Abstract
Background:
Asthma disproportionately affects African-American/Black (AA/B) and Hispanic/Latinx (H/L) patients and individuals with low socioeconomic status (SES), but the relationship between SES and asthma morbidity within these racial/ethnic groups is inadequately understood.
Objective:
To determine the relationship between SES and asthma morbidity among AA/B and H/L adults with moderate-severe asthma using multi-domain SES frameworks and mediation analyses.
Methods:
We analyzed enrollment data from the PREPARE randomized trial, evaluating inhaled corticosteroid supplementation to rescue therapy. We tested for direct and indirect relationships between SES and asthma morbidity using structural equation models. For SES we used a latent variable defined by poverty, education, and unemployment. For asthma morbidity we used self-reported asthma exacerbations in the year before enrollment (corticosteroid bursts, emergency room (ER)/urgent care (UC) visits, or hospitalizations), and asthma control test (ACT®) scores. We tested for mediation via health literacy, perceived stress, and self-reported discrimination. All models adjusted for age, sex, BMI, ethnicity, and comorbidities.
Results:
Among 990 AA/B and H/L adults, low SES (latent variable) was directly associated with hospitalizations (β=0.24) and worse ACT scores (β=0.20). Stress partially mediated the relationship between SES and increased ER/UC visits and worse asthma control (β=0.03 and =0.05, respectively). Individual SES domains were directly associated with asthma morbidity. Stress mediated indirect associations between low educational attainment and unemployment with worse asthma control (β=0.05 and =0.06, respectively).
Conclusion:
Lower SES is directly, and indirectly through stress, associated with asthma morbidity among AA/B and H/L adults. Identification of stressors and relevant management strategies may lessen asthma-related morbidity among these populations.
Keywords: Minority health, mediation analysis, structural equation modeling, income, employment, education, severe persistent asthma, stress, discrimination, health literacy, asthma exacerbations
Introduction
Asthma affects more than 25 million individuals in the United States (US)1 and is associated with substantial morbidity1 and healthcare costs.2 African American/Black (AA/B) and Hispanic/Latinx (H/L) patients disproportionately bear this burden, and experience higher rates of asthma morbidity compared to Whites.3 These higher asthma morbidity rates amongst AA/B and H/L are likely due to multifactorial causes related to inequitable distribution of social determinants of health.4–14 For example, a recent pediatric study found that associations between hospital readmissions for asthma exacerbations and racial genetic ancestry are mostly mediated by socioeconomic status (SES).15 SES is a construct that is variably defined by multiple indicators, including income, education, and employment, among others.16,17 Several of these SES-related indicators are associated with worse asthma outcomes,18–21 including low income,22,23 unemployment,24 and low educational attainment.25 Relationships between SES and asthma outcomes may be mediated by other factors in the causal pathway.26 Perceived stress, inadequate health literacy, and perceived discrimination are associated with both low SES27–29 and worse asthma outcomes.30–33 However, empirical data about potential mediators of the association between low SES and asthma morbidity are lacking, particularly among AA/B and H/L adults. SES is correlated with race and ethnicity in the US,34 where AA/B and H/L individuals tend to have lower incomes and attain lower levels of education.35 Thus, most research on the role of SES in asthma morbidity has been unable to disentangle the independent contributions from SES and race/ethnicity on asthma morbidity.36 Moreover, the studies that have accounted for both race/ethnicity and SES have included few AA/B and H/L participants, have been conducted in children, have recruited participants from a single center, have assessed a single SES domain (e.g., only assessed income), or have evaluated a limited number of asthma morbidity measures.37–40 Therefore, an in-depth analysis of SES among AA/B and H/L adults with asthma is warranted. For that reason, we analyzed poverty, unemployment, and education; and the mediatory effects of health literacy, perceived stress, and perceived discrimination on asthma morbidity measures among AA/B and H/L adults with moderate to severe persistent asthma.
Methods
We used enrollment data from the Patient-Centered Outcomes Research Institute (PCORI)-funded PeRson EmPowered Asthma RElief (PREPARE) trial; a description of the study population, trial aims and methods, and data collected at enrollment has been published.41 One thousand two hundred and one participants were recruited from November 2017 through March 2020 from 19 study sites located across the United States, including Puerto Rico, encompassing a broad range of practice sizes, healthcare systems, and specialties (e.g., allergy/immunology, pulmonology, and primary care clinics), with the majority recruited from primary care. Recruitment methods varied by site, but participants were commonly directly enrolled from affiliated clinicians’ practices. Participants had a physician’s diagnosis of asthma for ≥1 years prior to enrollment, and either uncontrolled disease (ACT<= 19) or ≥1 asthma exacerbations requiring systemic corticosteroids in the year prior enrollment. To summarize, PREPARE is a multi-institutional, pragmatic, open-label, randomized trial investigating whether a “Patient-Activated Reliever-Triggered Inhaled CorticoSteroid” (PARTICS) intervention can reduce asthma exacerbations relative to usual care among AA/B and H/L adults with moderate to severe persistent asthma.
For this cross-sectional ancillary study, we used a multi-domain SES framework which included a latent variable with three measures: poverty, unemployment, and low educational attainment. Poverty status was calculated using income and household size compared against the federal poverty income guidelines.42 Poverty guidelines are established for the 48 continental states and typically extended to US territories like Puerto Rico.43,44 Participant incomes were captured in $10,000/year increments, starting at “less than $10,000/year” through “more than $75,000/year”. Educational attainment was divided into three levels: no high school diploma, high school diploma, or greater than a high school diploma. Employment status was classified as either employed or unemployed.
We tested for effect mediation by perceived stress, health literacy, and perceived discrimination on the effect of SES on asthma morbidity. Self-perceived stress was measured using the 4-item short form of the Perceived Stress Scale;45 a participant was classified as having high perceived stress if his/her score was in the cohort’s top quintile. Health literacy adequacy was determined using the 3-question version of the Brief Health Literacy Scale (BHLS).46 Self-perceived discrimination was measured using the Everyday Discrimination Scale;47 a participant was classified as having high perceived discrimination if his/her score was in the cohort’s top quintile.
We measured our study outcome, asthma morbidity, in several ways. First, we used measures of self-reported asthma exacerbations [outpatient corticosteroid bursts for asthma, emergency room (ER)/urgent care (UC) visits and hospitalizations] in the year prior to enrollment. We defined steroid bursts and ER/UC visits for asthma as ordinal variables (0, 1, 2, ≥3), while asthma hospitalizations were dichotomized as 0 vs. ≥1. Secondly, we assessed scores from three participant-completed surveys: the Asthma Control Test (ACT®48), a validated 5-item questionnaire which assesses level of asthma control; the Asthma Symptom Utility Index (ASUI),49 a validated 10-item questionnaire which assesses preference-based quality of life; and the asthma Activities, Persistent triGgers, Asthma medications and Response to therapy (APGAR),50 a validated 5-domain questionnaire which assesses asthma control and is designed for use in primary care.
Statistical Analysis
Demographic and clinical characteristics were categorized by poverty status and presented using descriptive statistics, with Chi-square tests for categorical variables, and student’s t-tests for continuous variables, where appropriate. We fitted structural equation models (SEM) in order to determine the relationship of SES (as a 3-domain latent variable, and individually with each measure) and mediators with asthma morbidity measures. The SES latent variable was estimated by analyzing the variance and covariance of its 3 domains--poverty, unemployment, and low educational attainment. We used mediation analyses to assess for indirect (i.e., effect of the SES latent variable on the asthma morbidity outcome via perceived stress, health literacy, or perceived discrimination) and direct pathways (i.e., the effect of the SES latent variable directly on the asthma morbidity outcome). Unmeasured mediators may be reflected in the error terms for pathway effect estimates but are otherwise unaccounted for in these models. The strength of association of a pathway was determined using model-based beta-coefficients. Beta coefficients for direct pathway estimates equal the standard deviation change in asthma morbidity outcome for one standard deviation change in the SES latent variable (or individual SES domain variables) score. Beta coefficients for indirect pathway estimates equal the product of the beta for the pathway between the SES latent variable to the mediator multiplied by the beta for the pathway from the mediator to the asthma morbidity outcome (Figure 1). The contribution of indirect effects through mediation was determined from the product of path coefficients and standard error estimates using the delta method proposed by Sobel.51 Model adequacy was tested through chi-square and adjusted goodness of fit measures.52 ACT, ASUI, and APGAR were analyzed as continuous outcomes. Steroid bursts, ER/UC visits, and hospitalizations were grouped according to the categories described earlier and analyzed as continuous, ordinal variables within their respective models. All models were adjusted for age, sex, H/L ethnicity, body mass index (BMI), and medical comorbidity counts (heart disease, cancer [excluding non-melanoma skin], stroke, diabetes, chronic kidney disease, COPD, HIV/AIDS, depression, and/or sleep disorders). Additional models adjusted by smoking status, smoke exposure, and depression (assessed by the Patient Health Questionnaire-2 [PHQ-2] depression screen53. To account for federal poverty guidelines being established for the 48 continental states42 even if typically extended to Puerto Rico,43,44 we conducted sensitivity analyses excluding Puerto Rican residents (n=101).
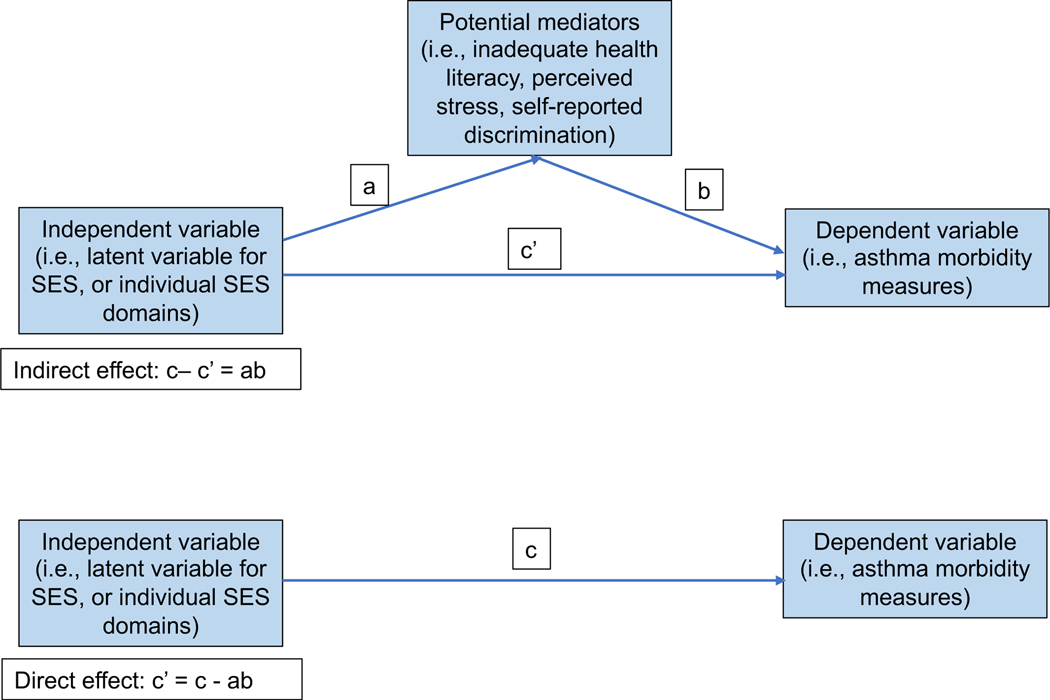
Figure 1 legend.
Conceptual diagram of the Structural Equation Model (SEM) depicting direct and indirect associations between independent (socioeconomic status latent variable or its individual domains) and dependent variables (asthma morbidity measures) and potential mediators (inadequate health literacy, perceived stress, and self-reported discrimination). The variable “C” refers to the total effect of the independent variable on the dependent variable, including direct and indirect effects.
Results
Data on SES domains, mediators and asthma morbidity measures were available for 990 PREPARE trial participants; 211 participants preferred not to disclose their income and were thus excluded from this analysis. Table 1 shows the demographic and clinical characteristics of study participants included in the current analysis, categorized by poverty status (i.e., one of the three SES latent variable domains). Most (83.9%) participants were female, obese (69.6%), and had ≥1 medical comorbidity (71.0%). Most participants also lived in poverty (N=517, 52.2%), were unemployed (58.0%), had adequate health literacy (83.8%), experienced ≥1 asthma exacerbation(s) and one asthma-related ER visit(s) in the previous year (60.2%), and had uncontrolled asthma (i.e., ACT score<20; 86.2%). Those living in poverty, relative to those who were not, were more likely to speak Spanish (26.9% vs. 14.0%, p<0.001), be unemployed (73.7% vs. 40.8%, p<0.001) and not a high school graduate (22.2% vs. 5.3%, p<0.001), and to have multiple medical comorbidities (i.e., two or more: 54.7% vs. 42.1%, p<0.001), inadequate health literacy (23.8% vs. 7.8%, p<0.001), to be depressed (32.7% vs. 19.0%, p<0.001), and to have high self-perceived stress levels (36.9% vs. 23.7%, p<0.001). In addition, those living in poverty, relative to those who were not, were more likely to have been hospitalized for asthma (23.6% vs. 14.0%, p<0.001) and to have worse asthma control (mean ACT score =14.0 vs. 15.3, p<0.001) but similar rates of ER/UC visits (39.3% vs. 35.7% with ≥2 ER/UC visits in the year prior to enrollment, p=0.405) and corticosteroid bursts for asthma (36.6% vs. 37.0% with 2+ corticosteroid bursts for asthma in the year prior to enrollment, p=0.669).
Table 1.
Demographic and clinical characteristics of PREPARE trial participants, categorized by poverty guideline thresholds
| Characteristic | Overall cohort | Poverty^ | P-value | |
|---|---|---|---|---|
| N=990 | No (n=473) | Yes (n=517) | ||
| Demographics | ||||
| Age, in years | 49 (13) | 49 (13) | 48 (13) | 0.284 |
| Female | 831 (83.9%) | 393 (83.1%) | 438 (84.7%) | 0.4846 |
| Race/ethnicity | ||||
| H/L | 486 (49.1%) | 217 (45.9%) | 269 (52.0%) | 0.053 |
| AA/B | 504 (50.9%) | 256 (54.1%) | 248 (48.0%) | |
| Preferred language | ||||
| English | 785 (79.3%) | 407 (86.0%) | 378 (73.1%) | <0.001 |
| Spanish | 205 (20.7%) | 66 (14.0%) | 139 (26.9%) | |
| Clinical features | ||||
| BMI | ||||
| Overweight (25–29.9) | 207 (20.9%) | 105 (22.2%) | 102 (19.7%) | 0.457 |
| Obese (≥30) | 689 (69.6%) | 323 (68.3%) | 366 (70.8%) | |
| Medical comorbidities | ||||
| 0 | 276 (27.9%) | 154 (32.6%) | 122 (23.6%) | <0.001 |
| 1 | 222 (22.4%) | 120 (25.4%) | 102 (19.7%) | |
| 2+ | 482 (48.6%) | 199 (42.1%) | 283 (54.7%) | |
| Smoking Status | ||||
| Current smoker | 75 (7.6%) | 30(6.3%) | 45(8.7%) | 0.079 |
| Former smoker | 239 (24.1%) | 104(22.0%) | 135(26.1%) | |
| Non-smoker | 676 (68.3%) | 339(71.7%) | 337(65.2%) | |
| Living in a smoking environment | 216 (21.8%) | 91 (19.2%) | 125 (24.2%) | 0.06 |
| PHQ-2# | 259 (26.2%) | 90 (19.0%) | 169 (32.7%) | <0.001 |
| SES domains | ||||
| Educational attainment | ||||
| High school or more | 850 (85.9%) | 448 (94.7%) | 402 (77.8%) | <0.001 |
| Less than high school | 140 (14.1%) | 25 (5.3%) | 115 (22.2%) | |
| Employment | ||||
| Employed | 416 (42.0%) | 280 (59.2%) | 136 (26.3%) | <0.001 |
| Not Employed | 574 (58.0%) | 293 (40.8%) | 381 (73.7%) | |
| SES mediators | ||||
| Inadequate Health Literacy* | 160 (16.2%) | 37 (7.8%) | 123 (23.8%) | <0.001 |
| High Stress** | 303 (30.6%) | 112 (23.7%) | 191 (36.9%) | <0.001 |
| High Discrimination*** | 297 (30.0%) | 141 (29.8%) | 156 (30.2%) | 0.901 |
Continuous variables are presented as means, (standard deviation); categorical variables are presented as counts, (proportions).
Participants whose household size-adjusted income is below the federal poverty threshold28 were considered to be living in poverty.
Patient Health Questionnaire-2 (PHQ-2) screens for depression with a 2-week recall.
Total score ranges from 0–6, and scores of ≥3 suggest depression.53
Inadequate health literacy is based on participant responses to the Brief Health Literacy Scale.32
High stress was defined as having a score in the cohort’s top quintile of the 4-item Perceived Stress Scale.31
High discrimination was defined as having a score in the cohort’s top quintile in the Everyday Discrimination Scale.33 Significant values are bolded. AA/B: African-American/Black; BMI: body mass index; H/L: Hispanic/Latinx.
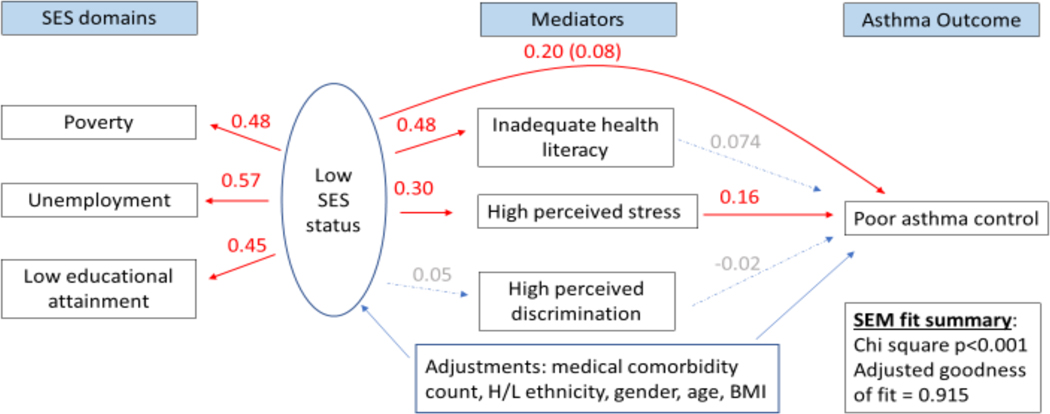
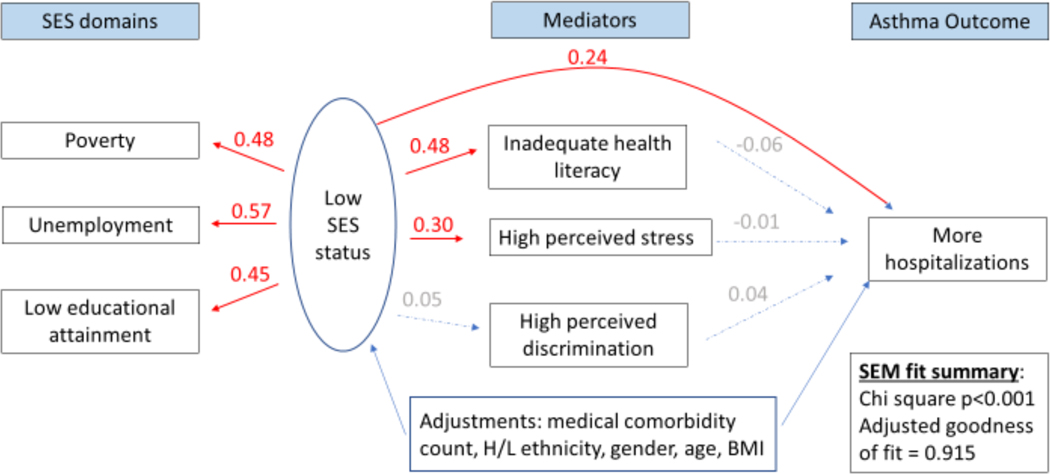
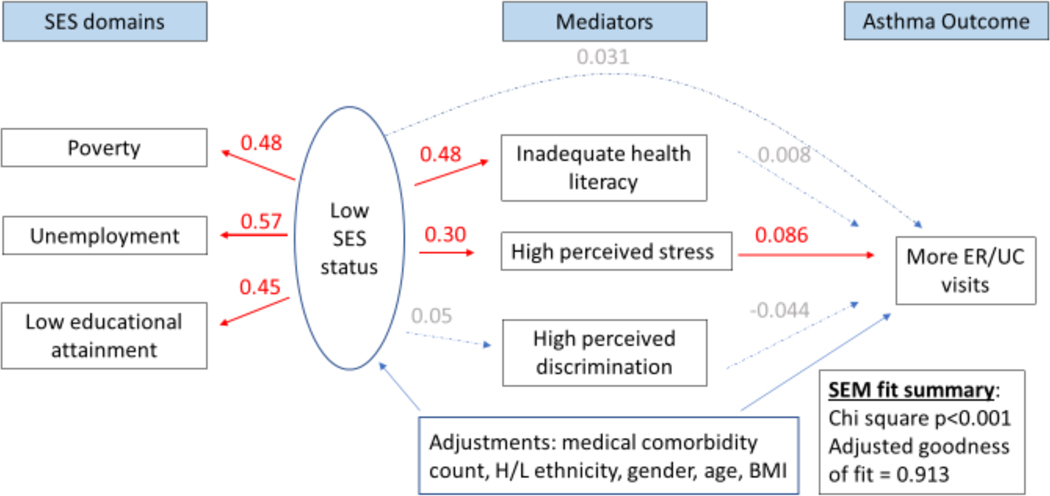
In multivariable analyses, the SEM showed that low SES (as a 3-measure latent variable) was directly associated with a lower ACT score (β=0.20; 95% confidence interval (CI): 0.07–0.33). Specifically, a one standard deviation change in the SES latent variable was associated with a 0.20 standard deviation change in ACT scores. An indirect relationship between low SES and lower ACT scores mediated by stress was also observed (β=0.05; 95%CI: 0.026–0.07) (Figure 2), after adjustment for other covariates. The contributions of direct and indirect effects of SES on ACT scores were 71%, and 29%, respectively. Similar direct and indirect associations between low SES and worse asthma control and preference-based quality of life were identified using the asthma APGAR scores and ASUI respectively (Supplementary figures 1 and 2). We did not find evidence for mediation of the effect of SES through health literacy or self-perceived discrimination on asthma control. Low SES was directly associated with asthma hospitalizations (β=0.24; 95%CI: 0.11–0.38; Figure 3). A one standard deviation change in the low SES latent variable was directly associated with 0.09 additional asthma hospitalizations. No direct association with asthma ER/UC visits was observed, however. No indirect association with asthma hospitalizations was observed through any mediator, yet low SES was indirectly associated with asthma ER/UC visits through an effect mediated by stress (β=0.026; 95%CI: 0.003–0.048) (Figure 4). We did not detect an association between low SES and corticosteroid bursts for asthma exacerbations. All of the above models that evaluated the SES latent variable on multiple asthma morbidity measures were adequately fit, with adjusted goodness of fit >90%, Chi-square for the models p<0.001. Finally, all of these results were robust to additional adjustment by smoking status, smoking environment, and depression.
Figure 2 legend.
Structural equation model (SEM) depicting direct and indirect associations between low socioeconomic status (SES) and poor asthma control mediated by high self-perceived stress. Numbers shown indicate pathway beta coefficients within the SEM. Those in red denote significant associations, while those in gray denote insignificant ones. The latent variable labeled “Low SES status” is composed of three SES domains: income- and household size-determined poverty status,42,43 unemployment, and low educational level attainment. Poor asthma control is indicated by lower Asthma Control Test® scores.48
Figure 3 legend.
Structural equation model (SEM) depicting a direct association between low socioeconomic status (SES) and asthma-related hospitalizations. Numbers shown indicate pathway beta coefficients within the SEM. Those in red denote significant associations, while those in gray denote insignificant ones. The latent variable labeled “Low SES status” is composed of three SES domains: income- and household size-determined poverty status,42,43 unemployment, and low educational level attainment. Hospitalizations are asthma related.
Figure 4 legend.
Structural equation model (SEM) depicting an indirect association between low socioeconomic status (SES) and asthma-related emergency room (ER)/urgent care (UC) visits mediated by high self-perceived stress. Numbers shown indicate pathway beta coefficients within the SEM. Those in red denote significant associations, while those in gray denote insignificant ones. The latent variable labeled “Low SES status” is composed of three SES domains: income- and household size-determined poverty status,42,43 unemployment, and low educational level attainment. ER/UC visits are asthma related.
We found that individual SES measures (i.e., including only poverty, low educational attainment, or unemployment) had similar adjusted goodness of fit (>90%) and chi-square tests (p-values <0.001) for all asthma morbidity measures that were similar to those of the models with the SES latent variable. A one standard deviation change in poverty, however, was directly associated with 0.19 additional asthma hospitalizations (β=0.48; 95%CI: 0.15–0.80) and 1.28-point lower asthma control scores (ACT score β= −0.29; 95%CI: −0.004 to −0.59) but did not show any stress-mediated indirect associations with asthma morbidity outcomes (unadjusted associations between poverty and asthma morbidity measures appear in Supplementary Table 1). Both unemployment and education showed stress-mediated indirect associations with asthma control scores [β=0.06, 95%CI 0.03–0.09; β=0.05; 95%CI: 0.02–0.07, respectively], but only unemployment maintained a stress-mediated indirect association with ER/UC visits [β=0.03; 95%CI: 0.01–0.06]. Analyses excluding Puerto Rican residents (n=101) yielded results consistent with those that analyzed the complete cohort. All of these results were robust to additional adjustment by smoking status, smoking environment and depression.
Discussion
We identified associations between a multi-domain model of SES which included poverty, education, and unemployment and several important asthma morbidity outcomes in a cohort of AA/B and H/L adults with moderate to severe asthma. To our knowledge, this is the first study to identify a relationship between SES and asthma morbidity outcomes using a multi-domain latent variable in adults, and to identify perceived stress as an important mediator of the association between low SES and ER visits for asthma exacerbations and several measures of worse asthma control in adults. Our findings are also novel in that they are based on data from two highly impacted racial/ethnic groups, thus implicating low SES as a risk factor for worse asthma morbidity outcomes independently of race/ethnicity and highlighting the need for policies that address these socioeconomic issues.
Using a SEM modelling approach, we uncovered direct and indirect pathways connecting SES with asthma morbidity outcomes. Both pathways affected asthma control as determined by ACT and asthma APGAR scores, as well as preference-based quality of life (ASUI). We found that the majority of the effect of SES on asthma control was due to direct effects, while high stress mediated 17% of the effect of SES on asthma control. This finding suggests that there are other exposures associated with low SES beyond stress, health literacy and discrimination—for example, access to medical care, access to nutritious foods, pollution, and housing and built environment, among others—that could explain its association with asthma outcomes. The mediator effect of stress was particularly strong, contributing to roughly half of the overall effect of SES on asthma-related ED visits within the constraints of our model. Stress may result from psychological, behavioral (e.g., unhealthy diet, poor sleep)54 or environmental (e.g., pollution, unsafe neighborhoods)30,55 factors, social isolation,56 or systemic racism, all of which could exert an influence on asthma.19,57 We captured perceived stress using the 4-item version of the Perceived Stress Scale, which does not delve into specific stressors. However, single SES component analyses indicated that only unemployment had stress as a mediator on ER visits for asthma, suggesting that unemployment may be an important stressor. We cannot rule out the possibility that employed individuals may be reluctant to leave work for medical care and self-medicate asthma deteriorations at home, resulting in fewer ER/UC visits. Because of our cross-sectional study design we cannot also assess the temporality between stress and unemployment, and thus cannot exclude the possibility that stress leads to unemployment instead of, or in addition to, unemployment leading to stress as a mediator on ER visits for asthma.
The magnitude of our results should be interpreted from a population-level and not a patient-level perspective. For example, we found that a one-standard deviation decrease in the low SES latent variable was associated with a 0.88-point decrease in the ACT score, which is below the minimal clinically important difference (MCID) of 3 points48. However, value thresholds that are clinically meaningful at patient level may be much larger than value thresholds at the population level. For example, decreases in salt consumption on average decrease systolic blood pressure by 4mmHg58 which is a clinically small change in systolic blood pressure at the individual patient-level. But public health strategies to reduce salt consumption decrease all-cause mortality at the population level.59 That being stated, our results also show that individuals with the lowest SES latent variable score (10% of our cohort) on average had 2.7 lower ACT score points relative to those with the highest SES latent variable score (28% of our cohort), which in the range of the individual level MCID. Results in other asthma morbidity measures also further substantiate our findings connecting lower SES with worse asthma control.
The connection between low SES and worse asthma morbidity outcomes has long been established. For example, Schyllert et al confirmed the association between individual SES components (i.e., education and unemployment) and asthma morbidity.38 Yet like most of the literature, this study employed linear analytical models between single SES components and asthma outcomes and did not incorporate mediation. In contrast, latent variables are useful in approximating the complex nature of multidimensional concepts like SES, while mediation analysis helps with understanding mechanism of action especially of abstract concepts like SES. While many studies on SES and asthma do not include AA/B and H/L participants, the 2019 study by Seibert et al analyzed prospective data on 342 adults with asthma from Chicago where 85% of participants were AA/B or H/L, and looked at income, health literacy and asthma outcomes using SEM and mediation.39 The authors found that AA/B and H/L patients, relative to Whites, had poorer asthma quality of life and higher rates of ER visits for asthma, and that these results were indirectly mediated by low health literacy and low income. Our findings complement these results showing that income, analyzed as poverty status, is directly associated with worse asthma morbidity, and expand upon them with our larger sample size representative of several regions of the US and Puerto Rico. Seibert and colleagues sought to understand SES as the mediator of racial/ethnic disparities, while ours sought to identify mediators of a multidomain SES variable on asthma outcomes within AA/B and H/L populations. Their study identified health literacy as a significant mediator between race/ethnicity and worse asthma outcomes while health literacy was not significant in our model. This discrepancy may relate to the different surveys used to capture health literacy [Rapid Estimate of Adult Literacy in Medicine (REALM)60 vs BHLS,46 and single city vs multi-city cohort recruitment. We caution against concluding that inadequate health literacy is not associated with worse asthma outcomes in general, and may reflect our cohort and ascertainment methods, as the majority of participants (84%) in the current study had adequate health literacy. Consistent with our results, Yakubovich et al showed that South African children living in poverty had greater asthma prevalence and asthma “attack” rates and that this effect was mediated by greater psychological stress.40 Our results expand on theirs with our analysis of an adult American cohort, and validation through multiple asthma morbidity outcomes. A prominent role for stress as a mediator of the association between low SES and worse asthma outcomes in adults had not been shown.
Our study has some limitations. First, our results may not be generalizable to children. However, the literature has better addressed the dynamics of SES and asthma outcomes in children compared to adults. Second, the PREPARE trial only enrolled AA/B and H/L participants, and therefore our results may not be generalizable to other racial or ethnic groups. The lack of a White comparison group is a drawback which prevented comparing SES and asthma morbidity between racial/ethnic populations differentially affected by systemic racism, and should be addressed in future studies. However, even without a White comparator group our study design allowed us to disentangle the effects of SES from those of race/ethnicity on asthma morbidity outcomes among a cohort of AA/B and H/L patients—a difficulty which had thus far limited much of the existing literature. By investigating a large cohort of AA/B and H/L individuals, we were able to determine that low SES, regardless of race/ethnicity, is an important driver of worse asthma morbidity outcomes. Third, our cohort was disproportionately burdened by asthma severity and comorbidities, partly the result of a selection bias from the PREPARE trial eligibility criteria. Our results on the relationship between SES and asthma morbidity may not be generalizable to other racial/ethnic adult minority populations. Fourth, we used a definition of poverty that was stipulated for the 48 continental states even if typically extended to US territories like Puerto Rico. However, sensitivity analysis excluding Puerto Rican residents recapitulated findings from the larger cohort. Fifth, we had missing income data from 18% (n=211) of our participants, as is common in many studies.61 These participants were significantly different from those with available income data in terms of demographic and clinical characteristics and asthma morbidity outcomes, and therefore multiple imputation analysis was not appropriate. Sixth, our findings are limited to the variables entered into the SEM model. Although our study is first to use a multi-domain SES latent variable in adults with asthma, there are many other components to SES aside from poverty, education and unemployment, and there are other mediators aside from stress, discrimination, and health literacy which could be important drivers of asthma morbidity. Indeed, the large direct contributions of SES to asthma morbidity may be indicative of unmeasured mediation pathways, which could potentially be due to other social or political determinants of health, such as structural racism, or unmeasured individual determinants, such as patient mental health (in domains not captured by the PHQ-2 depression screen), access to primary care, or material hardship. Our findings, however, were robust to adjustment by important covariates including age, sex, ethnicity, BMI, medical comorbidities, smoking status, smoking environment and depression, all of which have an impact on asthma morbidity. Seventh, this is a cross-sectional study and therefore cannot assess temporality or causality. Eighth, our surveys have not been validated in Spanish except for the ACT. Efforts should be dedicated to validating our other surveys in Spanish. Lastly, much of our data is self-reported, which may be subject to recall and other biases, though this concern is partly mitigated by our reliance on validated surveys, in addition to the multiple, complementary ways we measured asthma morbidity outcomes.
This study identified that a multidomain measure of SES associates with worse asthma morbidity among AA/B and H/L adults, and that this effect is partly mediated by stress. These findings support the notion that race/ethnicity alone does not impart worse asthma morbidity risk, and that differences in SES might account for a substantial portion of asthma morbidity in these highly impacted patient populations. Clinicians should be aware of the greater risk of asthma morbidity imparted by poverty, low educational attainment, unemployment, and stress and should seek to address these factors when and if possible. For example, clinicians should consider, where appropriate, counseling patients on stress reduction strategies and optimizing support and resilience as part of a comprehensive person-centered approach to asthma management. Investigators should identify stressors experienced by AA/B and H/L patients within these low SES groups and test stress management interventions optimized for these populations and inform population-level policy changes that will improve employment and education rates and minimize stressors.
Supplementary Material
Key Messages:
Low socioeconomic status is directly and indirectly associated with worse asthma morbidity measures among Black and Latinx adults with moderate to severe persistent asthma.
Stress is an important mediator of the association between low SES and asthma morbidity measures in these racial/ethnic groups.
Capsule summary:
Lower socioeconomic status is directly and indirectly associated with worse asthma morbidity measures among Black and Latinx adults with moderate to severe persistent asthma, with high stress as an important mediator of this effect.
Acknowledgments
We acknowledge all study participants and their families for trusting us with their time and dedication; Julia Harder, PharmD who provided medical writing assistance.
We also acknowledge the stakeholders listed below who were active in developing the study design and implementation of this study, offering expert advice throughout this project. Our Patient Partner Stakeholders (Alex Colon Moya, MPH; Aracelis Diaz; Bridget Hickson; Margarita Lorenzi, MS; Suzanne Madison, PhD, MPH; Kathy Monteiro; Wilfredo Morales-Cosme, MPH; Alexander Muniz Ruiz; Addie Perez; Richard Redondo; Dennis Reid; Janet Robles; Marsha Santiago; Opal Thompson; Joyce Wade; and Mary White) ensured that the patient voice is heard and incorporated into all aspects of the PREPARE study. Our Professional Society Stakeholders (Rubin Cohen, MD, MSc, FACP, FCCP, FCCM; Patricia Finn, MD; Michael Foggs, MD; Robert Lemanske, MD; Folashade Omole, MD, FAAFP) provided their expertise in asthma and the population being enrolled. Our Patient Advocacy Stakeholders (Mary K. Hart, MS, RRT, RCP, AEC, FAARC, FCCP; Mario Herrera, MD, MPH; Barbara M. Kaplan, MPH; Sharon Schumack, MEd) contributed their expertise regarding the populations of interest and affirmed the patient voice is being heard. Our Expert Scientific Advisors (Michelle M. Cloutier, MD; Giselle Mosnaim, MD; Cynthia S. Rand, PhD; Michael E. Wechsler, MD; Barbara P. Yawn, MD, MSc) ensured all aspects of PREPARE are scientifically valid and relevant. Our Health Policy Experts (Sarah Alwardt, PhD; Tangita Daramola, MD, CFMM; Gretchen Hammer, MPH; Arif M. Khan, MD; Troy Trygstad, MD, CCNC; Sreekanth Chaguturu, MD) strengthened our pragmatic approach to the introduction of PARTICS into the daily flow of health care. Our Study Site Investigators (Andrea J. Apter, MD; Ahmet Baydur, MD, FACP, FCCP; Paula J. Busse, MD; Rafael A. Calderon-Candelario, MD, MSc; Thomas B. Casale, MD; Geoffrey Chupp, MD; Michelle L. Hernandez, MD; Laura P. Hurley, MD, MPH; Sunit Jariwal, MD; Elina Jerscow, MD; David C. Kaelber, MD, PhD, MPH; Sybille M. Liautaud, MD; M. Diane McKee, MD, MS; Sylvette Nazario, MD; Magdalena Pasarica, MD, PhD; Victor Pinto-Plata, MD; Isaretta L. Riley, MD, MPH; Paul M. Stranges, PharmD; Kartik Shenoy, MD; Hazel Tapp, PhD; Jennifer Trevor, MD) who helped with study implementation and recruited the population of interest. Also, our Coordinators from the American Academy of Family Physicians (Alicia Brooks-Greisen, BA; Ileana Cepeda, MP; Angie Lanigan, MPA, RD, LD; Cory Lutgen, BA; Elizabeth Staton, MSTC; Carolyn Valdez, BSN, RN) who ensured monthly surveys were completed and followed up on reported exacerbations and our colleagues from the DartNet Institute, (Shaddai Amolitos, BS; Gabriela Gaona Villarreal, MPH) who designed and maintained the database and provided data support.
Summary conflict of interest statements:
Dr. Cardet reports receiving honoraria from AstraZeneca, Genentech, and GSK for work in advisory boards. Dr. Coyne-Beasley has served on an external advisory board for GSK in the past 12 months. Dr. Fuhlbrigge is an unpaid consultant to AstraZeneca for the development of outcome measures for asthma and COPD clinical trials and a consultant to Novartis on epidemiologic analyses related to asthma control. Dr. Israel received corticosteroid inhalers for the intervention arm of the PREPARE trial free of charge from Teva, NIOX VERO machines and supplies for FENO assessments from Circassia, royalties from UpToDate, and consulting fees from AB Science, Allergy and Asthma Network, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Avillion, Biometry, Equillium, Genentech, GlaxoSmithKline, Merck, NHLBI, Novartis, Pneuma Respiratory, PPS Health, Regeneron, Sanofi Genzyme, Sienna Biopharmaceuticals, TEVA and Cowen. Dr. Pace has received research grants from and is a consultant for Boehringer Ingelheim and a research grant from AstraZeneca. Dr. Wisnivesky received grants from Sanofi, Regeneron, Arnold Consulting and consulting fees from Banook, Sanofi, and Atea. Dr. Phipatanakul received grants from Genentech, Regeneron, Novartis, Sanofi, Merck, and GSK; consulting fees from Regeneron, Sanofi, GSK, Genentech, and Novartis; and honoraria for educational events from Genentech, Sanofi, Regeneron, and GSK. The rest of the authors have no conflicts of interest to disclose.
Funding information:
This work was supported by a Patient-Centered Outcomes Research Institute (PCORI) Project Program Award to Dr. Israel (PCS-1504-30283). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. Additionally, this work was supported by grant K23AI125785 from NIAID and the ALA/AAAAI Allergic Respiratory Diseases Award (AI-835475) to Dr. Cardet; grant L30 HL143781 and the Brigham and Women’s Hospital Minority Faculty Career Development Award to Dr. Louisias; K24 AI 106822 to Dr. Phipatanakul; and the Gloria M. and Anthony C. Simboli Distinguished Chair in Asthma Research to Dr. Israel.
Abbreviations:
- AA/B
African American/Black
- BHLS
Brief Health Literacy Scale
- BMI
Body mass index
- ER
Emergency room
- H/L
Hispanic/Latinx
- ICS
Inhaled corticosteroid
- MCID
Minimal clinically important difference
- PARTICS
Patient-Activated Reliever-Triggered Inhaled CorticoSteroid
- PCORI
Patient-Centered Outcomes Research Institute
- PREPARE
PeRson EmPowered Asthma RElief
- REALM
Rapid Estimate of Adult Literacy in Medicine
- SEM
Structural equation models
- SES
Socioeconomic status
- US
United States
Footnotes
Clinical Trial Registration: NCT02995733
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Centers for Disease Control and Prevention. Asthma: Most Recent National Asthma Data [Internet]. 2021. [cited 2021 Aug 28]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
- 2.Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018. Mar;15(3):348–356. [DOI] [PubMed] [Google Scholar]
- 3.Homa DM, Mannino DM, Lara M. Asthma Mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban Heritage, 1990–1995. Am J Respir Crit Care Med. 2000. Feb;161(2):504–509. [DOI] [PubMed] [Google Scholar]
- 4.Baptist AP, Lowe D, Sarsour N, Jaffee H, Eftekhari S, Carpenter LM, et al. Asthma Disparities During the COVID-19 Pandemic: A Survey of Patients and Physicians. J Allergy Clin Immunol Pract. 2020. Dec;8(10):3371–3377.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Federico MJ, Denlinger LC, Corren J, Szefler SJ, Fuhlbrigge AL. Exacerbation-Prone Asthma: A Biological Phenotype or a Social Construct. J Allergy Clin Immunol Pract. 2021. Jul;9(7):2627–34. [DOI] [PubMed] [Google Scholar]
- 6.Horner SD. Examining Social Determinants of Health in Childhood Asthma Management. Clin Nurse Spec CNS. 2020. Oct;34(5):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louisias M, Phipatanakul W. Managing Asthma in Low-Income, Underrepresented Minority, and Other Disadvantaged Pediatric Populations: Closing the Gap. Curr Allergy Asthma Rep. 2017. Sep 15;17(10):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zárate RA, Zigler C, Cubbin C, Matsui EC. Neighborhood-level variability in asthma-related emergency department visits in Central Texas. J Allergy Clin Immunol. 2021. Sep 8;S0091–6749(21)01360–9. [Google Scholar]
- 9.Lucas JA, Marino M, Fankhauser K, Bazemore A, Giebultowicz S, Cowburn S, et al. Role of social deprivation on asthma care quality among a cohort of children in US community health centres. BMJ Open. 2021. Jun 23;11(6):e045131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leibel S, Geng B, Phipatanakul W, Lee E, Hartigan P. Screening Social Determinants of Health in a Multidisciplinary Severe Asthma Clinical Program. Pediatr Qual Saf. 2020. Oct;5(5):e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009. Mar;123 Suppl 3:S174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet Lond Engl. 2017. Apr 8;389(10077):1453–63. [DOI] [PubMed] [Google Scholar]
- 13.Le TP, Sutherlin TK, Teverbaugh LA, Gleason MM, Carlson JC. The impact of socioeconomic risk factors and mental health on asthma. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2021. May;126(5):453–7. [DOI] [PubMed] [Google Scholar]
- 14.Matsui EC, Adamson AS, Peng RD. Time’s up to adopt a biopsychosocial model to address racial and ethnic disparities in asthma outcomes. J Allergy Clin Immunol. 2019. Jun;143(6):2024–5. [DOI] [PubMed] [Google Scholar]
- 15.Mersha TB, Qin K, Beck AF, Ding L, Huang B, Kahn RS. Genetic ancestry differences in pediatric asthma readmission are mediated by socioenvironmental factors. J Allergy Clin Immunol. 2021. Jul;S0091674921010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong S, Lobb E, Khan R, Abu-Rayya H, Byun R, Jalaludin B. Neighbourhood safety and area deprivation modify the associations between parkland and psychological distress in Sydney, Australia. BMC Public Health. 2013. Dec;13(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker EH. Socioeconomic Status, Definition. In: Cockerham WC, Dingwall R, Quah S, editors. The Wiley Blackwell Encyclopedia of Health, Illness, Behavior, and Society [Internet]. Chichester, UK: John Wiley & Sons, Ltd; 2014. [cited 2021 Aug 28]. p. 2210–4. Available from: 10.1002/9781118410868.wbehibs395 [DOI] [Google Scholar]
- 18.Bacon SL, Bouchard A, Loucks EB, Lavoie KL. Individual-level socioeconomic status is associated with worse asthma morbidity in patients with asthma. Respir Res. 2009. Dec;10(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forno E, Celedón JC. Health Disparities in Asthma. Am J Respir Crit Care Med. 2012. May 15;185(10):1033–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen E, Shalowitz MU, Story RE, Ehrlich KB, Manczak EM, Ham PJ, et al. Parents’ childhood socioeconomic circumstances are associated with their children’s asthma outcomes. J Allergy Clin Immunol. 2017. Sep;140(3):828–835.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Lynd LD, FitzGerald JM, Sadatsafavi M. Influences of Socioeconomic Status on Costs of Asthma Under Universal Health Coverage. Med Care. 2016. Aug;54(8):789–795. [DOI] [PubMed] [Google Scholar]
- 22.Cardet JC, Louisias M, King TS, Castro M, Codispoti CD, Dunn R, et al. Income is an independent risk factor for worse asthma outcomes. J Allergy Clin Immunol. 2018. Feb;141(2):754–760.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. J Allergy Clin Immunol. 2015. Mar;135(3):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taponen S, Lehtimäki L, Karvala K, Luukkonen R, Uitti J. Correlates of employment status in individuals with asthma: a cross-sectional survey. J Occup Med Toxicol. 2017. Dec;12(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilmarinen P, Stridsman C, Bashir M, Tuomisto LE, Vähätalo I, Goksör E, et al. Level of education and asthma control in adult-onset asthma. J Asthma. 2021. Mar 10;1–20. [DOI] [PubMed] [Google Scholar]
- 26.Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun Monogr. 2009. Dec;76(4):408–420. [Google Scholar]
- 27.Businelle MS, Mills BA, Chartier KG, Kendzor DE, Reingle JM, Shuval K. Do stressful events account for the link between socioeconomic status and mental health? J Public Health. 2014. Jun 1;36(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stormacq C, Van den Broucke S, Wosinski J. Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promot Int. 2019. Oct 1;34(5):e1–17. [DOI] [PubMed] [Google Scholar]
- 29.Stepanikova I, Oates GR. Perceived Discrimination and Privilege in Health Care: The Role of Socioeconomic Status and Race. Am J Prev Med. 2017. Jan;52(1):S86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landeo-Gutierrez J, Forno E, Miller GE, Celedón JC. Exposure to Violence, Psychosocial Stress, and Asthma. Am J Respir Crit Care Med. 2020. Apr 15;201(8):917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soones TN, Lin JL, Wolf MS, O’Conor R, Martynenko M, Wisnivesky JP, et al. Pathways linking health literacy, health beliefs, and cognition to medication adherence in older adults with asthma. J Allergy Clin Immunol. 2017. Mar;139(3):804–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur N, Barcelo NE, Borrell LN, Singh S, Eng C, Davis A, et al. Perceived Discrimination Associated With Asthma and Related Outcomes in Minority Youth. Chest. 2017. Apr;151(4):804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopel LS, Petty CR, Gaffin JM, Sheehan WJ, Baxi SN, Kanchongkittiphon W, et al. Caregiver stress among inner-city school children with asthma. J Allergy Clin Immunol Pract. 2017. Aug;5(4):1132–1134.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright RJ, Subramanian SV. Advancing a Multilevel Framework for Epidemiologic Research on Asthma Disparities. Chest. 2007. Nov;132(5):757S–769S. [DOI] [PubMed] [Google Scholar]
- 35.Pew Research Center. On Views of Race and Inequality, Blacks and Whites are Worlds Apart: Demographic trends and economic well-being [Internet]. 2016. [cited 2021 Aug 28]. Available from: https://www.pewresearch.org/social-trends/2016/06/27/1-demographic-trends-and-economic-well-being/
- 36.Burchard EG. Medical research: Missing patients. Nature. 2014. Sep;513(7518):301–302. [DOI] [PubMed] [Google Scholar]
- 37.Law H-Z, Oraka E, Mannino DM. The Role of Income in Reducing Racial and Ethnic Disparities in Emergency Room and Urgent Care Center Visits for Asthma— United States, 2001–2009. J Asthma. 2011. May;48(4):405–413. [DOI] [PubMed] [Google Scholar]
- 38.Schyllert C, Lindberg A, Hedman L, Stridsman C, Andersson M, Ilmarinen P, et al. Low socioeconomic status relates to asthma and wheeze, especially in women. ERJ Open Res. 2020. Jul;6(3):00258–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seibert RG, Winter MR, Cabral HJ, Wolf MS, Curtis LM, Paasche-Orlow MK. Health Literacy and Income Mediate Racial/Ethnic Asthma Disparities. Health Lit Res Pract. 2019. Jan;3(1):e9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yakubovich AR, Cluver LD, Gie R. Socioeconomic factors associated with asthma prevalence and severity among children living in low-income South African communities. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2016. Mar 9;106(4):57. [DOI] [PubMed] [Google Scholar]
- 41.Israel E, Cardet JC, Carroll JK, Fuhlbrigge AL, Pace WD, Maher NE, et al. A randomized, open-label, pragmatic study to assess reliever-triggered inhaled corticosteroid in African American/Black and Hispanic/Latinx adults with asthma: Design and methods of the PREPARE trial. Contemp Clin Trials. 2021. Feb;101:106246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Office of the Assistant Secretary for Planning and Education (ASPE). HHS Poverty Guidelines for 2021 [Internet]. 2021. [cited 2021 Aug 28]. Available from: https://aspe.hhs.gov/topics/poverty-economic-mobility/poverty-guidelines
- 43.Health Resources & Services Administration (HRSA). Federal Poverty Guidelines [Internet]. 2021. [cited 2021 Aug 28]. Available from: https://www.hrsa.gov/get-health-care/affordable/hill-burton/poverty-guidelines.html
- 44.Association of American Medical Colleges (AAMC). Who is eligible to participate in the fee assistance program? [Internet]. 2021. [cited 2021 Aug 28]. Available from: https://students-residents.aamc.org/fee-assistance-program/who-eligible-participate-fee-assistance-program
- 45.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983. Dec;24(4):385–396. [PubMed] [Google Scholar]
- 46.Wallston KA, Cawthon C, McNaughton CD, Rothman RL, Osborn CY, Kripalani S. Psychometric Properties of the Brief Health Literacy Screen in Clinical Practice. J Gen Intern Med. 2014. Jan;29(1):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams DR, Yan Yu, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol. 1997. Jul;2(3):335–351. [DOI] [PubMed] [Google Scholar]
- 48.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test☆A survey for assessing asthma control. J Allergy Clin Immunol. 2004. Jan;113(1):59–65. [DOI] [PubMed] [Google Scholar]
- 49.Revicki DA, Kline Leidy N, Brennan-Diemer F, Sorensen S, Togias A. Integrating Patient Preferences Into Health Outcomes Assessment. Chest. 1998. Oct;114(4):998–1007. [DOI] [PubMed] [Google Scholar]
- 50.Rank MA, Bertram S, Wollan P, Yawn RA, Yawn BP. Comparing the Asthma APGAR System and the Asthma Control Test™ in a Multicenter Primary Care Sample. Mayo Clin Proc. 2014. Jul;89(7):917–925. [DOI] [PubMed] [Google Scholar]
- 51.Sobel ME. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociol Methodol. 1982;13:290. [Google Scholar]
- 52.Yung Y-F. Introduction to Structural Equation Modeling Using the CALIS Procedure in SAS/STAT® Software [Internet]. Computer Technology Workshop presented at the Joint Statistical Meeting; 2010. Aug 4 [cited 2021 Sep 14]; Vancouver, Canada. Available from: https://support.sas.com/rnd/app/stat/papers/JSM2010_Yung.pdf [Google Scholar]
- 53.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003. Nov;41(11):1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. PMID: 14583691. [DOI] [PubMed] [Google Scholar]
- 54.Latkin CA, Curry AD, Hua W, Davey MA. Direct and Indirect Associations of Neighborhood Disorder With Drug Use and High-Risk Sexual Partners. Am J Prev Med. 2007. Jun;32(6):S234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kopel LS, Gaffin JM, Ozonoff A, Rao DR, Sheehan WJ, Friedlander JL, et al. Perceived neighborhood safety and asthma morbidity in the School Inner-City Asthma Study: Perceived Neighborhood Safety and Asthma Morbidity. Pediatr Pulmonol. 2015. Jan;50(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beutel ME, Klein EM, Brähler E, Reiner I, Jünger C, Michal M, et al. Loneliness in the general population: prevalence, determinants and relations to mental health. BMC Psychiatry. 2017. Dec;17(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landeo-Gutierrez J, Celedón JC. Chronic stress and asthma in adolescents. Ann Allergy Asthma Immunol. 2020. Oct;125(4):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013. Apr 3;346:f1325. doi: 10.1136/bmj.f1325. PMID: 23558162. [DOI] [PubMed] [Google Scholar]
- 59.He FJ, Tan M, Ma Y, MacGregor GA. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020. Feb 18;75(6):632–647. doi: 10.1016/j.jacc.2019.11.055. PMID: 32057379. [DOI] [PubMed] [Google Scholar]
- 60.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993. Jun;25(6):391–395. [PubMed] [Google Scholar]
- 61.Kim S, Egerter S, Cubbin C, Takahashi ER, Braveman P. Potential Implications of Missing Income Data in Population-Based Surveys: An Example from a Postpartum Survey in California. Public Health Rep. 2007. Nov;122(6):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.