Abstract
BACKGROUND
Asymptomatic infections and mild symptoms are common in patients infected with the Omicron variant, and data on liver test abnormalities are rare.
AIM
To evaluated the clinical characteristics of asymptomatic and mild coronavirus disease 2019 (COVID-19) patients with abnormal liver test results.
METHODS
This retrospective study included 661 laboratory-confirmed asymptomatic and mild COVID-19 patients who were treated in two makeshift hospitals in Ningbo from April 5, 2022 to April 29, 2022. Clinical information and viral shedding time were collected, and univariate and multivariate logistic regression models were performed in statistical analyses.
RESULTS
Of the 661 patients, 83 (12.6%) had liver test abnormalities, and 6 (0.9%) had liver injuries. Abnormal liver tests revealed a reliable correlation with a history of liver disease (P < 0.001) and a potential correlation with male sex and obesity (P < 0.05). Elevated alanine aminotransferase was reliably associated with obesity (P < 0.05) and a history of liver disease (P < 0.001). Elevated aspartate transaminase (AST) was reliably correlated with a history of liver disease (P < 0.001), and potentially correlated with age over 30 years (P < 0.05). There was a reliable correlation between AST ≥ 2× the upper limit of normal and a longer viral shedding time, especially in mild cases.
CONCLUSION
Obesity and a history of liver disease are risk factors for liver test abnormalities. Being male and an older age are potential risk factors. Attention should be given to liver tests in asymptomatic and mild COVID-19 patients, which has crucial clinical significance for evaluating the viral shedding time.
Keywords: COVID-19, Liver test abnormalities, Asymptomatic carriers, Mild cases, Viral shedding time, Risk factors
Core Tip: This is the first clinical study focusing on liver test abnormalities in asymptomatic and mild coronavirus disease 2019 patients. Unlike studies concerning severe cases, we focused on the association between liver test results and viral shedding time in patients infected with the Omicron BA2.2 variant, with a relatively high proportion of asymptomatic carriers and mild cases. The viral shedding time for patients with elevated aspartate transaminase were significantly longer, especially in mild patients. This provides crucial evidence for identifying high-risk patients with a prolonged viral shedding time.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This highly contagious disease poses a great threat to global public health. By September 2, 2022, the disease had resulted in 601189435 infections and 6475346 deaths[1]. In addition to respiratory symptoms and fever, 14%-69% of patients with COVID-19 have abnormal liver function tests, mainly manifested by elevations of hypoalbuminemia, gamma-glutamyl transferase, alanine aminotransferase (ALT) and aspartate aminotransferase (AST)[2,3]. Patients with severe diseases are more likely to develop elevated liver tests, suggesting an association between liver injury and the severity of disease[2,4]. Possible mechanisms of COVID-19-related liver injury include direct SARS-CoV-2-induced cytopathic injury to hepatocytes and cholangiocytes, immune dysregulation and hypoxic liver injury, and drug-induced liver injury[5].
The Omicron B.1.1.529 (BA.1) variant was first discovered in South Africa on November 9, 2021[6]. Omicron variants have rapidly replaced delta variants due to their strong interpersonal infectivity, which has caused a worldwide pandemic. Compared with patients infected with previous variants, those infected with the Omicron variants are younger, have fewer comorbidities, and develop lower severity and mortality, and asymptomatic infections and mild symptoms are more common[7-9]. However, no studies have systematically focused on liver abnormalities in asymptomatic carriers or mild cases. Previous studies have revealed that liver test abnormalities are associated with prolonged hospitalization time and viral shedding time in COVID-19 patients[10,11], indicating a prognostic indicator of poor outcome. At present, no studies have examined the association between liver abnormalities and viral shedding time in asymptomatic and mild COVID-19 patients.
The epidemic prevention policies of different countries are established based on their own political, economic and health conditions, which are in line with the national circumstances and none is superior to others. This study was carried out under the policy of Chinese government, aiming to determine the clinical characteristics of liver test abnormalities in asymptomatic and mild COVID-19 patients infected with the Omicron BA2.2 variant[12] and their association with the viral shedding time. Our research provides suggestions for health policymakers and medical practitioners.
MATERIALS AND METHODS
Participants
In this cross-sectional study, we recruited patients with COVID-19 who were treated in Ningbo Makeshift Hospital and Dapengshan Makeshift Hospital in Ningbo, Zhejiang from April 5, 2022 to April 29, 2022. All patients were transferred from Shanghai. The inclusion criteria were as follows: (1) Age over 14 years; (2) complete clinical information; and (3) asymptomatic carriers or mild cases of COVID-19. Asymptomatic carriers were those who had positive etiological tests of COVID-19 but with no clinical symptoms or imaging features of COVID-19. Mild cases were defined as confirmed cases with mild clinical symptoms and no pneumonia manifestation on imaging[13]. The exclusion criteria were as follows: (1) Ordinary cases whose pneumonia manifestation could be seen in imaging, or severe cases with respiratory distress[13]; and (2) patients who were transferred to designated hospitals for further diagnosis and treatment due to underlying diseases or other reasons. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Ningbo First Hospital (No. 2022RS069).
Measures
The clinical information of patients, including age, sex, height, weight, symptoms, medical history (hypertension, diabetes, and liver diseases), and laboratory tests (complete blood count, liver tests, chest computed tomography (CT) scans, and nucleic acid tests for COVID-19), were retrospectively collected. Obesity was defined as a body mass index (BMI) ≥ 28 kg/m2. Symptoms included fever, fatigue (weakness and muscle soreness), respiratory symptoms (sore throat, dry throat, cough, and chest stuffiness), and gastrointestinal symptoms (nausea, anorexia, and diarrhea). The patients’ symptoms were collected from the electronic medical records, and were double-checked on the day of discharge. Liver diseases included chronic hepatitis B, alcoholic/nonalcoholic fatty liver disease (NAFLD) and other liver diseases. NAFLD was defined as hepatic steatosis detected by ultrasound or CT, excluding secondary causes and excessive alcohol consumption (> 30 g/d for males, and > 20 g/d for females). Hepatitis B was defined as positive serum hepatitis B surface antigen. Complete blood counts, liver and kidney tests, and chest CT scans were performed on the admission day, and nasal swabs for SARS-CoV-2 were tested every two days. All patients received Chinese medicine and symptomatic therapy (if necessary), but not antivirals or monoclonal antibodies.
Real-time reverse transcription PCR was used to detect SARS-CoV-2, and primers targeting the open reading frame 1ab (ORF1ab) and nucleocapsid protein N were used for amplification and detection. The corresponding sequences for ORF1ab were 5'-CCCTGTGGGTTTTACACTTAA-3'(F), 5'-ACGATTGTGCATCAGCTGA-3' (R), and 5'-CY3-CCGTCTGCGGTATGTGGGAAAGGTTATGG-BHQ1-3' (probe), and those for N were 5'-GGGGAACTTCTCCTGCTAGAAT-3' (F), 5'-CAGACATTTTGCTCTCAAGCTG-30' (R), and 5'-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3' (probe). The methods were based on the criteria provided by the National Health Commission of the People’s Republic of China[14].
Liver test abnormalities were defined as elevations of the following liver enzymes in serum: ALT > 50 U/L (male) or 40 U/L (female), AST > 40 U/L (male) or 35 U/L (female), and total bilirubin (TBIL) > 17.1 μmol/L. Liver injury was defined as ALT and/or AST more than 3× the upper limit of normal (ULN) and/or TBIL more than 2 × ULN[15].
Viral shedding was defined as the cycle threshold (Ct) values of both ORF1ab and N greater than 35. The viral shedding time was defined as the duration from the first positive nucleic acid test to the first negative result (two consecutive negatives). Patients were discharged with significantly recovered respiratory symptoms and two consecutive negative nucleic acid tests.
Statistical analysis
Data were analyzed by IBM SPSS (version 26.0). Continuous variables were described by the means ± standard deviation (SD) or medians and interquartile range (IQR), while categorical variables were described by the frequency and percentage. For continuous variable comparison, independent sample T tests or one-way analysis of variance were used for normally distributed data, and Mann–Whitney U tests or Kruskal–Wallis tests were used for nonnormally distributed data. For categorical variable comparison, chi-square tests or Fisher's exact tests were applied. Ordinal logistic regression analyses were used to identify risk factors associated with liver test abnormalities, and multiple linear regression analyses were used to identify factors associated with the viral shedding time. P < 0.05 (two-sided) was considered statistically significant. The results of statistical ayalyses can be re-verified if any party wishes to confirm the credibility.
RESULTS
Study design and participant criteria
The participant recruitment process is shown in Figure 1. In total, 429 patients in Ningbo Makeshift Hospital and 256 patients in Dapengshan Makeshift Hospital were selected according to the inclusion criteria. Excluding 17 ordinary cases and 7 patients who were transferred to designated hospitals (4 cases of pulmonary tuberculosis diagnosed with CT scan, one case of acute asthma attack, one case of acute appendicitis, and one case of persistent abdominal pain), 661 participants were included in the final sample.
Figure 1.
Inclusion flow chart.
As shown in Table 1, the median age was 33 years (IQR 27-44 years), 331 (50.1%) were male, and the median BMI was 22.8 kg/m2 (IQR 20.7-25.2). A total of 130 (19.7%) had underlying diseases, of whom 57 (8.6%) had liver diseases. 45 (6.8%) had NAFLD, 11 (1.7%) had hepatitis B, and 1 (0.2%) had both. A total of 425 (64.3%) patients developed a fever, and 619 (93.6%) patients had mild cases. Eighty-three (12.6%) patients had liver test abnormalities and 6 (0.9%) had liver injuries. The number of patients with elevations in ALT, AST and TBIL was 53 (8.0%), 61 (9.2%), and 4 (0.6%), respectively, with a majority of mild liver test abnormalities (Supplementary Table 1). Some patients reported medicines taken before admission, including traditional Chinese medicine (382, 57.8%), nonsteroidal anti-inflammatory drugs (NSAIDs) (89, 13.5%), and other medicines for cold (91, 13.8%).
Table 1.
Characteristics of 661 patients with coronavirus disease 2019 by liver tests
|
Liver tests
|
||||
|
Characteristics
|
Normal
|
Abnormal
|
Injury
|
Total
|
| Number (%) | 578 (87.4) | 77 (11.6) | 6 (0.9) | 661 |
| Age, yr, median (IQR) | 33 (27-44) | 33 (29.5-40.5) | 32.5 (30-48) | 33 (27-44) |
| 14-29 | 209 (36.1) | 19 (24.7) | 1 (16.7) | 229 (34.6) |
| 30-49 | 271 (46.9) | 43 (55.8) | 4 (66.7) | 318 (48.1) |
| ≥ 50 | 98 (17.0) | 15 (19.5) | 1 (16.7) | 114 (17.2) |
| Males, n (%) | 280 (48.4) | 47 (61.0) | 4 (66.7) | 331 (50.1) |
| BMI, kg/m2, median (IQR) | 22.7 (20.4-25.0) | 23.5 (21.2-26.5) | 25.8 (23.0-28.8) | 22.8 (20.7-25.2) |
| < 18.5 | 33 (5.7) | 3 (3.9) | 0 (0) | 36 (5.4) |
| 18.5-23.9 | 320 (55.4) | 37 (48.1) | 3 (50) | 360 (54.5) |
| 24-27.9 | 149 (25.8) | 24 (31.2) | 0 (0) | 173 (26.2) |
| > 28 | 38 (6.6) | 10 (13.0) | 3 (50) | 51 (7.7) |
| Comorbidities, n (%) | ||||
| Hypertension | 36 (6.2) | 3 (3.9) | 1 (16.7) | 40 (6.1) |
| Diabetes | 22 (3.8) | 2 (2.6) | 2 (33.3) | 26 (3.9) |
| Liver disease | 30 (5.2) | 24 (31.2) | 3 (50) | 57 (8.6) |
| Disease type, n (%) | ||||
| Asymptomatic cases | 37 (6.4) | 5 (6.4) | 0 (0) | 42 (6.4) |
| Mild cases | 541 (93.6) | 72 (93.5) | 6 (100) | 619 (93.6) |
| Symptoms, n (%) | ||||
| Fever | 371 (64.2) | 51 (66.2) | 3 (50) | 425 (64.3) |
| Fatigue | 276 (47.8) | 37 (48.1) | 4 (33.3) | 317 (48.0) |
| Respiratory symptoms | 483 (83.6) | 64 (83.1) | 6 (100) | 553 (83.7) |
| GI symptoms | 170 (29.4) | 26 (33.8) | 1 (16.7) | 197 (29.8) |
| Medication | ||||
| Chinese medicine | 336 (58.1) | 40 (51.9) | 6 (100) | 382 (57.8) |
| NSAIDs | 77 (13.3) | 11 (14.3) | 1 (16.7) | 89 (13.5) |
| Other medicines for cold | 82 (14.2) | 9 (11.7) | 0 (0) | 91 (13.8) |
IQR: Interquartile range; BMI: Body mass index; GI: Gastrointestinal; NSAIDs: Nonsteroidal anti-inflammatory drugs.
Clinical features of COVID-19 patients with liver test abnormalities
As shown by a univariate logistic regression model, liver test abnormalities were significantly associated with male sex [odds ratio (OR) 1.699, 95% confidence interval (CI) 1.061-2.721, P = 0.027], obesity (OR 2.707, 95%CI 1.384-5.291, P = 0.004) and a history of liver disease (OR 2.707, 95%CI 1.384-5.291, P = 0.004) but not with age, disease type, clinical manifestations or medication history (Figure 2). The correlated factors in the univariate model were taken as key factors. A multivariate logistic regression model for key factors indicated that liver test abnormalities were only associated with a history of liver disease (OR 8.004, 95%CI 4.319-14.835, P < 0.001). A multivariate logistic regression model for all factors showed that liver test abnormalities were significantly associated with the age of 30-49 years (compared with age 14-30 years, OR 1.970, 95%CI 1.073-3.618, P = 0.029), male sex (OR 1.728, 95%CI 1.005-2.971, P = 0.048), and a history of liver disease (OR 8.265, 95%CI 4.315-15.831, P < 0.001). Based on the above models, liver test abnormalities had a reliable correlation with a history of liver disease, and a potential correlation with male sex and obesity.
Figure 2.
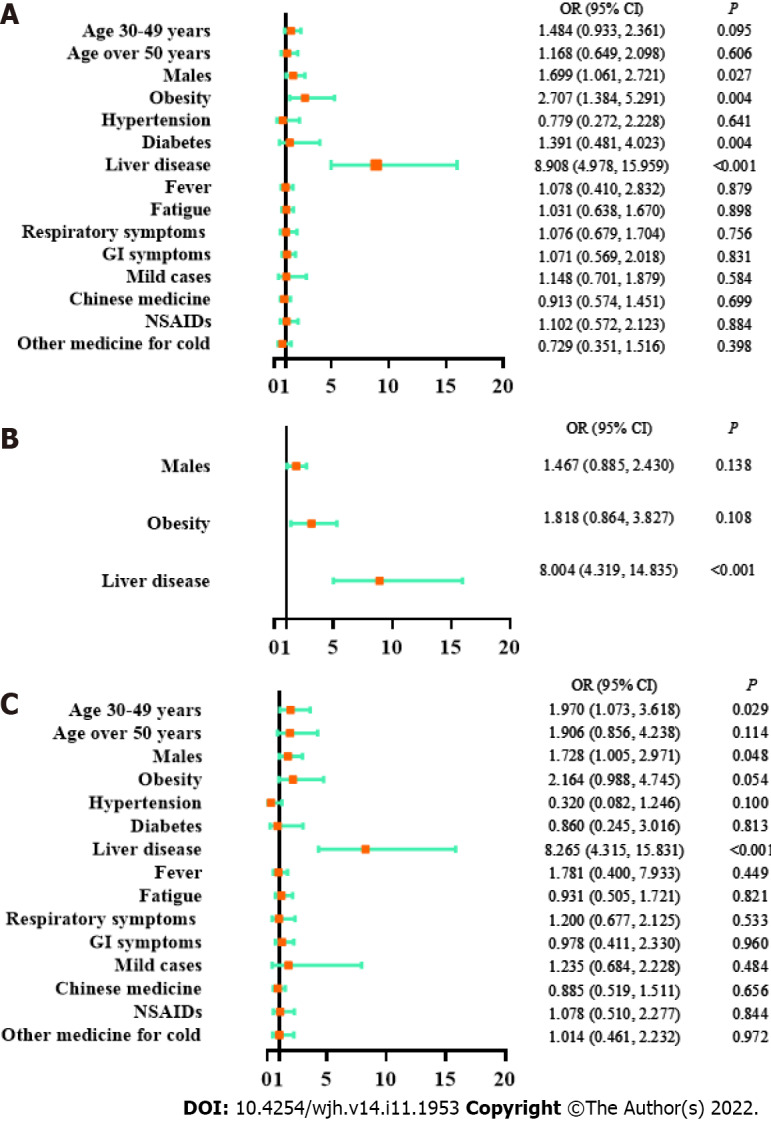
Logistic regression models on factors associated with liver tests. A: Univariate logistic regression model; B: Multivariate logistic regression model on key factors; C: Multivariate logistic regression model on all factors; age: vs age 14-29 years; GI: Gastrointestinal; NSAIDs: Nonsteroidal anti-inflammatory drugs; OR: Odds ratio; CI: Confidence interval.
As revealed by all three models in Supplementary Figure 1, elevated ALT had a firm correlation with obesity (univariate logistic regression: OR 3.82, 95%CI 1.84-7.94, P < 0.001; multivariate logistic regression for key factors: OR 2.57, 95%CI 1.15-5.70, P = 0.021; multivariate logistic regression for all factors: OR 3.31, 95%CI 1.37-7.98, P = 0.009) and a history of liver disease (univariate logistic regression: OR 9.54, 95%CI 5.01-18.16, P < 0.001; multivariate logistic regression for key factors: OR 8.30, 95%CI 4.21-16.36, P < 0.001; multivariate logistic regression for all factors: OR 9.05, 95%CI 4.31-19.01, P < 0.001). Therefore, ALT elevation was reliably correlated with obesity and a history of liver disease.
A univariate logistic regression model indicated that elevated AST was associated with a history of liver disease (OR 8.18, 95%CI 4.40-15.24, P < 0.001, Supplementary Figure 2). A multivariate logistic regression model on all factors showed that AST elevation was associated with older age (age of 30-49 years vs age of 14-29 years: OR 2.48, 95%CI 1.18-5.20, P = 0.016; age over 50 years vs. age of 14-29 years: OR 2.81, 95%CI 1.14-6.97, P = 0.025) and a history of liver disease (OR 7.84, 95%CI 3.89-15.78, P < 0.001) (Supplementary Figure 2). Therefore, the AST elevation had a reliable correlation with a history of liver disease, and a potential correlation with age over 30 years.
In summary, obesity and a history of liver disease were risk factors for abnormal liver test results, and male sex and age over 30 years were potential risk factors. These findings are of great clinical significance for evaluating high-risk subjects with liver abnormalities among asymptomatic and mild COVID-19 patients.
Association between viral shedding time and liver test abnormalities
The association between the viral shedding time and abnormal liver test results was analyzed in 657 participants after excluding 4 subjects with missing data. As shown in Table 2, the viral shedding time of patients with AST ≥ 2 × ULN were significantly prolonged (< 2 × ULN vs ≥ 2 × ULN, viral shedding time: 10.51 ± 2.76 d vs 12.40 ± 3.57 d, P = 0.033). As suggested by three models in Figure 3, AST ≥ 2 × ULN had a reliable correlation with the viral shedding time (univariate linear regression: coefficient 1.893, 95%CI 0.158-3.628, P = 0.033; multivariate linear regression of key factors: coefficient 1.862, 95%CI 0.140-3.584, P = 0.034; multivariate linear regression for all factors: coefficient 2.778, 95%CI 0.615-4.942, P = 0.012). In contrast, there was no significant correlation between ALT elevation and the viral shedding time. In addition, mild cases or female sex were associated with a longer viral shedding time (Table 2, Figure 3).
Table 2.
Comparison of viral shedding time by liver tests and other characteristics
|
Characteristics
|
|
Viral shedding time (day, mean ± SD)
|
P
value
|
| Age, yr | 14-29 | 10.31 ± 2.60 | 0.129 |
| 30-49 | 10.55 ± 2.78 | ||
| ≥ 50 | 10.96 ± 3.11 | ||
| Gender | Male | 10.27 ± 2.77 | 0.015 |
| Female | 10.80 ± 2.78 | ||
| BMI, kg/m2 | < 27.9 | 10.62 ± 2.67 | 0.487 |
| ≥ 28 | 10.90 ± 3.11 | ||
| Comorbidities | Hypertension | 10.51 ± 3.16 | 0.958 |
| Diabetes | 11.04 ± 3.37 | 0.364 | |
| Liver disease | 10.41 ± 3.01 | 0.725 | |
| Disease type | Asymptomatic cases | 9.26 ± 3.16 | 0.002 |
| Mild cases | 10.62 ± 2.73 | ||
| ALT | < 2×ULN | 10.54 ± 2.78 | 0.983 |
| ≥ 2×ULN | 10.56 ± 2.74 | ||
| AST | < 2×ULN | 10.51 ± 2.76 | 0.033 |
| ≥ 2×ULN | 12.40 ± 3.57 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; ULN: Upper limit of normal; SD: Standard deviation.
Figure 3.
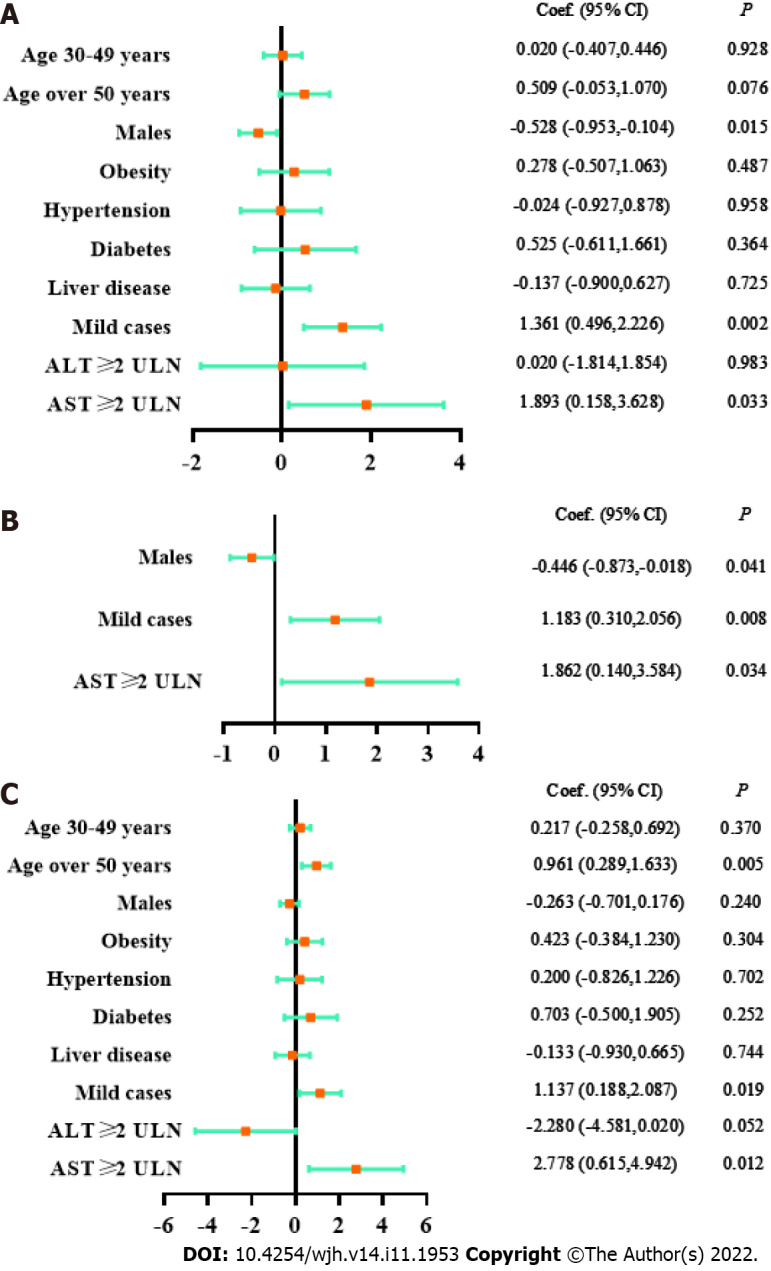
Linear regression models on factors associated with viral shedding time. A: Univariate logistic regression model; B: Multivariate logistic regression model on key factors; C: Multivariate logistic regression model on all factors; age: vs age 14-29 years; ULN: Upper limit of normal; Coef.: Coefficient; CI: Confidence interval.
The correlations between the AST elevation or disease types and the viral shedding time were further analyzed. As shown in Supplementary Table 2, the viral shedding time of mild cases with AST ≥ 2 × ULN was significantly prolonged (AST ≥ 2 × ULN & mild cases vs AST < 2 × ULN & mild cases vs AST < 2 × ULN & asymptomatic carriers: 12.40 ± 3.57 d vs 10.59 ± 2.71 d vs 9.26 ± 3.16 d, P = 0.001). Therefore, it is suggested that great attention should be given to the liver test results of asymptomatic and mild COVID-19 patients, which has crucial clinical significance for predicting the viral shedding time.
DISCUSSION
To the best of our knowledge, this is the first clinical study focusing on liver test abnormalities in asymptomatic and mild COVID-19 patients. In the current epidemic of the Omicron BA2.2 variant, 12.6% of COVID-19 patients developed liver test abnormalities, and 0.9% developed liver injuries. The majority of the liver test abnormalities were mild. The ratio of abnormal liver tests in our study was lower than previous data of 14%-69%[2,3]. One possible reason for this was that our participants were all asymptomatic carriers or mild cases, among whom abnormal liver test results were uncommon. Previous studies revealed normal ALT and AST levels in asymptomatic patients, which were significantly lower than those with symptomatic infection[16]. In contrast, our study showed no differences in liver tests between asymptomatic carriers and mild cases. The probable reason was that all of the symptomatic patients in our study were mild cases, which weakened the difference between the two groups.
As shown in our study, the risk factors for liver test abnormalities in asymptomatic and mild COVID-19 patients included obesity and a history of liver disease, and the potential risk factors included male sex and age over 30 years. Previous studies suggested that preexisting chronic liver diseases, such as NAFLD, chronic hepatitis B, and alcoholic liver disease, were high-risk factors for liver injury in COVID-19[17-19]. Consistent with these findings, our study verified a strong correlation between abnormal liver test results and a history of liver disease. Virus-induced cytokine storms, impaired mitochondrial activity, or endoplasmic reticulum stress may aggravate the preexisting liver disease, which further leads to the progression of liver injury[20]. In addition, liver test abnormalities were more common in males, which was similar to a previous study[21]. The higher estrogen level in females may play a protective role[22]. In addition, elevated ALT was strongly associated with obesity. This may be due to a high ratio of NAFLD patients among obese individuals, and fatty liver diseases can result in elevated ALT levels[18]. There was a potential correlation between elevated AST and age over 30 years. Since AST is known to reflect the injury of organs, such as myocardium and skeletal muscle, these organic injuries may occur more frequently in elderly patients with COVID-19.
Medications for COVID-19 treatment, such as antibiotics and antiviral medications (e.g., lopinavir/ritonavir) have been reported to cause liver injury[10]. This study focused on the liver test features of COVID-19 patients in the early stage of the disease. These patients had rarely taken antibiotics or antiviral medications, while some had taken Chinese medicine, NSAIDs or other cold medicines for a short period. Importantly, no association was found between these medications and liver test abnormalities in COVID-19 patients.
As reported in hospitalized COVID-19 patients, abnormal liver test results at admission were associated with a prolonged hospitalization time and viral shedding time[10,11]. Early elevation of AST was closely related to mortality, suggesting that it might be a predictor of poor prognosis for COVID-19[23]. Similarly, we also found a reliable association between the increase in AST and a prolonged viral shedding time in asymptomatic and mild COVID-19 patients. We speculate that the lower immunity of patients with elevated AST levels might affect the viral clearance. The viral shedding time was even longer in mild cases with elevated AST. Similar results have been previously revealed in which the viral shedding duration was longer in symptomatic patients than in asymptomatic patients[16,24]. Lymphocyte-mediated immune responses may play a vital role[15].
Our study has two advantages. First, unlike studies concerning severe cases[2,4,15,23], we focused on the association between liver test results and viral shedding time, especially in patients infected with the Omicron BA2.2 variant, with a relatively high proportion of asymptomatic carriers and mild cases. The viral shedding time for patients with elevated AST were significantly longer, especially in mild patients. This provides crucial evidence for identifying high-risk patients with a prolonged viral shedding time. Second, we analyzed the risk factors for liver test abnormalities. Male sex, older age, obesity, and a history of liver disease may increase the risk of liver abnormalities, which can be used to identify high-risk patients with abnormal liver test results.
One limitation of our research is that we only measured the levels of ALT, AST, and TBIL, but failed to obtain other liver test results, such as γ-glutamyl transpeptidase and alkaline phosphatase, and we did not monitor the dynamic alterations of the liver tests. The absence of these indicators may have led to an incomplete assessment of liver abnormalities. Another limitation is that we only recruited participants from two makeshift hospitals in Ningbo, but not from multiple regions. This may have led to a limited sample size and underrepresentation of the sample.
CONCLUSION
We evaluated the liver test features of asymptomatic and mild COVID-19 patients during the epidemic of the Omicron BA2.2 variant. Patients with a history of liver disease or male patients were more likely to develop liver test abnormalities or liver injury. Those who were obese or had a history of liver disease tended to develop ALT elevation, and those who were aged over 30 years or had a history of liver disease tended to develop AST elevation. The increase in AST in the early stage was closely associated with a prolonged viral shedding time, especially in mild cases. Attention should be given to the liver test data of asymptomatic and mild COVID-19 patients, which has important clinical significance for evaluating the viral shedding time.
ARTICLE HIGHLIGHTS
Research background
Data on liver test abnormalities in asymptomatic and mild coronavirus disease 2019 (COVID-19) patients are rare.
Research motivation
This study evaluated the clinical characteristics of asymptomatic and mild COVID-19 patients with abnormal liver test results.
Research objectives
We aimed to determine the liver test abnormalities in asymptomatic and mild COVID-19 patients and their association with the viral shedding time, providing suggestions for health policymakers and medical practitioners.
Research methods
Clinical information and viral shedding time were collected retrospectively from 661 laboratory-confirmed asymptomatic and mild COVID-19 patients. Univariate and multivariate logistic regression models were performed in statistical analyses.
Research results
Elevated alanine aminotransferase was associated with obesity and a history of liver disease. Elevated aspartate transaminase (AST) was correlated with a history of liver disease age over 30 years. There was a correlation between AST ≥ 2× the upper limit of normal and a longer vital shedding time.
Research conclusions
Obesity and a history of liver disease are risk factors for liver test abnormalities. Liver test abnormality in asymptomatic and mild COVID-19 patients has clinical correlation with the viral shedding time.
Research perspectives
Attention should be given to liver tests in asymptomatic and mild COVID-19 patients, which has crucial clinical significance for evaluating the viral shedding time.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee of Ningbo First Hospital (No. 2022RS069).
Informed consent statement: The data of our study was collected retrospectively, and the informed consent was exempted by the Ethics Committee of Ningbo First Hospital.
Conflict-of-interest statement: All the authors declare that they have no conflict of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 1, 2022
First decision: October 17, 2022
Article in press: November 21, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia; Nasa P, United Arab Emirates S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Si-Yi Yu, Department of Gastroenterology, Ningbo First Hospital, Ningbo 315010, Zhejiang Province, China.
Jia-Rong Xie, Department of Gastroenterology, Ningbo First Hospital, Ningbo 315010, Zhejiang Province, China.
Jun-Jun Luo, Department of Cardiology, Ningbo First Hospital, Ningbo 315010, Zhejiang Province, China.
Hong-Peng Lu, Department of Gastroenterology, Ningbo First Hospital, Ningbo 315010, Zhejiang Province, China.
Lei Xu, Department of Gastroenterology, Ningbo First Hospital, Ningbo 315010, Zhejiang Province, China.
Jun-Jie Wang, Department of Information Technology, Ningbo First Hospital, Ningbo 315010, Zhejiang Province, China.
Xue-Qin Chen, Department of Traditional Medicine, Ningbo First Hospital, Ningbo 315010, Zhejiang Province, China. cxq2316@163.com.
Data sharing statement
No additional data are available.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. (accessed on 3 Sep 2022). Available online: https://covid19.who.int/
- 2.Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol. 2021;13:522–532. doi: 10.4254/wjh.v13.i5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890–900. doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herta T, Berg T. COVID-19 and the liver - Lessons learned. Liver Int. 2021;41 Suppl 1:1–8. doi: 10.1111/liv.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, Pillay S, San EJ, Msomi N, Mlisana K, von Gottberg A, Walaza S, Allam M, Ismail A, Mohale T, Glass AJ, Engelbrecht S, Van Zyl G, Preiser W, Petruccione F, Sigal A, Hardie D, Marais G, Hsiao NY, Korsman S, Davies MA, Tyers L, Mudau I, York D, Maslo C, Goedhals D, Abrahams S, Laguda-Akingba O, Alisoltani-Dehkordi A, Godzik A, Wibmer CK, Sewell BT, Lourenço J, Alcantara LCJ, Kosakovsky Pond SL, Weaver S, Martin D, Lessells RJ, Bhiman JN, Williamson C, de Oliveira T. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 7.Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. JAMA. 2022;327:583–584. doi: 10.1001/jama.2021.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyer O. Covid-19: Omicron is causing more infections but fewer hospital admissions than delta, South African data show. BMJ. 2021;375:n3104. doi: 10.1136/bmj.n3104. [DOI] [PubMed] [Google Scholar]
- 9.Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, Espy N, Wallis CL, Randhawa AK, Miner MD, Ketter N, Yacovone M, Goga A, Huang Y, Hural J, Kotze P, Bekker LG, Gray GE, Corey L Ubuntu Study Team. High Asymptomatic Carriage With the Omicron Variant in South Africa. Clin Infect Dis. 2022;75:e289–e292. doi: 10.1093/cid/ciac237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang S, Wang R, Li L, Hong D, Ru R, Rao Y, Miao J, Chen N, Wu X, Ye Z, Hu Y, Xie M, Zuo M, Lu X, Qiu Y, Liang T. Liver Injury in Critically Ill and Non-critically Ill COVID-19 Patients: A Multicenter, Retrospective, Observational Study. Front Med (Lausanne) 2020;7:347. doi: 10.3389/fmed.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Zhang W, Chen S. Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. 2022;399:2011–2012. doi: 10.1016/S0140-6736(22)00838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Health Commission of the People’s Republic of China, National Administration of Traditional Chinese Medicine. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (trial version eighth) (in Chinese), Chinese Medicine, 2022(4) [Google Scholar]
- 14.National Health Commission of the People’s Republic of China, Guidelines for the prevention and control of coronavirus disease 2019 (trial version eighth) (in Chinese), 2021 (accessed on 3 Sep 2022). Available from: http://www.gov.cn/xinwen/2021-05/14/content_5606469.htm .
- 15.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han H, Xu Z, Cheng X, Zhong Y, Yuan L, Wang F, Li Y, Liu F, Jiang Y, Zhu C, Xia Y. Descriptive, Retrospective Study of the Clinical Characteristics of Asymptomatic COVID-19 Patients. mSphere. 2020;5 doi: 10.1128/mSphere.00922-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study- NCT 04345640) Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, Hsu CY, Steiner CA, Louissaint J, Gunaratnam NT, Sharma P. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig Dis Sci. 2021;66:3192–3198. doi: 10.1007/s10620-020-06618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160–2163. doi: 10.1111/liv.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Zhang YH, Wang F, Liu B, Li H, Tang GD, Chang ZG, Liu AH, Fu CY, Gao J, Li J. Sex differences in clinical findings among patients with coronavirus disease 2019 (COVID-19) and severe condition. MedRxiv. 2020. (accessed on 3 Sep 2022). Available from: https://www.medrxiv.org/content/10.1101/2020.02.27.20027524v1 .
- 22.Jacobsen H, Klein SL. Sex Differences in Immunity to Viral Infections. Front Immunol. 2021;12:720952. doi: 10.3389/fimmu.2021.720952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B Tongji Multidisciplinary Team for Treating COVID-19 (TTTC) Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295–1302. doi: 10.1016/j.jhep.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Li P, Ding Y, Liu M, Liu L, Yi B, Wu T, Dong H, Lao X, Ding K, Wang H, Zhang D, Tan X, Wang Z, Xu G, Cao G. Epidemiological feature, viral shedding, and antibody seroconversion among asymptomatic SARS-CoV-2 carriers and symptomatic/presymptomatic COVID-19 patients. J Infect Public Health. 2021;14:845–851. doi: 10.1016/j.jiph.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.