Abstract
Herein, we report a one-pot synthesis of styrene derivatives via a novel B(C6F5)3-catalyzed E-selective isomerization of readily accessible allyl silanes and subsequent Hiyama coupling of the versatile alkenyl silane intermediates. This one-pot, two-step approach enables access to a broad range of styrene derivatives, including those containing Lewis basic functional groups, that cannot be accessed via the previously developed B(C6F5)3-catalyzed isomerization of allyl benzenes.
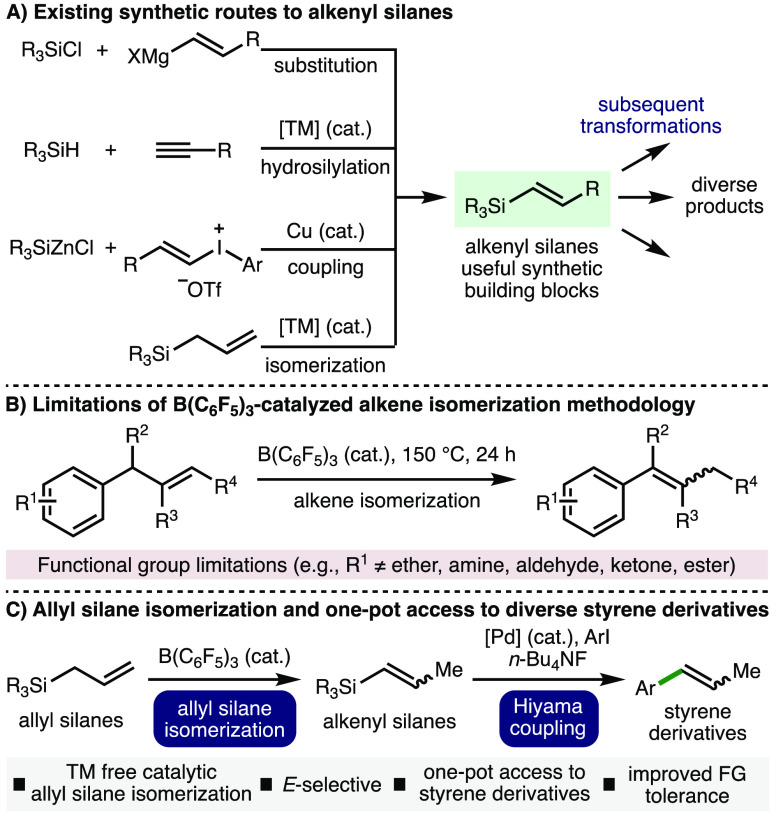
Alkenyl silanes are useful building blocks in organic synthesis, polymer chemistry, and materials science.1 They participate in a diverse array of transformations, including electrophilic substitution,2 polymerization,3 and cross-coupling reactions.4 Alkenyl silanes can be accessed by various methods, including nucleophilic substitution of chlorosilanes with alkenyl magnesium reagents,5 transition metal-catalyzed hydrosilylation of alkynes6 and allenes,7 dehydrogenative silylation of alkenes,8 and Cu-catalyzed silylation of alkenyl iodonium salts (Scheme 1A).9 An attractive alternative approach for the formation of substituted alkenyl silanes is the isomerization of allyl silanes, due to their relative ease of synthesis and commercial availability.10 A variety of catalytic approaches for the isomerization of allyl silanes to alkenyl silanes have been developed, which employ catalysts based on both precious metals (e.g., Ru, Pd, and Ir)11 and more abundant first-row transition metals (e.g., Fe, Co, and Ni).12
Scheme 1. Context.
The development and application of main group catalysts in synthesis continues to be an active area of investigation in organic chemistry.13 This can be attributed to the desire to understand further the reactivity and capabilities of main group catalysts, combined with the increasing drive to reduce the dependence upon finite precious metals.14 Among main group catalysts, fluorinated triarylboranes such as commercially available B(C6F5)3 have garnered significant attention.15 These species have been employed as catalysts in a variety of transformations, including hydrosilylation, frustrated Lewis pair (transfer) hydrogenation, and various C–C bond-forming reactions.16 As part of our ongoing interest in the use of boranes as catalysts in synthesis,17 we recently reported the B(C6F5)3-catalyzed E-selective isomerization of alkenes (Scheme 1B).18 Although the method could be applied across a broad range of alkene-containing substrates, the high Lewis acidity of B(C6F5)3 resulted in a number of limitations with respect to the incorporation of Lewis basic functional groups (e.g., ethers, amines, aldehydes, ketones, and esters). To address these limitations, herein we report the B(C6F5)3-catalyzed isomerization of allyl silanes to alkenyl silanes, which undergo Hiyama coupling in a one-pot, two-step process to access a more diverse array of valuable substituted styrene derivatives (Scheme 1C). Examples of biologically active molecules that contain substituted styrene motifs include anethole (food additive), isoeugenol (fragrance), and licarin A (antimycobacterial).
To commence our studies, the B(C6F5)3-catalyzed isomerization of allyl triphenyl silane 1 to form triphenyl(prop-1-en-1-yl)silane 2 was selected for reaction optimization (Table 1).19 Employing commercially available B(C6F5)3 (5 mol %) as a catalyst and toluene ([1] = 0.25 M) as a solvent in a sealed tube at 140 °C for 48 h under argon gave 2 in 85% NMR yield (80% isolated yield) with high selectivity for the E-alkene isomer (97:3 E:Z) (entry 1). No alkene isomerization was observed in the absence of B(C6F5)3 (entry 2). Decreasing the reaction time or the reaction temperature each reduced the NMR yield of 2 (entries 3 and 4), as did variation of the concentration and solvent (entries 5–8). Decreasing the catalyst loading to 2.5 mol % resulted in only 21% conversion to 2 (entry 9).
Table 1. Reaction Optimizationa.
| entry | variation from “standard” conditions | yieldb (%) | E:Z ratiob |
|---|---|---|---|
| 1 | none | 85 (80) | 97:3 |
| 2 | no B(C6F5)3 | <2 | – |
| 3 | reaction time of 24 h | 72 | >98:<2 |
| 4 | 130 °C | 6 | 84:16 |
| 5 | [1] = 0.1 M | 65 | 98:2 |
| 6 | [1] = 0.5 M | 72 | 94:6 |
| 7 | chlorobenzene as the solvent | 76 | 96:4 |
| 8 | xylenes as the solvent | 30 | >98:<2 |
| 9 | B(C6F5)3 (2.5 mol %) | 21 | >98:<2 |
Reactions performed using 0.1 mmol of 1.
Determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethylbenzene as the internal standard. Isolated yield in parentheses.
With the optimized reaction conditions in hand, the scope of the B(C6F5)3-catalyzed allyl isomerization process was explored (Scheme 2). It was found that various aryl/alkyl substitutions on silicon were tolerated, which provided access to the corresponding internal alkene products in high yields (≤91%), and with good selectivity for the E-alkene isomer (products 2–13). Commonly employed silicon-based protecting groups could be incorporated into the products, including tert-butyldiphenylsilyl (TBDPS) 3, tert-butyldimethylsilyl (TBS) 8, triisopropylsilyl (TIPS) 12, and trimethylsilyl (TMS) 13. The reaction performed well on a 2 mmol scale, which gave 7 in 80% yield and with 93:7 E:Z selectivity. It was found that allyltriphenylgermane and allyltriethylgermane also underwent B(C6F5)3-catalyzed isomerization to form products 14 and 15 in 58% and 45% yields, respectively.
Scheme 2. Scope of Allyl Silane Isomerization.
Reactions performed using 0.1 mmol of allyl silane. Yields determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethylbenzene as the internal standard.
At 130 °C.
With 2 mmol of substrate.
At 24 h.
At 72 h.
Having established the scope of the B(C6F5)3-catalyzed isomerization of allyl silanes, we investigated the synthetic utility of the corresponding prop-1-en-1-yl silane products. Of particular interest was the Pd-catalyzed Hiyama coupling between prop-1-en-1-yl silanes and aryl iodides,4 as it was envisaged that this strategy would generate substituted styrene derivatives that could not be accessed using our previously developed B(C6F5)3-catalyzed isomerization of allyl benzenes.18 Employing benzyldimethylsilane 7,20 alkene isomerization was followed by Hiyama coupling via the addition of Pd(dba)2 (4 mol %), n-Bu4NF (2 equiv), and the desired aryl iodide (1 equiv) to the same reaction vessel, which was heated at 40 °C for 24 h under N2 (Scheme 3A). This one-pot, two-step process provided access to a broad range of substituted styrene derivatives in good yields with high E selectivity, bearing various functional groups, including ethers, amines, acetals, esters, nitriles, ketones, and sulfonamides (Scheme 3B, products 16–27). Alkenyl-substituted heterocycles, including indole, benzofuran, and pyridine, were also formed in good yields with high E selectivity (products 28–31). A majority of these products could not be accessed using our previously developed B(C6F5)3-catalyzed alkene isomerization methodology due to several competing processes, including the coordination of B(C6F5)3 to basic functionalities (e.g., pyridines), B(C6F5)3-mediated C–H hydride abstraction (e.g., benzylic and α-amino positions), and undesired reduction of susceptible functional groups (e.g., ketones). As an alternative demonstration of alkenyl silane derivatization, the stereospecific epoxidation of prop-1-en-1-yl silane 2 with mCPBA gave the corresponding trans-epoxide 32 as a single observable isomer in 65% isolated yield (Scheme 3C).
Scheme 3. One-Pot Isomerization–Hiyama Coupling.
Reactions performed using 0.1 mmol of 7. Yields determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethylbenzene as the internal standard.
At 50 °C.
Isolated yield.
Our investigation of the B(C6F5)3-catalyzed isomerization of allyl benzenes involved a detailed synthetic and computational mechanistic investigation,18 which revealed that multiple competing reaction mechanisms may be operative, namely, (i) hydride abstraction, (ii) 1,2-hydride shift, and (iii) 1,3-hydride shift. By analogy, it is proposed that the isomerization of allyl silanes may proceed via the same pathways (Scheme 4A). With a view to providing supporting evidence for plausible reaction intermediates, the B(C6F5)3-catalyzed isomerization of allyltriisopropyl silane 33 was performed in the presence of 1,2-dimethylindole 34 (1.2 equiv), which gave C(3)-alkylated indole 35 in 13% NMR yield alongside alkenyl silane 12 (Scheme 4B). This product indicates the presence of a β-silyl cation intermediate, formed via alkene activation by B(C6F5)3 (cf., proposed 1,2-hydride shift mechanism), which in this case is intercepted by nucleophilic indole 34.
Scheme 4. Reaction Mechanism.
In conclusion, a one-pot synthesis of styrene derivatives has been developed via a novel B(C6F5)3-catalyzed E-selective isomerization of readily accessible allyl silanes and subsequent Hiyama coupling of the versatile alkenyl silane intermediates. This one-pot, two-step approach enables access to a broad range of styrene derivatives, including those containing Lewis basic functional groups that cannot be accessed via the previously developed B(C6F5)3-catalyzed isomerization of allyl benzenes. Ongoing work in our laboratory is focused on further applications of Lewis acid triarylborane catalysts in organic synthesis.
Acknowledgments
The authors gratefully acknowledge the School of Chemistry, Cardiff University, for generous support and the Schlumberger Foundation for a Faculty of the Future Fellowship (B.A.K.).
The data underlying this study are openly available in the Cardiff University data catalogue at 10.17035/d.2022.0232368584.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c03584.
Optimization data, experimental procedures, characterization of new compounds, and spectral data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Luh T.-Y.; Liu S.-T.. Synthetic Applications of Allylsilanes and Vinylsilanes. In The Chemistry of Organic Silicon Compounds, Vol. 2; Rappoport Z., Apeloig Y., Eds.; Wiley: Weinheim, Germany, 1998; pp 1793–1868. [Google Scholar]
- Fleming I.; Dunoguès J.; Smithers R. The Electrophilic Substitution of Allylsilances and Vinylsilanes. Org. React. 1989, 37, 57–575. 10.1002/0471264180.or037.02. [DOI] [Google Scholar]
- Itoh M.; Iwata K.; Kobayashi M.; Takeuchi R.; Kabeya T. Preparations and Properties of Poly(vinylsilane)s. Macromolecules 1998, 31, 5609–5615. 10.1021/ma980488c. [DOI] [Google Scholar]
- Foubelo F.; Nájera C.; Yus M. The Hiyama Cross-Coupling Reaction: New Discoveries. Chem. Rec. 2016, 16, 2521–2533. 10.1002/tcr.201600063. [DOI] [PubMed] [Google Scholar]
- Murakami K.; Yorimitsu H.; Oshima K. Zinc-Catalyzed Nucleophilic Substitution Reaction of Chlorosilanes with Organomagnesium Reagents. J. Org. Chem. 2009, 74, 1415–1417. 10.1021/jo802433t. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Zhao X.; Yang D.; Zhang Y.; Wang B.; Qu J. Highly β(Z)-Selective Hydrosilylation of Terminal Alkynes Catalyzed by Thiolate-Bridged Dirhodium Complexes. Org. Lett. 2018, 20, 5357–5361. 10.1021/acs.orglett.8b02267. [DOI] [PubMed] [Google Scholar]; b Liang H.; Ji Y.-X.; Wang R.-H.; Zhang Z.-H.; Zhang B. Visible-Light-Initiated Manganese-Catalyzed E-Selective Hydrosilylation and Hydrogermylation of Alkynes. Org. Lett. 2019, 21, 2750–2754. 10.1021/acs.orglett.9b00701. [DOI] [PubMed] [Google Scholar]; c Hu M.-Y.; He P.; Qiao T.-Z.; Sun W.; Li W.-T.; Lian J.; Li J.-H.; Zhu S.-F. Iron-Catalyzed Regiodivergent Alkyne Hydrosilylation. J. Am. Chem. Soc. 2020, 142 (39), 16894–16902. 10.1021/jacs.0c09083. [DOI] [PubMed] [Google Scholar]; d Wang Z.-L.; Zhang F.-L.; Xu J.-L.; Shan C.-C.; Zhao M.; Xu Y.-H. Copper-Catalyzed Anti-Markovnikov Hydrosilylation of Terminal Alkynes. Org. Lett. 2020, 22, 7735–7742. 10.1021/acs.orglett.0c02952. [DOI] [PubMed] [Google Scholar]
- Miller Z. D.; Li W.; Belderrain T.; Montgomery J. Regioselective Allene Hydrosilylation Catalyzed by N-Heterocyclic Carbene Complexes of Nickel and Palladium. J. Am. Chem. Soc. 2013, 135, 15282–15285. 10.1021/ja407749w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S.; Glavic M.; Stöger B.; Pittenauer E.; Podewitz M.; Veiros L. F.; Kirchner K. Manganese-Catalyzed Dehydrogenative Silylation of Alkenes Following Two Parallel Inner-Sphere Pathways. J. Am. Chem. Soc. 2021, 143, 17825–17832. 10.1021/jacs.1c09175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Oestreich M. Copper-Catalyzed Cross-Coupling of Vinyliodonium Salts and Zinc-Based Silicon Nucleophiles. Org. Lett. 2018, 20, 8061–8063. 10.1021/acs.orglett.8b03714. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see:; a Hilt G. Double Bond Isomerisation and Migration—New Playgrounds for Transition Metal-Catalysis. ChemCatChem. 2014, 6, 2484–2485. 10.1002/cctc.201402341. [DOI] [Google Scholar]; b Larionov E.; Li H.; Mazet C. Well-defined transition metal hydrides in catalytic isomerizations. Chem. Commun. 2014, 50, 9816–9826. 10.1039/C4CC02399D. [DOI] [PubMed] [Google Scholar]; c Molloy J. J.; Morack T.; Gilmour R. Positional and Geometrical Isomerisation of Alkenes: The Pinnacle of Atom Economy. Angew.Chem. Int. Ed. 2019, 58, 13654–13664. 10.1002/anie.201906124. [DOI] [PubMed] [Google Scholar]; d Liu X.; Li B.; Liu Q. Base-Metal-Catalyzed Olefin Isomerization Reactions. Synthesis 2019, 51, 1293–1310. 10.1055/s-0037-1612014. [DOI] [Google Scholar]; e Massad I.; Marek I. Alkene Isomerization through Allylmetals as a Strategic Tool in Stereoselective Synthesis. ACS Catal. 2020, 10, 5793–5804. 10.1021/acscatal.0c01174. [DOI] [Google Scholar]
- For selected examples, see:; a Urata H.; Suzuki H.; Moro-oka Y.; Ikawa T. Catalytic Conversion of Allylic Esters to Corresponding Allylic Silanes with Hexamethyldisilane and Palladium(0) or Rhodium(I) Complexes. Bull. Chem. Soc. Jpn. 1984, 57, 607–608. 10.1246/bcsj.57.607. [DOI] [Google Scholar]; b Manzini S.; Nelson D. J.; Nolan S. P. A Highly Active Cationic Ruthenium Complex for Alkene Isomerisation: A Catalyst for the Synthesis of High Value Molecules. ChemCatChem. 2013, 5, 2848–2851. 10.1002/cctc.201300396. [DOI] [Google Scholar]; c Becica J.; Glaze O. D.; Wozniak D. I.; Dobereiner G. W. Selective Isomerization of Terminal Alkenes to (Z)-2-Alkenes Catalyzed by an Air-Stable Molybdenum(0) Complex. Organometallic 2018, 37, 482–490. 10.1021/acs.organomet.7b00914. [DOI] [Google Scholar]; d Poitiers N. E.; Giarrana L.; Huch V.; Zimmer M.; Scheschkewitz D. Exohedral Functionalization vs. Core Expansion of Siliconoids with Group 9 Metals: Catalytic Activity in Alkene Isomerization. Chem. Sci. 2020, 11, 7782–7788. 10.1039/D0SC02861D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples, see:; a Kobayashi T.; Yorimitsu H.; Oshima K. Cobalt-Catalyzed Isomerization of 1-Alkenes to (E)-2-Alkenes with Dimethylphenylsilylmethylmagnesium Chloride and Its Application to the Stereoselective Synthesis of (E)-Alkenylsilanes. Chem. Asian. J. 2009, 4, 1078–1083. 10.1002/asia.200900111. [DOI] [PubMed] [Google Scholar]; b Chen C.; Dugan t. R.; Brennessel W. W.; Weix D. J.; Holland P. L. Z-Selective Alkene Isomerization by High-Spin Cobalt(II) Complexes. J. Am. Chem. Soc. 2014, 136, 945–955. 10.1021/ja408238n. [DOI] [PubMed] [Google Scholar]; c Basu D.; Gilbert-Wilson R.; Gray D. L.; Rauchfuss T. B.; Dash A. K. Fe and Co Complexes of Rigidly Planar Phosphino-Quinoline- Pyridine Ligands for Catalytic Hydrosilylation and Dehydrogenative Silylation. Organometallics 2018, 37, 2760–2768. 10.1021/acs.organomet.8b00416. [DOI] [Google Scholar]; d Kapat A.; Sperger T.; Guven S.; Schoenebeck F. E-Olefins Through Intramolecular Radical Relocation. Science 2019, 363, 391–396. 10.1126/science.aav1610. [DOI] [PubMed] [Google Scholar]; e Garhwal S.; Kaushansky A.; Fridman N.; de Ruiter G. Part per Million Levels of an Anionic Iron Hydride Complex Catalyzes Selective Alkene Isomerization via Two-State Reactivity. Chem. Catal. 2021, 1, 631–647. 10.1016/j.checat.2021.05.002. [DOI] [Google Scholar]; f Kawamura K. E.; Chang A. S.; Martin D. J.; Smith H. M.; Morris P. T.; Cook A. K. Modular Ni(0)/Silane Catalytic System for the Isomerization of Alkenes. Organometallics 2022, 41, 486–496. 10.1021/acs.organomet.2c00010. [DOI] [Google Scholar]
- For selected reviews, see:; a Power P. P. Main-group elements as transition metals. Nature 2010, 463, 171–177. 10.1038/nature08634. [DOI] [PubMed] [Google Scholar]; b Melen R. L. Frontiers in Molecular p-Block Chemistry: From structure to reactivity. Science 2019, 363, 479–484. 10.1126/science.aau5105. [DOI] [PubMed] [Google Scholar]
- Aldridge S.; Jones C. Modern Main Group Chemistry. Chem. Soc. Rev. 2016, 45, 763–764. 10.1039/C6CS90014C. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see:; a Erker G. Tris(pentafluorophenyl)borane: A Special Boron Lewis Acid for Special Reactions. Dalton Trans. 2005, 1883–1890. 10.1039/b503688g. [DOI] [PubMed] [Google Scholar]; b Patrick E. A.; Piers W. E. Twenty-Five years of Bis-Pentafluorophenyl Borane: A Versatile Reagent for Catalyst and Materials Synthesis. Chem. Commun. 2020, 56, 841–853. 10.1039/C9CC08338C. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see:; a Oestreich M. Transfer Hydrosilylation. Angew. Chem., Int. Ed. 2016, 55, 494–499. 10.1002/anie.201508879. [DOI] [PubMed] [Google Scholar]; b Lawson J. R.; Melen R. L. Tris(pentafluorophenyl)borane and Beyond: Modern Advances in Borylation Chemistry. Inorg. Chem. 2017, 56, 8627–8643. 10.1021/acs.inorgchem.6b02911. [DOI] [PubMed] [Google Scholar]; c Keess S.; Oestreich M. Cyclohexa-1,4-dienes in Transition-Metal-Free Ionic Transfer Processes. Chem. Sci. 2017, 8, 4688–4695. 10.1039/C7SC01657C. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Stephan D. W. Catalysis, FLPs, and Beyond. Chem. 2020, 6, 1520–1526. 10.1016/j.chempr.2020.05.007. [DOI] [Google Scholar]; e Basak S.; Winfrey L.; Kustiana B. A.; Melen R. L.; Morrill L. C.; Pulis A. P. Electron Deficient Borane-Mediated Hydride Abstraction in Amines: Stoichiometric and Catalytic Processes. Chem. Soc. Rev. 2021, 50, 3720–3737. 10.1039/D0CS00531B. [DOI] [PubMed] [Google Scholar]
- a Khan I.; Manzotti M.; Tizzard G. J.; Coles S. J.; Melen R. L.; Morrill L. C. Frustrated Lewis Pair (FLP)-Catalyzed Hydrogenation of Aza-Morita–Baylis–Hillman Adducts and Sequential Organo-FLP Catalysis. ACS Catal. 2017, 7, 7748–7752. 10.1021/acscatal.7b03077. [DOI] [Google Scholar]; b Khan I.; Reed-Berendt B. G.; Melen R. L.; Morrill L. C. FLP-Catalyzed Transfer Hydrogenation of Silyl Enol Ethers. Angew. Chem., Int. Ed. 2018, 57, 12356–12359. 10.1002/anie.201808800. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Basak S.; Alvarez-Montoya A.; Winfrey L.; Melen R. L.; Morrill L. C.; Pulis A. P. B(C6F5)3-Catalyzed Direct C3 Alkylation of Indoles and Oxindoles. ACS Catal. 2020, 10, 4835–4840. 10.1021/acscatal.0c01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustiana B. A.; Elsherbeni S. A.; Linford-Wood T. G.; Melen R. L.; Grayson M. N.; Morrill L. C. B(C6F5)3-Catalyzed E-Selective Isomerization of Alkenes. Chem. - Eur. J. 2022, e202202454. 10.1002/chem.202202454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See the Supporting Information for full experimental details.
- Denmark S. E.; Tymonko S. A. Sequential Cross-Coupling of 1,4-Bissilylbutadienes: Synthesis of Unsymmetrical 1,4-Disubstituted 1,3-Butadienes. J. Am. Chem. Soc. 2005, 127, 8004–8005. 10.1021/ja0518373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.