Abstract
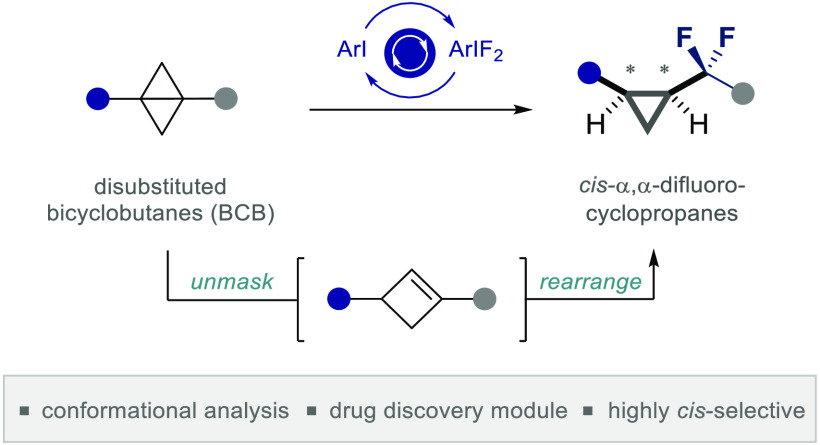
The clinical success of α,α-difluorocyclopropanes, combined with limitations in the existing synthesis portfolio, inspired the development of an operationally simple, organocatalysis-based strategy to access cis-configured derivatives with high levels of stereoselectivity (up to >20:1 cis:trans). Leveraging an I(I)/I(III)-catalysis platform in the presence of an inexpensive HF source, it has been possible to exploit disubstituted bicyclobutanes (BCBs) as masked cyclobutene equivalents for this purpose. In situ generation of this strained alkene, enabled by Brønsted acid activation, facilitates an unprecedented 4 → 3 fluorinative ring contraction, to furnish cis-α,α-difluorinated cyclopropanes in a highly stereoselective manner (up to 88% yield). Mechanistic studies are disclosed together with conformational analysis (X-ray crystallography and NMR) to validate cis-α,α-difluorocyclopropanes as isosteres of the 1,4-dicarbonyl moiety. Given the importance of this unit in biology and the foundational no → π* interactions that manifest themselves in this conformation (e.g., collagen), it is envisaged that the title motif will find application in focused molecular design.
Keywords: cyclopropanes, fluorination, hypervalent iodine, isosteres, stereoelectronic effects
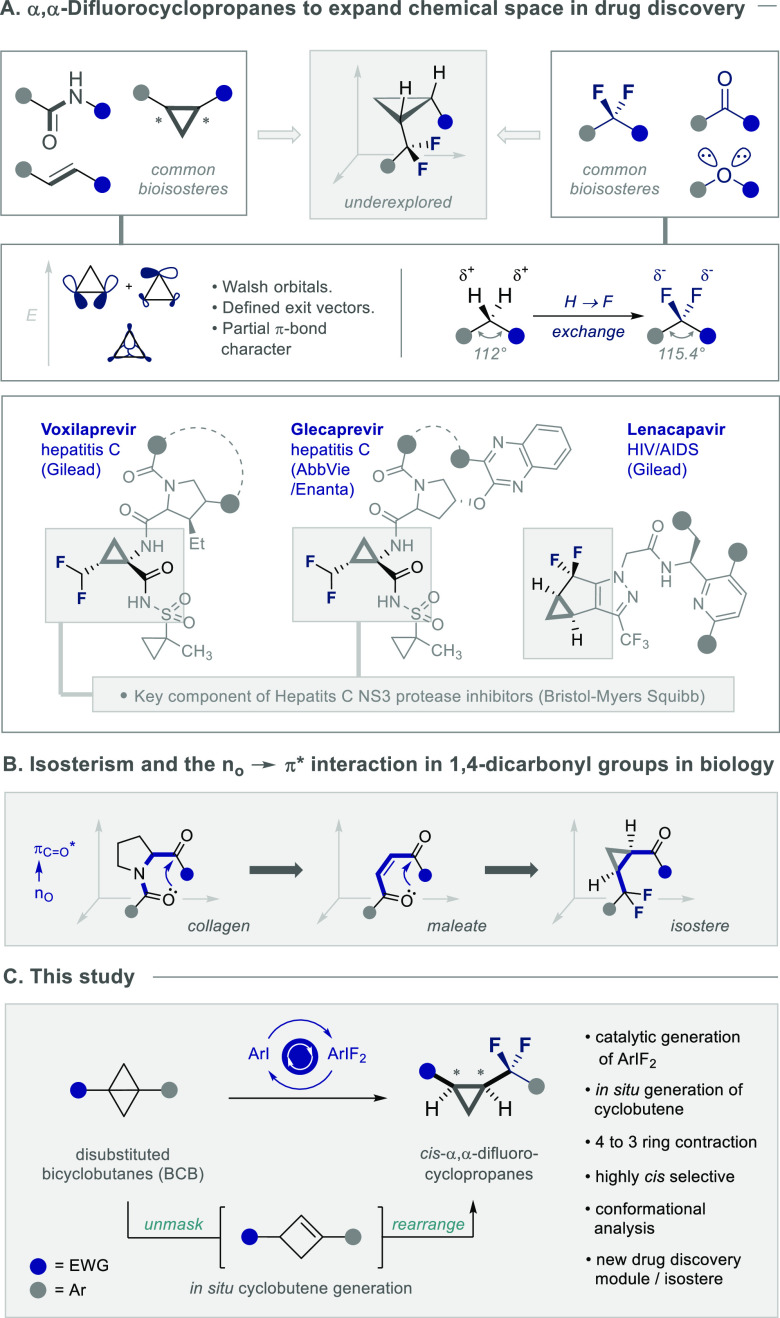
The emergent success of cyclopropanes in advancing lead drug candidates to the clinic is a compelling incentive to further explore this area of chemical space.1 First validated as a pharmacophore over 50 years ago,2 the inclusion of this carbocycle in marketed pharmaceuticals continues to follow a steep trajectory.3 Contracted C(sp3)–H bonds and deviation from an idealized tetrahedral geometry reveal a structural dichotomy that traverses the saturated/unsaturated functional group continuum, and this ultimately manifests itself in the venerable Walsh bonding description (Scheme 1A).4,5 The rigidity of the carbocycle mitigates conformational isomerism, ensuring that substrate exit vectors are well-defined for bioisostere design,6,7 and this provides a unique platform to tailor physicochemistry in the context of focused molecular design.8 This is exemplified by the “Janus-face” (syn)-fluorinated cyclopropanes developed by O’Hagan and co-workers to modulate log P values9 and the success of fluorinated cyclopropanes in medicinal chemistry and peptidomimetics in a broader sense.10 Motivated by the popularity of cyclopropyl isosteres, coupled with the popularity of fluorine in contemporary medicinal chemistry to tailor ADMET properties,11 attention was directed to an underexplored constitutional isomer series,12 the α,α-difluorocyclopropanes (Scheme 1A, top). This motif has gained distinction in recent years due to its successful deployment in the development of next-generation antivirals,13 which include voxilaprevir (Vosevi)13a and glecaprivir (Mavyret)13b for the treatment of chronic hepatitis C (Scheme 1, top), the newly approved lenacapivir (Sunlenca) for HIV/AIDS,13c and Bristol-Myers Squibb’s potent inhibitors of Hepatits C NS3 protease.13d
Scheme 1. (A) Evolution of α,α-Difluorocyclopropanes as New Drug Discovery Modules; (B) n → π* Interaction in Biology and Isostere Design.; (C) Catalysis-Based Strategy to Enable the Direct Conversion of Bicyclobutanes to cis-α,α-Difluorocyclopropanes Enabled by I(I)/I(III) Organocatalysis.
Given their highly preorganized topology, relaxation of the internal C–C–C angle14 and oxidative resilience of the gem-difluoromethylene group,15 a strategy to access the cis-configured products was deemed to be particularly appealing: this would generate conformationally restricted isosteres of 1,4-dicarbonyl groups that are ubiquitous in biology (Scheme 1B).16 Importantly, a structural analysis of the C(sp2)=O → C(sp3)F2 replacement would provide a platform from which to interrogate the foundational no → π* interaction17 that underpins collagen structure18 and manifests itself in maleate to fumarate isomerization.19
It was envisaged that the target scaffold might derive from a fluorinative ring contraction of aryl-substituted cyclobutene derivatives under the auspices of I(I)/I(III) catalysis (Scheme 1C). Confidence in this strategy stemmed from a seminal report by Hara and Yoneda describing the synthesis of α,α-difluorocyclopentanes from cyclohexenes upon treatment with stoichiometric ArIF2 species and HF sources.20 Furthermore, easily accessible disubstituted bicyclobutanes (BCBs)21 were explored as cyclobutene equivalents that would be unmasked under the Brønsted acidic reaction conditions.22 If successful, the strategy would expand upon the existing routes23,24 and introduce direct direct difluorocyclopropane formation to the ever-growing portfolio of transformations enabled by I(I)/I(III) catalysis.25−28
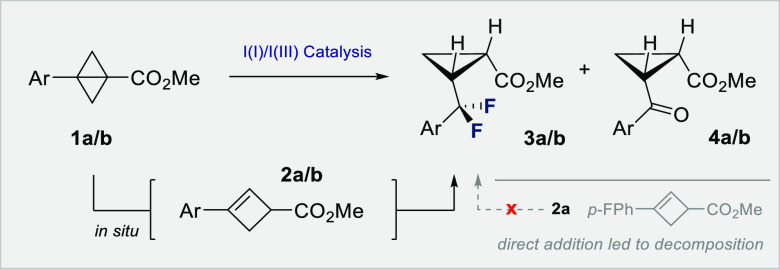
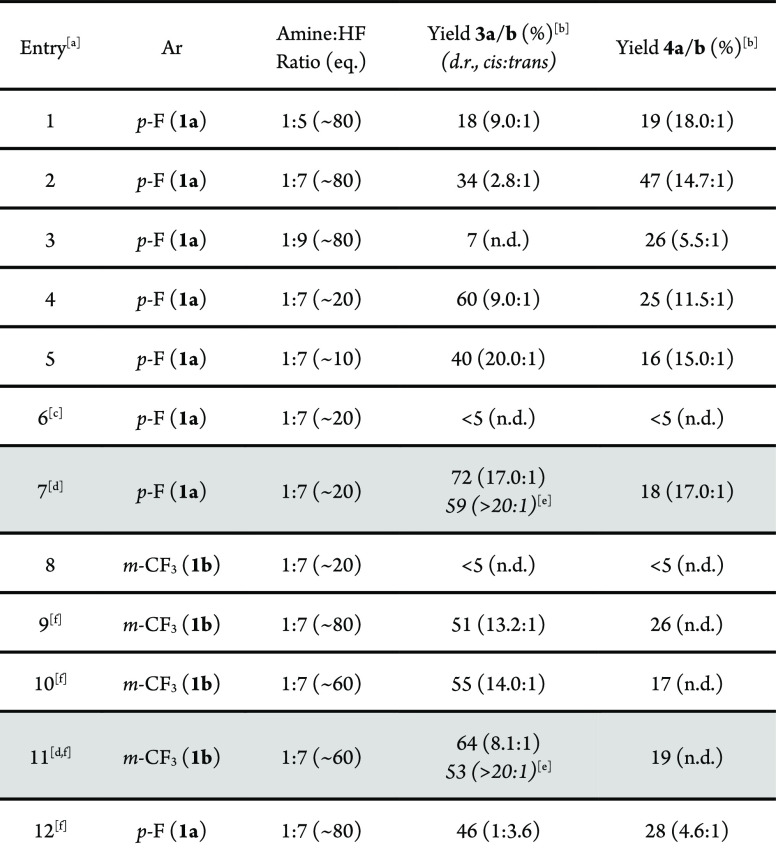
To validate the working hypothesis delineated in Scheme 1 (bottom), bicyclobutanes 1a,b were exposed to oxidative fluorination conditions with p-TolI functioning as an inexpensive organocatalyst, Selectfluor as the terminal oxidant, amine·HF complexes as fluoride reservoirs, and DCE as the reaction medium (Table 1). It is pertinent to note that exposing cyclobutene 2a directly to the reaction conditions led to rapid decomposition, rendering this approach impractical.29 However, bicyclobutane reagents30 proved to be compatible with the reaction conditions, and their propensity to rapidly isomerize to the corresponding cyclobutene under acidic conditions22 rendered them ideally suited as masked reagents. When substrate 1a (p-F-Ph) was exposed to 20 mol % of the catalyst in the presence of an amine·HF complex (ratio 1:5), it was possible to generate the desired product 3a (dr 9.0:1 cis:trans, Table 1, entry 1), albeit with comparable quantities of the 1,4-diketone 4a (dr 18.0:1 cis:trans). Variation in the amine:HF ratio revealed 1:7 to be optimal, and to suppress the competing hydrolysis (entries 1–4), the water content was reduced by lowering the stoichiometry to 20 equiv. (dr 9.0:1, Table 1, entry 4). Further reduction to 10 equiv. improved the dr but at the expense of catalysis efficiency (entry 5). Reactions performed in the absence of p-TolI were not productive, thereby supporting the involvement of an I(I)/I(III) cycle (entry 6). Moreover, increasing the scale to 0.5 mmol generated 3a in 72% yield (dr 17.0:1) (Table 1, entry 7). In the case of the more electron deficient substrate 1b (m-CF3-Ph), direct translation of these optimized conditions was not productive (entry 8). However, elevated temperatures and amounts of amine·HF (entries 9–11) allowed the target cis-α,α-difluorocyclopropane to be formed in 64% yield (dr 8.1:1). In a reversal of circumstances, exposure of bicyclobutane 1a (p-F-Ph) to these conditions resulted in an inversion of diastereoselectivty to favor the trans product (Table 1, entry 12). This comparative optimization process proved valuable in identifying variables that allow the product distribution and diastereoselectivity to be regulated.
Table 1. Reaction Optimizationa.
Standard reaction conditions: 1 (0.20 mmol), p-TolI (20 mol %), Selectfluor (0.30 mmol), Py·HF (4–16 mmol), Et3N (0.22–1.50 mmol), DCE (0.50 mL), 24 h, room temperature.
Yield and dr determined by 19F NMR with α,α,α-trifluorotoluene as an internal standard.
p-TolI was excluded from the reaction mixture.
0.50 mmol scale.
Isolated yield.
Heated to 50 °C.
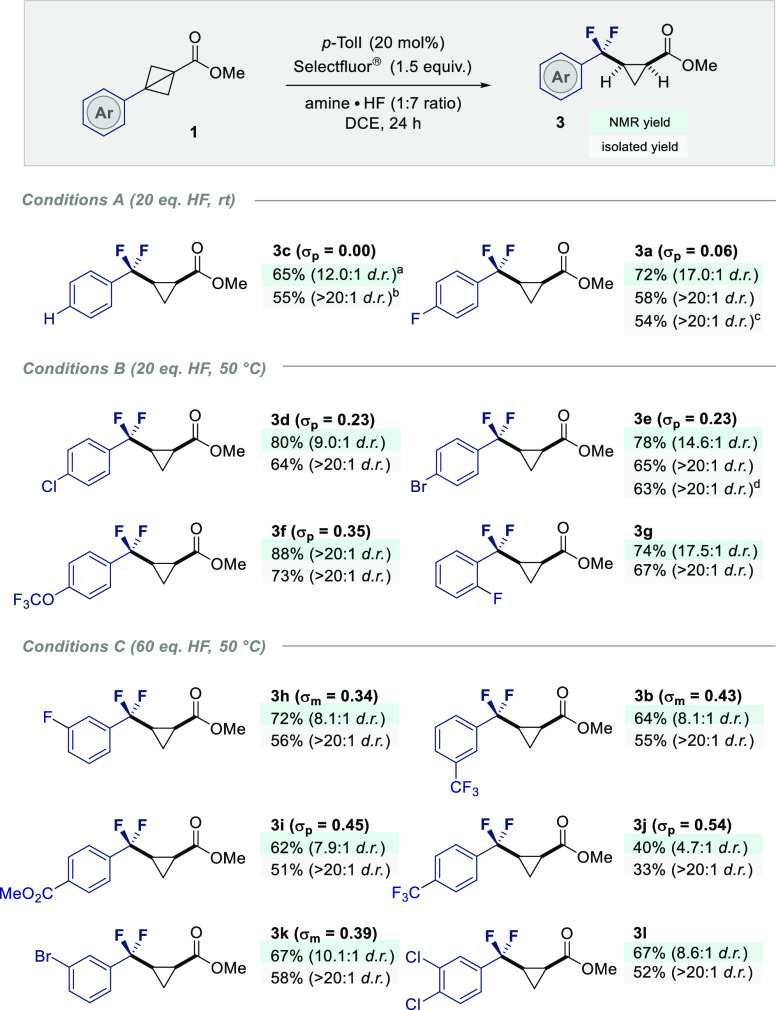
To establish the scope and limitations of the transformation, a series of BCBs with electronically modulated aryl rings were investigated (Scheme 2). Guided by the findings summarized in Table 1, three sets of reaction conditions were employed, varying only the temperature and equivalents of amine·HF complex. As a convenient method to categorize this substrate set, the Hammett σ value of the aryl substituent was used. This revealed a clear trend linking the increased HF/temperature with the electronic nature of the ring (vide infra).
Scheme 2. Substrate Scope of the Difluorinative Cyclopropanation of BCBs by I(I)/I(III) Catalysis.
Green box: combined yield of both cis and trans isomers and dr determined by 19F NMR with α,α,α-trifluorotoluene as an internal standard.
Gray box: isolated yield following column chromatography, average of 2 runs, 0.50 mmol scale.
Reaction performed on a 4.00 mmol scale.
Reaction performed on a 3.75 mmol scale.
Whereas conditions A were sufficient to process the p-H and p-F substrates to products 3c and 3a, respectively, (up to 72%, 17:1 cis:trans), the conversion of BCBs 1d–g to α,α-difluorocyclopropanes 3d–g required an increased reaction temperature of 50 °C (up to 88%, >20:1 cis:trans). Although challenging, it was possible to induce the fluorinative skeletal rearrangement of highly electron deficient substrates, to generate products 3b,h–l (up to 72%, up to 10.1:1 cis:trans) by increasing the amounts of HF. In all cases, product isolation proved facile by column chromatography, enabling the target cis-α,α-difluorocyclopropanes to be isolated in >20:1 dr.
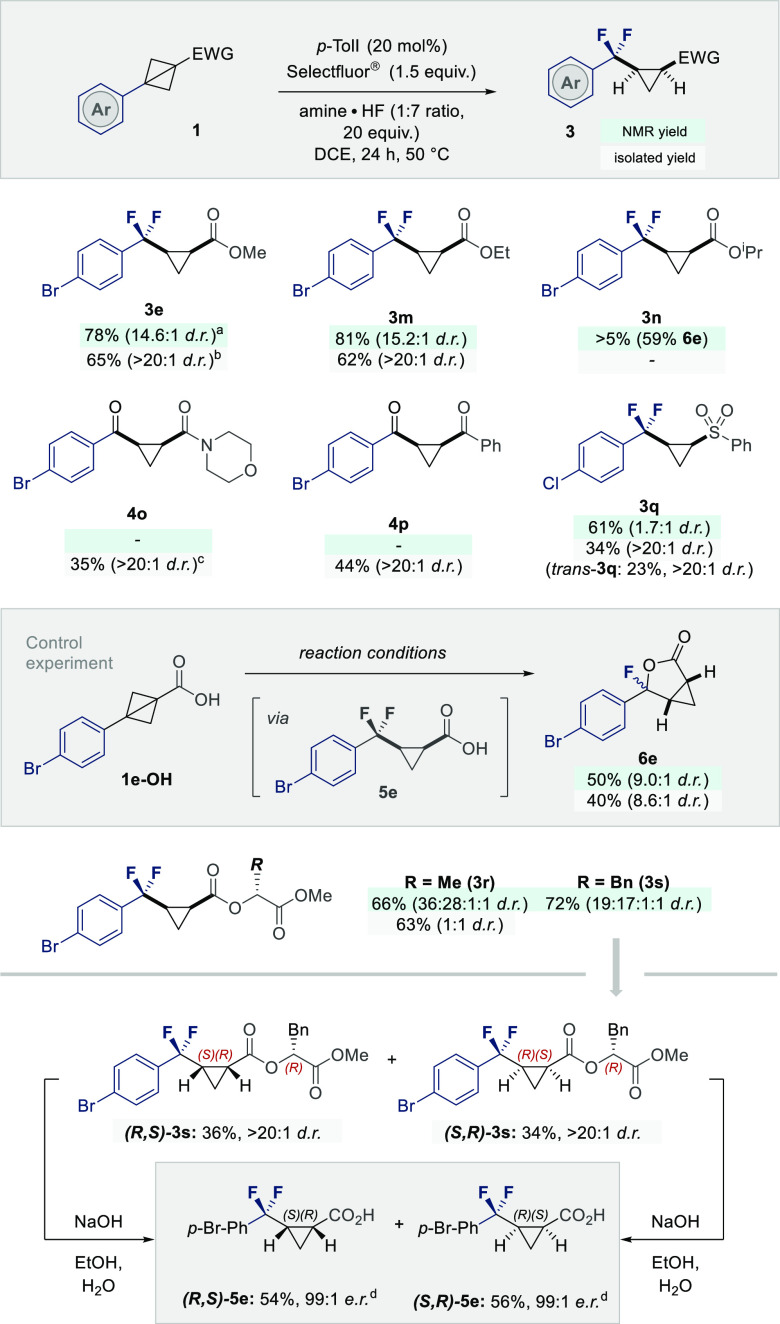
To further expand the reaction scope, the impact of modifying the pendant methyl ester was investigated (Scheme 3, top). Whereas the methyl and ethyl derivatives were smoothly processed to 3e,m (up to 81%, dr 15.2:1), the iPr ester (1n) hydrolyzed under the reaction conditions, furnishing the bicyclic lactone 6e as the major product (59%). As a control experiment, the carboxylic acid 1e-OH and intermediate 5e were independently exposed to the reaction conditions and this led to comparable lactone formation (please see the Supporting Information for full details). Intriguingly, replacement of the ester with an amide or a ketone led almost exclusively to 1,4-ketones 4o,p (35% and 44% isolated yields, respectively). In both cases, traces of the expected difluorocyclopropane were visible in the 19F NMR spectra, demonstrating postreaction hydrolysis of the geminal CF2 group. Gratifyingly, the process proved to be compatible with sulfones, enabling 3q to be forged in 61% yield. To access enantiomerically pure materials via this strategy, the bicyclobutanes 1r,s were prepared in which the methyl ester was replaced by an auxiliary. Both substrates were compatible with the reaction conditions, enabling 3r,s to be prepared in synthetically useful yields (66% and 72%, respectively). In the case of cyclopropane 3s, a facile separation/saponification sequence afforded both enantiomers of cis-5e (er 99:1 for both enantiomers).
Scheme 3. Investigating the Impact of the Electron Withdrawing Group and the Generation of Optically Active Derivatives.
Green box: combined yield of both cis and trans isomers and dr determined by 19F NMR with α,α,α-trifluorotoluene as an internal standard.
Gray box: isolated yield following column chromatography, average of 2 runs, 0.50 mmol scale.
0.20 mmol scale.
er determined by esterification to form 3e, followed by analysis by HPLC.
To interrogate the mechanism, a series of control experiments were conducted (Scheme 4). As the reaction hinges on the in situ generation of cyclobutenes from bicyclobutanes in the presence of HF, this initial process was investigated. Exposure of bicyclobutane 1a to amine·HF, in the absence of Selectfluor or the organocatalyst, generated cyclobutene 2a and a diastereomeric mixture of cyclobutane 7a as determined by NMR analyses (Scheme 4A).
Scheme 4. (A) Identification of Cyclobutene 2 and Fluorinated Cyclobutane 7 as Intermediates within the Reaction Pathway; (B) Validating Alternative Cyclobutene Precursors; (C) Correlating Hydrolytic Stability with Hammett σp+ Parameter; (D) Correlating Aryl Group Electronics with Diastereoselectivity; (E) Proposed Reaction Mechanism.
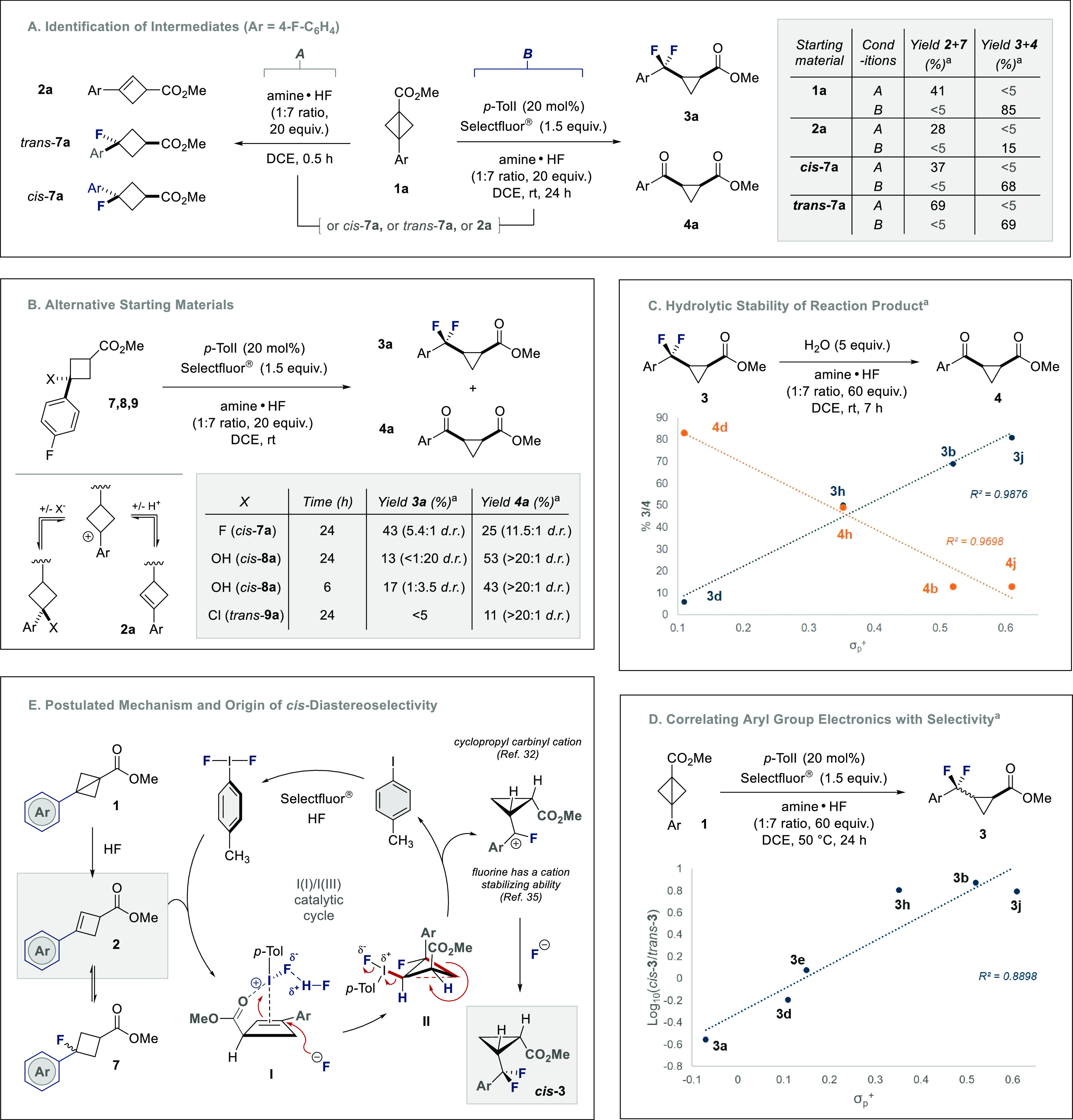
Yields determined by 1H and 19F NMR analyses.
The isolation of these compounds and subsequent exposure to the amine·HF complex afforded similar mixtures, as judged by NMR analysis, which indicates a dynamic equilibrium. The product distribution shifts to the generation of 3a and 4a upon addition of the catalyst p-TolI and Selectfluor (conditions A versus conditions B; see table in the inset).
To further support the involvement of cyclobutane 7a, additional cyclobutane derivatives were validated as cyclobutene sources (Scheme 4B). It is interesting to note that exposure of cis-8a (X = OH) to the standard reaction conditions did indeed generate the ring-contracted product (3a) in 13% yield, but substantial hydrolysis was observed even when employing reduced reaction times. This is likely due to the residual water that is generated upon elimination to generate the cyclobutene 2a. This observation and the degradation of the chlorinated derivative 9a under the reaction conditions underscore the value of bicyclobutanes for this transformation. To establish the electronic influence of the aryl substituent on the relative stabilities of the product cis-α,α-difluorocyclopropanes, representative examples were exposed to modified conditions in the presence of water (5 equiv) and absence of catalyst. A plot of the starting material and product after 7 h against the Hammett σp+ parameter confirmed a linear dependence (Scheme 4C). This demonstrates that, while diketone 4a is stable under the reaction conditions (see the Supporting Information for full details), difluorocyclopropanes (3) slowly hydrolyze over time. It is pertinent to note that HF has been leveraged to enable the Brønsted acid activation of benzylic fluorides.31 In this study, the contribution of the cyclopropyl Walsh orbitals cannot be discounted, given their contribution to the stability of the cyclopropinyl carbinyl cation.4,32 This trend was mirrored in the plot of selectivity (log10[cis-3/trans-3]) versus σp+ (Scheme 4D) and demonstrated that an erosion of diastereoselectivity occurred at higher temperatures and HF equivalents.
Collectively, these data allow a mechanistic scenario to be postulated that is contingent on in situ generation of 2; a species that exists in dynamic equilibrium with the HF adduct 7 (Scheme 4E). Simultaneously, p-TolIIIIF2 is generated via Selectfluor-mediated oxidation of p-TolI in the presence of HF.33 The dominant formation of the cis isomer suggests that the ester functionality may play a role in coordinating the iodine(III) species to the same face of the alkene. This coordinating role of the ester is well-established in the I(I)/I(III)-catalyzed fluorohydration of alkynes (the fluoro-Kucherov reaction)34 and supported by the observation that CO2Me → SO2Ph exchange erodes stereoselectivity (3q; Scheme 3). Fluorination to generate the cyclobutane II would enable a stereospecific ring contraction to liberate the catalyst and generate a cis-configured cyclopropyl carbinyl cation. In addition to being benzylic, this cation is stabilized by the cyclopropyl Walsh orbitals and the proximal fluorine atom.35 The stereochemical course of this reaction is complementary to a recent study by Aggarwal and co-workers on trans-selective cyclopropane formation enabled by treating bicyclo[1.1.0]butyl pinacol boronic esters with sterically hindered nucleophiles.30h
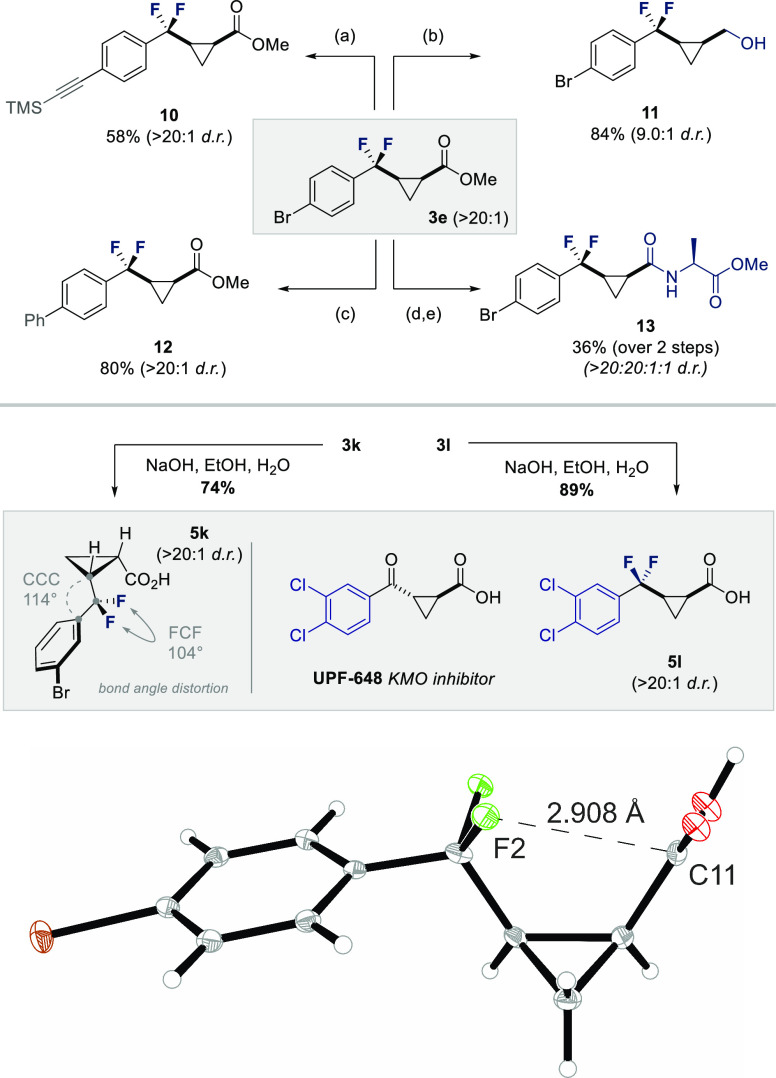
To demonstrate the synthetic utility of the difluorocyclopropyl motif, derivative 3e was subjected to standard Sonogashira and Suzuki cross-coupling conditions to afford the products 10 and 12, respectively. Modification of the ester motif was also facile, as was demonstrated by the formation of alcohol 11 and amide 13 (Scheme 5). To facilitate conformational analysis of the cis-α,α-difluorocyclopropane moiety by single-crystal diffraction, compounds 3k,l were saponified to generate the acids 5k,l, respectively (Scheme 5, bottom).36 Compound 5l is a novel isostere of UPF-648, which is a widely studied kynurenine 3-monooxygenase (KMO) inhibitor that has a range of clinical applications in translational neurology.37,38 In the case of 5k, the X-ray analysis reveals a C–C–C bond angle of 114° and a F–C–F angle of 104°: this further demonstrates the distorting impact of fluorination on an idealized tetrahedral geometry. In addition, the proximity of one fluorine atom to the carbonyl group (2.99 Å) is reminiscent of the preferential conformation adopted by 1,4-carbonyl groups in collagen (nO → πC=O*).
Scheme 5. (Top) Derivatization of Substrate 3e; (Bottom) X-ray Crystallographic Analysis of 5k and Preparation of a Fluorinated Analogue of the API, UPF-648.
Reaction conditions: (a) TMS-acetylene (1.5 equiv), Pd(PPh3)2Cl2 (10 mol %), CuI (20 mol %), iPr2NH, 80 °C, 3 h; (b) LiAlH4 (2.2 equiv), THF, 0 °C, 1 h; (c) PhB(OH)2 (1.5 equiv), Pd(PPh3)4 (5 mol %), Cs2CO3 (2.0 equiv), H2O, 1,4-dioxane, 80 °C, 3 h; (d) NaOH (6.0 equiv), MeOH, rt, 5 h; (e) H-Ala-OMe·HCl (1.1 equiv), EDC (1.1 equiv), DMAP (5 mol %), DCM, rt, 16 h. Yields and dr values refer to isolated products.
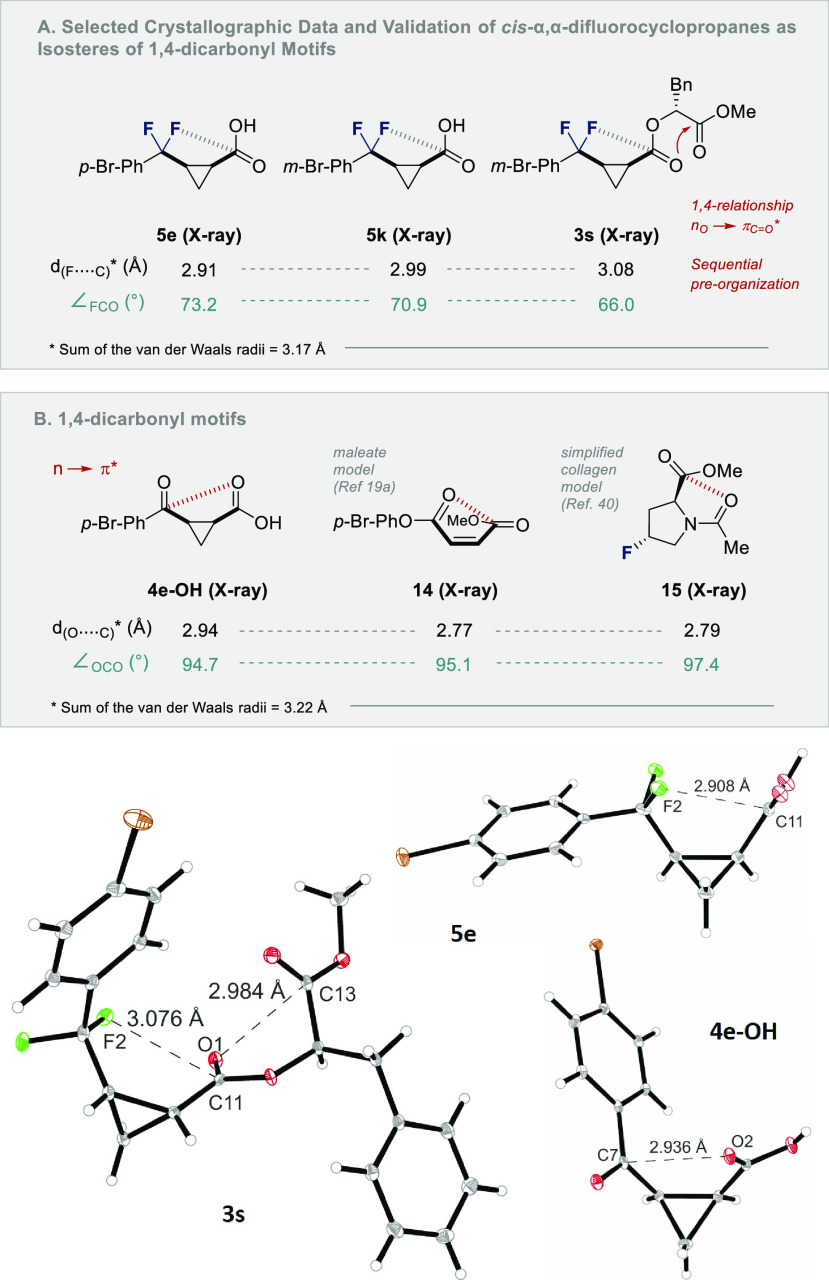
To further explore α,α-difluorocyclopropanes as structural mimics, the interatomic distances of crystalline samples of 5e,k and 3s were compared39 with the 1,4-diketone 4e-OH and two known compounds in which no → π* interactions are operational (14 and 15) (Scheme 6). In the case of 5e,k and 3s, the distances between one of the F atoms and the C(sp2) center were found to be less than the sum of the van der Waals radii. This was also noted for the ketone derived from 5e (4e-OH). These values (2.91–3.08 Å) are in good agreement with the structural analyses of a model maleate (14, 2.77 Å)19a and Raines’ simplified collagen model 15 (2.79 Å).40 This study contributes to the current interest in C–F···C=O(amide) interactions, as is exemplified by a recent crystallographic and spectroscopic study by Lectka and co-workers.41
Scheme 6. Selected X-ray Structural Data and Validation of cis-α,α-Difluorocyclopropanes 3s and 5s as 1,4-Dicarbonyl Bioisosteres (4e-OH).
Clinical success is an effective driver for the conception and development of synthetic methodology. Motivated by the emergence of α,α-difluorocyclopropanes on the drug discovery landscape, and the conspicuous dearth of methods to facilitate their construction, a fluorinated skeletal rearrangement of disubstituted bicyclobutanes (BCBs) has been developed that leverages I(I)/I(III) catalysis. The Brønsted acidity of the HF serves to unmask the BCB and reveal a cyclobutene: this then engages with in situ generated p-TolIF2. A fluorination/stereospecific ring contraction/fluorination sequence then ensues to liberate the cis product with high levels of selectivity. It is postulated that this unprecedented 4 → 3 rearrangement proceeds via a cation in which all three substituents confer a stabilizing effect. The route facilitates access to structural isosteres of 1,4-dicarbonyl compounds, and an X-ray analysis indicates that similar conformational behavior is observed in the solid state. In addition to expanding the pharmacophore discovery arsenal, this transformation enables the generation of fluorinated isosteres and peptidomimetics. It is envisaged that this study will stimulate interest in the activation of strained-ring systems by hypervalent iodine catalysis.
Acknowledgments
We thank the analytical departments of the Organisch-Chemisches Institut (WWU Münster) for support.
Glossary
Abbreviations
- BCBs
bicyclobutanes
- HPLC
high-performance liquid chromatography
- NMR
nuclear magnetic resonance
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.2c04511.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
We acknowledge generous financial support from the WWU Münster, the European Research Council (ERC Consolidator Grant RECON 818949), and the Alexander von Humboldt Foundation (fellowships to K.L. and V.M.-H.).
The authors declare no competing financial interest.
Supplementary Material
References
- a Taylor R. D.; MacCoss M.; Lawson A. D. G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]; b Talele T. T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756. 10.1021/acs.jmedchem.6b00472. [DOI] [PubMed] [Google Scholar]; c Shearer J.; Castro J. L.; Lawson A. D. G.; MacCoss M.; Taylor R. D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. 10.1021/acs.jmedchem.2c00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A.Cyclopropane Compounds of Biological Interest. In Progress in Drug Research; Jucker E., Ed.; Birkhäuser: 1971; Vol. 15; pp 227–270. [Google Scholar]
- a Salaün J.Cyclopropane Derivatives and their Diverse Biological Activities In Topics in Current Chemistry; De Meijere A., Ed; Springer, 2000; Vol 207 (Small Ring Compounds in Organic Synthesis VI), pp 1–67. [Google Scholar]; b Driscoll J. P.; Sadlowski C. M.; Shah N. R.; Feula A. Metabolism and Bioactivation: It’s Time to Expect the Unexpected. J. Med. Chem. 2020, 63, 6303–6314. 10.1021/acs.jmedchem.0c00026. [DOI] [PubMed] [Google Scholar]; c Bauer M. R.; Di Fruscia P.; Lucas S. C. C.; Michaelides I. N.; Nelson J. E.; Storer R. I.; Whitehurst B. C. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 2021, 12, 448–471. 10.1039/D0MD00370K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Walsh A. D. Structures of ethylene oxide and cyclopropane. Nature 1947, 159, 165. 10.1038/159165a0. [DOI] [PubMed] [Google Scholar]; b Robinson R. Structures of Ethylene Oxide and Cyclopropane. Nature 1947, 159, 400–401. 10.1038/159400b0. [DOI] [PubMed] [Google Scholar]; c McDowell C. A. Structures of Ethylene Oxide and Cyclopropane. Nature 1947, 159, 508–509. 10.1038/159508b0. [DOI] [PubMed] [Google Scholar]; d Walsh A. D. Structures of Ethylene Oxide and Cyclopropane. Nature 1947, 159, 712–713. 10.1038/159712a0. [DOI] [PubMed] [Google Scholar]; e Robinson R. Formulæ for Ethylene Oxide and Cyclopropane. Nature 1947, 160, 162. 10.1038/160162a0. [DOI] [PubMed] [Google Scholar]; f Linnett J. W. Structure of Ethylene Oxide and Cyclopropane. Nature 1947, 160, 162–163. 10.1038/160162b0. [DOI] [PubMed] [Google Scholar]; g Walsh A. D. The structures of ethylene oxide, cyclopropane, and related molecules. Trans. Faraday Soc. 1949, 45, 179–190. 10.1039/tf9494500179. [DOI] [Google Scholar]; h Sparr C.; Gilmour R. Cyclopropyl Iminium Activation: Reactivity Umpolung in Enantioselective Organocatalytic Reaction Design. Angew. Chem., Int. Ed. 2011, 50, 8391–8395. 10.1002/anie.201103360. [DOI] [PubMed] [Google Scholar]
- De Meijere A. Bonding Properties of Cyclopropane and Their Chemical Consequences. Angew. Chem., Int. Ed. 1979, 18, 809–826. 10.1002/anie.197908093. [DOI] [Google Scholar]
- a Meanwell N. A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]; b Kumari S.; Carmona A. V.; Tiwari A. K.; Trippier P. C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. 10.1021/acs.jmedchem.0c00530. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Mondal R.; Agbaria M.; Nairoukh Z. Fluorinated Rings: Conformation and Application. Chem. - Eur. J. 2021, 27, 7193–7213. 10.1002/chem.202005425. [DOI] [PubMed] [Google Scholar]
- Mizuno A.; Matsui K.; Shuto S. From Peptides to Peptidomimetics: A Strategy Based on the Structural Features of Cyclopropane. Chem. - Eur. J. 2017, 23, 14394–14409. 10.1002/chem.201702119. [DOI] [PubMed] [Google Scholar]
- a Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; b Landry M. L.; Crawford J. J. LogD Contributions of Substituents Commonly Used in Medicinal Chemistry. ACS Med. Chem. Lett. 2020, 11, 72–76. 10.1021/acsmedchemlett.9b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fang Z.; Cordes D. B.; Slawin A. M. Z.; O’Hagan D. Fluorine containing cyclopropanes: synthesis of aryl substituted all-cis 1,2,3-trifluorocyclopropanes, a facially polar motif. Chem. Commun. 2019, 55, 10539–10542. 10.1039/C9CC05749H. [DOI] [PubMed] [Google Scholar]; For related facially polarized rings, see:; b Thomson C. J.; Zhang Q.; Al-Maharik N.; Bühl M.; Cordes D. B.; Slawin A. M. Z.; O′Hagan D. Fluorinated cyclopropanes: synthesis and chemistry of the aryl α,β,β-trifluorocyclopropane motif. Chem. Commun. 2018, 54, 8415–8418. 10.1039/C8CC04964E. [DOI] [PubMed] [Google Scholar]; c Keddie N. S.; Slawin A. M. Z.; Lebl T.; Philp D.; O′Hagan D. All-cis 1,2,3,4,5,6-hexafluorocyclohexane is a facially polarized cyclohexane. Nat. Chem. 2015, 7, 483–488. 10.1038/nchem.2232. [DOI] [PubMed] [Google Scholar]; d Santschi N.; Gilmour R. A Janus cyclohexane ring. Nat. Chem. 2015, 7, 467–468. 10.1038/nchem.2240. [DOI] [PubMed] [Google Scholar]; e Ziegler B. E.; Lecours M.; Marta R. A.; Featherstone J.; Fillion E.; Hopkins W. S.; Steinmetz V.; Keddie N. S.; O′Hagan D.; McMahon T. B. Janus Face Aspect of All-cis 1,2,3,4,5,6-Hexafluorocyclohexane Dictates Remarkable Anion and Cation Interactions In the Gas Phase. J. Am. Chem. Soc. 2016, 138, 7460–7463. 10.1021/jacs.6b02856. [DOI] [PubMed] [Google Scholar]; f Rodil A.; Bosisio S.; Ayoup M. S.; Quinn L.; Cordes D. B.; Slawin A. M. Z.; Murphy C. D.; Michel J.; O′Hagan D. Metabolism and hydrophilicity of the polarised ‘Janus face’ all-cis tetrafluorocyclohexyl ring, a candidate motif for drug discovery. Chem. Sci. 2018, 9, 3023–3028. 10.1039/C8SC00299A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dolbier W. R.; Battiste M. A. Structure, Synthesis, and Chemical Reactions of Fluorinated Cyclopropanes and Cyclopropenes. Chem. Rev. 2003, 103, 1071–1098. 10.1021/cr010023b. [DOI] [PubMed] [Google Scholar]; b Abele S.; Seiler P.; Seebach D. Synthesis, Crystal Structures, and Modelling of β-Oligopeptides Consisting of 1-(Aminomethyl)cyclopropanecarboxylic Acid: Ribbon-Type Arrangement of Eight-Membered H-Bonded Rings. Helv. Chim. Acta 1999, 82, 1559–1571. . [DOI] [Google Scholar]; c Grygorenko O. O.; Artamonov O. S.; Komarov I. V.; Mykhailiuk P. K. Trifluoromethyl-substituted cyclopropanes. Tetrahedron 2011, 67, 803–823. 10.1016/j.tet.2010.11.068. [DOI] [Google Scholar]; d Časar Z. Synthetic Approaches to Contemporary Drugs that Contain the Cyclopropyl Moiety. Synthesis 2020, 52, 1315–1345. 10.1055/s-0039-1690058. [DOI] [Google Scholar]; e Pons A.; Delion L.; Poisson T.; Charette A. B.; Jubault P. Asymmetric Synthesis of Fluoro, Fluoromethyl, Difluoromethyl, and Trifluoromethylcyclopropanes. Acc. Chem. Res. 2021, 54, 2969–2990. 10.1021/acs.accounts.1c00261. [DOI] [PubMed] [Google Scholar]
- a Müller K.; Faeh C.; Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; b O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]; c Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; d Zimmer L. E.; Sparr C.; Gilmour R. Fluorine Conformational Effects in Organocatalysis: An Emerging Strategy for Molecular Design. Angew. Chem., Int. Ed. 2011, 50, 11860–11871. 10.1002/anie.201102027. [DOI] [PubMed] [Google Scholar]; e Gillis E. P.; Eastman K. J.; Hill M. D.; Donnelly D. J.; Meanwell N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]; f Meanwell N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]; g Molnár I. G.; Thiehoff C.; Holland M. C.; Gilmour R. Catalytic, Vicinal Difluorination of Olefins: Creating a Hybrid, Chiral Bioisostere of the Trifluoromethyl and Ethyl Groups. ACS Catal. 2016, 6, 7167–7173. 10.1021/acscatal.6b02155. [DOI] [Google Scholar]; h Richardson P. Applications of fluorine to the construction of bioisosteric elements for the purposes of novel drug discovery. Expert Opin. Drug Discovery 2021, 16, 1261–1286. 10.1080/17460441.2021.1933427. [DOI] [PubMed] [Google Scholar]
- Fedoryński M. Syntheses of gem-Dihalocyclopropanes and Their Use in Organic Synthesis. Chem. Rev. 2003, 103, 1099–1132. 10.1021/cr0100087. [DOI] [PubMed] [Google Scholar]
- a Taylor J. G.; Zipfel S.; Ramay K.; Vivian R.; Schrier A.; Karki K. K.; Katana A.; Kato D.; Kobayashi T.; Martinez R.; Sangi M.; Siegel D.; Tran C. V.; Yang Z.-Y.; Zablocki J.; Yang C. Y.; Wang Y.; Wang K.; Chan K.; Barauskas O.; Cheng G.; Jin D.; Schultz B. E.; Appleby T.; Villaseñor A. G.; Link J. O. Discovery of the pan-genotypic hepatitis C virus NS3/4A protease inhibitor voxilaprevir (GS-9857): A component of Vosevi®. Bioorg. Med. Chem. Lett. 2019, 29, 2428–2436. 10.1016/j.bmcl.2019.03.037. [DOI] [PubMed] [Google Scholar]; b Lawitz E. J.; O’Riordan W. D.; Asatryan A.; Freilich B. L.; Box T. D.; Overcash J. S.; Lovell S.; Ng T. I.; Liu W.; Campbell A.; Lin C. W.; Yao B.; Kort J. Potent Antiviral Activities of the Direct-Acting Antivirals ABT-493 and ABT-530 with Three-Day Monotherapy for Hepatitis C Virus Genotype 1 Infection. Antimicrob. Agents Chemother. 2016, 60, 1546–1555. 10.1128/AAC.02264-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Link J. O.; Rhee M. S.; Tse W. C.; Zheng J.; Somoza J. R.; Rowe W.; Begley R.; Chiu A.; Mulato A.; Hansen D.; Singer E.; Tsai L. K.; Bam R. A.; Chou C.-H.; Canales E.; Brizgys G.; Zhang J. R.; Li J.; Graupe M.; Morganelli P.; Liu Q.; Wu Q.; Halcomb R. L.; Saito R. D.; Schroeder S. D.; Lazerwith S. E.; Bondy S.; Jin D.; Hung M.; Novikov N.; Liu X.; Villaseñor A. G.; Cannizzaro C. E.; Hu E. Y.; Anderson R. L.; Appleby T. C.; Lu B.; Mwangi J.; Liclican A.; Niedziela-Majka A.; Papalia G. A.; Wong M. H.; Leavitt S. A.; Xu Y.; Koditek D.; Stepan G. J.; Yu H.; Pagratis N.; Clancy S.; Ahmadyar S.; Cai T. Z.; Sellers S.; Wolckenhauer S. A.; Ling J.; Callebaut C.; Margot N.; Ram R. R.; Liu Y.-P.; Hyland R.; Sinclair G. I.; Ruane P. J.; Crofoot G. E.; McDonald C. K.; Brainard D. M.; Lad L.; Swaminathan S.; Sundquist W. I.; Sakowicz R.; Chester A. E.; Lee W. E.; Daar E. S.; Yant S. R.; Cihlar T. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. 10.1038/s41586-020-2443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zheng B.; D'Andrea S. V.; Sun L.-Q.; Wang A. X.; Chen Y.; Hrnciar P.; Friborg J.; Falk P.; Hernandez D.; Yu F.; Sheaffer A. K.; Knipe J. O.; Mosure K.; Rajamani R.; Good A. C.; Kish K.; Tredup J.; Klei H. E.; Karuchuri M.; Ng A.; Gao Q.; Rampulla R. A.; Mathur A.; Meanwell N. A.; McPhee F.; Scola P. M. Potent Inhibitors of Hepatitis C Virus NS3 Protease: Employment of a Difluoromethyl Group as a Hydrogen-Bond Donor. ACS Med. Chem. Lett. 2018, 9, 143–148. 10.1021/acsmedchemlett.7b00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jung M. E.; Piizi G. gem-Disubstituent Effect: Theoretical Basis and Synthetic Applications. Chem. Rev. 2005, 105, 1735–1766. 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]; b O′Hagan D.; Wang Y.; Skibinski M.; Slawin A. M. Z. Influence of the difluoromethylene group (CF2) on the conformation and properties of selected organic compounds. Pure Appl. Chem. 2012, 84, 1587–1595. 10.1351/PAC-CON-11-09-26. [DOI] [Google Scholar]
- Dossetter A. G. A statistical analysis of in vitro human microsomal metabolic stability of small phenyl group substituents, leading to improved design sets for parallel SAR exploration of a chemical series. Bioorg. Med. Chem. 2010, 18, 4405–4414. 10.1016/j.bmc.2010.04.077. [DOI] [PubMed] [Google Scholar]
- Fischer F. R.; Wood P. A.; Allan F. H.; Diederich F. Orthogonal dipolar interactions between amide carbonyl groups. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 17290–17294. 10.1073/pnas.0806129105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hodges J. A.; Raines R. T. Energetics of an n → π* Interaction that Impacts Protein Structure. Org. Lett. 2006, 8, 4695–4697. 10.1021/ol061569t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shoulders M. D.; Raines R. T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Choudhary A.; Gandla D.; Krow G. R.; Raines R. T. Nature of Amide Carbonyl–Carbonyl Interactions in Proteins. J. Am. Chem. Soc. 2009, 131, 7244–7246. 10.1021/ja901188y. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Newberry R. W.; Raines R. T. Acc. Chem. Res. 2017, 50, 1838–1846. 10.1021/acs.accounts.7b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kilgore H. R.; Raines R. T. n→π* Interactions Modulate the Properties of Cysteine Residues and Disulfide Bonds in Proteins. J. Am. Chem. Soc. 2018, 140, 17606–17611. 10.1021/jacs.8b09701. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Sahariah B.; Sarma B. K. Relative orientation of the carbonyl groups determines the nature of orbital interactions in carbonyl–carbonyl short contacts. Chem. Sci. 2019, 10, 909–917. 10.1039/C8SC04221G. [DOI] [PMC free article] [PubMed] [Google Scholar]; g León I.; Alonso E. R.; Cabezas C.; Mata S.; Alonso J. L. Unveiling the n→π* interactions in dipeptides. Commun. Chem. 2019, 2, 3. 10.1038/s42004-018-0103-2. [DOI] [Google Scholar]
- a Bretscher L. E.; Jenkins C. L.; Taylor K. M.; DeRider M. L.; Raines R. T. Conformational Stability of Collagen Relies on a Stereoelectronic Effect. J. Am. Chem. Soc. 2001, 123, 777–778. 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]; b DeRider M. L.; Wilkens S. J.; Waddell M. J.; Bretscher L. E.; Weinhold F.; Raines R. T.; Markley J. L. Collagen Stability: Insights from NMR Spectroscopic and Hybrid Density Functional Computational Investigations of the Effect of Electronegative Substituents on Prolyl Ring Conformations. J. Am. Chem. Soc. 2002, 124, 2497–2505. 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]; c Jakobsche C. E.; Choudhary A.; Miller S. J.; Raines R. T. n → π* Interaction and n)(π Pauli Repulsion Are Antagonistic for Protein Stability. J. Am. Chem. Soc. 2010, 132, 6651–6653. 10.1021/ja100931y. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wilhelm P.; Lewandowski B.; Trapp N.; Wennemers H. A Crystal Structure of an Oligoproline PPII-Helix, at Last. J. Am. Chem. Soc. 2014, 136, 15829–15832. 10.1021/ja507405j. [DOI] [PubMed] [Google Scholar]; e Dobitz S.; Aronoff M. R.; Wennemers H. Oligoprolines as Molecular Entities for Controlling Distance in Biological and Material Sciences. Acc. Chem. Res. 2017, 50, 2420–2428. 10.1021/acs.accounts.7b00340. [DOI] [PubMed] [Google Scholar]
- a Neveselý T.; Molloy J. J.; McLaughlin C.; Brüss L.; Daniliuc C. G.; Gilmour R. Leveraging the n→π* Interaction in Alkene Isomerization by Selective Energy Transfer Catalysis. Angew. Chem., Int. Ed. 2022, 61, e202113600. 10.1002/anie.202113600. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Neveselý T.; Wienhold M.; Molloy J. J.; Gilmour R. Advances in the E → Z Isomerization of Alkenes Using Small Molecule Photocatalysts. Chem. Rev. 2022, 122, 2650–2694. 10.1021/acs.chemrev.1c00324. [DOI] [PubMed] [Google Scholar]
- Hara S.; Nakahigashi J.; Ishi-i K.; Fukuhara T.; Yoneda N. Fluorinative ring-contraction of cyclic alkenes with p-iodotoluene difluoride. Tetrahedron Lett. 1998, 39, 2589–2592. 10.1016/S0040-4039(98)00276-7. [DOI] [Google Scholar]
- a Wiberg K. B.; Lampman G. M.; Ciula R. P.; Connor D. S.; Schertler P.; Lavanish J. Bicyclo[1.1.0]butane. Tetrahedron 1965, 21, 2749–2769. 10.1016/S0040-4020(01)98361-9. [DOI] [Google Scholar]; b Walczak M. A. A.; Krainz T.; Wiff P. Ring-Strain-Enabled Reaction Discovery: New Heterocycles from Bicyclo[1.1.0]butanes. Acc. Chem. Res. 2015, 48, 1149–1158. 10.1021/ar500437h. [DOI] [PubMed] [Google Scholar]; c Kelly C. B.; Milligan J.; Tilley L. J.; Sodano T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci. 2022, 13, 11721–11737. 10.1039/D2SC03948F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nilsen N. O.; Skattebøl L.; Baird M. S.; Buxton S. R.; Slowey P. D. A simple route to 1-bromobicyclo[1.1.0]butanes by intramolecular trapping of 1-bromo-1-lithiocyclopropanes. Tetrahedron Lett. 1984, 25, 2887–2890. 10.1016/S0040-4039(01)81317-4. [DOI] [Google Scholar]; b Baird M. S.; Hussain H. H. The preparation and decomposition of alkyl 2-diazopent-4-enoates and 1-trimethylsilyl-1-diazobut-3-enes. Tetrahedron 1987, 43, 215–224. 10.1016/S0040-4020(01)89947-6. [DOI] [Google Scholar]; c McNamee R. E.; Haugland M. M.; Nugent J.; Chan R.; Christensen K. E.; Anderson E. A. Synthesis of 1,3-disubstituted bicyclo[1.1.0]butanes via directed bridgehead functionalization. Chem. Sci. 2021, 12, 7480–7485. 10.1039/D1SC01836A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Chen H.; Hamilton G.; Patel S.; Zhao G.; Daniels B.; Stivala C.. Bicyclic Compounds for Use as RIP1 Inhibitors. WO2019/072942A1, 2019;.; b Baccei J. M.; Bravo Y.; Chen A. C.-Y.; Roppe J.; Schrader T.; Xiong Y.. Preparation of Diazabicyclooctanyl Nicotinonitriles as Muscarinic Acetylcholine M1 Receptor Antagonists. WO2021/071843A1, 2021.
- An L.; Tong F.-F.; Zhang S.; Zhang X. Stereoselective Functionalization of Racemic Cyclopropylzinc Reagents via Enantiodivergent Relay Coupling. J. Am. Chem. Soc. 2020, 142, 11884–11892. 10.1021/jacs.0c04462. [DOI] [PubMed] [Google Scholar]
- For selected reviews of I(III)-catalyzed fluorination, see:; a Kohlhepp S. V.; Gulder T. Hypervalent iodine(III) fluorinations of alkenes and diazo compounds: new opportunities in fluorination chemistry. Chem. Soc. Rev. 2016, 45, 6270–6288. 10.1039/C6CS00361C. [DOI] [PubMed] [Google Scholar]; b Arnold A. M.; Ulmer A.; Gulder T. Advances in Iodine(III)-Mediated Halogenations: A Versatile Tool to Explore New Reactivities and Selectivities. Chem. - Eur. J. 2016, 22, 8728–8739. 10.1002/chem.201600449. [DOI] [PubMed] [Google Scholar]; c Meyer S.; Häfliger J.; Gilmour R. Expanding organofluorine chemical space: the design of chiral fluorinated isosteres enabled by I(I)/I(III) catalysis. Chem. Sci. 2021, 12, 10686–10695. 10.1039/D1SC02880D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of 1,1-difluorination, see:; a Ilchenko N. O.; Tasch B. O. A.; Szabó K. J. Mild Silver-Mediated Geminal Difluorination of Styrenes Using an Air- and Moisture-Stable Fluoroiodane Reagent. Angew. Chem., Int. Ed. 2014, 53, 12897–12901. 10.1002/anie.201408812. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kitamura T.; Muta K.; Oyamada J. Hypervalent Iodine-Mediated Fluorination of Styrene Derivatives: Stoichiometric and Catalytic Transformation to 2,2-Difluoroethylarenes. J. Org. Chem. 2015, 80, 10431–10436. 10.1021/acs.joc.5b01929. [DOI] [PubMed] [Google Scholar]; c Banik S. M.; Medley J. W.; Jacobsen E. N. Catalytic, asymmetric difluorination of alkenes to generate difluoromethylated stereocenters. Science 2016, 353, 51–54. 10.1126/science.aaf8078. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ilchenko N. O.; Szabó K. J. Geminal difluorination of α,α′- disubstituted styrenes using fluoro-benziodoxole reagent. Migration aptitude of the α-substituents. J. Fluorine Chem. 2017, 203, 104–109. 10.1016/j.jfluchem.2017.07.006. [DOI] [Google Scholar]; e Scheidt F.; Neufeld J.; Schäfer M.; Thiehoff C.; Gilmour R. Catalytic Geminal Difluorination of Styrenes for the Construction of Fluorine-rich Bioisosteres. Org. Lett. 2018, 20, 8073–8076. 10.1021/acs.orglett.8b03794. [DOI] [PubMed] [Google Scholar]; f Zhao Z.; Racicot L.; Murphy G. K. Fluorinative Rearrangements of Substituted Phenylallenes Mediated by (Difluoroiodo)toluene: Synthesis of α-(Difluoromethyl)styrenes. Angew. Chem., Int. Ed. 2017, 56, 11620–11623. 10.1002/anie.201706798. [DOI] [PubMed] [Google Scholar]; g Kitamura T.; Yoshida K.; Mizuno S.; Miyake A.; Oyamada J. Difluorination of Functionalized Aromatic Olefins Using Hypervalent Iodine/HF Reagents. J. Org. Chem. 2018, 83, 14853–14860. 10.1021/acs.joc.8b02473. [DOI] [PubMed] [Google Scholar]; h Lv W.-X.; Li Q.; Li J.-L.; Li Z.; Lin E.; Tan D.-H.; Cai Y.-H.; Fan W.-X.; Wang H. gem-Difluorination of Alkenyl N-methyliminodiacetyl Boronates: Synthesis of α- and β-Difluorinated Alkylborons. Angew. Chem., Int. Ed. 2018, 57, 16544–16548. 10.1002/anie.201810204. [DOI] [PubMed] [Google Scholar]; i Levin M. D.; Ovian J. M.; Read J. A.; Sigman M. S.; Jacobsen E. N. Catalytic Enantioselective Synthesis of Difluorinated Alkyl Bromides. J. Am. Chem. Soc. 2020, 142, 14831–14837. 10.1021/jacs.0c07043. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Häfliger J.; Livingstone K.; Daniliuc C. G.; Gilmour R. Difluorination of α-(bromomethyl)styrenes via I(I)/I(III) catalysis: facile access to electrophilic linchpins for drug discovery. Chem. Sci. 2021, 12, 6148–6152. 10.1039/D1SC01132D. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Neufeld J.; Stünkel T.; Mück-Lichtenfeld C.; Daniliuc C. G.; Gilmour R. Trifluorinated Tetralins via I(I)/I(III)-Catalysed Ring Expansion: Programming Conformation by [CH2CH2] → [CF2CHF] Isosterism. Angew. Chem., Int. Ed. 2021, 60, 13647–13651. 10.1002/anie.202102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of 1,2-difluorination, see:; a Banik S. M.; Medley J. W.; Jacobsen E. N. Catalytic, Diastereoselective 1,2-Difluorination of Alkenes. J. Am. Chem. Soc. 2016, 138, 5000–5003. 10.1021/jacs.6b02391. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Molnár I. G.; Gilmour R. Catalytic Difluorination of Olefins. J. Am. Chem. Soc. 2016, 138, 5004–5007. 10.1021/jacs.6b01183. [DOI] [PubMed] [Google Scholar]; c Scheidt F.; Schäfer M.; Sarie J. C.; Daniliuc C. G.; Molloy J. J.; Gilmour R. Enantioselective, Catalytic Vicinal Difluorination of Alkenes. Angew. Chem., Int. Ed. 2018, 57, 16431–16435. 10.1002/anie.201810328. [DOI] [PubMed] [Google Scholar]; d Haj M. K.; Banik S. M.; Jacobsen E. N. Catalytic, Enantioselective 1,2-Difluorination of Cinnamamides. Org. Lett. 2019, 21, 4919–4923. 10.1021/acs.orglett.9b00938. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Doobary S.; Sedikides A. T.; Caldora H. P.; Poole D. L.; Lennox A. J. J. Electrochemical Vicinal Difluorination of Alkenes: Scalable and Amenable to Electron-Rich Substrates. Angew. Chem., Int. Ed. 2020, 59, 1155–1160. 10.1002/anie.201912119. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Meyer S.; Häfliger J.; Schäfer M.; Molloy J. J.; Daniliuc C. G.; Gilmour R. A Chiral Pentafluorinated Isopropyl Group via Iodine(I)/(III) Catalysis. Angew. Chem., Int. Ed. 2021, 60, 6430–6434. 10.1002/anie.202015946. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Martín-Heras V.; Daniliuc C. G.; Gilmour R. An I(I)/I(III) Catalysis Route to the Heptafluoroisopropyl Group: A Privileged Module in Contemporary Agrochemistry. Synthesis 2021, 53, 4203–4212. 10.1055/a-1485-4916. [DOI] [Google Scholar]
- For I(III)-mediated fluorinative ring opening of cyclopropanes, see:; a Ilchenko N. O.; Hedberg M.; Szabó K. J. Fluorinative ring-opening of cyclopropanes by hypervalent iodine reagents. An efficient method for 1,3-oxyfluorination and 1,3-difluorination. Chem. Sci. 2017, 8, 1056–1061. 10.1039/C6SC03471C. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Banik S. M.; Mennie K. M.; Jacobsen E. N. Catalytic 1,3-Difunctionalization via Oxidative C–C Bond Activation. J. Am. Chem. Soc. 2017, 139, 9152–9155. 10.1021/jacs.7b05160. [DOI] [PMC free article] [PubMed] [Google Scholar]; c For a study on cyclopropene activation, see:Meyer S.; Göbel L.; Livingstone K.; Roblick C.; Daniliuc C. G.; Gilmour R. Cyclopropene activation via I(I)/I(III) catalysis: Proof of principle and application in direct tetrafluorination. Tetrahedron 2022, 126, 132925. 10.1016/j.tet.2022.132925. [DOI] [Google Scholar]
- For a report of the oxidative contraction of cyclobutenes using m-CPBA, see:Baumann A. N.; Schüppel F.; Eisold M.; Kreppel A.; Vivie-Riedle R. de; Didier D. Oxidative Ring Contraction of Cyclobutenes: General Approach to Cyclopropylketones including Mechanistic Insights. J. Org. Chem. 2018, 83, 4905–4921. 10.1021/acs.joc.8b00297. [DOI] [PubMed] [Google Scholar]
- For selected examples of BCB reactivity, see:; a Gianatassio R.; Lopchuk J. M.; Wang J.; Pan C.-M.; Malins L. R.; Prieto L.; Brandt T. A.; Collins M. R.; Gallego G. M.; Sach N. W.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-release amination. Science 2016, 351, 241–246. 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lopchuk J. M.; Fjelbye K.; Kawamata Y.; Malins L. R.; Pan C.-M.; Gianatassio R.; Wang J.; Prieto L.; Bradow J.; Brandt T. A.; Collins M. R.; Elleraas J.; Ewanicki J.; Farrell W.; Fadeyi O. O.; Gallego G. M.; Mousseau J. J.; Oliver R.; Sach N. W.; Smith J. K.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity. J. Am. Chem. Soc. 2017, 139, 3209–3226. 10.1021/jacs.6b13229. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Fawcett A.; Biberger T.; Aggarwal V. K. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem. 2019, 11, 117–122. 10.1038/s41557-018-0181-x. [DOI] [PubMed] [Google Scholar]; d Silvi M.; Aggarwal V. K. Radical Addition to Strained σ-Bonds Enables the Stereocontrolled Synthesis of Cyclobutyl Boronic Esters. J. Am. Chem. Soc. 2019, 141, 9511–9515. 10.1021/jacs.9b03653. [DOI] [PubMed] [Google Scholar]; e Pratt C. J.; Aycock R. A.; King M. D.; Jui M. T. Radical α-C-H Cyclobutylation of Aniline Derivatives. Synlett 2020, 31, 51–54. 10.1055/s-0039-1690197. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Bennett S. H.; Fawcett A.; Denton E. H.; Biberger T.; Fasano V.; Winter N.; Aggarwal V. K. Difunctionalization of C–C σ-Bonds Enabled by the Reaction of Bicyclo[1.1.0]butyl Boronate Complexes with Electrophiles: Reaction Development, Scope, and Stereochemical Origins. J. Am. Chem. Soc. 2020, 142, 16766–16775. 10.1021/jacs.0c07357. [DOI] [PubMed] [Google Scholar]; g Tokunaga K.; Sato M.; Kuwata K.; Miura C.; Fuchida H.; Matsunaga N.; Koyanagi S.; Ohdo S.; Shindo N.; Ojida A. Bicyclobutane Carboxylic Amide as a Cysteine-Directed Strained Electrophile for Selective Targeting of Proteins. J. Am. Chem. Soc. 2020, 142, 18522–18531. 10.1021/jacs.0c07490. [DOI] [PubMed] [Google Scholar]; h Guo L.; Noble A.; Aggarwal V. K. α-Selective Ring-Opening Reactions of Bicyclo[1.1.0]butyl Boronic Ester with Nucleophiles. Angew. Chem., Int. Ed. 2021, 60, 212–216. 10.1002/anie.202011739. [DOI] [PubMed] [Google Scholar]; i Lewis-Borrell L.; Sneha M.; Clark I. P.; Fasano V.; Noble A.; Aggarwal V. K.; Orr-Ewing A. J. Direct Observation of Reactive Intermediates by Time-Resolved Spectroscopy Unravels the Mechanism of a Radical-Induced 1,2-Metalate Rearrangement. J. Am. Chem. Soc. 2021, 143, 17191–17199. 10.1021/jacs.1c07964. [DOI] [PubMed] [Google Scholar]; j McNamee R. E.; Thompson A. L.; Anderson E. A. Synthesis and Applications of Polysubstituted Bicyclo[1.1.0]butanes. J. Am. Chem. Soc. 2021, 143, 21246–21251. 10.1021/jacs.1c11244. [DOI] [PubMed] [Google Scholar]; k Michalland J.; Casaretto N.; Zard S. Z. A Modular Access to 1,2- and 1,3-Disubstituted Cyclobutylboronic Esters by Consecutive Radical Additions. Angew. Chem., Int. Ed. 2022, 61, e202113333. 10.1002/anie.202113333. [DOI] [PubMed] [Google Scholar]
- Champagne P. A.; Benhassine Y.; Desroches J.; Paquin J.-F. Friedel-Crafts reaction of benzyl fluorides: selective activation of C-F bonds as enabled by hydrogen bonding. Angew. Chem., Int. Ed. 2014, 53, 13835–13839. 10.1002/anie.201406088. [DOI] [PubMed] [Google Scholar]
- a Pittman C. U. Jr; Olah G. A. Stable Carbonium Ions. XVII.1a Cyclopropyl Carbonium Ions and Protonated Cyclopropyl Ketones. J. Am. Chem. Soc. 1965, 87, 5123–5132. 10.1021/ja00950a026. [DOI] [Google Scholar]; b Sparr C.; Gilmour R. Cyclopropyl Iminium Activation: Reactivity Umpolung in Enantioselective Organocatalytic Reaction Design. Angew. Chem., Int. Ed. 2011, 50, 8391–8395. 10.1002/anie.201103360. [DOI] [PubMed] [Google Scholar]; c Holland M. C.; Gilmour R. Deconstructing Covalent Organocatalysis. Angew. Chem., Int. Ed. 2015, 54, 3862–3871. 10.1002/anie.201409004. [DOI] [PubMed] [Google Scholar]
- Sarie J. C.; Thiehoff C.; Mudd R. J.; Daniliuc C. G.; Kehr G.; Gilmour R. Deconstructing the Catalytic, Vicinal Difluorination of Alkenes: HF-Free Synthesis and Structural Study of p-TolIF2. J. Org. Chem. 2017, 82, 11792–11798. 10.1021/acs.joc.7b01671. [DOI] [PubMed] [Google Scholar]
- Neufeld J.; Daniliuc C. G.; Gilmour R. Fluorohydration of alkynes via I(I)/I(III) catalysis. Beilstein J. Org. Chem. 2020, 16, 1627–1635. 10.3762/bjoc.16.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Johnson W. S.; Chenera B.; Tham F. S.; Kullnig R. K. Cation-stabilizing auxiliaries in polyene cyclizations. 4. The fluorine atom as a cation-stabilizing auxiliary in biomimetic polyene cyclizations. 1. Background and exploratory experiments. J. Am. Chem. Soc. 1993, 115, 493–497. 10.1021/ja00055a019. [DOI] [Google Scholar]; b Johnson W. S.; Fletcher V. R.; Chenera B.; Bartlett W. R.; Tham F. S.; Kullnig R. K. Cation-stabilizing auxiliaries in polyene cyclizations. 5. The fluorine atom as a cation-stabilizing auxiliary in biomimetic polyene cyclizations. 2. Asymmetric synthesis of a steroid. J. Am. Chem. Soc. 1993, 115, 497–504. 10.1021/ja00055a020. [DOI] [Google Scholar]; c Johnson W. S.; Buchanan R. A.; Bartlett W. R.; Tham F. S.; Kullnig R. K. Cation-stabilizing auxiliaries in polyene cyclizations. 6. The fluorine atom as a cation-stabilizing auxiliary in biomimetic polyene cyclizations. 3. Use to effect regiospecific control. J. Am. Chem. Soc. 1993, 115, 504–515. 10.1021/ja00055a021. [DOI] [Google Scholar]; d Johnson W. S.; Plummer M. S.; Reddy S. P.; Bartlett W. R. Cation-stabilizing auxiliaries in polyene cyclizations. 7. The fluorine atom as a cation-stabilizing auxiliary in biomimetic polyene cyclizations. 4. Total synthesis of dl-β-amyrin. J. Am. Chem. Soc. 1993, 115, 515. 10.1021/ja00055a022. [DOI] [Google Scholar]
- CCDC 2194272 contains supplementary crystallographic data for 5k. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- a Ceresoli-Borroni G.; Guidetti P.; Amori L.; Pellicciari R.; Schwarcz R. Perinatal kynurenine 3-hydroxylase inhibition in rodents: pathophysiological implications. J. Neurosci. Res. 2007, 85, 845–854. 10.1002/jnr.21183. [DOI] [PubMed] [Google Scholar]; b Amori L.; Guidetti P.; Pellicciari R.; Kajii Y.; Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem. 2009, 109, 316–325. 10.1111/j.1471-4159.2009.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Amaral M.; Levy C.; Heyes D. J.; Lafite P.; Outeiro T. F.; Giorgini F.; Leys D.; Scrutton N. S. Structural basis of kynurenine 3-monooxygenase inhibition. Nature 2013, 496, 382–385. 10.1038/nature12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vécsei L.; Szalárdy L.; Fülöp F.; Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug Discovery 2013, 12, 64–82. 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- CCDC 2194271 (5e), 2194273 (3s), and 2194274 (4e-OH) contains supplementary crystallographic data for this study. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Panasik N.; Eberhardt E. S.; Edison A. S.; Powell D. R.; Raines R. T. Inductive effects on the structure of proline residues. Int. J. Peptide Protein Res. 1994, 44, 262–269. 10.1111/j.1399-3011.1994.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Harry S. A.; Kazim M.; Nguyen P. M.; Zhu A.; Xiang M. R.; Catazaro J.; Siegler M.; Lectka T. The Close Interaction of a C-F Bond with an Amide Carbonyl: Crystallographic and Spectroscopic Characterization. Angew. Chem., Int. Ed. 2022, 61, e202207966. 10.1002/anie.202207966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.