outcomes for asthma, with pollen relevant to risk throughout the year. Knowledge of this pattern is important for the appropriate timing of public health initiatives regarding allergic asthma. Also, asthma triggered by pollen sensitivity or mechanical irritation by pollen is preventable through regular use of inhaled corticosteroid preventer medicines. These medicines work by reducing the sensitivity of the airways to asthma triggers such as pollen and reduce the swelling and mucus production which occurs when the airways are irritated.39 Public health messaging should consider the range of preventive measures for allergic asthma, including reducing pollen exposure and use of preventive medications.
Conclusion
This study was designed to explore the relationship between grass, weed and tree pollen counts and asthma in Adelaide, SA, specifically hospital admissions, ED presentations and ambulance callouts. Time series analysis was used to explore associations by month rather than within a predefined pollen season, given previous research has indicated the presence of pollen year-round. The results are mixed but demonstrate increased asthma-related hospital, emergency and ambulance health outcomes associated with exposure to grass, weed and tree pollen at various time points across the year. The monthly risk estimates showed two main periods of asthma-related events during the yearly cycle. They occurred in late summer-autumn and in late winter-spring, indicating the need for asthma vigilance across the year rather than a focus of preventive activity during spring as is currently the case. Weed pollen was mainly responsible for asthma morbidity in children during the first event from February to May and in adults in February. The second event was dominated by tree-pollen morbidity in children in August and September and by grass pollen in October in adults. No tree pollen-related asthma morbidity was observed for adults. Grass pollen was related to asthma in children in May while for adults it was October.
The discrepancy in asthma morbidity in relation to tree and grass pollen in Adelaide for children and adults should be investigated in future studies including a population-based assessment of sensitivity to tree, grass and weed pollen in the population. This should be accompanied by year-round monitoring of the relevant pollen taxa, if possible, including subspecies identification at various locations in Adelaide and in country areas, where pollen monitoring does not currently occur. This should be followed up over time with studies combining health outcomes and pollen measurements.
The results of this and future studies can inform public health responses. Asthma and hay fever campaigns currently start mid-September in SA, simultaneously with monitoring of pollen, which is restricted to grass pollen. Future preventive measures should mirror the identified risk periods with monitoring extended to all pollen taxa, preferably all year round, and appropriately timed programmes to alert the public to pollen-related risk and encourage preventive action.
Supplementary Material
Acknowledgments
We thank Dr Richard Evans from SA Health for extracting weather data from the BoM database. We also thank Dr Pushan Shah from the Environment Protection Authority for providing air pollution data.
Footnotes
Twitter: @srsly?
Contributors: MN is the guarantor and was involved in designing the study, analysed and interpreted the statistical results, and wrote the draft research paper. KBGD and KV conducted the statistical analysis. HPAJ and NS provided medical advice and took part in the development of the study design. KMRL assisted with the draft writing and interpretation of data. DLS assisted with the design, draft and interpretation.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Restrictions apply to the availability of these data. Data were obtained from several third parties including the South Australian Department for Health and Wellbeing, Asthma Australia (SA branch), SA Environment Protection Authority (EPA) and the Australian Bureau of Meteorology (BoM). On reasonable request, the authors may be able to facilitate sharing the data with the permission of the third parties.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethics approval (HREC/18/SAH/16) was received from the Human Research Ethics Committee (HREC), SA Department for Health and Wellbeing for the conduct of the pollen and asthma study and for access to and use of asthma data for hospital admissions, emergency presentations and ambulance callouts, the latter from SA Ambulance Services.
References
- 1.Erbas B, Jazayeri M, Lambert KA, et al. Outdoor pollen is a trigger of child and adolescent asthma emergency department presentations: a systematic review and meta-analysis. Allergy 2018;73:1632–41. 10.1111/all.13407 [DOI] [PubMed] [Google Scholar]
- 2.Shrestha SK, Lambert KA, Erbas B. Ambient pollen concentrations and asthma hospitalization in children and adolescents: a systematic review and meta-analysis. J Asthma 2021;58:1155–68. 10.1080/02770903.2020.1771726 [DOI] [PubMed] [Google Scholar]
- 3.Kitinoja MA, Hugg TT, Siddika N, et al. Short-term exposure to pollen and the risk of allergic and asthmatic manifestations: a systematic review and meta-analysis. BMJ Open 2020;10:e029069. 10.1136/bmjopen-2019-029069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilbert A, Cox B, Bruffaerts N, et al. Relationships between aeroallergen levels and hospital admissions for asthma in the brussels-capital region: a daily time series analysis. Environ Health 2018;17:35. 10.1186/s12940-018-0378-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne NJ, Alcock I, Wheeler BW, et al. Pollen exposure and hospitalization due to asthma exacerbations: daily time series in a European city. Int J Biometeorol 2017;61:1837–48. 10.1007/s00484-017-1369-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobias A, Galan I, Banegas JR. Short term effects of airborne pollen concentrations on asthma epidemic. Thorax 2003;58:708–10. 10.1136/thorax.58.8.708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Weinberger KR, Robinson GS, et al. The associations between daily spring pollen counts, over-the-counter allergy medication sales, and asthma syndrome emergency department visits in New York city, 2002-2012. Environmental Health 2015;14:71. 10.1186/s12940-015-0057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erbas B, Chang J-H, Dharmage S, et al. Do levels of airborne grass pollen influence asthma hospital admissions? Clin Exp Allergy 2007;37:1641–7. 10.1111/j.1365-2222.2007.02818.x [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Waller A, Yeatts KB, et al. Pollen concentration and asthma exacerbations in wake County, North Carolina, 2006-2012. Sci Total Environ 2016;544:185–91. 10.1016/j.scitotenv.2015.11.100 [DOI] [PubMed] [Google Scholar]
- 10.Gleason JA, Bielory L, Fagliano JA. Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: a case-crossover study. Environ Res 2014;132:421–9. 10.1016/j.envres.2014.03.035 [DOI] [PubMed] [Google Scholar]
- 11.Suphioglu C, Singh MB, Taylor P, et al. Mechanism of grass-pollen-induced asthma. The Lancet 1992;339:569–72. 10.1016/0140-6736(92)90864-Y [DOI] [PubMed] [Google Scholar]
- 12.Taylor PE, Flagan RC, Valenta R, et al. Release of allergens as respirable aerosols: a link between grass pollen and asthma. J Allergy Clin Immunol 2002;109:51–6. 10.1067/mai.2002.120759 [DOI] [PubMed] [Google Scholar]
- 13.Taylor PE, Jacobson KW, House JM, et al. Links between pollen, atopy and the asthma epidemic. Int Arch Allergy Immunol 2007;144:162–70. 10.1159/000103230 [DOI] [PubMed] [Google Scholar]
- 14.(DAC) Deloitte Access Economic . The hidden cost of asthma. Report for asthma Australia and national asthma Council Australia. Canberra; 2015. https://www2.deloitte.com/content/dam/Deloitte/au/Documents/Economics/deloitte-au-economics-hidden-cost-asthma-241115.pdf [Accessed 21 Mar 2021]. [Google Scholar]
- 15.Australian Commission on Safety and Quality in Health Care . Australian Atlas of Healthcare Variation 2015 Australian Commission on Safety and Quality in Health Care. Australian Atlas of Healthcare Variation 2015: Chapter 6.4 - Asthma and related respiratory hospital admissions 3-19 years, 2015. Available: https://www.safetyandquality.gov.au/sites/default/files/migrated/SAQ201_07_Chapter6_v7_FILM_tagged_merged_6-4.pdf [Accessed 21 Mar 2022].
- 16.Prevention and Population Health Directorate Wellbeing SA . South Australian population health survey. 2019 annual report children. Adelaide: Government of South Australia; 2019. https://das7nagdq54z0.cloudfront.net/downloads/SAPHS/SAPHS2019AnnualReport-Children.pdf [Accessed 21 Mar 2022]. [Google Scholar]
- 17.Prevention and Population Health Directorate Wellbeing SA . South Australian population health survey. 2019 annual Report-Adults. Adelaide: Government of South Australia; 2019. https://das7nagdq54z0.cloudfront.net/downloads/SAPHS/SAPHS2019AnnualReport-Adults.pdf [Accessed 21 Mar 2022]. [Google Scholar]
- 18.Australian Bureau of Statistics . National health survey: first results 2017-18. Canberra: ABS; 2018. [Accessed 21 Mar 2022]. [Google Scholar]
- 19.Erbas B, Akram M, Dharmage SC, et al. The role of seasonal grass pollen on childhood asthma emergency department presentations. Clin Exp Allergy 2012;42:799–805. 10.1111/j.1365-2222.2012.03995.x [DOI] [PubMed] [Google Scholar]
- 20.Chen K, Glonek G, Hansen A, et al. The effects of air pollution on asthma hospital admissions in Adelaide, South Australia, 2003-2013: time-series and case-crossover analyses. Clin Exp Allergy 2016;46:1416–30. 10.1111/cea.12795 [DOI] [PubMed] [Google Scholar]
- 21.Shrestha SK, Katelaris C, Dharmage SC, et al. High ambient levels of grass, weed and other pollen are associated with asthma admissions in children and adolescents: a large 5-year case-crossover study. Clin Exp Allergy 2018;48:1421–8. 10.1111/cea.13225 [DOI] [PubMed] [Google Scholar]
- 22.Lambert KA, Katelaris C, Burton P, et al. Tree pollen exposure is associated with reduced lung function in children. Clin Exp Allergy 2020;50:1176–83. 10.1111/cea.13711 [DOI] [PubMed] [Google Scholar]
- 23.Idrose NS, Lodge CJ, Erbas B, et al. A review of the respiratory health burden attributable to short-term exposure to pollen. Int J Environ Res Public Health 2022;19:7541. 10.3390/ijerph19127541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirst JM. An automatic volumetric spore trap. Ann Applied Biology 1952;39:257–65. 10.1111/j.1744-7348.1952.tb00904.x [DOI] [Google Scholar]
- 25.Bhaskaran K, Gasparrini A, Hajat S, et al. Time series regression studies in environmental epidemiology. Int J Epidemiol 2013;42:1187–95. 10.1093/ije/dyt092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stata Statistical Software . Release 17. College Station. TX: StataCorp LLC.StataCorp; 2019. [Google Scholar]
- 27.Dobson AJ, Barnett AG. An introduction to generalized linear models. 4th ed. New YorkP: Chapman and Hall/CRC, 2018. [Google Scholar]
- 28.Gasparrini A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med 2014;33:881–99. 10.1002/sim.5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Héguy L, Garneau M, Goldberg MS, et al. Associations between grass and weed pollen and emergency department visits for asthma among children in montreal. Environ Res 2008;106:203–11. 10.1016/j.envres.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 30.De Roos AJ, Kenyon CC, Zhao Y, et al. Ambient daily pollen levels in association with asthma exacerbation among children in Philadelphia, Pennsylvania. Environ Int 2020;145:106138. 10.1016/j.envint.2020.106138 [DOI] [PubMed] [Google Scholar]
- 31.Sousa-Silva R, Smargiassi A, Kneeshaw D, et al. Strong variations in urban allergenicity riskscapes due to poor knowledge of tree pollen allergenic potential. Sci Rep 2021;11:10196. 10.1038/s41598-021-89353-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price D, Hughes KM, Thien F, et al. Epidemic thunderstorm asthma: lessons learned from the storm down-under. J Allergy Clin Immunol 2021;9:1510–5. 10.1016/j.jaip.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 33.Pakkasela J, Ilmarinen P, Honkamäki J, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med 2020;20. 10.1186/s12890-019-1040-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beggs P, Katelaris C, Medek D. Differences in grass pollen allergen exposure across Australia. Aust N Z J Public Health 2014;39. 10.1111/1753-6405.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haberle SG, Bowman DMJS, Newnham RM, et al. The macroecology of airborne pollen in Australian and New Zealand urban areas. PLoS One 2014;9:e97925. 10.1371/journal.pone.0097925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beggs PJ. Climate change and allergy in Australia: an innovative, high-income country, at potential risk. Public Health Res Pract 2018;28:e2841828. 10.17061/phrp2841828 [DOI] [PubMed] [Google Scholar]
- 37.Cecchi L. From pollen count to pollen potency: the molecular era of aerobiology. Eur Respir J 2013;42:898–900. 10.1183/09031936.00096413 [DOI] [PubMed] [Google Scholar]
- 38.Addison-Smith B, Milic A, Dwarakanath D, et al. Medium-term increases in ambient grass pollen between 1994-1999 and 2016-2020 in a subtropical climate zone. Frontiers in Allergy 2021;2:38. 10.3389/falgy.2021.705313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Asthma Council Australia . Australian asthma Handbook, version 2.1. Melbourne: National Asthma Council Australia, 2020. https://www.asthmahandbook.org.au/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066851supp001.pdf (56KB, pdf)
bmjopen-2022-066851supp002.pdf (57.3KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Restrictions apply to the availability of these data. Data were obtained from several third parties including the South Australian Department for Health and Wellbeing, Asthma Australia (SA branch), SA Environment Protection Authority (EPA) and the Australian Bureau of Meteorology (BoM). On reasonable request, the authors may be able to facilitate sharing the data with the permission of the third parties.