Abstract
Introduction
Non-adherence to antiretroviral therapy (ART) is the main cause of viral non-suppression and its risk is increased by depression. In countries with high burden of HIV, there is a lack of trained professionals to deliver depression treatments. This paper describes the protocol for a 2-arm parallel group superiority 1:1 randomised controlled trial, to test the effectiveness and cost effectiveness of the TENDAI stepped care task-shifted intervention for depression, ART non-adherence and HIV viral suppression delivered by lay interventionists.
Methods and analysis
Two hundred and ninety people living with HIV aged ≥18 years with probable depression (Patient Health Questionnaire=>10) and viral non-suppression (≥ 1000 HIV copies/mL) are being recruited from HIV clinics in towns in Zimbabwe. The intervention group will receive a culturally adapted 6-session psychological treatment, Problem-Solving Therapy for Adherence and Depression (PST-AD), including problem-solving therapy, positive activity scheduling, skills to cope with stress and poor sleep and content to target barriers to non-adherence to ART. Participants whose score on the Patient Health Questionnaire-9 remains ≥10, and/or falls by less than 5 points, step up to a nurse evaluation for possible antidepressant medication. The control group receives usual care for viral non-suppression, consisting of three sessions of adherence counselling from existing clinic staff, and enhanced usual care for depression in line with the WHO Mental Health Gap intervention guide. The primary outcome is viral suppression (<1000 HIV copies/mL) at 12 months post-randomisation.
Ethics and dissemination
The study and its tools were approved by MRCZ/A/2390 in Zimbabwe and RESCM-18/19–5580 in the UK. Study findings will be shared through the community advisory group, conferences and open access publications.
Trial registration number
Keywords: HIV & AIDS, Depression & mood disorders, HEALTH ECONOMICS, MENTAL HEALTH, PUBLIC HEALTH
Strengths and limitations of this study.
The first randomised controlled trial in a low-income country to test an intervention to improve adherence to antiretroviral therapy (ART) (primary outcome) and depression (as a secondary outcome) in people living with HIV.
Culturally adapted and culturally appropriate intervention to address barriers to adherence to ART and to treat depression, based on extensive preliminary work.
Assessment of the cost effectiveness of the TENDAI stepped care task-shifted intervention.
Stepped care intervention is delivered through task-shifting to non-specialist staff, allowing for future scale up.
Limited scope to assess implementation science questions given the individually randomised design.
Introduction
Over 27% of people in sub-Saharan Africa currently receiving antiretroviral therapy (ART) are non-adherent,1 and non-adherence to ART is the main cause of viral non-suppression.2 Achieving and maintaining viral suppression are not only effective HIV treatment strategies, but also effective HIV prevention approaches, preventing transmission of the virus to sexual partners, and from mother to child.3 4 Depression is among the strongest correlates of non-adherence and affects over 15% of people living with HIV (PLHIV) attending HIV out-patient clinics in sub-Saharan Africa.1 5 6 Depression is linked to non-adherence through the reduced motivation and forgetting to take ART,7 through impaired problem-solving ability,8 and may interfere with uptake of existing adherence support programmes as part of clinical care.9 10 Depression may also impact adherence through its association with structural factors, such as poverty, and interpersonal difficulties, which impede access to HIV medication.7 10 11 Non-adherence to ART may also precede and increase risk of depression.12 Evidence-based interventions for depression include psychological interventions based on cognitive behavioural approaches and antidepressant medication.13
Countries with high burden of HIV, such as Zimbabwe, have a dearth of trained mental health professionals. Given the public health importance of viral suppression,14 and the strong association with depression, adherence interventions must address comorbid psychological factors and be able to be delivered through task-shifting to non-specialists.15 Systematic reviews of mental health interventions in PLHIV in low-resource settings have been unable to report effects on HIV outcomes as, to date, these have not been studied.16 17
The most promising evidence for the effectiveness and utility of integrated treatments for depression and ART adherence for PLHIV has come from the development of Cognitive Behavioural Therapy for Adherence and Depression (CBT-AD) in the USA.18 19 CBT-AD, which includes the ‘life steps’ intervention for addressing barriers to medication adherence, has been shown to improve rates of ART adherence, and to reduce depression severity among men in the USA.20 21 In contrast, interventions for people living with HIV (PLWH) with depression and poor adherence which only target mood have not been found to improve viral suppression.22 Recent reports, including from our team in Zimbabwe, support the acceptability and feasibility of culturally adapted cognitive behavioural interventions for low-resource settings.23–25 However, there have yet to be any definitive RCTs from low-resource settings focused on treatment of depression and ART adherence to improve viral suppression.26 Thirteen per cent of the adult population in Zimbabwe is living with HIV, with 22% of those virally non-suppressed.22 The objective of this trial is to test the effectiveness and cost-effectiveness of the TENDAI stepped care psychological intervention for adherence to ART and depression (Stepped Care-AD), compared with enhanced usual care (EUC), for PLHIV in Zimbabwe with viral non-suppression and depression. TENDAI is derived from principles of problem-solving and psychoeducation for depression and adherence, and motivational interviewing.
Methods and analysis
Study design and setting
The study is a two-arm parallel group superiority 1:1 randomised effectiveness trial (n=290). PLHIV receive care according to the standard national guidelines.27 Viral load is monitored every 12 months, with more frequent screening every 3 months for those who are virally non-suppressed. Participants are being recruited from two sites in Zimbabwe providing HIV services for those initiated on ART. These are the Marondera Provincial Hospital and Chitungwiza Central Hospital, along with satellite clinics for each hospital. Marondera is the capital of Mashonaland East province, situated in the north east of Zimbabwe. The town and its surrounding district have a total population of approximately 224 000.28 Chitungwiza is an urban town, divided into five townships, with a total population of approximately 391 000.29 Taken together, both sites combined have approximately 25 000 adults registered as receiving ART.
Patient and public involvement
No patient involvement in the design of the study. Results will be disseminated to study participants and community members via local advisory groups.
Eligibility criteria
Inclusion criteria were: (1) HIV positive and initiated on ART for at least 6 months; (2) clinically significant depression symptoms (operationalised as a score of 10 or more on the locally validated Patient Health Questionnaire-9 (PHQ-9), which has been validated for adults in a primary care population with high HIV prevalence in Zimbabwe6); (3) virally non-suppressed in the past 2 months (viral load >/= 1000 copies/mL); (4) able to provide informed consent and (5) if prescribed antidepressants, have been on a stable antidepressant regimen for at least 2 months. Exclusion criteria were: (1) unwilling or unable to provide informed consent; (2) major untreated or undertreated mental illness (eg, untreated psychosis or mania, actively suicidal assessed using the MINI International Neuropsychatric Inventory (MINI) and P4 suicide screener), major or advanced physical disease (assessed using clinic records) or severe cognitive impairment (assessed using the International HIV Dementia Scale), which would interfere with engagement in Stepped Care-AD; (3) have received a course of problem solving therapy or cognitive-behavioural psychological therapy for depression or (4) <18 years old.
Study procedures
Recruitment and informed consent
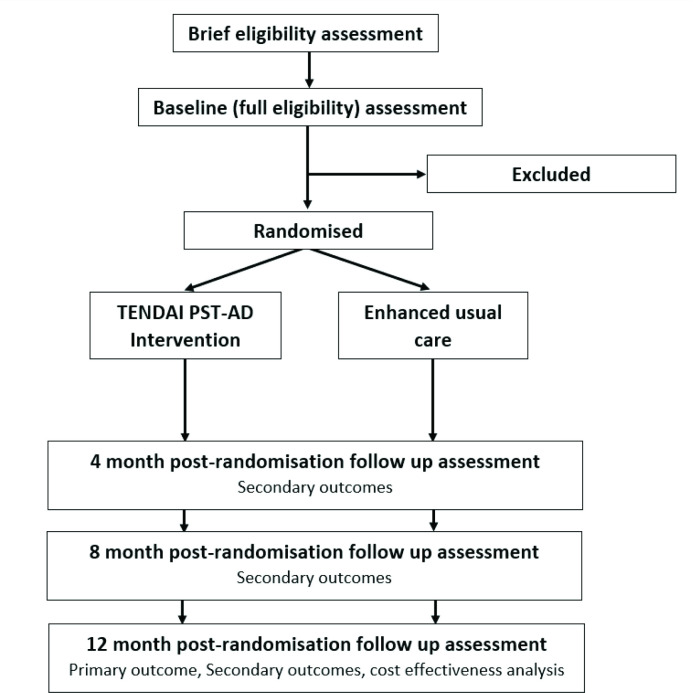
See figure 1 for study flow. Research assistants (RAs) in the ART clinic approach patients identified by clinic staff as having a detectable viral load (>1000 copies/mL) in the past 2 months or at high risk of viral non-suppression, for example, self-reported poor adherence. Potentially eligible individuals will complete a brief screen for inclusion and exclusion criteria. Those meeting initial screening eligibility criteria are invited to complete informed consent procedures and the full baseline assessment. Informed consent will include consent to access participants’ medical records, and for telephone calls and home visits if needed for follow-up. Capacity to provide consent is assessed by a licensed psychiatrist (WM) for any participant indicating consent but suspected of being unable to fully understand and/or retain information provided.
Figure 1.
Flow of participants through TENDAI study.
Baseline
A trained RA collects data by face-to-face interview, including: demographics, measures of socioeconomic position (employment status, educational history and ownership of household assets),30 31 depression using the PHQ-9,6 anxiety using the Hospital Anxiety and Depression Scale32 quality of life using the EuroQol-5 Dimension-3 Level assessment (EQ-5D-3L),33 use of alcohol and substances,34 35 cognitive impairment using the International HIV Dementia Scale,36 psychiatric diagnosis using the MINI International Neuropsychiatric Interview,37 use of health services in the last 4 months using a modified version of the Client Services Receipt Inventory31 and several additional exploratory measures. Current ART regimen and recent CD4 test results are taken directly from participants medical records.
Viral load in the past 60 days is ascertained from the participants medical records, or, for those who have not been tested in the past 2 months, by testing plasma.
Eligibility
The study team, including a clinical psychologist or psychiatrist, will meet weekly via teleconference to discuss each baseline assessment and confirm that participants meet eligibility criteria.
Randomisation
Approximately 2 weeks after the baseline assessment, eligible participants return for a randomisation visit and are randomly assigned to Stepped Care-AD or EUC. Randomisation is determined by a computer-generated chart and is conducted via the Research Electronic Data Capture system (REDCap) system randomisation module by the Zimbabwe site Programme Manager.
Follow-up assessments
In addition to baseline, there are 3 major study assessments: 4 months, 8 months and 12 months post-randomisation. An independent assessor (IA) who is blind to study condition will administer the PHQ-9, EQ-5D-3L and self-report medication adherence measures only. RAs will collect all other self-report data, including use of alcohol and substances,34 35 Hospital Anxiety and Depression Scale for Anxiety,32 quality of life33 and use of other healthcare services.38 At the final 12-month follow-up, we will also extract chart information from medical records for pharmacy refill data and HIV viral load results. Participants without a viral load test in the past 30 days are invited to undergo venepuncture for viral load testing, which is in addition to the venepuncture for ART detection.
Interventions
Active intervention arm: task-sharing stepped care intervention for adherence and depression (TENDAI Stepped Care-AD)
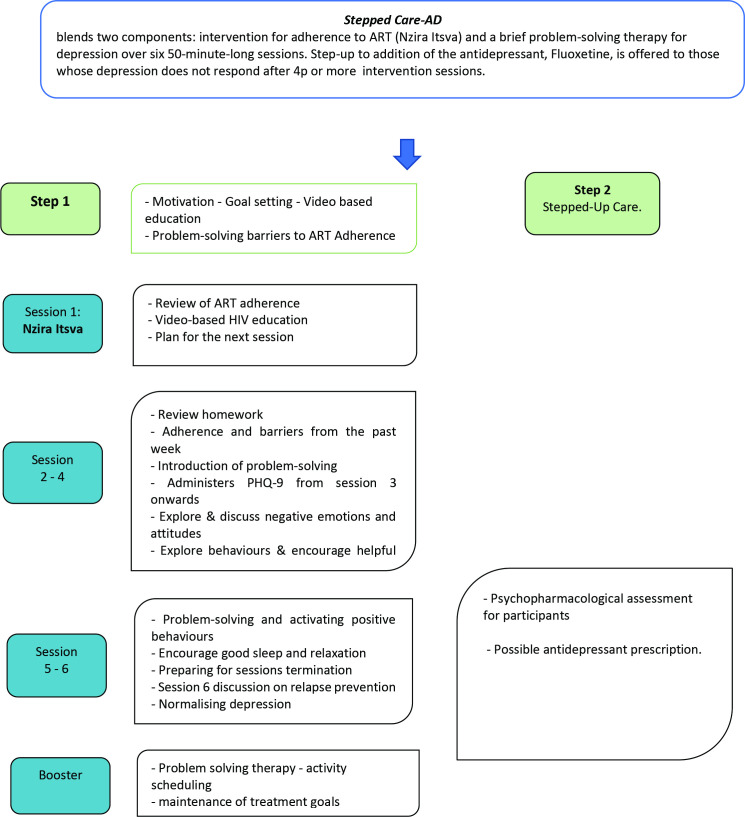
As shown in figure 2, all participants in the TENDAI arm receive six 50-minute sessions of a culturally adapted intervention for depression and non-adherence to ART delivered weekly, followed by one booster session 6 weeks later. The intervention is based on cognitive behavioural principles and includes problem-solving therapy, positive activity scheduling, skills to cope with stress and poor sleep and content to target barriers to non-adherence to ART. Training of interventionists emphasises common elements of effective psychological interventions, including empathy, active listening and creating realistic hope.39 The training is conducted by the principal investigators and other clinical psychologists with expertise in the intervention. Refresher trainings and ongoing supervision will be conducted throughout the study.
Figure 2.
Stepped Care-AD intervention flow diagram. Stepped Care-AD, stepped care psychological intervention for adherence to ART and depression.
Adherence to ART
Session one comprises a locally adapted version of the LifeSteps adherence intervention called Nzira Itsva.18 40 This includes motivation, goal setting, video-based education and problem-solving. Motivational interviewing is used to identify the participants’ life goals and to tie adherence to achieving these goals. Education about on time adherence is provided using an animated video in the Shona language. Barriers to adherence are assessed through a culturally adapted checklist. During each of the subsequent sessions 2–6 targeting depression, 5–10-minute adherence boosters are included to review adherence to ART and the participants’ experience with strategies to overcome barriers to adherence.
Psychological intervention for depression
Sessions 2 and 3 focus on psychoeducation about depression and problem-solving therapy (PST),41 incorporating storytelling and illustrations and training in problem-solving. A goal for each session is to identify a defined specific problem to work on, to collaboratively agree a solution to work on and to schedule homework. An intervention based on PST has been shown to be acceptable and effective for depression in Zimbabwe.42 43 In Session 4, participants are encouraged to choose and schedule at least four adaptive activities in which to engage: an activity that promotes a sense of achievement, a physical activity, a pleasurable activity and a social activity. Homework is mutually agreed as part of every session, to test out participants’ implementation of solutions to problems and of positive activities. Thorough review of homework is done at each session, including barriers to doing homework. Skills to promote good sleep and relaxation are taught in Sessions 5 and 6. A relapse prevention plan is developed in session 6, including triggers for relapse of depression, warning signs, coping strategies and self-care activities. Fidelity of the intervention will be assessed through rating 10% of audio-recorded sessions for adherence to the intervention protocol and for therapist competence.44
Booster session
About 6 weeks after the 6th session, participants are invited to a 50-minute booster session. This includes a review of depressive symptoms, and of adherence to HIV treatment and, where appropriate, adherence to antidepressant medication. The session includes ongoing positive activity scheduling to promote recovery from depressive symptoms.
Stepped up care
Participants with persistent depression (depression score continuing above cut-off (PHQ-9 ≥10) or if they have less than a 5-point improvement in PHQ-9 score) after at least 4 sessions receive step up to a nurse-evaluation for antidepressant medication. The antidepressant fluoxetine is offered for those with confirmed depression.
Control arm: EUC
All participants in the control arm receive EUC comprising usual care for viral non-suppression, and EUC for depression in line with the WHO Mental Health Gap (mhGAP) intervention guide. Usual care for those with viral non-suppression includes three sessions of adherence counselling provided by an adherence counsellor, nurse or NGO support worker based at the clinic. These sessions include establishing the participants knowledge about HIV and ART, providing information about use of ART, encouraging adherence and describing barriers to adherence. Strategies commonly used include encouraging use of an alarm and a treatment supporter, linking ART taking to daily routines, and disclosure of HIV status. The first session is given on the day of receiving viral load results, with two subsequent sessions scheduled on a monthly basis. Referrals may be made to local support groups or organisations for social or economic support, general psychological support or to an HIV clinician. The HIV operational strategy recommends that all patients living with HIV and registered at facility should be screened for common mental disorders (CMD) annually. Patients with high viral load or those initiating ART should be screened for CMD at their appointment.45 Patients exhibiting symptoms of CMD or psychological distress should be managed with counselling interventions and are usually referred to the outpatient’s department to be assessed by a psychiatric nurse. If they require further treatment, they will be seen by a psychiatrist. Patients can also be referred to community-based organisation to receive psychosocial services.45 Usual care for depression is enhanced in three ways: (1) the study team will train all health service providers in the study sites on psychological and antidepressant management of depression using the WHO mhGAP intervention guide46; (2) we will provide a letter for each participant communicating the patients’ PHQ-9 score and probable depression to their HIV-care provider and (3) we will provide those in the EUC condition with access to Stepped Care-AD on completion of their 12-month follow-up visit.
Outcomes
Primary outcome
The primary outcome for the trial is viral suppression at 12-month post-randomisation follow-up (defined as <1000 copies/mL), measured through blood (plasma). This measure will be taken from the medical record if viral load was collected within 30 days of the expected visit date or through study specific assay if not in the medical record.
Secondary outcomes
Depression at 12-month post-randomisation measured as the total score on the PHQ-9.6
Adherence to ART medication at 4-month, 8-month and 12-month post-randomisation assessed as the proportion of the sample achieving at least 90% adherence in the past month assessed through pharmacy refill.47
Self-reported adherence to ART medication at 4-month, 8-month and 12-month post-randomisation assessed as the frequency of adherence in the past 30 days measured using a score derived from a three-item questionnaire adapted from Wilson et al.48
Viral load copies/mL at 12-month post-randomisation follow-up measured as mean log viral load.
Tertiary outcomes
The total costs of the healthcare services used by each study participant will be calculated using service use information collected from hospital records and from participant self-report (via a modified version of the Client Services Receipt Inventory suitable for use in sub-Saharan Africa38) at 4-month, 8-month and 12-month follow-up and with unit costs identified and calculated using locally available data. Detailed information on the use of Stepped Care-AD and EUC will be collected from therapist records. Quality of life at 4 months, 8 months and 12 months is measured using EQ-5D-3L.33 Quality-adjusted life years will be calculated using Zimbabwe-specific health states.49
Data collection and management
Trial data are collected and stored in REDCap, a data management tool designed for collection and protection of patient health information. The REDCap database is hosted and routinely audited at Massachusetts General Hospital (MGH), with access restricted via user roles. Data extracts are sent to the study statisticians, via secure file transfer. To ensure accuracy of collected data, MGH staff generate weekly error reports. These error reports are then sent to staff in Zimbabwe, who correct any discrepancies and document changes made to the database.
Strategies to improve participant retention
Procedures to maximise participant retention include sending text message reminders before scheduled appointments and collection of locator information (eg, contact information of two significant others with whom the participant is in regular contact). Participants are also provided with refreshments at study visits and reimbursed transport costs. Although those in the EUC arm have fewer scheduled clinical sessions, the same total amount ($46) will be provided to participants attending all clinical sessions and research assessments in both arms. We will make efforts to retain individuals who move to a non-study site for their HIV care and are willing to complete follow-up. Where participants are unable to travel to the clinic to complete follow-up assessments (eg, because of COVID-19 lockdown travel restriction), participants will be offered phone assessments. Where participants can not be reached by phone, a home visit may be conducted.
Confidentiality
Participants are given a study-specific identification number at screening that is used on all forms and data collection instruments, excluding the consent form. Participants are referred to only by their identification number during eligibility and supervision meetings. A file that links participant names to identification numbers is stored in a locked file at the University of Zimbabwe.
Blinding
To reduce bias and maximise the validity of the findings, the IA for the primary and secondary outcomes, and the lead study statistician, are blinded to randomisation condition. To ensure blinding, the IA will explain their role to participants and ask that they do not give the IA information about the treatment they received. The lead study statistician will not attend meetings where randomisation or clinical issues are discussed, and their access in REDCap is restricted so that they cannot view any data or report that may unblind them. A second statistician, who will conduct the analyses and will review data for thoroughness and completeness, will not be blinded. This trial does not have procedures for unblinding IAs or the lead study statistician. If the IA is concerned about the safety of a participant, they will communicate the concern to study staff, who will contact the clinical supervisor.
Data safety and monitoring board
A data safety and monitoring board (DSMB), consisting of members with experience in clinical trials for mental disorders, biostatistics, HIV in African settings and human subject protection issues, will function independent of the sponsor and monitor safety of study participants and integrity of data. The DSMB will meet annually and receive safety information in an unblinded manner. Expedited review by the DSMB will occur for all serious adverse events (SAEs) as defined as any fatal, immediately life-threatening or substantially disabling event; event requiring or prolonging inpatient hospitalisation or any congenital anomaly.
Statistical methods
Sample size
Using two-sided Fisher’s exact test, α=0.05 and 20% attrition, a sample size of 290 participants will provide 85% power to detect an absolute difference of 20% or more in achieving viral suppression (eg, 45% in the EUC arm vs 65% in the intervention arm) at 12-month follow-up. Pilot data showed a larger difference between arms (50% in the EUC arm vs 75% in the intervention arm),24 suggesting this should be a conservative sample size estimate.
Statistical methods for primary and secondary outcomes
Baseline and outcome variables will be summarised using appropriate statistics; no baseline statistical comparisons will be made. The main analysis will follow intention to treat principles, reporting appropriate 95% CIs and use a 5% significance level. All models will include a site stratification variable. In the mixed models, we will have random intercepts at the participant level, and random slopes if warranted (assessed via likelihood ratio test).
Twelve-month primary outcome viral load will be coded as suppressed (<1000 copies/mL) versus not suppressed (≥1000 copies/mL). If these data are missing and we have no further information, they will be left as missing. If we do have information from medical or death records that the individual died from high viral load or AIDS-related reasons, we will code them as not being virally suppressed. Suppressed/not suppressed will be the dependent variable in a logistic regression model estimating the TENDAI versus EUC OR, with trial arm as the independent variable. The mean difference in PHQ-9 depression and self-report adherence will each be estimated using a linear mixed effects model with the 4-month, 8-month and 12-month measures as dependent variables, with the baseline measure of the outcome, time and trial arm by time interaction terms as independent variables. The OR at 4 months, 8 months and 12 months for ≥90% adherence versus 90% adherence by pharmacy refill in the past 30 days will be estimated using a logistic mixed effects model with the 4-month, 8-month and 12-month measures as dependent variables, and independent variables as described for PHQ-9 and self-report adherence. The mean difference in log copies/mL of viral load at 12 months will be estimated using a linear regression model with log viral load at 12 months as the dependent variable, trial arm and baseline log viral load as independent variables. In line with other recent and similar trials in HIV and methodology literature,50–56 we do not plan to undertake secondary outcome adjustment for multiple comparisons. Rather, we will focus on our single prespecified primary outcome to assess effectiveness and take a ‘precise interpretation’/separate hypotheses approach for the secondary outcomes in combination with appropriate reporting of effect sizes and CIs to support transparent interpretation.52 56
A ‘per protocol’ analysis for the primary viral load outcome only will exclude participants not completing at least four TENDAI sessions, and anyone found to be ineligible post randomisation. No interim or formal powered subgroup analyses are planned; however, we will explore moderation by gender for viral suppression, self-report adherence and depression outcomes by adding a sex by trial arm (by time, where appropriate) interaction term to the final outcome analysis models. Additional exploratory mediation analysis (eg, to examine changes in both depression and adherence as mediators of treatment related changes in viral load) is planned but will not be reported on in the main paper. This analysis should help elucidate whether intervention effects were due to improving depression or more directly by changing adherence. Missing baseline measures will be imputed using simple mean imputation.57 Missing repeatedly measured outcome data will be handled using mixed models/maximum likelihood methods, including baseline variables predicting missing outcome data. If there is more than 10% missing primary outcome data and post-randomisation variables (completion of therapy in the TENDAI arm only and ART adherence at 12 months) predict whether these data are missing, we will consider multiple imputation.58
Cost-effectiveness results will be reported following CHEERS guidelines.59 Economic evaluations can be used to inform healthcare decision-makers on the total budget needed to treat people with a particular disease or condition; it is only the mean cost that allows for this calculation to be made. Thus, it is the arithmetic mean cost that is the relevant summary statistic in pragmatic trials with economic evaluations and the mean average total cost in each randomised group will be calculated and compared between the two groups using standard t-tests, despite the likely skewed nature of the data.60 As is common in the analysis of cost data, the robustness of the mean cost comparisons will be confirmed through the calculation of non-parametric bootstrapped CIs.61 The primary cost effectiveness will consider costs together with the dichotomous primary outcome measure (viral suppression <1000 copies/mL), generating information on the incremental cost per successful case (in the form of an incremental cost-effectiveness ratio) and the probability that the TENDAI is cost effective compared with EUC given available information. A secondary cost–utility analysis will also be completed, which will report the cost per quality-adjusted life-year (QALY) of the TENDAI intervention compared with EUC via incremental cost-effectiveness ratios. Analyses will be adjusted for costs and outcomes. Sensitivity analyses will be carried out to test the robustness of costing assumptions to variation.
Discussion
In Zimbabwe, both HIV and comorbid depression are common, yet, as in other low-resource settings with high HIV burden, there is a lack of evidence on interventions to improve both ART adherence and depression.62 Due to resource limitations, interventions that allow for task-shifting and administration by community health workers are particularly well placed to be effective and sustainable. Our treatment, Stepped Care-AD, blends active ingredients of treatment for depression with a culturally adapted LifeSteps intervention to enhance adherence to ART.24 40 If successful, the Stepped Care-AD intervention represents a useful model for policy and for further research. As the primary outcome of the trial is viral suppression, its implementation in Zimbabwe and other low-resource settings may further the UNAIDS goal of ending the AIDS epidemic by 2030, through optimising viral suppression.63 Results gathered in a Zimbabwean context may be leveraged for testing and implementation of similar task-shifted stepped care interventions in other sub-Saharan African settings.
Ethics and dissemination
All study procedures were reviewed and approved by ethics committees at King’s College London (RESCM-17/18–5580), MGH (IRB00012706) and the Medical Research Council of Zimbabwe (MRCZ/A/2390). SAEs will be reported to research ethics committees at King’s College London, MGH and the Medical Research Council of Zimbabwe within 72 hours.
Dissemination of findings will involve three primary papers describing the study outcomes, as well as submitting to lead workshops on the treatment approach at relevant national meetings and conferences. Additionally, data will be available to external parties after publication of the outcome papers via Principal Investigator-approved application. Data will be stored indefinitely.
Trial status
This trial began recruitment and enrolment on 2 July 2019.
Supplementary Material
Acknowledgments
Mercy Mundwa, Oline Chivere, Sheila Marezva, Vimbai Gwata, Rumbidzai Nyanda, Moreblessing Phiri, Megan R Wirtz, Rachel Holland, Dr Tsitsi Apollo, Dr Celestino Dhege, Dr Michael Chiwanga, Tinashe Macheka and Isaac Masarira, KG contributions to this paper represent independent research part funded by the NIHR Biomedical Research Centre (South London and Maudsley NHS Foundation Trust and KCL) and the NIHR Applied Research Collaboration South London (King’s College Hospital NHS Foundation Trust). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. This work is funded by National Institute of Mental Health.
Footnotes
Twitter: @melanieabas
Deceased: Dr. Hakim passed away in 2020
Contributors: MA is the King’s College London's Principal Investigator for the trial and engaged in substantial writing of this manuscript and gaining funding for the study. WM is the University of Zimbabwe's Principal Investigator for the trial and engaged in revision of this manuscript and gaining funding for the study. PN is the University of Zimbabwe's project director for the trial and engaged in revision of this manuscript. RJ is the King’s College London's project director for the trial and engaged in substantial writing of this manuscript. TB is the University of Zimbabwe's clinical supervisor for the trial and engaged in substantial writing of this manuscript. SMM is the trial data manager and engaged in substantial writing of this manuscript. KG is the trial statistician, engaged in substantial writing of this manuscript and gaining funding for the study. CF is the Massachusetts General Hospital's clinical supervisor for the trial and engaged in revision of this manuscript. ES, TM and DG are research assistants for this trial and engaged in substantial writing of this manuscript. BMB is the trial health economist, engaged in revision of this manuscript and gaining funding for the study. DC and SAS is a co-investigator on this trial and provided guidance around the development and execution of this manuscript and gaining funding for the study. JH is a co-investigator on this trial, assisted in gaining funding for the study and provided guidance around the development and execution of this manuscript up until his untimely death in 2020. COC is the Massachusetts General Hospital's Principal Investigator for the trial and engaged in substantial writing of this manuscript and assisted in gaining funding for the study.
Sponsor: the National Institute of Mental Health provides funding for this trial and monitors progress on the project through yearly Research Performance Progress Reports.
Funding: Grant number 1R01MH114708 was awarded to MA and COC by the National Institute of Mental Health, NIH.
Disclaimer: The National Institute of Mental Health provides funding for this trial and monitors progress on the project through yearly Research Performance Progress Reports.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Heestermans T, Browne JL, Aitken SC, et al. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-Saharan Africa: a systematic review. BMJ Glob Health 2016;1:e000125. 10.1136/bmjgh-2016-000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis 2008;197 Suppl 3:S272–8. 10.1086/533415 [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 2002;29:484–94. 10.1097/00042560-200204150-00009 [DOI] [PubMed] [Google Scholar]
- 5.Lofgren SM, Bond DJ, Nakasujja N, et al. Burden of depression in outpatient HIV-infected adults in sub-Saharan Africa; systematic review and meta-analysis. AIDS Behav 2020;24:1752–64. 10.1007/s10461-019-02706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chibanda D, Verhey R, Gibson LJ, et al. Validation of screening tools for depression and anxiety disorders in a primary care population with high HIV prevalence in Zimbabwe. J Affect Disord 2016;198:50–5. 10.1016/j.jad.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Wagner GJ, Slaughter M, Ghosh-Dastidar B. Depression at treatment initiation predicts HIV antiretroviral adherence in Uganda. J Int Assoc Provid AIDS Care 2017;16:91–7. 10.1177/2325957416677121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safren SA, Radomsky AS, Otto MW, et al. Predictors of psychological well-being in a diverse sample of HIV-positive patients receiving highly active antiretroviral therapy. Psychosomatics 2002;43:478–85. 10.1176/appi.psy.43.6.478 [DOI] [PubMed] [Google Scholar]
- 9.Andersen L, Kagee A, O'Cleirigh C, et al. Understanding the experience and manifestation of depression in people living with HIV/AIDS in South Africa. AIDS Care 2015;27:59–62. 10.1080/09540121.2014.951306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidia K, Machando D, Bere T, et al. 'I was thinking too much': experiences of HIV-positive adults with common mental disorders and poor adherence to antiretroviral therapy in Zimbabwe. Trop Med Int Health 2015;20:903–13. 10.1111/tmi.12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalichman SC, Kalichman MO, Cherry C. Forget about forgetting: structural barriers and severe non-adherence to antiretroviral therapy. AIDS Care 2017;29:418–22. 10.1080/09540121.2016.1220478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wing RR, Phelan S, Tate D. The role of adherence in mediating the relationship between depression and health outcomes. J Psychosom Res 2002;53:877–81. 10.1016/S0022-3999(02)00315-X [DOI] [PubMed] [Google Scholar]
- 13.Abas MA. Combining active ingredients to treat depression in the wake of COVID-19. Lancet Psychiatry 2022;9:190–1. 10.1016/S2215-0366(21)00436-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNAIDS . Prevailing againt pandemics by putting people at the centre. Geneva; 2020. [Google Scholar]
- 15.Abas M, O'Cleirigh C. Global mental health and the ambition to end AIDS by 2030. Lancet Psychiatry 2018;5:867–9. 10.1016/S2215-0366(18)30385-7 [DOI] [PubMed] [Google Scholar]
- 16.Nakimuli-Mpungu E, Musisi S, Smith CM, et al. Mental health interventions for persons living with HIV in low- and middle-income countries: a systematic review. J Int AIDS Soc 2021;24 Suppl 2:e25722. 10.1002/jia2.25722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sikkema KJ, Dennis AC, Watt MH, et al. Improving mental health among people living with HIV: a review of intervention trials in low- and middle-income countries. Glob Ment Health 2015;2:e19. 10.1017/gmh.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther 2001;39:1151–62. 10.1016/S0005-7967(00)00091-7 [DOI] [PubMed] [Google Scholar]
- 19.Safren SA, Hendriksen ES, Mayer KH, et al. Cognitive-behavioral therapy for HIV medication adherence and depression. Cogn Behav Pract 2004;11:415–24. 10.1016/S1077-7229(04)80058-0 [DOI] [Google Scholar]
- 20.Safren SA, O'Cleirigh C, Skeer MR, et al. Demonstration and evaluation of a peer-delivered, individually-tailored, HIV prevention intervention for HIV-infected MSM in their primary care setting. AIDS Behav 2011;15:949–58. 10.1007/s10461-010-9807-8 [DOI] [PubMed] [Google Scholar]
- 21.Safren SA, Bedoya CA, O'Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV 2016;3:e529–38. 10.1016/S2352-3018(16)30053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pence BW, Gaynes BN, Adams JL, et al. The effect of antidepressant treatment on HIV and depression outcomes: results from a randomized trial. AIDS 2015;29:1975–86. 10.1097/QAD.0000000000000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen LS, Magidson JF, O'Cleirigh C, et al. A pilot study of a nurse-delivered cognitive behavioral therapy intervention (Ziphamandla) for adherence and depression in HIV in South Africa. J Health Psychol 2018;23:776–87. 10.1177/1359105316643375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abas M, Nyamayaro P, Bere T, et al. Feasibility and acceptability of a task-shifted intervention to enhance adherence to HIV medication and improve depression in people living with HIV in Zimbabwe, a low income country in sub-Saharan Africa. AIDS Behav 2018;22:86–101. 10.1007/s10461-016-1659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyamayaro P, Bere T, Magidson J. A task-shifted intervention for depression integrated with counselling for antiretroviral therapy adherence for people living with HIV/AIDS in Zimbabwe. Global Mental health 2016. 10.1016/j.cbpra.2018.10.003 [DOI] [Google Scholar]
- 26.Jopling R, Nyamayaro P, Andersen LS, et al. A cascade of interventions to promote adherence to antiretroviral therapy in African countries. Curr HIV/AIDS Rep 2020;17:529–46. 10.1007/s11904-020-00511-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Medicines and Therapeutics Policy Advisory Committee (NMTPAC), The AIDS and TB Directorate Ministry of Health and Child Care Zimbabwe . Guidelines for antiretroviral therapy for the prevention and treatment of HIV in Zimbabwe. Harare Zimbabwe; 2016. [Google Scholar]
- 28.Zimbabwe National Statistics Agency . Mashonaland East Province district population projections report. Harare; 2020. [Google Scholar]
- 29.PopulationStat . Chitungwiza Zimbabwe Population, 2021. Available: https://populationstat.com/zimbabwe/chitungwiza
- 30.Falkingham J, Namazie C. Measuring health and poverty: a review of approaches to identifying the poor Department for International Development Health Systems Resource Centre; 2002. [Google Scholar]
- 31.Wambogo EA, Ghattas H, Leonard KL, et al. Validity of the food insecurity experience scale for use in sub-Saharan Africa and characteristics of food-insecure individuals. Curr Dev Nutr 2018;2:nzy062. 10.1093/cdn/nzy062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 33.Jelsma J, Hansen K, De Weerdt W, et al. How do Zimbabweans value health states? Popul Health Metr 2003;1:11. 10.1186/1478-7954-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babor TF, De La Fuente MF, Saunders JB. AUDIT - The Alcohol use Disorders Identification Test: Guidelines for use in Primary Health Care. Geneva, Switzerland: World Health Organization, 1989. [Google Scholar]
- 35.Hildebrand M. The psychometric properties of the drug use disorders identification test (DUDIT): a review of recent research. J Subst Abuse Treat 2015;53:52–9. 10.1016/j.jsat.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 36.Sacktor NC, Wong M, Nakasujja N, et al. The International HIV dementia scale: a new rapid screening test for HIV dementia. AIDS 2005;19:1367–74. [PubMed] [Google Scholar]
- 37.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59 Suppl 20:4–57. [PubMed] [Google Scholar]
- 38.Mogga S, Prince M, Alem A, et al. Outcome of major depression in Ethiopia: population-based study. Br J Psychiatry 2006;189:241–6. 10.1192/bjp.bp.105.013417 [DOI] [PubMed] [Google Scholar]
- 39.Wampold BE. How important are the common factors in psychotherapy? an update. World Psychiatry 2015;14:270–7. 10.1002/wps.20238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bere T, Nyamayaro P, Magidson JF, et al. Cultural adaptation of a cognitive-behavioural intervention to improve adherence to antiretroviral therapy among people living with HIV/AIDS in Zimbabwe: nzira Itsva. J Health Psychol 2017;22:1265–76. 10.1177/1359105315626783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Zurilla T, Nezu A, Therapy P-S. Problem-Solving Therapy. In: Dobson KS, ed. Handbook of cognitive-behavioral therapies. Third Edition. United States of America: The Guilford Press, 2010. [Google Scholar]
- 42.Abas M, Bowers T, Manda E, et al. ‘Opening up the mind’: problem-solving therapy delivered by female lay health workers to improve access to evidence-based care for depression and other common mental disorders through the friendship bench project in Zimbabwe. Int J Ment Health Syst 2016;10:1–8. 10.1186/s13033-016-0071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chibanda D, Weiss HA, Verhey R, et al. Effect of a primary care-based psychological intervention on symptoms of common mental disorders in Zimbabwe: a randomized clinical trial. JAMA 2016;316:2618–26. 10.1001/jama.2016.19102 [DOI] [PubMed] [Google Scholar]
- 44.Kohrt BA, Jordans MJD, Rai S, et al. Therapist competence in global mental health: development of the enhancing assessment of common therapeutic factors (ENACT) rating scale. Behav Res Ther 2015;69:11–21. 10.1016/j.brat.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.AIDS & TB Programme Ministry of Health and Child Care Zimbabwe . Operational and service delivery manual for the prevention, care and treatment of HIV in Zimbabwe. Zimbabwe: Ministry of Health and Child Care, 2017. [Google Scholar]
- 46.WHO . mhGAP mental health gap action programme: scaling up care for mental, neurological and substance use disorders. Geneva: World Health Organisation, 2010. [PubMed] [Google Scholar]
- 47.Nau D. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Pharm Qual Alliance, 2012. [Google Scholar]
- 48.Wilson IB, Lee Y, Michaud J, et al. Validation of a new three-item self-report measure for medication adherence. AIDS Behav 2016;20:2700–8. 10.1007/s10461-016-1406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szende A, Janssen B, Cabases J. Self-Reported population health: an international perspective based on EQ-5D. Dordrecht (NL): Springer, 2014. [PubMed] [Google Scholar]
- 50.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005;365:1591–5. 10.1016/S0140-6736(05)66461-6 [DOI] [PubMed] [Google Scholar]
- 51.Cramer AOJ, van Ravenzwaaij D, Matzke D, et al. Hidden multiplicity in exploratory multiway ANOVA: prevalence and remedies. Psychon Bull Rev 2016;23:640–7. 10.3758/s13423-015-0913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Althouse AD. Adjust for multiple comparisons? it's not that simple. Ann Thorac Surg 2016;101:1644–5. 10.1016/j.athoracsur.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 53.Fox MP, Pascoe SJ, Huber AN, et al. Assessing the impact of the national department of health's national adherence guidelines for chronic diseases in South Africa using routinely collected data: a cluster-randomised evaluation. BMJ Open 2018;8:e019680. 10.1136/bmjopen-2017-019680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joska JA, Andersen LS, Smith-Alvarez R, et al. Nurse-delivered cognitive behavioral therapy for adherence and depression among people living with HIV (the Ziphamandla study): protocol for a randomized controlled trial. JMIR Res Protoc 2020;9:e14200. 10.2196/14200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saidi F, Mutale W, Freeborn K, et al. Combination adherence strategy to support HIV antiretroviral therapy and pre-exposure prophylaxis adherence during pregnancy and breastfeeding: protocol for a pair of pilot randomised trials. BMJ Open 2021;11:e046032. 10.1136/bmjopen-2020-046032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker RA, Weir CJ. Multiple secondary outcome analyses: precise interpretation is important. Trials 2022;23:27. 10.1186/s13063-021-05975-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993–1007. 10.1002/sim.1981 [DOI] [PubMed] [Google Scholar]
- 58.Dong P. Principled missing data methods for researchers. SpringerPlus, 2013: 2–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (cheers) statement. BMJ 2013;346:f1049. 10.1136/bmj.f1049 [DOI] [PubMed] [Google Scholar]
- 60.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000;320:1197–200. 10.1136/bmj.320.7243.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219–36. [DOI] [PubMed] [Google Scholar]
- 62.Remien RH, Patel V, Chibanda D, et al. Integrating mental health into HIV prevention and care: a call to action. J Int AIDS Soc 2021;24 Suppl 2:e25748. 10.1002/jia2.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.UNAIDS . End inequalities. end AIDS. global AIDS strategy 2021-2026. Geneva: UNAIDS; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.