Abstract
Purpose
Adults with diabetes mellitus (DM) suffer often from chronic pain, yet evidence-based interventions for comorbid pain and DM are scarce. We tested the effect of a peer-led cognitive behavioral training (CBT) intervention on pain self-efficacy (PSE), pain intensity, and pain-related functional limitations (PRFL) in adults with DM, 1 year after trial initiation.
Methods
The yearlong “Living Healthy” cluster-randomized trial included 230 residents of rural Alabama with DM, who reported pain in the past month; communities were treated as clusters. Intervention participants received a peer-delivered 8-session structured CBT intervention in the context of diabetes self-management; attention control arm participants received a peer-delivered 8-session general health education program. Outcomes included PSE (Arthritis Self-Efficacy Scale, range 10–100); pain intensity (McGill Pain Questionnaire, range 0–45); and PRFL (Western Ontario and McMaster Universities Osteoarthritis Index scale, range 0–100). We examined control-intervention differences in changes in outcome scores from baseline to 3-month and 12-month follow-up, adjusted for clustering.
Findings
The 195 participants with follow-up data were aged 59 ± 10.4 years, 96% were African American, 79% were women, and 80% reported pain on the day of baseline data collection. At 3-month follow-up, PSE increased more for intervention (21-point increase) than control (5-point increase) participants (P for control-intervention (C-I) difference in change < .001); pain intensity decreased for both groups; and PRFL decreased only for intervention participants (–11 score; P for C-I difference in change < .001). Results were sustained at 12 months, and pain intensity significantly improved in only the intervention arm (P for C-I difference in change = .01).
Conclusions
This peer-delivered CBT intervention improved pain self-efficacy, pain-related functional limitations, and pain intensity over 12 months among rural participants with DM and chronic pain.
About 50%−70% of adults with diabetes mellitus (DM) suffer from chronic pain,1–6 compared with 15%−20% of the general population.5 The source of chronic pain in DM is multifactorial. Approximately 15%−30% of patients with DM experience pain related to diabetic polyneuropathy, a specific complication of diabetes.7–11 Up to 80% of persons with DM report widespread musculoskeletal pain, most frequently located in the joints, neck, or lower back.1, 5, 7 Diabetes often co-occurs with osteoarthritis:12 about 30% of patients with DM also had osteoarthritis,13 and recent data suggest that diabetes may also predispose one to the development of metabolic osteoarthritis13 and various rheumatologic conditions.14
Co-occurrence of diabetes and chronic pain may lead to a number of deleterious consequences if left unmanaged. Patients with diabetes and chronic pain report worse quality of life,8, 9 physical functioning,11, 12 general health,5, 6, 12 and anxiety,6, 10 and they are less likely to engage in diabetes self-management, particularly related to maintaining regular exercise or a healthy diet.3 There are so-called triads of interrelated symptoms including chronic pain-diabetes-sleep disorders10, 15 or chronic pain-diabetes-depression.6, 10 Together all this may result in worsening of glycemic control and poor diabetic outcomes.2
Chronic pain management in patients with diabetes remains a challenge despite the existence of clinical recommendations.8, 9, 16 Recent epidemiological data suggest that even if patients are aware of pain, seek care, and take pain control medications, many still experience unacceptable levels of pain.1 Furthermore, a lot of patients with DM and chronic pain are offered predominantly pharmacological modalities and elect to rest and decrease physical activity,1 whereas an increase in physical activity may actually be more beneficial in the long-term control of pain.1, 7 While opioids, acetaminophen, and nonsteroidal anti-inflammatory drugs (NSAIDS) are an important part of pain management, frequently they are not sufficient pain control options for patients with diabetes and have potential life-threatening side effects. Opioids have well-documented risks for overdose17 and potential substance use disorder; NSAIDS are best avoided in diabetes due to risks of renal insufficiency,18, 19 especially in African Americans; and chronic use of acetaminophen is associated with liver damage. Therefore, nonpharmacologic options, such as cognitive behavioral therapy (CBT),20 are attractive for chronic pain management in diabetes. However, nonpharmacological pain management services are not widely available, especially for rural low-income African American populations.2 Therefore, effective interventions to manage chronic pain in diabetes are needed, especially in under-resourced areas where chronic diseases like diabetes are also often more common than in the general population.21
Engagement of health coaches or peer supporters to help patients with complex self-management of chronic conditions, such as diabetes, recently has demonstrated increasing evidence of effectiveness.22–24 Peer supporters with minimal training have successfully delivered complex interventions; for example, in a cluster-randomized trial, lady health workers in Pakistan delivered an intervention based on principles of cognitive behavioral therapy (CBT) and reduced postpartum depression by 50%.25, 26 However, this type of intervention has not been studied in remote rural areas of the United States, and not for diabetes and chronic pain. Therefore, the purpose of this study was to examine the effectiveness of a peer support intervention incorporating principles of CBT in the context of a diabetes self-management intervention and to conduct secondary analysis to examine the intervention effect specifically on chronic pain intensity, pain-related functioning, and pain-related self-efficacy. We conducted a cluster-randomized, pragmatic, community-based trial among predominantly low-income African American adults with diabetes and chronic pain in rural Alabama. Chronic pain was defined as joint pain that interfered with daily activities for at least 2 weeks in the preceding month. The trial compared the CBT-based intervention with a general health education (GHE) program. We tested the hypothesis that compared to the GHE group participants, intervention arm participants would experience greater pain self-efficacy, decreased pain intensity, and increased pain-related functioning. Pain self-efficacy served as the most important outcome of the study because previous research has underscored a relationship between high pain self-efficacy and the ability to perform self-management tasks among patients with diabetes.
RESEARCH DESIGN AND METHODS
Setting, Participants, and Procedures
This study is a secondary analysis of data from the yearlong cluster-randomized community-based “Living Healthy” pragmatic trial, conducted in 2013–2015 in rural Alabama counties that are part of a region known as the Black Belt. Rural counties were defined using population density criteria by the US Census. For example, Wilcox County, Alabama, one of the included counties, had a population density of 15 people per square mile. The Black Belt is an area with a predominantly low-income African American population, high prevalence of diabetes, and limited access to medical resources.22 We recruited 230 participants with both diabetes and chronic pain, living in an 8-county area via respondent-driven sampling and previously established community-based networks.22, 27 Participants were eligible if they were told by their health care provider that they had diabetes and also reported chronic pain that interfered with daily activities for at least 2 weeks during the past month, and if they were willing to work with a trained peer coach to help with diabetes self-management. Exclusions were the absence of a primary care provider (PCP), advanced medical illness that limited life expectancy, plans to move out of the area within the next year, and inability or unwillingness to participate in a walking program. Peer supporters were recruited from the same communities as participants, and they had to have diabetes themselves or to have personal experience caring for someone with diabetes; they also partnered with the study team on a previous diabetes self-management study and had a 2-year experience in motivational interviewing.22, 28 All participants provided written informed consent, and the Institutional Review Board approved the study protocol.
Data on demographics and questionnaire data were collected at baseline via a 45-minute telephone interview and an in-person visit in the participant’s home or a community location conducted by a trained, certified, and quality-controlled research assistant following a standardized protocol. Questionnaire data at 3-month and 12-month follow-up were obtained via participant’s phone interview. Participants received a portable DVD player worth approximately $150 for participating in the program. Peer supporters received $100 for every participant who completed the 8-session 12-week program in either the intervention or GHE arms of the study.
Study Design
This study was a cluster-randomized trial with towns serving as clusters blocked on small, medium, and large community size, with participants nested within communities. A cluster randomized design was used because the communities are tightly knit and randomization at the individual level would have increased the chances of contamination across trial arms. Clusters were block randomized to a trial arm by a random number generator and balance across blocks was monitored. Since the intervention acted at the individual level, analyses were conducted at the individual level and adjusted for clustering. Peer advisors and participants were not blinded to the arm assignment for practical reasons. The primary trial outcomes included health-related quality of life, functional status, glycated hemoglobin (A1c), systolic blood pressure (SBP), and body mass index.29 The study was designed to provide 80% power to detect clinically important differences in A1c (0.4%) and SBP (4 mm Hg). The sample size calculations included 20% attrition and a variance inflation factor to account for the cluster randomized design. The trial is registered with Clinicaltrials.gov (#NCT02538055).
Intervention Arm Program
Ten experienced peer advisors were engaged early in the intervention development process, resulting in a culturally acceptable, practical, collaboratively developed intervention. The intervention was adapted from the “Thinking Healthy” program delivered by lady health workers in Pakistan that successfully halved postpartum depression.25, 26 The details of the intervention were described elsewhere,30 but briefly peer advisors received training on the protocol over 10 weeks, including one 6-hour in-person session emphasizing motivational interviewing skills and goal setting and 3 hours per week on each session of the intervention. Peer advisors were certified on each session by delivering the intervention on the telephone to a research assistant playing the role of a participant.
Each study participant in the intervention arm was paired with a certified peer advisor and received the 8-session telephone-delivered intervention over a period of 12 weeks. Intervention participants also received an activity book, a DVD, and a health calendar that was used to track their homework and daily self-monitoring activities. Prior to the session with the peer advisor, the participant watched a 15- to 30-minute video created by the study team that provided that week’s educational content. Each session lasted between 30 and 60 minutes and incorporated 6 diabetes and pain self-management areas (healthy eating, physical activity, stress management, communication with health care provider, medication adherence, and social support). Following the principles of CBT, each session emphasized identifying and replacing negative or unhealthy thoughts with positive or healthy thoughts and practicing healthy actions. Session activities and homework included daily self-monitoring of mood and pain levels, deep breathing and stress reduction activities, and setting and monitoring individualized behavioral goals for healthy eating and physical activity. The intervention program concluded with 2 sessions aimed at maintaining new activities and skills after the end of the program.
General Health Arm Program
A separate cadre of experienced peer supporters was trained on the GHE program during three 2-hour sessions. Similar to the intervention, the GHE was scripted and interspersed with opportunities for questions or discussion. No diabetes self-management related to healthy eating or physical activity or principles of CBT were included in the GHE program. Control group participants received an equal number of sessions of similar length delivered by a trained peer supporter and, similar to the intervention arm, GHE arm participants received an activity book, a DVD player, and program DVD with educational content developed by the study team. The GHE program consisted of the following topics: dementia and Alzheimer’s disease, breast cancer, colorectal cancer, osteoporosis and fall prevention, oral health, eye health, foot care, and driving safety.
MEASURES
Pain Outcomes
The outcomes for this report included (1) change in pain intensity, (2) change in pain-related functional limitations, and (3) change in pain self-efficacy from baseline to 3 months (right after completing the program) and from baseline to 1 year of follow-up. Pain self-efficacy was assessed by the self-efficacy pain subscale of the Arthritis Self-Efficacy Scale with a summary score ranging from 10 to 100 and higher scores indicating higher self-efficacy (Cronbach’s alpha for 3 subscales were 0.89, 0.87, and 0.76).31 Pain intensity in the past 7 days was measured using the 19-item McGill Pain Questionnaire (MPQ) that assessed severity, sensory, and affective descriptive properties of pain, as well as the presence of current pain during the data collection interview.32, 33 MPQ scores ranged from 0 to 45 with higher scores indicative of worse pain intensity (test-retest reliability 0.70).33 Pain-related functional limitations were ascertained by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scale. WOMAC scores ranged from 0 to 1 and examined (1) patient-reported stiffness, (2) pain when resting or moving, and (3) difficulties with performing daily activities and different regular movements such as rising from a bed, sitting, standing, etc. (Cronbach’s alpha 0.81, 0.91, and 0.84, respectively).34, 35 Higher scores on the WOMAC scale indicated worse pain-related functional limitations.34 All these questionnaires were administered by a trained research assistant.
Baseline Characteristics
Baseline sociodemographics included age, gender, race, self-reported annual household income, and education. Health behaviors assessed current smoking status (if participant reported smoking at least 1 cigarette in the past 30 days); physical activity level compared to others of similar age (“less,” “similar,” or “more active”); and high-fat food consumption, which was assessed by asking, “How many days during the past 7 days did you eat high-fat foods?” Physiological characteristics entailed the average of 2 systolic blood pressures taken by aneroid sphygmomanometer; hemoglobin A1c measured using point-of-service equipment and capillary finger stick blood (National Glycohemoglobin Standardization Project-compliant DCA2000), and body mass index (BMI) calculated as weight in kilograms divided by the square of height in meters.
Psychological characteristics included depressive symptoms on the 11-item Center for Epidemiologic Studies Depression (CES-D) scale36 with scores ranging from 0 to 22 and score ≥9 indicative of at least moderate depressive symptoms.37 Other covariates included pain coping assessed by the Pain Coping Strategies Questionnaire (CSQ-24), ranging from 0 to 6 with higher scores indicating better coping; social support;38 and the physical component summary score of the Short Form 12.39 Use of prescription pain medications was self-reported and included NSAIDS, muscle relaxants, prescription opioids, anticonvulsants, and antidepressants for diabetic polyneuropathy, other anti-inflammatory drugs, and biologic drugs for musculoskeletal/rheumatoid conditions.
Statistical Analyses
We used Student’s t-tests for continuous variables and chi-square tests for categorical variables to compare intervention and GHE arm participants on baseline characteristics. We compared intervention and GHE arms on the 3 main outcome measures (pain self-efficacy, pain intensity, and pain-related functional limitations) using Student’s t-tests. Mean scores and standard deviations were estimated for pain self-efficacy, MPQ, and WOMAC scales at baseline, 3-month, and 12-month follow-up for the intervention and control arms separately.
We also constructed sequentially adjusted Generalized Estimating Equations (GEE) models in parallel for all of the 3 pain-related outcomes and examined control-intervention (C-I) differences in the change of mean scores, separately, first from baseline to 3 months and second from baseline to 1-year follow-up. GEEs estimated (1) least square mean scores for control and intervention group with 95% confidence interval (CI) and (2) a C-I mean difference in change in the score from baseline to 3-month and, separately, to 12-month follow-up and a P value for these differences. Model 1 adjusted for the baseline score and clustering. Model 2 added adjustment for characteristics, where there was potentially a difference at baseline between the intervention and GHE groups, demonstrating trends toward imbalance across treatment arms at baseline and portrayed by P value < .15 (Table 1). These covariates included age, gender, body mass index, smoking, consumption of high-fat diet, pain coping, and depressive symptoms. SAS version 9.4 was utilized to conduct the analyses (SAS Institute Inc., Cary, NC). All analyses were intention-to-treat and included all participants available at 3-month follow-up.
Table 1.
Baseline Characteristics of the “Living Healthy” Participants
| General Health Education Group (n = 99) | Intervention Group (n = 96) | ||
|---|---|---|---|
| n (%) | n (%) | P | |
| Sociodemographics | |||
| Age, M, SD | 57.9, 10.8 | 60.0, 9.9 | .15 |
| Female | 74 (74.7) | 81 (84.4) | .10 |
| African American | 94 (94.9) | 94 (97.9) | .27 |
| Annual household income | .98 | ||
| < $40,000 | 83 (83.8) | 81 (84.4) | |
| ≥ $40,000 | 8 (8.1) | 7 (7.3) | |
| Declined to provide | 8 (8.1) | 8 (8.3) | |
| Education | .38 | ||
| Less than high school | 32 (32.3) | 23 (24.0) | |
| High school | 32 (32.3) | 38 (39.6) | |
| Some college or greater | 35 (35.4) | 35 (36.5) | |
| Health behaviors | |||
| Smoked at least 1 cigarette in past 30 days | 1 (1.1) | 4 (4.8) | .15 |
| Physical activity level, compared to others of similar age: | .42 | ||
| Less active | 44 (44.4) | 34 (35.4) | |
| Same as others similar age | 28 (28.3) | 30 (31.3) | |
| More active | 27 (27.3) | 32 (33.3) | |
| Number of days, consuming high-fat food in the past 7 days | 3.2, 2.1 | 2.6, 1.8 | .05 |
| Medications | |||
| Use of any prescription pain medication | 54 (55.7) | 46 (48.4) | .31 |
| Physiological | |||
| Body mass index, kg/m2, M, SD | 36.7, 7.1 | 38.3, 8.8 | .15 |
| Systolic blood pressure, mmHg, M, SD | 133.4, 21.4 | 133.2, 20.3 | .94 |
| Hemoglobin A1c %, M, SD | 8.4, 2.2 | 8.2, 2.1 | .40 |
| Psychosocial | |||
| Pain coping (range 0–6) M, SD | 2.0, 0.9 | 1.8, 0.9 | .15 |
| CES-D score, short form, (range 0–22) M, SD | 8.2, 4.8 | 7.2, 4.1 | .13 |
| CES-D score ≥ 9 n, % | 51 (51.5) | 41 (42.7) | .22 |
| Social support (range 4–12) M, SD | 6.0, 1.8 | 6.0, 2.0 | .97 |
| Physical component score of SF-12 M, SD | 37.5, 8.8 | 38.8, 8.9 | .33 |
RESULTS
Participant Characteristics
The CONSORT diagram for “Living Healthy” study is presented in Figure 1. Of 230 enrolled trial participants, 195 (84.8%) were available at 3-month follow-up and 175 (76.1%) at 12-month follow-up. There were no significant differences in the baseline characteristics between the intervention and GHE arm participants (Table 1). The majority of the sample was African American (95% among the GHE and 98% among the intervention arm) and female (75% among the GHE and 84% among the intervention arm) with mean age 58 years among the GHE and 60 years among the intervention arm. In both trial arms, 84% of participants reported annual household income below $40,000. More than a third of the sample considered their level of physical activity below others of similar age (44% among GHE and 35% among intervention arm), and about a half of the participants reported clinically significant depressive symptoms (52% among GHE and 43% among intervention arm). Glycemic control was suboptimal in both groups with mean HbA1c of 8% (Table 1).
Figure 1.
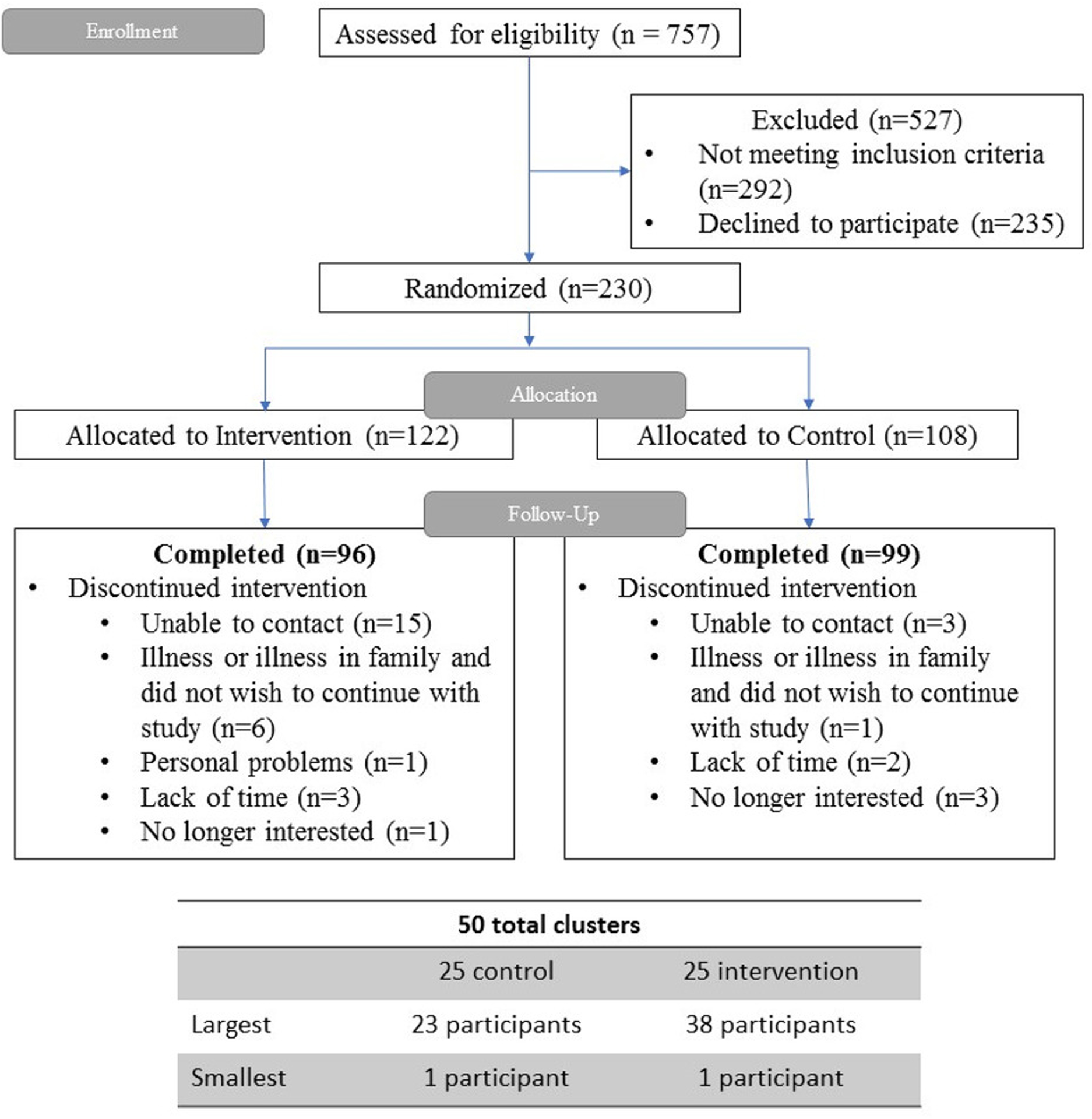
Consort Diagram of the Living Healthy Trial.
Only 56% among the GHE and 48% among the intervention participants reported use of any prescription medication for pain (Table 1).
Pain at Baseline
At baseline 84% of GHE and 75% of intervention participants (P = .17) reported presence of current mild to severe pain at the time of the interview for the baseline data collection; 63% of GHE and 56% of intervention participants (P = .36) reported that pain at least moderately interfered with their work and/or household activities in the past 4 weeks.
Participants in the GHE arm demonstrated slightly higher pain intensity in the last 7 days than intervention arm participants at baseline (mean MPQ scores 16.5 and 13.7, P = .04) (Figure 2). There were no differences between the trial arms in baseline pain-related functional limitations (WOMAC Index) or pain self-efficacy (Figure 2).
Figure 2.
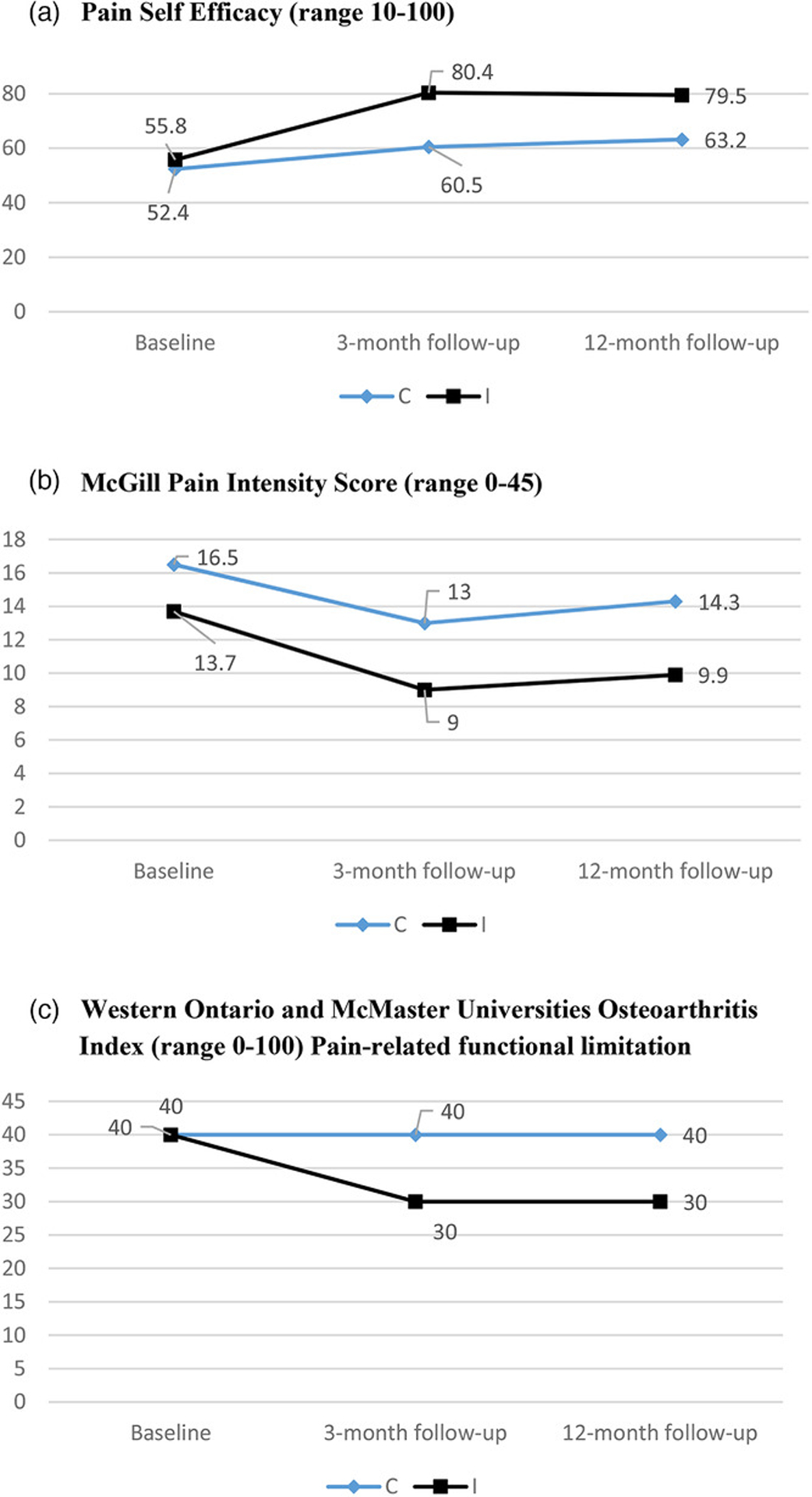
Change in Unadjusted Mean Scores for Pain-Related Outcomes from Baseline to 3- and 12-Month Follow-Ups for the Intervention and General Health Education (Control) Arm.
Abbreviations: C, control; I, intervention.
Effect of the Peer-Delivered CBT-Based Intervention on Pain at Follow-Up
The proportion of participants reporting any current pain at follow-up decreased from baseline, but more so in the intervention arm: at 3-month follow-up 57% of intervention arm versus 76% of GHE arm participants reported any pain at the phone interview (P = .005). This decrease in the presence of current pain was sustained at 12-month follow-up: 60% of intervention arm versus 80% of GHE arm reported any pain at the time of the phone interview (P = .07).
Both trial arms experienced improved pain self-efficacy from baseline to 3-month follow-up; this improvement was sustained at 12-month follow-up (Figure 2). However, the intervention arm demonstrated a much greater increase in self-efficacy scores (+21 points) than the GHE arm (+4.6 points), C-I difference P < .001 at 3-month follow-up after adjustment for all covariates (Table 2, panel A). The same C-I difference in the change in pain self-efficacy scores remained significant at 12-month follow-up, C-I difference P = .005 (Table 2, panel A).
Table 2.
Changes in Control-Intervention Differences From Baseline to 3- and 12-Month Follow-Up, the Living Healthy Trial
| Panel A. Pain Self-Efficacy Score (Range 10–100) | ||||||
|---|---|---|---|---|---|---|
| 3-Month change | ||||||
| GHE group (n = 99) | Intervention group (n = 96) | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | C-I mean difference in change | P value for difference | |
| Model 1 | 6.5 | (2.3–10.7) | 25.2 | (20.9–29.4) | −18.7 | <.001 |
| Model 2 | 4.6 | (−0.04 to 9.3) | 21.5 | (16.1–26.3) | −16.5 | <.001 |
| 12-Month change | ||||||
| GHE group (n = 86)* | Intervention group (n = 89)* | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | C-I mean difference in change | P value for difference | |
| Model 1 | 7.4 | (1.8–12.9) | 23.9 | (19.0–28.8) | −16,6 | <.001 |
| Model 2 | 6.8 | (−0.1 to 13.8) | 23.7 | (17.1–30.4) | −16,9 | .0005 |
| Panel B. McGill Pain Intensity Score (Range 0–45) | ||||||
| 3-Month change | ||||||
| GHE group (n = 99) | Intervention group (n = 96) | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | C-I mean difference in change | P value for difference | |
| Model 1 | −3.0 | (−4.4 to −1.5) | −5.4 | (−6.9 to −4.0) | 2.5 | .02 |
| Model 2 | −2,2 | (−4.0 to −0.5) | −4.4 | (−6.3 to −2.5) | 2.1 | .07 |
| 12-Month change | ||||||
| GHE group (n = 86) | Intervention group (n = 89) | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | C-I mean difference in change | P value for difference | |
| Model 1 | 1.0 | (−2.3 to 2.5) | −4.3 | (−6.0 to −2.6) | 4.4 | .004 |
| Model 2 | 0.4 | (−2.2 to 2.3) | −4.0 | (−6.4 to −1.6) | 4.0 | .01 |
| Panel C. WOMAC Osteoarthritis Index Score (Range 0–100) | ||||||
| 3-Month change | ||||||
| GHE group (n = 99) | Intervention group (n = 96) | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | C-I mean difference in change | P value for difference | |
| Model 1 | −4.2 | (−7.9 to 0.4) | −10.3 | (−13.1 to −7.5) | 6.2 | .01 |
| Model 2 | −3.1 | (−7.3 to 1.0) | −12.0 | (−16.1 to −7.8) | 8.8 | .002 |
| 12-Month change | ||||||
| GHE group (n = 86) | Intervention group (n = 89) | |||||
| LS-mean | (95% CI) | LS-mean | (95% CI) | C-I mean difference in change | P value for difference | |
| Model 1 | 0.0 | (−3.7 to 4.2) | −11.7 | (−14.5 to −8.8) | 11.9 | < .001 |
| Model 2 | 1.8 | (−2.5 to 6.2) | −10.7 | (−14.7 to −6.7) | 12.6 | < .001 |
Abbreviations: C-I, control-intervention difference or difference between intervention arm and GHE arm; GHE, general health education; LS-mean, least square mean.
Model 1 adjusted for baseline score and clustering.
Model 2 adjusts for age, sex, body mass index, current smoking, high-fat food intake, pain coping score, depressive symptoms, all of which demonstrated trends toward imbalance across treatment arms at baseline (Table 1).
175 participants were available at 12-month follow-up.
P values < .05 are bolded.
Pain intensity on the MPQ scale decreased for both trial arms at 3-month follow-up and remained decreased at 12-month follow-up (Figure 2). However, the intervention group experienced a 4.7 unit decrease (5.0 unit decrease is a minimal clinically important difference)40 in pain intensity, whereas the GHE group had only 3.5 unit decrease, which was not sustained at 12 months. In the fully adjusted models, decreased pain intensity was greater for the intervention group at both 3-month and 12-month follow-up (C-I difference in change 2.1 [P value for difference = .07] and 4.0 [P for difference = .01], respectively) (Table 2, panel B).
In terms of pain-related functional limitations, only the intervention group demonstrated a decrease in the mean WOMAC score from baseline (M = 40) to follow-up (M = 30) at both follow-up time points (Figure 2). Among the intervention group participants, after adjustment for covariates, the mean decrease in functional limitations on the WOMAC scale was −12 at 3 months and −10 units at 12 months, which approached the previously established minimal clinically important difference of −15 units.41 This C-I difference in the change of WOMAC scores from baseline to follow-up remained significant after adjustment for all covariates: C-I mean difference in change 8.8 (P value for difference = .002) at 3-month follow-up and 12.6 (P value for difference < .001) at 12-month follow-up (Table 2, panel C).
Discussion
This report analyzed data from a community-based cluster-randomized pragmatic trial conducted in a rural area with limited medical resources. We evaluated the effect of the CBT-based peer support “Living Healthy” intervention on pain-related outcomes among adults with diabetes and chronic pain, one of the first efforts combining CBT and peer support for chronic pain management. The intervention did not lead to change in A1c or BMI at 3-month follow-up,29 but it did produce greater improvement in pain-related self-efficacy, pain intensity, and functioning, compared to the general health education group. Importantly, these results were sustained at 12-month follow-up after the intervention completion.
One of the most unique features of the “Living Healthy” intervention was that it was culturally adapted and targeted specifically to rural African American adults with diabetes in partnership with area residents. African American adults with diabetes face worse outcomes than whites2, 42 and there is a lack of low-cost interventions specifically developed for this group.42 A recent systematic review found only 3 published studies evaluating the effectiveness of diabetes interventions delivered in the community for African Americans.42 Similar to previous data, our predominantly African American study sample demonstrated relatively suboptimal glycemic control with a mean hemoglobin A1c of 8%. Moreover, there is a limited number of existing treatment interventions targeting both diabetes and chronic pain simultaneously. The “Living Healthy” program, adapted from the Pakistani CBT-based intervention delivered by Lady Health Workers and designed to prevent postpartum depression, is one of the first interventions that combined peer support and CBT for managing chronic pain in adults with diabetes. Both peer support43 and CBT16 have been previously shown to be effective in the treatment of chronic pain44, 45 or diabetes22, 23, 46 separately, but not concurrently. For example, Moore et al conducted a CBT-based psychologist-led intervention for primary care patients with chronic back pain.20 Compared to the control group, intervention participants demonstrated significant reduction in pain-related overall worry.20 Unlike the “Living Healthy” program, which improved pain-related functional limitations, Moore et al did not report sustainable improvement in pain interference with daily activities or pain-related disability. Unlike the study by Moore et al, the CBT-based intervention in the “Living Healthy” study demonstrated significant improvement in pain intensity.
Another line of previous research has examined the effectiveness of peer support in the management of chronic pain.47, 48 A peer support intervention targeting veterans with chronic musculoskeletal pain resulted in nonsignificant improvement in pain severity, pain interference, and pain self-efficacy over a 4-month follow up.48 In contrast to Mathias et al, the “Living Healthy” intervention resulted in a significant and sustainable improvement in pain-related functional limitations and pain self-efficacy.
The “Living Healthy” intervention participants demonstrated improvement in 3 important pain-related domains: pain-related self-efficacy, pain intensity, and functional limitations. All of these domains might serve as important mechanisms for improving overall health outcomes in these patients. For example, pain self-efficacy plays an important intervening role in patients with both diabetes and chronic pain. In previous reports self-efficacy has been shown to mediate the relationship between chronic pain and diabetes self-management.4 Therefore, an increase in pain self-efficacy may lead to a higher probability of performing self-management tasks such as adherence to an exercise schedule. An increase in pain self-efficacy is very important given the fact that some patients with diabetes have serious damage in muscle and nervous tissue related to a prolonged hyperglycemia and insulin resistance that is hard to manage only by pharmacological treatment.14, 49 A decrease in pain intensity was accompanied by improvements in functional limitations due to pain, opening the possibility for a patient to increase physical activity and ultimately improve glycemic control. The fact that these gains were observed without medications is also noteworthy, as the nation continues to confront the crisis of prescription opioid use disorder and overdose.8, 50 Additionally, although self-management was not a primary outcome of the study, we anticipate that our CBT-based program will improve self-management skills via such techniques as cognitive restructuring, adaptive coping, patient empowerment, skills building, setting realistic and achievable behavioral goals, and stress reduction.
Participants in the control GHE arm also demonstrated a moderate improvement in pain-related self-efficacy and pain intensity, but not functional limitations during the study period. Such improvements were more pronounced at 3-month follow-up with a trend of worsening pain intensity at 12 months. Improvements in pain-related self-efficacy and pain intensity among the controls were likely a result of a combination of working with supportive peer coaches and the placebo effect.
This study’s strengths include its rigorous experimental design and the community-partnered approach to every stage of the research. Over the course of the trial, we established a community network of fully trained community health advisors who were able to participate in all stages of the intervention development, delivery, and evaluation. With the help of the peer supporters, we were able to engage a very hard-to-reach population with very limited financial and medical resources and at high risk of diabetes complications. The statistical analyses accounted for imbalance across treatment arms, a common problem in cluster-randomized trials. By its nature, this highly personable and individualized intervention that established relationships between peer supporters and their clients may contribute to a higher level of sustainability of the results over time.
Several limitations of this study should also be noted. We did not determine which medical conditions were contributing to the chronic pain burden experienced by participants, but we note that CBT has been shown to work in a host of chronic pain conditions, suggesting that the target of this type of treatment is not etiologically but rather functionally based. The study sample was modest in size with some data collected through self-report; that is, data on health behaviors, physical activity, medications, food consumption, with known limitations, including recall bias. Due to the nature of the intervention both peer supporters and study participants were not blinded to the treatment conditions, which may have introduced an allocation bias: if intervention participants know that they will receive an intervention, they may change their behavior, resulting in bias away from the null and exaggerated effect size. We minimized allocation bias and intervention contamination effects by randomizing rural towns instead of individual participants, and by delivering an active control intervention, allowing us to attribute intervention effects to the CBT intervention.
The single geographic region may limit generalizability. Because the intervention was delivered in the community, we could not account for differences among peer supporter in peer-coaching style or intensity of use of skills acquired during training.
In conclusion, a peer-delivered telephone CBT-based intervention, the “Living Healthy” intervention, was successfully culturally adapted and delivered to African American adults suffering from diabetes and chronic pain. The intervention resulted in significantly increased pain self-efficacy, decreased pain intensity, and decreased pain-related functional limitations over 1 year. A combination of peer support and cognitive-behavioral approaches may be an effective strategy for pain control among adults with diabetes, without resorting to opioids, especially in areas with limited medical resources.
Funding:
Funding for this research was provided by the American Academy of Family Physicians Foundation through the Peers for Progress program with support from the Eli Lilly and Company Foundation. Representatives of the funding agency have not been involved in the collection, management, analysis, or interpretation of the data. This research was supported in part by an Agency for Healthcare Research and Quality (AHRQ) training grant to the University of Alabama at Birmingham (2T32HS013852-16). Drs Monika Safford and Yulia Khodneva had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosures: All authors report no potential or actual conflicts of interest pertaining to this manuscript publication.
REFERENCES:
- 1.Butchart A, Kerr EA, Heisler M, Piette JD, Krein SL. Experience and management of chronic pain among patients with other complex chronic conditions. Clin J Pain. 2009; 25(4): 293– 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbert MS, Varley AL, Andreae SJ, Goodin BR, Bradley LA, Safford MM. Association of pain with HbA1c in a predominantly black population of community-dwelling adults with diabetes: a cross-sectional analysis. Diabetic Med. 2013; 30(12): 1466– 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krein SL, Heisler M, Piette JD, Makki F, Kerr EA. The effect of chronic pain on diabetes patients’ self-management. Diabetes Care. 2005; 28(1): 65– 70. [DOI] [PubMed] [Google Scholar]
- 4.Krein SL, Heisler M, Piette JD, Butchart A, Kerr EA. Overcoming the influence of chronic pain on older patients’ difficulty with recommended self-management activities. Gerontologist. 2007; 47(1): 61– 68. [DOI] [PubMed] [Google Scholar]
- 5.Molsted S, Tribler J, Snorgaard O. Musculoskeletal pain in patients with type 2 diabetes. Diabetes Res Clin Pract. 2012; 96(2): 135– 140. [DOI] [PubMed] [Google Scholar]
- 6.Bair MJ, Brizendine EJ, Ackermann RT, Shen C, Kroenke K, Marrero DG. Prevalence of pain and association with quality of life, depression and glycaemic control in patients with diabetes. Diabetic Med. 2010; 27(5): 578– 584. [DOI] [PubMed] [Google Scholar]
- 7.Jensen TM, Eriksen SBM, Larsen JS, et al. Exercise training is associated with reduced pains from the musculoskeletal system in patients with type 2 diabetes. Diabetes Res Clin Pract. 2019; 154: 124– 129. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler D Current concepts in the management of diabetic polyneuropathy. Curr Diabetes Rev. 2011; 7(3): 208– 220. [DOI] [PubMed] [Google Scholar]
- 9.Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabet Metab. 2009; 35(3): 206– 213. [DOI] [PubMed] [Google Scholar]
- 10.Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One. 2013; 8(9):e74195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006; 29(7): 1518– 1522. [DOI] [PubMed] [Google Scholar]
- 12.Miksch A, Hermann K, Rolz A, et al. Additional impact of concomitant hypertension and osteoarthritis on quality of life among patients with type 2 diabetes in primary care in Germany—a cross-sectional survey. Health Quality Life Outcomes. 2009; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015; 1(1):e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebiedz-Odrobina D, Kay J. Rheumatic manifestations of diabetes mellitus. Rheumat Dis Clin North Am. 2010; 36(4): 681– 699. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld V Sleep dysfunction, diabetes, and pain: a troublesome triad. J Family Pract. 2014; 63(6 Suppl): S18– S24. [PubMed] [Google Scholar]
- 16.Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014; 69(2): 119– 130. [DOI] [PubMed] [Google Scholar]
- 17.Khodneva Y, Muntner P, Kertesz S, Kissela B, Safford MM. Prescription opioid use and risk of coronary heart disease, stroke, and cardiovascular death among adults from a prospective cohort (REGARDS Study). Pain Med. 2016; 17(3): 444– 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harirforoosh S, Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Safety. 2009; 8(6): 669– 681. [DOI] [PubMed] [Google Scholar]
- 19.Wehling M Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol. 2014; 70(10): 1159– 1172. [DOI] [PubMed] [Google Scholar]
- 20.Moore JE, Von Korff M, Cherkin D, Saunders K, Lorig K. A randomized trial of a cognitive-behavioral program for enhancing back pain self care in a primary care setting. Pain. 2000; 88(2): 145– 153. [DOI] [PubMed] [Google Scholar]
- 21.Lepard MG, Joseph AL, Agne AA, Cherrington AL. Diabetes self-management interventions for adults with type 2 diabetes living in rural areas: a systematic literature review. Curr Diab Rep. 2015; 15(6): 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safford MM, Andreae S, Cherrington AL, et al. Peer coaches to improve diabetes outcomes in Rural Alabama: a cluster randomized trial. Ann Fam Med. 2015; 13(Suppl 1): S18– 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013; 11(2): 137– 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010; 153(8): 507– 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Challenges Rahman A. and opportunities in developing a psychological intervention for perinatal depression in rural Pakistan—a multi-method study. Arch Womens Ment Health. 2007; 10(5): 211– 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008; 372: 902– 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreae SJ, Halanych JH, Cherrington A, Safford MM. Recruitment of a rural, southern, predominantly African-American population into a diabetes self-management trial. Contemp Clin Trials. 2012; 33(3): 499– 506. [DOI] [PubMed] [Google Scholar]
- 28.Cherrington A, Martin MY, Hayes M, et al. Intervention mapping as a guide for the development of a diabetes peer support intervention in rural Alabama. Prev Chronic Dis. 2012; 9: E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreae SJ, Andreae LJ, Richman JS, Cherrington AL, Safford MM. Peer-delivered cognitive behavioral training reduced pain as a barrier to exercise in diabetes: a cluster randomized trial. Ann Fam Med. 2020; 18(1): 15– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreae SJ, Andreae LJ, Cherrington AL, et al. Development of a community health worker-delivered cognitive behavioral training intervention for individuals with diabetes and chronic pain. Fam Commun Health. 2018; 41(3): 178– 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989; 32(1): 37– 44. [DOI] [PubMed] [Google Scholar]
- 32.Melzack R The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975; 1(3): 277– 299. [DOI] [PubMed] [Google Scholar]
- 33.Burckhardt CS. The use of the McGill Pain Questionnaire in assessing arthritis pain. Pain. 1984; 19(3): 305– 314. [DOI] [PubMed] [Google Scholar]
- 34.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988; 15(12): 1833– 1840. [PubMed] [Google Scholar]
- 35.Salaffi F, Leardini G, Canesi B, et al. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthr Cartil. 2003; 11(8): 551– 560. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol. 1979; 88(2): 174– 181. [DOI] [PubMed] [Google Scholar]
- 37.Payne C, Hedberg EC, Kozloski M, Dale W, McClintock MK. Using and interpreting mental health measures in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014; 69 (Suppl 2): S99– 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang TS, Brown MB, Funnell MM, Anderson RM. Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. Diabetes Educ. 2008; 34(2): 266– 276. [DOI] [PubMed] [Google Scholar]
- 39.Andresen EM, Meyers AR. Health-related quality of life outcomes measures. Arch Phys Med Rehabil. 2000; 81(12 Suppl 2): S30– 45. [DOI] [PubMed] [Google Scholar]
- 40.Strand LI, Ljunggren AE, Bogen B, Ask T, Johnsen TB. The Short-Form McGill Pain Questionnaire as an outcome measure: test-retest reliability and responsiveness to change. Eur J Pain. 2008; 12(7): 917– 925. [DOI] [PubMed] [Google Scholar]
- 41.Escobar A, Quintana JM, Bilbao A, Aróstegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthr Cartil. 2007; 15(3): 273– 280. [DOI] [PubMed] [Google Scholar]
- 42.Ricci-Cabello I, Ruiz-Perez I, Nevot-Cordero A, Rodriguez-Barranco M, Sordo L, Goncalves DC. Health care interventions to improve the quality of diabetes care in African Americans: a systematic review and meta-analysis. Diab Care. 2013; 36(3): 760– 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher EB, Earp JA, Maman S, Zolotor A. Cross-cultural and international adaptation of peer support for diabetes management. Fam Pract. 2010; 27 (Suppl 1): i6– 16. [DOI] [PubMed] [Google Scholar]
- 44.Linden M, Scherbe S, Cicholas B. Randomized controlled trial on the effectiveness of cognitive behavior group therapy in chronic back pain patients. J Back Musculoskelet Rehabil. 2014; 27(4): 563– 568. [DOI] [PubMed] [Google Scholar]
- 45.Bjornsdottir SV, Arnljotsdottir M, Tomasson G, Triebel J, Valdimarsdottir UA. Health-related quality of life improvements among women with chronic pain: comparison of two multidisciplinary interventions. Disab Rehab. 2016; 38(9): 828– 836. [DOI] [PubMed] [Google Scholar]
- 46.Tang TS, Funnell MM, Sinco B, Spencer MS, Heisler M. Peer-Led, empowerment-based approach to self-management efforts in diabetes (PLEASED): a randomized controlled trial in an African American Community. Ann Fam Med. 2015; 13 (Suppl 1): S27– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz F Peer consultants: missing link in the treatment of chronic pain. Canad Fam Phys. 2015; 61(10): 837– 838, 844–835. [PMC free article] [PubMed] [Google Scholar]
- 48.Matthias MS, McGuire AB, Kukla M, Daggy J, Myers LJ, Bair MJ. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015; 16(1): 81– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhai X, Sun C, Rong P, et al. A correlative relationship between chronic pain and insulin resistance in Zucker Fatty rats: role of downregulation of insulin receptors. J Pain. 2016; 17(4): 404– 413. [DOI] [PubMed] [Google Scholar]
- 50.Kroenke K, Bair MJ, Damush TM, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009; 301(20): 2099– 2110. [DOI] [PMC free article] [PubMed] [Google Scholar]


