Abstract
CONTEXT
Preterm brain injuries are common; neurodevelopmental outcomes following contemporary neonatal care are continually evolving.
OBJECTIVE
To systematically review and meta-analyze neurodevelopmental outcomes among preterm infants after intraventricular hemorrhage (IVH) and white matter injury (WMI).
DATA SOURCES
Published and grey literature were searched across 10 databases between 2000 and 2021.
STUDY SELECTION
Observational studies reporting 3-year neurodevelopmental outcomes for preterm infants with IVH or WMI compared with preterm infants without injury.
DATA EXTRACTION
Study characteristics, population characteristics, and outcome data were extracted.
RESULTS
Thirty eight studies were included. There was an increased adjusted risk of moderate-severe neurodevelopmental impairment after IVH grade 1 to 2 (adjusted odds ratio 1.35 [95% confidence interval 1.05–1.75]) and IVH grade 3 to 4 (adjusted odds ratio 4.26 [3.25–5.59]). Children with IVH grade 1 to 2 had higher risks of cerebral palsy (odds ratio [OR] 1.76 [1.39–2.24]), cognitive (OR 1.79 [1.09–2.95]), hearing (OR 1.83 [1.03–3.24]), and visual impairment (OR 1.77 [1.08–2.9]). Children with IVH grade 3 to 4 had markedly higher risks of cerebral palsy (OR 4.98 [4.13–6.00]), motor (OR 2.7 [1.52–4.8]), cognitive (OR 2.3 [1.67–3.15]), hearing (OR 2.44 [1.42–4.2]), and visual impairment (OR 5.42 [2.77–10.58]). Children with WMI had much higher risks of cerebral palsy (OR 14.91 [7.3–30.46]), motor (OR 5.3 [3–9.36]), and cognitive impairment (OR 3.48 [2.18–5.53]).
LIMITATIONS
Heterogeneity of outcome data.
CONCLUSIONS
Mild IVH, severe IVH, and WMI are associated with adverse neurodevelopmental outcomes. Utilization of core outcome sets and availability of open-access study data would improve our understanding of the nuances of these outcomes.
Prematurity is the leading global cause of childhood morbidity and mortality.1,2 Internationally, 15 million infants are born preterm every year and this figure has remained static in high-income countries since the turn of the millenium.1,3,4 In the last 3 decades there have been substantial changes to routine neonatal care with the widespread uptake of treatments such as antenatal steroids, careful thermoregulation, use of novel approaches, such as postnatal exogenous surfactant administration, and use of less invasive ventilation strategies. These improvements have resulted in considerable survival gains for preterm infants, and many have also been neuroprotective, and as such, have reduced the rate and severity of preterm brain injuries.4–9 Therefore, the relationship between neurodevelopmental impairment and brain injuries of prematurity may be different to that described previously.
Neonatal trials and observational studies typically employ composite primary outcomes (using a combination of death and disability) at 2-years of age. There are widely acknowledged issues with the use of composite outcomes, including their lack of pragmatic utility for clinicians, that they are less meaningful to parents, and that they can both mask or inflate effect sizes.10–12 Additionally, neonatal studies with composite primary outcomes are typically not adequately powered to explore the risk of specific neurodevelopmental sequelae after preterm brain injury, which has been repeatedly highlighted as a priority question from parents.12,13 A meta-analysis exploring neurodevelopmental impairment after intraventricular hemorrhage (IVH) in 2014 was only able to explore a handful of neurodevelopmental outcomes because of such issues. The included studies, even on pooling in meta-analyses, were inadequately powered to explore key outcomes, such as hearing and visual impairment, and few studies provided adjusted effect estimates.14 As such, and in view of the evolution of neonatal care, we anticipated that an updated overview of the evidence would prove useful and that the additional power afforded by more recent population-based studies would enable more detailed exploration of the risk of specific neurodevelopmental sequelae after preterm brain injury. Therefore, we undertook a systematic review to explore neurodevelopmental outcomes up to 3 years of age after preterm brain injuries including IVH and white matter injury (WMI).
Methods
Study Selection
This review followed an a priori registered protocol (CRD 42021278572). It is reported in-line with the PRISMA and MOOSE statements. Observational studies published between 2000 and 2021 examining neurodevelopmental outcomes up to 3 years of age after preterm brain injury were included. Studies were required to have a non–brain injured preterm comparator group for inclusion. Preterm brain injuries included intracranial hemorrhage, such as IVH of any grade, and WMI, such as non-cystic and cystic periventricular leukomalacia (PVL), among neonates born at less than 37 weeks’ gestation. The primary review outcome was any neurodevelopmental impairment; secondary outcomes included: cognitive, motor, speech and language, behavioral and neuropsychological, visual, and hearing impairment (Table 1).
TABLE 1:
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Peer-reviewed observational studies | Noncomparative studies; opinions; commentaries; reviews; case-reports; animal studies. |
| Studies in all languages | Studies where the population includes adults and children and the data for children cannot be extracted. |
| Studies published after 2000 | Studies where comparable outcome data from those with and without preterm brain injury cannot be extracted. |
| Preterm children born at <37 wk’ gestation with a diagnosis of intracranial hemorrhage or white matter injury during the neonatal period as defined by authors (including on imaging review [cranial ultrasound or MRI] by neonatologists, radiologists or sonographers; or on clinical record review).71,72 | Studies reporting outcomes for children diagnosed with preterm brain injury beyond the neonatal period. |
| Studies focused on neurodevelopmental outcomes of children up to 3 y of age including: | Studies not reporting quantitative neurodevelopmental, health or educational outcomes. |
| Primary outcome(s): neurodevelopmental impairment, as defined by authors (including direct testing, clinical record review, and parental interview or survey). | |
| Secondary outcome(s): (1) Any cognitive impairment, as defined by authors (direct testing). (2) Mild cognitive impairment (developmental quotient or IQ from 2 to 1 standard deviations below the mean). (3) Moderate-severe cognitive impairment (developmental quotient or IQ more than 2 standard deviations below the mean). (4) Epilepsy, as defined by authors (including medical history taking, clinical record review and parental interview or survey). (5) Emotional-behavioral difficulty, as defined by authors (including direct testing, clinical record review, and parental interview or survey). (6) Speech and language impairment, as defined by authors (on direct testing). (7) Visual impairment, as defined by authors (including direct testing, clinical record review, and parental interview or survey). (8) Hearing impairment, as defined by authors (including direct testing, clinical record review, and parental interview or survey). (9) Motor impairment, as defined by authors (including direct testing, clinical record review, and parental interview or survey). (10) Visual-motor impairment, as defined by authors (on direct testing). |
Search Strategy
A comprehensive search strategy was developed in Medline Ovid consisting of 99 key terms and Mesh headings, which was adapted for other databases (Supplemental Fig 13). The published and gray literature were searched across 10 databases from January 1, 2000 to September 1, 2021 (Supplemental Fig 14). Searches were augmented with snowballing techniques, such as handsearching the reference lists of full-text articles.
Study Screening and Risk of Bias
Each record identified underwent screening by 2 reviewers (P.R., C.C., M.V., J.D., S.S.) independently. The full text articles of all potentially relevant studies were retrieved and reviewed in detail by 2 trained reviewers, independently. This review included a risk of bias assessment using the Newcastle Ottawa Tool for cohort or case-control studies.15 Studies were assessed against 3 key domains: population selection, the comparability of the “exposed” brain injured and “comparator” non-brain injured groups; and outcome assessment (for cohort studies) or exposure assessment (for case control studies). For each domain, studies were classified as poor, fair, or good, and given an overall classification of high, moderate, or low risk of bias. Disagreements were resolved through group discussion.
Data Extraction and Synthesis
A purpose-built Microsoft excel spreadsheet was created to extract data from included studies. Studies were stratified by brain injury type, age of outcome assessment, and outcome type. Specific outcomes for each brain injury type were described in a narrative synthesis. Where suitable data were available and studies demonstrated reasonable clinical and contextual homogeneity (in terms of population, injury type, outcome type, definitions, and assessment) data were pooled in random-effects meta-analyses using RevMan 5.4.16 Dichotomous data were pooled using the Mantel-Haenszel method. Where studies only presented analysis data (such as risk estimates), their data were pooled with dichotomous data from other studies using the generic inverse variance method.16 Statistical heterogeneity was assessed using the I2 statistic. Where meta-analyses demonstrated substantial heterogeneity (>85%), sensitivity analyses were undertaken to further explore the underlying explanation for the heterogeneity based on risk of bias assessments, outcome assessment tools, and year of cohort.
Results
Overview
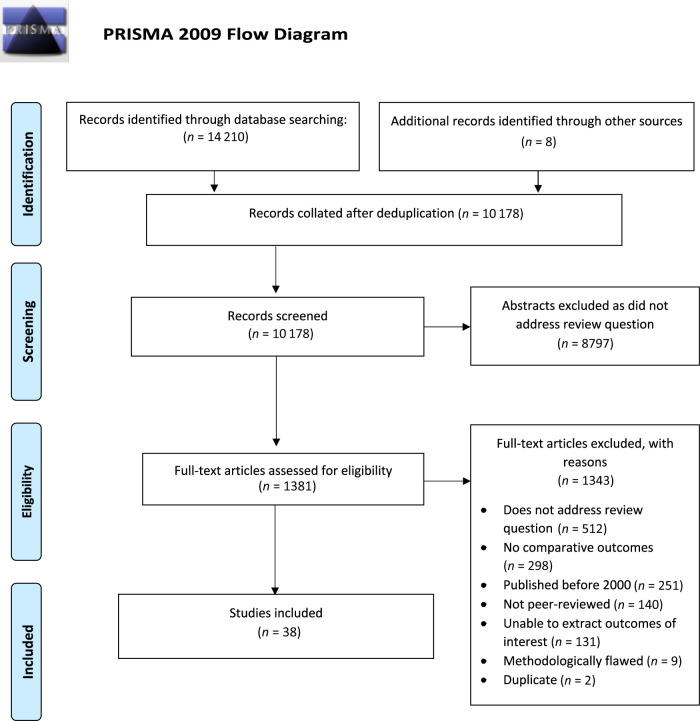
Of the 14 210 records identified, 10 178 were screened, 1381 full text articles were reviewed, and 38 studies included (Fig 1).17–54 Most (n = 35) included studies were retrospective or prospective cohort studies; 3 were case control studies. Studies were included from the United States (n = 17), Canada (n = 5), Taiwan (n = 4), Australia (n = 2) and many other countries (Supplemental Fig 15). Most studies were assessed as having a low risk of bias (n = 33), however 5 were deemed to have a moderate risk of bias (Supplemental Fig 16). Studies used 38 different types of outcome assessment tools and assessed outcomes at a variety of different time-points between 6 months to 3 years of age.
FIGURE 1.
PRISMA flow diagram of included and excluded studies.
IVH
Neurodevelopmental outcomes after IVH were explored by 34 included studies. Only 16 studies specified how IVH was confirmed: by radiologists (n = 8); neonatologists (n = 2); both neonatologists and radiologists (n = 3); or central reviewers and sonographers (n = 3). Five studies employed double-blinded image review. Most (n = 15) used the Papile classification.17,19,22,25,31, 35,36,38,41,42,44–48 No studies presented outcomes by laterality of IVH. In most studies, infants were born between 23 to 34 weeks’ gestation or had a birth weight of less than 1500g, and were born between 1985 and 2018, with most born after 2000 (n = 25).
Outcomes After IVH Grade 1 to 2
Of the 38 included studies, 15 explored outcomes after IVH grade 1 to 2, 11 of these presented only combined outcome data for IVH grade 1 and 2. Meta-analyses comparing outcomes following grade 1 IVH alone were therefore not possible.20,22,25,29,42,44,46,47,50,52,54
Neurodevelopmental Impairment
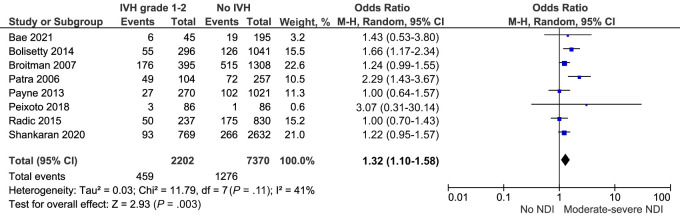
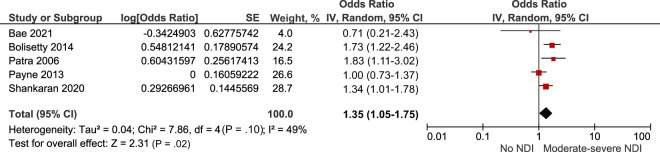
Nine studies explored moderate to severe neurodevelopmental impairment at 18 to 36 months of age after IVH grade 1 to 2.17,22,25,27,44,46–48,50 This composite outcome included cerebral palsy, visual, hearing, or cognitive impairment (defined as 1 or 2 standard deviations below the mean on either the Bayley Scale of Infant Development [BSID II] Mental Development Index [MDI], the Bayley Scales of Infant and Toddler Development Edition 3 [Bayley-III], or the Griffiths Scale of Child Development). Of these 9 studies, 8 were deemed sufficiently comparable for meta-analysis.22,25,27,44,46–48,50 The meta-analysis included 2202 preterm infants with IVH grade 1 to 2 and 7370 preterm infants without IVH. Compared with preterm infants without IVH, the combined crude risk of neurodevelopmental impairment after IVH grade 1 to 2 was higher, odds ratio (OR) 1.32 95% confidence interval (CI) (1.1–1.58) I2 = 41% (Fig 2; Table 2). This remained similar on sensitivity analyses exploring the impact of outcome assessment tools (Supplemental Fig 17). Additionally, the pooled adjusted risk of neurodevelopmental impairment was higher, adjusted odds ratio (aOR) 1.35 95% CI (1.05–1.75) I2 = 49% (Fig 3). Studies included in the adjusted meta-analysis accounted for several covariates, including gestation, sex, race, maternal education, and bronchopulmonary dysplasia.
FIGURE 2.
Forest plot of the crude risk of neurodevelopmental impairment after IVH grade 1 to 2. NDI, neurodevelopmental impairment.
TABLE 2:
Pooled Risks of Adverse Neurodevelopmental Outcomes After Preterm Brain Injury
| IVH Grade 1–2 | IVH Grade 3–4 | Cystic PVL | |
|---|---|---|---|
| Moderate-severe neurodevelopmental impairment | OR 1.32 (1.1–1.58) I2 = 41%, 8 studies; 9572 infants, aOR 1.35 (1.05–1.75) I2 = 49%, 5 studies | OR 3.27 (2.44–4.39) I2 = 81%, 7 studies, aOR 4.26 (3.25–5.59) I2 = 34%, 4 studies | OR 3.63 (2.49–5.31) I2 = 0%, 3 studies, aOR 2.38 (0.73–7.7) I2 = 94%, 3 studies |
| Motor | BSID II PDI < 70, OR 1.72(0.96–3.1) I2 = 82%, 3 studies; 2483 infants | BSID II PDI < 70, OR 2.7 (1.52–4.8) I2 = 73%, 2 studies | BSID II PDI < 70, OR 5.3 (3–9.36) I2 = 28%, 3 studies |
| Cerebral palsy | OR 1.76 (1.39–2.24) I2 = 52%, 10 studies; 11 018 infants | OR 4.98 (4.13–6.00) I2 =37%, 7 studies, moderate to severe cerebral palsy, OR 2.39 (1.49–3.85) I2 = 0%, 2 studies | OR 14.91 (7.3–30.46) I2 = 87%, 5 studies |
| Cognitive impairment | BSID II MDI < 70, OR 1.79 CI (1.09–2.95) I2 = 80%, 4 studies; 3646 infants | BSID II MDI < 70, OR 2.83 (1.54–5.2) I2 = 78%, 3 studies, Bayley-III scores <85, OR 2.3 (1.67–3.15) I2 = 0%, 2 studies | BSID II MDI < 70, OR 3.48 95% CI (2.18–5.53) I2 = 0%, 3 studies |
| Hearing impairment | OR 1.83 CI (1.03–3.24) I2=62%, 7 studies; 8273 infants | OR 2.44 (1.42–4.2) I2 = 52%, 5 studies; 7224 infants | — |
| Visual impairment | OR 1.77 (1.08–2.9) I2 = 0%, 6 studies; 7881 infants | OR 5.42 (2.77–10.58) I2 = 50%, 5 studies; 7203 infants | — |
BSID, Bayley Scale of Infant Development; MDI, Mental Development Index; PDI, Psychomotor Development Index; —, not applicable.
FIGURE 3.
Forest plot of the adjusted risk of neurodevelopmental impairment after IVH grade 1 to 2. NDI, neurodevelopmental impairment.
Motor and Cerebral Palsy Outcomes
Conflicting results on motor outcomes after IVH grade 1 to 2 were reported by 7 studies,22,24,32,36,44,46,50 3 of which were sufficiently comparable for meta-analysis. The combined crude risk of a BSID II Psychomotor Development Index (PDI) score < 70 after IVH grade 1 to 2 was not significantly higher compared with infants without IVH, OR 1.72 95% CI (0.96–3.1) I2 = 82% (Supplemental Fig 18). Shankaran 2020 reported that those with IVH grade 1 to 2 were equally likely to have “normal motor scores” as those without IVH after adjusting for confounders aOR 0.91, 95%CI (0.72–1.14). Payne 2013 also highlighted no increased adjusted risk of gross motor functional limitation after IVH grade 1 to 2 aOR 0.66, 95% CI (0.32–1.39). These studies could not be included in a meta-analysis because of heterogeneity in outcome selection and presentation.46,50
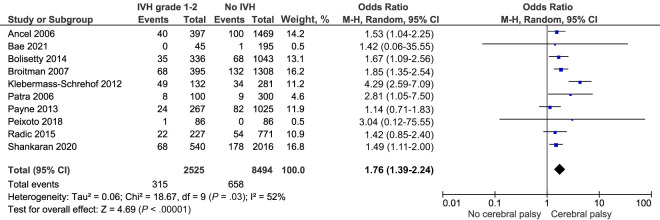
Risk of any cerebral palsy after IVH grade 1 to 2 was reported by 10 comparable studies which included 11 018 infants.20,22,25,27,36,44,46–48,50 Meta-analysis finds a crude higher risk of cerebral palsy after IVH grade 1 to 2 compared to infants without IVH, OR 1.76, 95% CI (1.39–2.24) I2 = 52% (Fig 4; Table 2). Sensitivity analyses exploring risk of cerebral palsy for infants born before and after 2000 did not highlight any significant differences (Supplemental Fig 19). There were insufficient data on severity of cerebral palsy and insufficient adjusted data for meta-analysis.
FIGURE 4.
Forest plot of the crude risk of cerebral palsy after IVH grade 1 to 2.
Cognitive Outcomes
Eleven included studies explored cognitive outcomes after IVH grade 1 to 2; 5 used the BSID II MDI, 4 used the Bayley-III, 1 used the Griffiths Mental Development Scales, and 1 used the Stanford Binet Intelligence Scale.19,22,25,27,36,44,46–48,50,54 Four studies were suitable for meta-analysis, indicating a higher crude risk of BSID II MDI <70 in infants with IVH grade 1 to 2 compared with controls, OR 1.79, 95% CI (1.09–2.95) I2=80% (Table 2; Supplemental Fig 20).25,27,36,44 Similar results were seen in studies not included in the meta-analysis: Peixoto 2018 reported that those with IVH grade 1 to 2 had significantly lower mean cognitive scores on the Griffiths Mental Development Scale (94.4 +/−12.7) compared with controls (98.6 +/− 9.8), but they were not more likely to have developmental quotients below 70. Payne 2013 reported that a higher risk of a BSID MDI II score <70 did not persist on adjusting for confounders aOR 1.03 (0.75–1.43).46 Similarly, Shankaran 2020 reported that those with IVH grade 1 to 2 had a similar risk to those without IVH of “normal” cognitive scores after adjusting for confounders (on Bayley-III): aOR 0.85, 95% CI (0.69–1.06).50
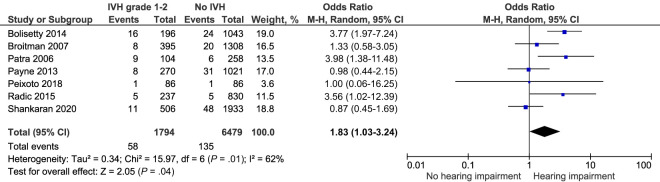
Hearing Impairment
Seven included studies explored hearing impairment after IVH grade 1 to 2 among 8273 infants.25,27,44,46–48,50 They found a higher combined crude risk of unilateral or bilateral hearing impairment OR 1.83, 95% CI (1.03–3.24) I2 = 62% (Fig 5; Table 2). Although this outcome was rare: reported in 3.2% of infants with IVH grade 1 to 2 and 2.1% of those without IVH.
FIGURE 5.
Forest plot of the crude risk of hearing impairment after IVH grade 1 to 2.
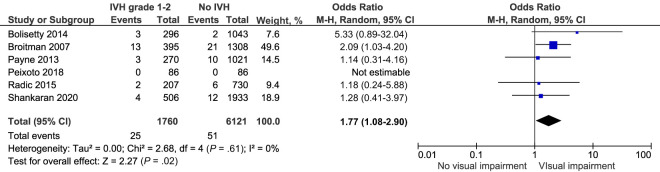
Visual Impairment
The pooled crude risk of visual impairment after IVH grade 1 to 2 was significantly higher in children following IVH grade 1 to 2 compared with controls, OR 1.77, 95% CI (1.08–2.9) I2 = 0% (Fig 6; Table 2), although the outcome was uncommon.25,27,46–48,50
FIGURE 6.
Forest plot of the crude risk of visual impairment after IVH grade 1 to 2.
Outcomes After IVH Grade 3 to 4
Outcomes after IVH grade 3 to 4 were presented by 23 studies, most (n = 17) combined results for those with IVH grade 3 and 4, therefore separate meta-analyses by grade of IVH were not possible.18,20,25,26,32–35,38,39, 41–43,46,50–52
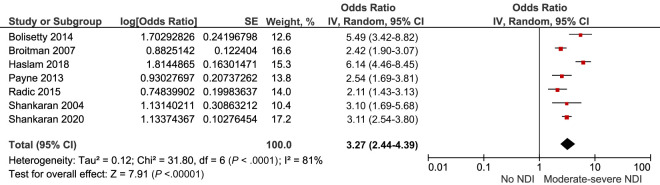
Neurodevelopmental Impairment
Neurodevelopmental impairment up to 3 years after IVH grade 3 to 4 was explored by 15 studies.17,18,23,25–27,34,35,39,41, 42,46,48,50,51 The crude pooled risk of moderate to severe neurodevelopmental impairment was OR 3.27, 95% CI (2.44–4.39) I2 = 81% (Fig 7; Table 2).
FIGURE 7.
Forest plot of the crude risk of neurodevelopmental impairment after IVH grade 3 to 4. NDI, neurodevelopmental impairment.
Four of these studies provided adjusted measures of effect, with the risk of moderate to severe neurodevelopmental impairment persisting on pooling adjusted data (aOR 4.26, 95% CI [3.25–5.59]) I2 = 34% [Fig 8; Table 2]).
FIGURE 8.
Forest plot of the adjusted risk of neurodevelopmental impairment after IVH grade 3 to 4. NDI, neurodevelopmental impairment.
Motor Outcomes and Cerebral Palsy
Motor outcomes after IVH grade 3 to 4 were explored by 13 studies.18,23,24,27,32,35,36,38,41,46,48,50,51 These studies used the BSID II PDI (n = 4), Bayley-III composite motor score (n = 6), and the Gross Motor Functional Classification System (n = 4). The Gross Motor Functional Classification System was used to assess the severity of functional motor impairment among those with cerebral palsy by some studies23; whereas others used it to assess motor function for the whole preterm study population.32,35,46 The combined crude risk of an abnormal BSID II PDI score (<70) across 2 comparable studies was OR 2.7, 95%CI (1.52–4.8) I2 = 73% (Supplemental Fig 21; Table 2). Klebermass-Schrehof 2012 and Banihani 2019 also highlighted a significant risk of motor impairment at 2 years of age.36,23 De Mauro 2020, Payne 2013, and Shankaran 2020 highlighted an increased adjusted risk of major motor impairment (aOR 2.83, 95% CI [1.99–4.01]), an increased adjusted risk of gross motor functional limitations (aOR 2.51, 95% CI [1.43–4.44]), and a decreased adjusted risk of normal Bayley-III motor scores (aOR 0.37, 95% CI [0.29–0.47]) respectively.32,46,50
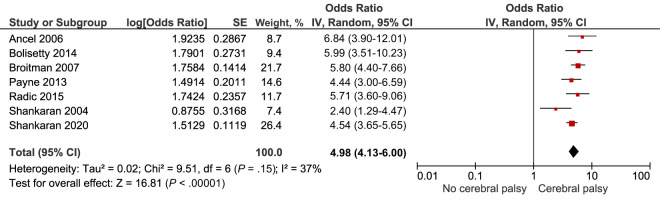
Cerebral palsy after IVH grade 3 to 4 was explored by 11 included studies.17,20,23,25,27,36,46,48,50–52 Of these, 7 were suitable for meta-analysis and they highlighted a combined crude risk of OR 4.98, 95% CI (4.13–6.00) I2 = 37% (Table 2; Fig 9).20,25,27,46,48,50,51
FIGURE 9.
Forest plot of the crude risk of cerebral palsy after IVH grade 3 to 4.
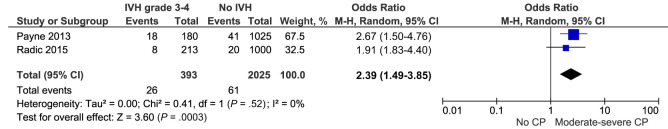
Sensitivity analyses exploring risk of cerebral palsy for infants born before and after 2000 did not highlight any significant differences (Supplemental Fig 22). Two comparable studies presented data on risk of moderate to severe cerebral palsy after IVH grade 3 to 4 and highlighted a combined crude risk of OR 2.39, 95%CI (1.49–3.85) I2 = 0% (Table 2; Fig 10).46,48
FIGURE 10.
Forest plot of the crude risk of moderate to severe cerebral palsy after IVH grade 3 to 4.
Cognitive Outcomes
Cognitive outcomes after IVH grade 3 to 4 were explored by 9 studies.18,23,25,27,36,46,48,50,51 The pooled crude risk of “abnormal” motor scores was significantly increased on BSID II (MDI < 70) OR 2.83, 95% CI (1.54–5.2) I2 = 78% and Bayley-III (<85) OR 2.3, 95% CI (1.67–3.15) I2 = 0% (Table 2; Supplemental Figs 23 and 24). Payne 2013 and Shankaran 2020 highlighted that this risk persisted after adjusting for confounders: the adjusted risk of a Bayley-III score <85 was aOR 1.82, 95% CI (1.26–2.64) and the adjusted risk of a normal BSID score was aOR 0.37, 95% CI (0.29–0.47) respectively.46,50
Hearing Impairment
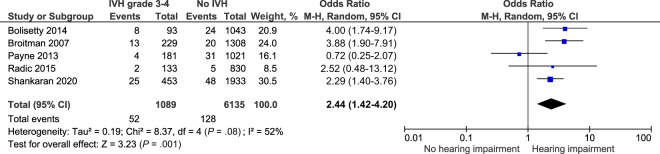
Hearing impairment after IVH grade 3 to 4 was explored by 8 included studies, 5 of these were suitable for meta-analysis.17,25,27,43,46,48,50,55 The combined crude risk of hearing impairment at 18 to 36 months was significantly increased OR 2.44, 95% CI (1.42–4.2) I2 = 52% (Fig 11; Table 2).25,27,46,48,50
FIGURE 11.
Forest plot of the crude risk of hearing impairment after IVH grade 3 to 4.
Visual Impairment
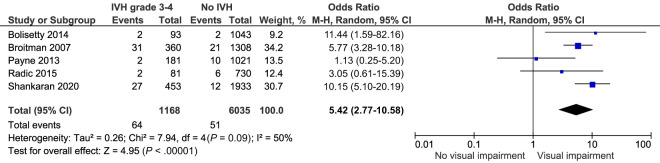
Visual outcomes after IVH grade 3 to 4 were reported by 5 comparable studies that included 7203 infants. They highlighted a significantly increased crude risk of visual impairment OR 5.42, 95% CI (2.77–10.58) I2 = 50% (Fig 12; Table 2).25,27,46,48,50
FIGURE 12.
Forest plot of the crude risk of visual impairment after IVH grade 3 to 4.
Outcomes After WMI
Neurodevelopmental Impairment
Neurodevelopmental impairment up to 3 years after preterm WMI (or IVH and WMI) was explored across 12 included studies. Included infants were born at less than 34 weeks’ gestation or weighing less than 1500 g, between 1993 and 2015.17,21,25,27,29,35,39,41,42,49,51,53 Of these studies, 8 provided data on neurodevelopmental impairment after cystic periventricular leukomalacia (cPVL) and 4 of these were suitable for meta-analysis.27,39,49,51 They highlighted a significantly increased crude risk of moderate to severe neurodevelopmental impairment: OR 3.63, 95% CI (2.49–5.31) I2 = 0% (Table 2; Supplemental Fig 25).27,49,51 This effect was attenuated on pooling studies that adjusted for key covariates, such as antenatal steroid exposure, gestation, sex, race, education, and bronchopulmonary dysplasia: aOR 2.38, 95% CI (0.73–7.7) I2 = 94% (Table 2; Supplemental Fig 26).39,49,51 However, there was high statistical heterogeneity.
Motor Outcomes
Ten studies explored motor impairment (other than cerebral palsy) after WMI.21,24,27,28,32,35,38,45,49,51 Four studies presented motor outcomes after cPVL, 3 of which were included in a meta-analysis highlighting a higher crude risk of motor impairment (BSID II PDI <70) OR 5.3, 95% CI (3–9.36) I2 = 28% (Table 2; Supplemental Fig 27).27,32,49,51 DeMauro 2020 also reported a higher risk of major motor abnormalities after cPVL or porencephalic cysts, which persisted after adjusting for covariates aOR 8.52, 95% CI (5.84–12.42).32
Cerebral Palsy
Nine studies reported cerebral palsy outcomes after WMI.17,20,25,27,35,40,49,51,53 Of these, 5 were suitable for meta-analysis and highlighted a considerably higher crude risk of cerebral palsy after cPVL OR 14.91, 95%CI (7.3–30.46) I2 = 87% (Table 2, Supplemental Fig 28). Although there was considerable statistical heterogeneity, studies consistently reported an increased risk of cerebral palsy.
Cognitive Outcomes
Ten included studies explored cognitive outcomes after preterm WMI.17,21,24,25,27,28,35,38,49,51 Three studies highlighted an increased combined crude risk of cognitive impairment (BSID II MDI score < 70) OR 3.48, 95% CI (2.18–5.53) I2 = 0% (Table 2, Supplemental Fig 29).
Behavioral and Speech and Language Outcomes
Only 1 study explored behavioral outcomes up to 3 years after WMI, and 3 explored speech and language outcomes.21,33,38,49 Lean 2019 and Sarkar 2018 both reported a higher crude risk of language impairment after WMI. Lean 2019 reported a higher crude risk after IVH grade 3 to 4 or posthemorrhagic hydrocephalus or cPVL OR 2.53, which did not persist on adjusting for confounders.38 Sarkar reported that those with disappearing cPVL (that was no longer present at 36 weeks’ gestation) had significantly lower mean language scores and as such, also had an increased crude risk of severe language impairment (Bayley-III < 70) OR 2.57, 95% CI (1.43–4.65).49
Hearing Impairment
Hearing impairment after WMI was evaluated by 3 included studies, however only 1 presented extractable outcome data.17,25,27 Broitman 2007 reported a higher crude risk of hearing impairment after cPVL OR 4.11, 95% CI (1.18–14.32), however this was not significant for those with noncystic PVL OR 2.5, 95% CI (0.92–6.76).27
Visual Impairment
Three studies explored visual outcomes after WMI.17,27,45 Broitman 2007 reported a higher crude risk of severe visual impairment after cPVL OR 13.45, 95% CI (5.8–31.18) and after PVL OR 7.15, 95% CI (3.54–14.42).27 Adams-Chapman 2018 combined infants with IVH grade 3 to 4 and PVL and reported a higher adjusted risk of bilateral blindness.17
Discussion
This review synthesizes the considerable evidence of higher crude and adjusted risks of moderate to severe neurodevelopmental impairment after preterm brain injury. The higher risk of adverse outcomes was also significant for individual neurodevelopmental domains, including cerebral palsy, cognitive impairment, hearing impairment, and visual impairment after preterm brain injury, and were seen following lower severity IVH grade 1 to 2. This review adds further support to previous reviews highlighting an increased crude risk of moderate to severe neurodevelopmental impairment after IVH grade 3 to 4 and new evidence that these risks are increased 4-fold and persist on adjusting for key covariates. This risk of neurodevelopmental impairment derives from two to five-fold increases in the individual risks of motor impairment, cerebral palsy, cognitive impairment, hearing impairment, and visual impairment after IVH grade 3 to 4. This review quantifies the higher crude risk of moderate to severe neurodevelopmental impairment after cPVL, although this did not persist on pooling adjusted measures of effect. We also reported markedly higher risks of motor impairment (OR 5.3, 95% CI (3–9.36), cerebral palsy OR 14.91, 95% CI (7.3–30.46) and cognitive impairment OR 3.48, 95% CI (2.18–5.53) after cPVL.
Strengths and Limitations
This review provides a comprehensive and up-to-date overview of existing evidence of neurodevelopmental outcomes after preterm brain injuries. An extensive search strategy was employed alongside a rigorous review process. Several recent population-based studies deemed to be low risk of bias were included. This enabled the review to expand on previous reviews in this area, provide stronger evidence of the risk of certain outcomes (for example by permitting new meta-analyses using adjusted data), and present novel evidence of associations with rarer outcomes, such as visual and hearing impairment. Despite this, the review findings were limited by the heterogeneity of included studies, particularly in relation to outcome assessment, outcome definitions (of neurodevelopmental impairment for example), how results were presented, and included population (with varying gestational age for example). There was also likely heterogeneity as a result of survival bias—differences in survival between studies—and potential publication bias. Unfortunately, studies combined their populations in different ways, explored varying outcomes measured with different neurodevelopmental assessment tools at different time-points, and presented their results in different ways, which limited the potential for meta-analyses and represents considerable research inefficiency.56 Several included studies were also not primarily designed to address our review question (for example by focusing on prematurity rather than brain injury) or did not report results for individual developmental domains, which limited the data that could be extracted. Outcomes in relation to the laterality of injury were not reported by studies, despite evidence that outcomes differ for those with bilateral and unilateral injuries.57,58 Because of the inclusion of studies published after 2000, included data are not completely representative of current neonatal care, for example delayed cord clamping and antenatal magnesium sulfate were not routine at the time of some studies.59,60 In addition, there were limited available data on key covariates, such as childhood environmental factors, which could act as important outcome modifiers. Many of the larger studies included in this review used data from neonatal networks consisting of specific tertiary units and are therefore not necessarily representative of population level care and outcomes, limiting their generalizability. Previous studies highlight poor interrater reliability in determining low grades of IVH on cranial ultrasound: this could have potentially attenuated or inflated the strength of the associations presented between low grade IVH and adverse neurodevelopmental outcomes.61 Finally, it is difficult to assess individual neurodevelopmental domains in isolation, which may affect results.62
Context of Current Literature
This review provides further evidence to support the findings of Mukerji 2015, who highlighted an increased risk of moderate to severe neurodevelopmental impairment, cerebral palsy, and cognitive impairment after IVH grade 1 to 2 and IVH grade 3 to 4.14 We included several additional studies in our crude and adjusted meta-analyses with resultantly reduced heterogeneity22,34,39,47,48, 50,51; we used random rather than fixed effects models as suggested for observational studies of heterogenous populations.16 We also provide new results, for example, we highlight that the risk of cognitive impairment after IVH grade 3 to 4 persists on adjusting for key confounders – previous reviews were unable to demonstrate this because of a lack of data. Previous studies and reviews in this area were also not powered to explore the risk of hearing or visual impairment after IVH grade 1 to 2 as presented in this review.
Gotardo 2019 also highlight an increased crude risk of cerebral palsy after IVH grade 2 to 3, PVL, and cPVL. However, their review was narrow and limited to older prospective studies (mostly including children from the presurfactant era).63 Our review, in-keeping with the findings of previous reviews in this area, therefore provides an updated overview of the literature.
Implications
Although this review provides evidence that preterm brain injuries are associated with a range of adverse neurodevelopmental outcomes, several questions could not be addressed. This was largely because of issues with how studies presented results rather than a paucity of research. Adoption of the core outcomes set and use of consistent definitions in neonatology offers a potential solution to this problem, alongside improved research transparency and provision of open access to study-data.13 This would increase the comparability of studies internationally and enable rigorous meta-analyses to address priority questions more efficiently. We would urge future studies to provide disaggregated outcome data based on site, laterality, severity of injury, and additional concurrent injuries to enable more granular analyses that would, in turn, inform more personalized counseling of parents.64
The continual evolution of neonatal care has meant that the risk of adverse neurodevelopmental sequelae for infants with preterm brain injuries born today is unclear; this is partially because of the time-lag between undertaking primary research, evidence synthesis, and publication. Improved used of routine data to monitor the incidence and outcomes of brain injuries for this population in real-time could address this problem and enable concurrent monitoring of the impact of quality improvement initiatives. Linkage to other data sources would also enable exploration of the impact of environmental factors on outcomes and efficient exploration of later childhood outcomes. In this review, we were unable to explore trajectories after preterm brain injuries, ie, to determine whether these adverse neurodevelopmental outcomes persist, worsen, or even improve throughout childhood. However, this should be a priority question in future studies as 3-year outcomes are not necessarily predictive of school-aged outcomes.65–68
Routine follow-up of preterm infants with these brain injuries is essential to support parents, detect signs of adverse neurodevelopmental outcomes, and intervene early to optimize outcomes. A recent Cochrane review highlighted that early developmental interventions can improve cognitive and motor outcomes of preterm infants.69 The potential of such interventions to exploit the neuroplasticity of the newborn brain, in the context of preterm brain injury, to mitigate adverse childhood outcomes also requires further exploration.70
Conclusion
This systematic review presents updated evidence of numerous adverse neurodevelopmental outcomes associated with preterm brain injuries, many of which persist on adjusting for confounders. Our findings were limited by the heterogeneity of reported outcomes and by the often limited data presented by studies. Population studies employing a core outcomes set are needed to enable international comparisons with a view to improving our understanding of changes in outcome over time, the role of confounders and effect modifiers, and the potential for early intervention to harness the neuroplasticity of the brain and ultimately improve outcomes.
Supplementary Material
Glossary
- aOR
adjusted OR
- Bayley III
Bayley Scales of Infant and Toddler Development edition 3
- BSID
Bayley Scale of Infant Development
- CI
confidence intervals
- cPVL
cystic periventricular leukomalacia
- IVH
intraventricular hemorrhage
- MDI
Mental Developmental Index
- PDI
psychomotor development index
- PVL
periventricular leukomalacia
- WMI
white matter injury
Footnotes
Dr Rees conceptualized and designed the review, reviewed and appraised studies, undertook data extraction and synthesis, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Callan conceptualized and designed the review, designed and oversaw the search strategy, reviewed and appraised studies, undertook data extraction, and reviewed and revised the manuscript; Drs Chadda, Vaal, Diviney, Sabti and Harnden reviewed and appraised studies, undertook data extraction, and reviewed and revised the manuscript; Dr Gardiner was the lead statistician for the review, advised on and oversaw the data analysis, and reviewed and revised the manuscript; Dr Battersby and Professors Gale and Professor Sutcliffe oversaw and supervised the review and critically revised the manuscript for important intellectual content; and all authors approve the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: This review was supported by a National Institute of Health Research Doctoral Fellowship award (NIHR301457). The National Institute of Health Research had no role in the design or conduct of the review.
CONFLICT OF INTEREST DISCLOSURES: Professor Gale is funded by the United Kingdom Medical Research Council through a Transition Support Award. In the past 5 years He has received support from Chiesi Pharmaceuticals to attend an educational conference and has been investigator on received research grants from Medical Research Council, National Institutes of Health Research, Canadian Institute of Health Research, Department of Health in England, Mason Medical Research Foundation, Westminster Medical School Research Trust, and Chiesi Pharmaceuticals. Professor Gale is funded by the United Kingdom National Institutes of Health Research Advanced Fellowship Award.
References
- 1. Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med. 2016;21(2):74–79 [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063): 3027–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172 [DOI] [PubMed] [Google Scholar]
- 4. Morgan AS, Mendonça M, Thiele N, David AL. Management and outcomes of extreme preterm birth. BMJ. 2022;376:e055924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rysavy MA, Li L, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serenius F, Blennow M, Maršál K, Sjörs G, Källen K, Group ES; EXPRESS Study Group . Intensity of perinatal care for extremely preterm infants: outcomes at 2.5 years. Pediatrics. 2015;135(5):e1163–e1172 [DOI] [PubMed] [Google Scholar]
- 7. Kramer KP, Minot K, Butler C, et al. Reduction of severe intraventricular hemorrhage in preterm infants: a quality improvement project. Pediatrics. 2022;149(3):e2021050652. [DOI] [PubMed] [Google Scholar]
- 8. Travers CP, Gentle S, Freeman AE, et al. A quality improvement bundle to improve outcomes in extremely preterm infants in the first week. Pediatrics. 2022;149(2):e2020037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helenius K, Longford N, Lehtonen L, Modi N, Gale C; Neonatal Data Analysis Unit and the United Kingdom Neonatal Collaborative . Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019;367:l5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janvier A, Farlow B, Baardsnes J, Pearce R, Barrington KJ. Measuring and communicating meaningful outcomes in neonatology: a family perspective. Semin Perinatol. 2016;40(8): 571–577 [DOI] [PubMed] [Google Scholar]
- 11. Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289(19):2554–2559 [DOI] [PubMed] [Google Scholar]
- 12. Marlow N. Is survival and neurodevelopmental impairment at 2 years of age the gold standard outcome for neonatal studies? Arch Dis Child Fetal Neonatal Ed. 2015;100(1):F82–F84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Webbe JWH, Duffy JMN, Afonso E, et al. Core outcomes in neonatology: development of a core outcome set for neonatal research. Arch Dis Child Fetal Neonatal Ed. 2019;105(4):425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukerji A, Shah V, Shah PS. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. 2015;136(6):1132–1143 [DOI] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Oxford, United Kingdom: Oxford; 2000 [Google Scholar]
- 16. Higgins JPT, Thomas J, Chandler Jet al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Available at: www.training.cochrane.org/handbook. Accessed October 7, 2022
- 17. Adams-Chapman I, Heyne RJ, DeMauro SB, et al. ; Follow-Up Study of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental impairment among extremely preterm infants in the neonatal research network. Pediatrics. 2018;141(5):e20173091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adant I, Miserez M, Naulaers G, et al. Long-term outcomes of very low birth weight infants with spontaneous intestinal perforation: a retrospective case-matched cohort study. J Pediatr Surg. 2019;54(10):2084–2091 [DOI] [PubMed] [Google Scholar]
- 19. Altendahl M, Sim MS, Kokhanov A, Gundlach B, Tsui I, Chu A. Severe retinopathy of prematurity is not independently associated with worse neurodevelopmental outcomes in preterm neonates. Front Pediatr. 2021;9:679546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ancel P-Y, Livinec F, Larroque B, et al. ; EPIPAGE Study Group . Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. 2006;117(3):828–835 [DOI] [PubMed] [Google Scholar]
- 21. Bae MH, Jang HJ, Lee NR, Han YM, Byun SY, Park KH. The clinical characteristics and neurodevelopmental outcome of preterm infants with persistent periventricular echogenicity. Pediatr Neonatol. 2018;59(6):606–610 [DOI] [PubMed] [Google Scholar]
- 22. Bae SP, Shin SH, Yoon YM, Kim E-K, Kim H-S. Association of severe retinopathy of prematurity and bronchopulmonary dysplasia with adverse neurodevelopmental outcomes in preterm infants without severe brain injury. Brain Sci. 2021;11(6):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Banihani R, Church PT, Luther M, Kiss A, Asztalos EV. Outcomes of preterm infants with a periventricular venous infarction in the neonatal period. Journal of Pediatric Neurology. 2019;17(2):057–064 [Google Scholar]
- 24. Benavente-Fernández I, Synnes A, Grunau RE, et al. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw Open. 2019;2(5):e192914–e192914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K; New South Wales and Australian Capital Territory Neonatal Intensive Care Units’ Data Collection . Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133(1):55–62 [DOI] [PubMed] [Google Scholar]
- 26. Bolisetty S, Tiwari M, Sutton L, Schindler T, Bajuk B, Lui K; New South Wales and the Australian Capital Territory Neonatal Intensive Care Units’ Data Registry . Neurodevelopmental outcomes of extremely preterm infants in New South Wales and the Australian Capital Territory. J Paediatr Child Health. 2019;55(8):956–961 [DOI] [PubMed] [Google Scholar]
- 27. Broitman E, Ambalavanan N, Higgins RD, et al. Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. Pediatrics. 2007;151(5): 500–505.e2, 505.e1–505.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen C-C, Huang C-B, Chung M-Y, Huang L-T, Yang C-Y. Periventricular echogenicity is related to delayed neurodevelopment of preterm infants. Am J Perinatol. 2004;21(8):483–489 [DOI] [PubMed] [Google Scholar]
- 29. Chmait RH, Chon AH, Schrager SM, Llanes A, Hamilton AH, Vanderbilt DL. Neonatal cerebral lesions predict 2-year neurodevelopmental impairment in children treated with laser surgery for twin-twin transfusion syndrome. J Matern Fetal Neonatal Med. 2019;32(1):80–84 [DOI] [PubMed] [Google Scholar]
- 30. Choi KY, Lee BS, Choi HG, Park S-K. Analysis of the risk factors associated with hearing loss of infants admitted to a neonatal intensive care unit: a 13-year experience in a university hospital in korea. Int J Environ Res Public Health. 2020;17(21):8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. da Silva LS, Ribeiro GE, Montovani JC, Silva DPCD. The effect of peri-intraventricular hemorrhage on the auditory pathway of infants. Int J Pediatr Otorhinolaryngol. 2018;112:24–26 [DOI] [PubMed] [Google Scholar]
- 32. DeMauro SB, Bann C, Flibotte J, Adams-Chapman I, Hintz SR. Cranial ultrasound and minor motor abnormalities at 2 years in extremely low gestational age infants. J Dev Behav Pediatr. 2020;41(4):308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duncan AF, Bann CM, Dempsey A, et al. Behavioral deficits at 18-22 months of age are associated with early cerebellar injury and cognitive and language performance in children born extremely preterm. Pediatrics. 2019;204:148–156.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haslam MD, Lisonkova S, Creighton D, et al. Severe neurodevelopmental impairment in neonates born preterm: impact of varying definitions in a Canadian cohort. J Pediatr. 2018;197:75–81.e4 [DOI] [PubMed] [Google Scholar]
- 35. Hintz SR, Barnes PD, Bulas D, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–e42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klebermass-Schrehof K, Czaba C, Olischar M, et al. Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants. Childs Nerv Syst. 2012;28(12):2085–2092 [DOI] [PubMed] [Google Scholar]
- 37. Kratimenos P, Christidis P, Kehinde F, et al. Association between hemoglobin concentrations at discharge from the neonatal intensive care unit with markers of neurodevelopmental outcomes in premature neonates. J Neonatal Perinatal Med. 2019;12(2):221–230 [DOI] [PubMed] [Google Scholar]
- 38. Lean RE, Han RH, Smyser TA, et al. Altered neonatal white and gray matter microstructure is associated with neurodevelopmental impairments in very preterm infants with high-grade brain injury. Pediatr Res. 2019;86(3):365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin C-Y, Hsu C-H, Chang J-H; Taiwan Premature Infant Follow-up Network . Neurodevelopmental outcomes at 2 and 5 years of age in very-low-birth-weight preterm infants born between 2002 and 2009: a prospective cohort study in Taiwan. Pediatr Neonatol. 2020;61(1):36–44 [DOI] [PubMed] [Google Scholar]
- 40. Logan JW, O’Shea TM, Allred EN, et al. ; ELGAN Study Investigators . Early postnatal hypotension is not associated with indicators of white matter damage or cerebral palsy in extremely low gestational age newborns. J Perinatol. 2011;31(8):524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsushita Y, Sakai Y, Torio M, et al. ; Neonatal Research Network of Japan (NRNJ) . Association of perinatal factors of epilepsy in very low birth weight infants, using a nationwide database in Japan. J Perinatol. 2019;39(11):1472–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–616 [DOI] [PubMed] [Google Scholar]
- 43. Nair V, Janakiraman S, Whittaker S, Quail J, Foster T, Loganathan PK. Permanent childhood hearing impairment in infants admitted to the neonatal intensive care unit: nested case-control study. Eur J Pediatr. 2021;180(7):2083–2089 [DOI] [PubMed] [Google Scholar]
- 44. Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149(2):169–173 [DOI] [PubMed] [Google Scholar]
- 45. Pavaine J, Young JM, Morgan BR, Shroff M, Raybaud C, Taylor MJ. Diffusion tensor imaging-based assessment of white matter tracts and visual-motor outcomes in very preterm neonates. Neuroradiology. 2016;58(3):301–310 [DOI] [PubMed] [Google Scholar]
- 46. Payne AH, Hintz SR, Hibbs AM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr. 2013;167(5):451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peixoto S, Amaral J, Resende C, Faria D, Taborda A. Low-grade intraventricular hemorrhage and neurodevelopment at 24 months of age. Sci Med (Porto Alegre). 2018;28(3):ID29354–ID29354 [Google Scholar]
- 48. Radic JA, Vincer M, McNeely PD. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J Neurosurg Pediatr. 2015;15(6):580–588 [DOI] [PubMed] [Google Scholar]
- 49. Sarkar S, Shankaran S, Barks J, et al. Outcome of preterm infants with transient cystic periventricular leukomalacia on serial cranial imaging up to term equivalent age. J Pediatr. 2018;195:59–65.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shankaran S, Bajaj M, Natarajan G, et al. Outcomes following post-hemorrhagic ventricular dilatation among infants of extremely low gestational age. J Pediatr. 2020;226:36–44.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shankaran S, Johnson Y, Langer JC, et al. Outcome of extremely-low-birth-weight infants at highest risk: gestational age < or =24 weeks, birth weight < or =750 g, and 1-minute Apgar < or =3. Am J Obstet Gynecol. 2004;191(4):1084–1091 [DOI] [PubMed] [Google Scholar]
- 52. Tu YF, Wang ST, Shih HI, Wu PM, Yu WH, Huang CC. Epilepsy occurrence after neonatal morbidities in very preterm infants. Epilepsia. 2019;60(10):2086–2094 [DOI] [PubMed] [Google Scholar]
- 53. Wang L-W, Lin Y-C, Tu Y-F, Wang S-T, Huang C-C, Group TPIDCS; Taiwan Premature Infant Developmental Collaborative Study Group . Isolated cystic periventricular leukomalacia differs from cystic periventricular leukomalacia with intraventricular hemorrhage in prevalence, risk factors and outcomes in preterm infants. Neonatology. 2017;111(1):86–92 [DOI] [PubMed] [Google Scholar]
- 54. Ann Wy P, Rettiganti M, Li J, et al. Impact of intraventricular hemorrhage on cognitive and behavioral outcomes at 18 years of age in low birth weight preterm infants. J Perinatol. 2015;35(7):511–515 [DOI] [PubMed] [Google Scholar]
- 55. Kaur A, Luu TM, Shah PS, Ayoub A, Auger N. Neonatal intraventricular hemorrhage and hospitalization in childhood. Pediatr Neurol. 2020;103:35–42 [DOI] [PubMed] [Google Scholar]
- 56. Webbe JWH, Ali S, Sakonidou S, et al. ; COIN Project Steering Committee . Inconsistent outcome reporting in large neonatal trials: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2020;105(1):69–75 [DOI] [PubMed] [Google Scholar]
- 57. Maitre NL, Marshall DD, Price WA, et al. Neurodevelopmental outcome of infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics. 2009;124(6):e1153–e1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Merhar SL, Tabangin ME, Meinzen-Derr J, Schibler KR. Grade and laterality of intraventricular haemorrhage to predict 18-22 month neurodevelopmental outcomes in extremely low birthweight infants. Acta Paediatr. 2012;101(4):414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. World Health Organization . WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Geneva, Switzerland: World Health Organization; 2015 [PubMed] [Google Scholar]
- 60. Zorn EP, Zhang L, Sandness K, et al. Preserved speed of processing and memory in infants with a history of moderate neonatal encephalopathy treated with therapeutic hypothermia. J Perinatol. 2018;38(12):1666–1673 [DOI] [PubMed] [Google Scholar]
- 61. Hintz SR, Slovis T, Bulas D, et al. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150(6):592–6, 596 e1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34(4):393–421 [DOI] [PubMed] [Google Scholar]
- 63. Gotardo JW, Volkmer NFV, Stangler GP, Dornelles AD, Bohrer BBA, Carvalho CG. Impact of peri-intraventricular haemorrhage and periventricular leukomalacia in the neurodevelopment of preterms: a systematic review and meta-analysis. PLoS One. 2019;14(10):e0223427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomaa N, Miller SP. Intraventricular haemorrhage in preterm children: viewing longer term with a wider lens. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):2–3 [DOI] [PubMed] [Google Scholar]
- 65. Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341 [DOI] [PubMed] [Google Scholar]
- 66. Koller H, Lawson K, Rose SA, Wallace I, McCarton C. Patterns of cognitive development in very low birth weight children during the first six years of life. Pediatrics. 1997;99(3):383–389 [DOI] [PubMed] [Google Scholar]
- 67. Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289(6):705–711 [DOI] [PubMed] [Google Scholar]
- 68. Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F380–F387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015;(11):CD005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morgan C, Fetters L, Adde L, et al. Early intervention for children aged 0 to 2 years with or at high risk of cerebral palsy: international clinical practice guideline based on systematic reviews. JAMA Pediatr. 2021;175(8):846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gale C, Stanikov E, Jawad S, Uthaya S, Modi N. Brain injury occurring during or soon after birth: a report for the national maternity ambition commissioned by the Department of Health. London, United Kingdom: Imperial College; 2017 [Google Scholar]
- 72. Gale C, Statnikov Y, Jawad S, Uthaya SN, Modi N; Brain Injuries expert working group . Neonatal brain injuries in England: population-based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F301–F306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.