Abstract
Listeria monocytogenes is a facultative intracellular bacterial pathogen that escapes from a host vacuolar compartment and grows rapidly in the cytosol. Listeriolysin O (LLO) is a secreted pore-forming protein essential for the escape of L. monocytogenes from the vacuole formed upon initial internalization. However, its role in intracellular growth and cell-to-cell spread events has not been testable by a genetic approach. In this study, purified six-His-tagged LLO (HisLLO) was noncovalently coupled to the surface of nickel-treated LLO-negative mutants. Bound LLO mediated vacuolar escape in approximately 2% of the mutants. After 5.5 h of growth, cytosolic bacteria were indistinguishable from wild-type bacteria with regard to formation of pseudopod-like extensions, here termed listeriopods, and spread to adjacent cells. However, bacteria in adjacent cells failed to multiply and were found in double-membrane vacuoles. Addition of bound LLO to mutants lacking LLO and two distinct phospholipases C (PLCs) also resulted in spread to adjacent cells, but these triple mutants became trapped in multiple-membrane vacuoles that are reminiscent of autophagocytic vacuoles. These studies show that neither LLO nor the PLCs are necessary for listeriopod formation and uptake of bacteria into neighboring cells but that LLO is required for the escape of L. monocytogenes from the double-membrane vacuole that forms upon cell-to-cell spread.
Listeria monocytogenes is a rapidly growing, facultatively intracellular bacterium, and it is a leading cause of food-borne illness that causes serious disease in immunocompromised individuals (5, 7). It is also highly amenable to experimental analysis and has emerged as a model intracellular pathogen with a well-characterized intracellular lifecycle (10, 14).
A primary virulence determinant of L. monocytogenes is the pore-forming protein listeriolysin O (LLO), a member of the family of cytolysins that also includes streptolysin O (1). LLO is essential for the escape of the bacterium from the host vacuole that is formed upon its initial entry. Wild-type bacteria rapidly escape from this vacuole and multiply in the host cytosol, but LLO-negative mutants remain trapped in the vacuole, do not grow intracellularly, and are avirulent in a murine model of listeriosis (14). Failure of LLO-negative mutants to escape the primary vacuole has prevented the study of the role of LLO in subsequent steps in pathogenesis. In this study, this block was bypassed by using purified six-His-tagged LLO to allow the escape of LLO-negative bacteria from the primary vacuole.
Purified LLO binds to LLO-negative bacteria.
LLO-negative bacteria were incubated with purified six-His-tagged listeriolysin O (HisLLO), and bound pore-forming activity was determined by hemolytic titration essentially as previously described (8). Several factors influenced the amount of purified protein that bound. The incubation of bacteria and HisLLO in a low-ionic-strength buffer increased binding fivefold compared to a normal-ionic-strength incubation. Preincubation of the bacteria with nickel ions increased binding twofold, whether ionic strength was normal or low. In addition, optimization of the bacterial culture medium and growth temperature increased binding threefold.
These findings led to the following protocol for the binding of HisLLO to LLO-negative strains. Approximately 4 × 108 bacteria that were grown to stationary phase at 37°C in Luria-Bertani medium, pH 7.4 (12), were resuspended in 200 μl of buffer A (20 mM HEPES [pH 7.5], 50 mM sodium chloride) and 1 mM nickel(II) chloride. After 10 min on ice, bacteria were pelleted and resuspended in 200 μl of fresh buffer A (without nickel). Twenty micrograms of HisLLO were added from a 4-mg/ml stock, and the suspension was again incubated for 10 min on ice. Bacteria were washed once in buffer A and were suspended in 200 μl of 20 mM HEPES (pH 7.5)–150 mM sodium chloride. Hemolytic activity of this suspension was determined.
L. monocytogenes mutants used in this study were in-frame deletions of targeted genes derived from wild-type strain 10403S by allelic exchange as previously described (3). Specifically, an LLO-, phosphatidylinositol-specific phospholipase C (PI-PLC)-, broad-range phospholipase C (PC-PLC)-negative strain was constructed from previously described strains (9), and an LLO-negative strain was constructed as previously reported (8). Construction and purification of HisLLO will be described elsewhere.
LLO-negative bacteria that were treated with HisLLO bound approximately 1 U of hemolytic activity per 106 bacteria. In comparison, washed wild-type bacteria had no detectable hemolytic activity. HisLLO binding was reversible, with most activity eluting from the bacteria in a 15-min, 37°C, normal-ionic-strength incubation. An increase of HisLLO binding to bacteria in low-ionic-strength buffer indicates that binding is mediated by charge-charge interactions, possibly between abundant negatively charged teichoic and lipoteichoic acids in the bacterial cell wall (6) and unidentified charged sites in LLO. Preincubation with nickel ions presumably causes the HisLLO six-His tag to bind to the bacterial surface. LLO does not contain GW repeats, which appear to mediate the binding of internalin B to the surface of InlB-negative L. monocytogenes (2).
Purified HisLLO bound to LLO-negative bacteria mediates escape from a vacuole.
To determine whether noncovalently bound HisLLO could mediate the escape of bacteria from macrophage primary vacuoles, J774 murine macrophage-like cells were infected with HisLLO-treated LLO-negative bacteria by a standard protocol (11) that was modified to decrease elution of hemolytic activity from the bacterial surface. The day before infection, 0.5 × 106 to 1.0 × 106 J774 cells were seeded onto glass coverslips in 35-mm dishes. To infect, a suspension of LLO-negative bacteria bound with HisLLO was diluted in fresh cold culture medium, placed over a cell monolayer, and centrifuged for 10 min at 800 × g at 4°C. Host cells internalized an average of 1 bacterium per cell. In contrast, the infection with a higher dilution of wild-type bacteria by a standard protocol (11) resulted in the internalization of about 1 bacterium per 20 host cells.
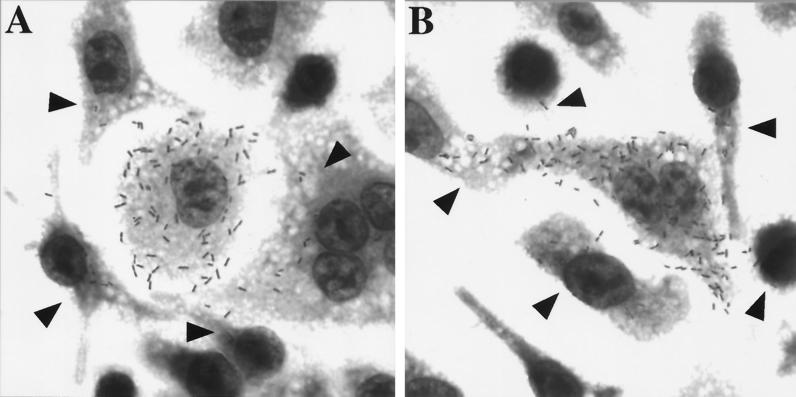
The escape of internalized LLO-negative bacteria from the host primary vacuoles was evaluated microscopically by using two criteria: (i) the number of bacteria present per host cell and (ii) the presence of bacteria in listeriopods (pseudopod-like extensions containing bacteria). Untreated LLO-negative bacteria do not form foci of replication and are not found in listeriopods (14). However, a small but consistent percentage of HisLLO-treated LLO-negative bacteria entered the host cytosol, replicated, nucleated actin, and entered listeriopods (Fig. 1B).
FIG. 1.
Growth and spread of wild-type and HisLLO-treated LLO-negative bacteria after 5.5 h of infection. J774 cells were infected for 5.5 h with wild-type (A) or HisLLO-treated LLO-negative (B) bacteria. Arrowheads indicate cells containing bacteria that have spread from the primary cell.
The efficiency of escape of HisLLO-treated LLO-negative bacteria from the primary vacuole was 2.0 ± 0.1% after 5 h of infection. Escape efficiency was determined by counting the foci of replicating bacteria (each representing one escaped bacterium) and bacteria not in foci (representing internalized, trapped bacteria) per area of monolayer. Efficiency was calculated as follows: (number of escaped bacteria)/(number of internalized plus number of escaped bacteria). To ensure the visualization of all bacteria, counts were performed on monolayers that were fluorescently stained as described in Table 1. Wild-type bacteria escape the primary vacuole and form foci with nearly 100% efficiency by this point during infection (4).
TABLE 1.
Roles of LLO, PI-PLC, and PC-PLC in spread of bacteria into secondary cellsa
| Strain | No. (mean ± SD) of:
|
Fraction of secondary-cell bacteria (mean % ± SD) | P value | |
|---|---|---|---|---|
| Primary-cell bacteria | Secondary-cell bacteria | |||
| Wild type | 80 ± 23 | 15 ± 8 | 19 ± 9 | |
| Δhly + LLO | 87 ± 28 | 26 ± 14 | 24 ± 14 | >0.05 |
| Δhly ΔplcA ΔplcB + LLO | 85 ± 19 | 21 ± 14 | 20 ± 12 | >0.05 |
J774 monolayers were infected with wild-type, HisLLO-treated LLO-negative, or HisLLO-treated LLO-, PlcA-, PlcB-negative strains for 5.5 h and were stained with fluorescein–anti-Listeria antibody and rhodamine-phalloidin as previously described (8). For each strain, 10 foci containing bacteria consistent with six to seven rounds of replication were identified. Bacteria associated with the primary cell and those lying within secondary cells were tallied. Bacteria lying within secondary cells were distinguished from those lying on them by determining the relation of each bacterium to the cell membrane by focusing through multiple focal planes and correlating cell surfaces visualized in the rhodamine channel with bacteria visualized in the fluorescein channel. Bacteria not lying within secondary cells were assumed to be associated with the primary cell. The background number of bacteria trapped in primary vacuoles or not internalized was less than one per cell. P values are for the one-tailed t test relative to the wild-type strain.
We have presumed that the LLO-negative bacteria present in the host cytosol in this study are LLO free, for the following reasons. The small amount of LLO bound to a bacterium, shown by the low escape efficiency to be very close to the minimum required, is expected to bind avidly to a vacuole and be consumed in the process of pore formation (1). Residual LLO on a bacterial surface would elute in minutes in the normal-ionic-strength environment of the cytosol and would additionally be diluted by bacterial replication in the hours that elapse before initiation of cell-to-cell spread. Accordingly, we view escaped bacteria as LLO-negative biochemically as well as genetically and as suitable for study of the role of LLO throughout the remainder of the L. monocytogenes life cycle. We cannot absolutely rule out any possible effects of LLO on the host cell physiology. However, the incubation of wild-type bacteria with LLO had no noticeable effect on their capacity to spread cell to cell (data not shown).
Growth in primary cells and spread into neighboring cells does not require LLO.
Progression of LLO-negative bacteria and that of wild-type bacteria through the intracellular life cycle were compared. After 5 h, J774 cells that were infected with either strain contained approximately 100 progeny that resulted from approximately 6 to 7 doublings. The numbers of bacteria in listeriopods were comparable for the 2 strains. LLO-negative bacteria also appeared to spread to neighboring cells to the same extent as wild-type bacteria (Fig. 1).
These impressions were evaluated by quantitating the spread of LLO-negative and wild-type bacteria into secondary cells. J774 monolayers were infected with wild-type or HisLLO-treated LLO-negative bacteria for 5.5 h, a time at which wild-type bacteria were observed to spread into secondary cells but not to replicate. Monolayers were fluorescently stained as described in Table 1, and the numbers of bacteria in primary cells and in secondary cells (those adjacent to primary cells) were tallied for 10 foci of each strain. Within each strain, the fraction of bacteria in secondary cells varied among the foci over a wide range. The average fractions for the two strains were statistically indistinguishable (Table 1). These data show that LLO plays no discernible role in the spreading of bacteria into secondary cells.
We examined the possibility that the spread of bacteria was mediated by 2 other membrane-active L. monocytogenes proteins, PI-PLC and PC-PLC. An LLO-, PI-PLC-, PC-PLC-negative strain that was treated with HisLLO also spread to neighboring cells with the same frequency as the wild-type strain (Table 1). Thus, the spreading was independent of all three of these membrane-active virulence factors.
Cell-to-cell spread of LLO-negative bacteria is abortive.
Cell-to-cell spread of L. monocytogenes is completed when a bacterium in a listeriopod that has spread into a neighboring cell escapes from the resulting double-membrane vacuole into the cytosol of a secondary cell (15). Bacteria in the secondary cell cytosol continue to grow, nucleate actin, and again form listeriopods. Thus, the appearance of listeriopods on secondary cells is evidence of successful cell-to-cell spread.
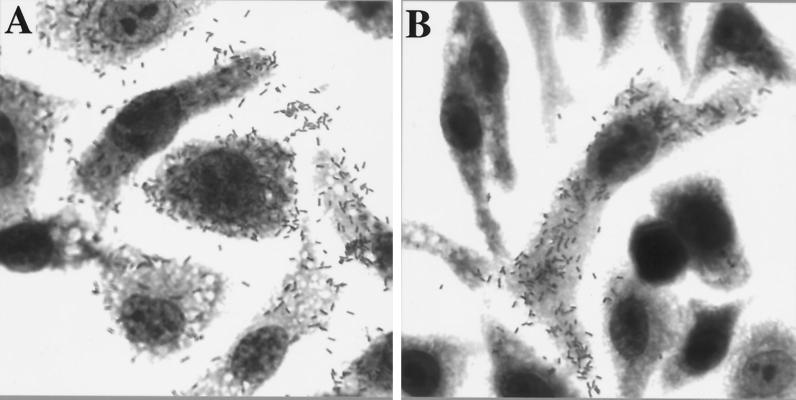
To determine whether LLO-negative bacteria could complete cell-to-cell spread, bacterial foci in J774 monolayers that were infected with HisLLO-treated LLO-negative bacteria were examined at time points up to 10 h for the presence of secondary cells bearing listeriopods. Secondary cells were taken operationally to be cells lying adjacent to primary cells. Nearly all foci resulting from growth of LLO-negative bacteria consisted of a single, central primary cell that was ringed by several secondary cells (shown for an 8-h infection in Fig. 2B). The primary cell had numerous bacteria dispersed in the cytosol and in listeriopods, while the secondary cells had few bacteria, and these were often clustered. Secondary cells had no listeriopods. It was concluded that LLO-negative bacteria are not able to complete cell-to-cell spread.
FIG. 2.
Cell-to-cell spread of wild-type and LLO-negative bacteria. J774 cells were infected for 8 h with wild-type (A) or HisLLO-treated LLO-negative (B) bacteria.
To quantitate this observation, secondary cells bearing listeriopods were counted in J774 monolayers infected for 9 h with wild-type or HisLLO-treated LLO-negative bacteria. Wild-type bacteria had spread through the monolayer so extensively that nearly all cells bore listeriopods and primary and secondary cells could not be distinguished (shown for an 8-h time point in Fig. 2A). In contrast, of 100 foci caused by LLO-negative bacteria, only four contained more than one cell with listeriopods. In these four cases, two central cells bore listeriopods, and these central cells contained comparable numbers of bacteria that together equaled the number of bacteria in a typical primary cell. Thus, these cases were consistent with a primary cell having divided after the replication of an escaped bacterium. An alternate possibility is that a rare LLO-negative bacterium may be able to escape into the cytosol of a secondary cell.
Cell-to-cell spread is abortive because LLO-negative bacteria are trapped in double-membrane vacuoles.
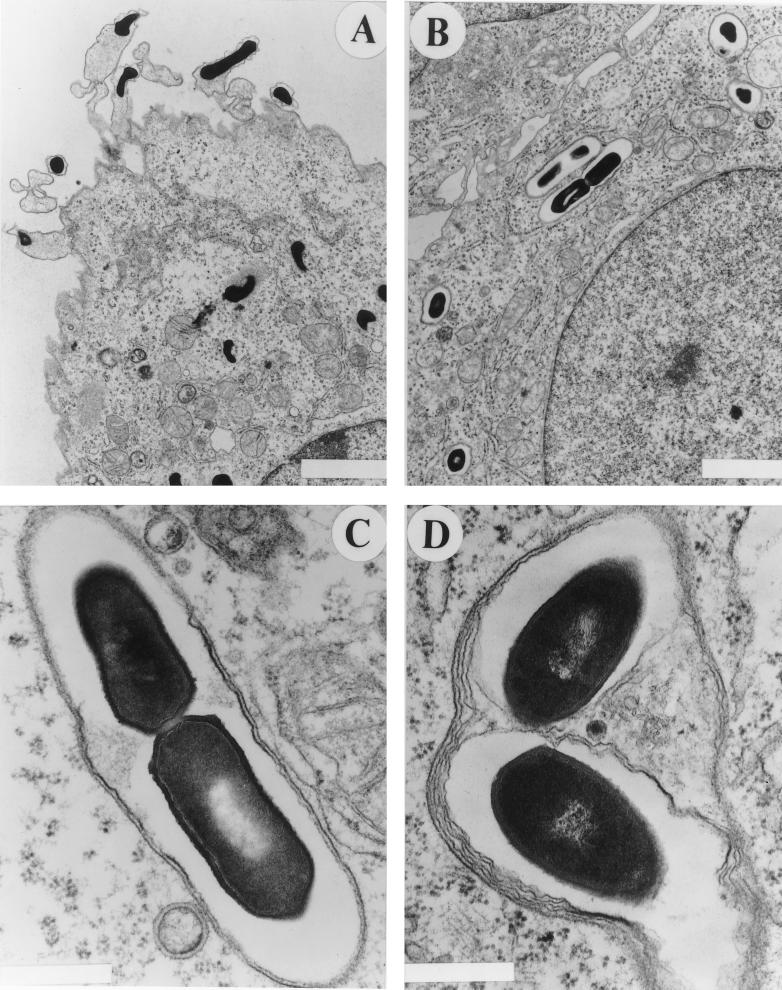
To visualize bacteria that had spread into secondary cells, J774 monolayers were infected for 8 h with HisLLO-treated LLO-negative bacteria and were examined by transmission electron microscopy as previously described (14). Primary cells were identified by the presence of free replicating bacteria in their cytosol (Fig. 3A), and then bacteria located in adjacent cells were examined (Fig. 3B). Bacteria in adjacent cells were surrounded by 2 membranes (Fig. 3C). This observation established that LLO-negative bacteria cannot complete cell-to-cell spread because they are trapped in spreading vacuoles. The result indicates that LLO is needed for lysis of both layers of the double-membrane vacuole.
FIG. 3.
Electron microscopy of LLO-negative bacteria 8 h after infection. J774 cells were infected for 8 h with HisLLO-treated LLO-negative bacteria and were prepared for electron microscopy as previously described (14). (A and B) Low magnification. (A) Primary infected cell with bacteria free in the cytosol; (B) secondary cell with bacteria in double-membrane vacuoles. Size bars = 2 μm. (C and D) High magnification. (C) LLO-negative bacterium in a double-membrane vacuole; (D) LLO-, PlcA-, PlcB-negative bacterium in a multiple-membrane vacuole. Size bars = 0.5 μm. A minority of both double- and multiple-membrane vacuoles contained more than one bacterium. Their appearance suggested that they originated from separate listeriopods that had spread together into the same secondary vacuole, or that had spread into separate secondary vacuoles that subsequently fused. It is also possible that some bacterial growth can occur in host vacuoles. The maximum number of bacteria seen within a vacuole was two.
Vacuoles formed during the spread of LLO-, PI-PLC-, PC-PLC-negative bacteria were also examined. Unexpectedly, many of these vacuoles had multiple membrane layers reminiscent of autophagocytic vesicles (13) (Fig. 3D). Spreading vacuoles of LLO-, PI-PLC-negative and LLO-, PC-PLC-negative double mutant strains also were examined. Multilayered vacuoles surrounded these strains as well (not shown). The multilayered vacuoles were never observed in infections with HisLLO-treated LLO-negative bacteria.
These results show that LLO is required for L. monocytogenes to complete the second step in cell-to-cell spread, escape from the double-membrane vacuole. Thus, LLO mediates the exit of this intracellular pathogen from both vacuolar compartments that are encountered in its intracellular life cycle. This finding supports work toward a detailed description of the mechanism of escape of L. monocytogenes from the spreading vacuole.
Acknowledgments
We gratefully acknowledge Pat Connelly's expert technical assistance with the electron microscopy experiments.
This work was supported by grants AI-27655 (to D. Portnoy) and HD-14474 (to L. Tilney) from the National Institutes of Health. M. Gedde was a Howard Hughes Medical Institute Postdoctoral Physician Fellow. D. Higgins was supported by a postdoctoral fellowship from The Helen Hay Whitney Foundation.
REFERENCES
- 1.Bayley H. Toxin structure: part of a hole? Curr Biol. 1997;7:R763–R767. doi: 10.1016/s0960-9822(06)00399-x. [DOI] [PubMed] [Google Scholar]
- 2.Braun L, Dramsi S, Dehoux P, Bierne H, Lindahl G, Cossart P. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol Microbiol. 1997;25:285–294. doi: 10.1046/j.1365-2958.1997.4621825.x. [DOI] [PubMed] [Google Scholar]
- 3.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabiri G A, Sanger J M, Portnoy D A, Southwick F S. Listeria monocytogenes moves rapidly through the host cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci USA. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiedler F. Biochemistry of the cell surface of Listeria strains: a locating general view. Infection. 1988;16(Suppl. 2):S92–S97. doi: 10.1007/BF01639729. [DOI] [PubMed] [Google Scholar]
- 7.Gellin B G, Broome C V. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 8.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquis H, Doshi V, Portnoy D A. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N. Y.: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 13.Seglen P O, Berg T O, Blankson H, Fengsrud M, Holen I, Stromhaug P E. Structural aspects of autophagy. In: Suzuki K, Bond J, editors. Intracellular protein catabolism. Vol. 389. New York, N. Y.: Plenum Press; 1996. pp. 103–111. [DOI] [PubMed] [Google Scholar]
- 14.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vazquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]