Abstract
Endotoxin (lipopolysaccharide [LPS]) tolerance is characterized by a reduced capacity of monocytes to produce proinflammatory cytokines upon restimulation in vitro. To determine whether LPS exposure induces a change in lymphocyte cytokine production and whether this results in a shift in the T-helper 1 (Th1)/Th2 balance, whole blood obtained from seven healthy subjects before and after an intravenous injection of LPS (4 ng/kg) was stimulated in vitro with the T-cell stimulus anti-CD3/CD28 or staphylococcal enterotoxin B. Whole-blood production of the Th1 cytokines gamma interferon (IFN-γ) and interleukin-2 (IL-2) was markedly reduced at 3 and 6 h, while the production of the Th2 cytokines IL-4 and IL-5 was not influenced or was slightly increased. The IFN-γ/IL-4 ratio was strongly decreased at 6 h. Serum obtained after LPS exposure could slightly inhibit the release of IFN-γ but increased IL-4 production during stimulation of blood drawn from subjects not previously exposed to LPS. Normal serum also inhibited IFN-γ production, albeit to a lesser extent. LPS exposure influences lymphocyte cytokine production, resulting in a shift toward a Th2 cytokine response, an effect that may be mediated in part by soluble factors present in serum after LPS administration in vivo.
Endotoxin (lipopolysaccharide [LPS]), a component of the outer cell membrane of gram-negative bacteria, is considered to be a central mediator in the pathogenesis of gram-negative sepsis. Intravenous injection of LPS into healthy humans not only initiates a cascade of inflammatory pathways but also induces a temporary refractory state, generally referred to as LPS tolerance (12, 32). LPS tolerance is characterized by decreased production of tumor necrosis factor (TNF), interleukin-1β (IL-1β), IL-6, and IL-10 and concurrently increased production of IL-1 receptor antagonist upon ex vivo restimulation of whole blood or peripheral blood mononuclear cells (PBMCs) with LPS. The same alterations in the capacity to produce cytokines have been found in whole blood or monocytes isolated from sepsis patients (10, 15, 23, 34) or from patients after surgery (16). Therefore, LPS tolerance can be considered an adaptive host immune response rather than a generalized hyporesponsiveness which is not specific for prior exposure to LPS.
Most research on LPS tolerance has focused on the reduced reactivity of monocytes. Only recently has it become apparent that other cell types may also display a reduced responsiveness during in vitro stimulation. Indeed, neutrophilic granulocytes isolated from patients with sepsis produced less IL-1β and IL-8 upon ex vivo stimulation with LPS (17, 20). The effects of endotoxemia on the production of cytokines by T lymphocytes are unknown. It has been reported that major surgery results in a severe defect in T-cell proliferation and cytokine secretion (8, 14). In addition, surgical stress may induce a shift in the T-helper 1 (Th1)/Th2 balance toward a Th2-type immune response (8, 26). A recent study with mice indicated that in vivo administration of LPS may result in a reduced ability of splenocytes to produce the T-cell cytokines IL-2, IL-4, and gamma interferon (IFN-γ) upon ex vivo stimulation with concanavalin A, as reflected by a diminished capacity to accumulate mRNA encoding these cytokines (7). Therefore, in the present study we sought to determine whether LPS exposure in healthy subjects induces a change in cytokine production by lymphocytes and whether this results in a shift in the Th1/Th2 balance, as indicated by the production of Th1 and Th2 cytokines during in vitro whole-blood stimulation with T-cell stimuli.
MATERIALS AND METHODS
Study design.
Seven healthy male volunteers (mean age, 21 years; range, 19 to 25 years) were admitted to the Clinical Research Unit, Academic Medical Center, University of Amsterdam. Written informed consent was obtained from all study subjects. The study was approved by the research and ethical committees of the Academic Medical Center. Medical history, physical and routine laboratory examinations, chest X-ray, and electrocardiogram of all volunteers were normal. Each volunteer received an intravenous bolus injection of Escherichia coli LPS, lot G (U.S. Pharmacopeia, Rockville, Md.), administered over 1 min in an antecubital vein at a dose of 4 ng/kg of body weight. Heparinized blood for in vitro stimulation and FACScan analysis was obtained directly before LPS injection (0 h) and at 3, 6, and 24 h thereafter. In addition, blood was collected in Vacutainer tubes and, after clotting, serum was collected after centrifugation (10 min at 1,600 × g at 4°C) and stored at −20°C.
Whole-blood stimulation.
Heparinized blood, diluted 1:1 in pyrogen-free RPMI 1640 (BioWhittaker, Verviers, Belgium), was stimulated for 24 h at 37°C in the presence or absence of the T-cell stimulus anti-CD3/anti-CD28 (αCD3/αCD28) (Central Laboratory of the The Netherlands Red Cross Blood Transfusion Service [CLB], Amsterdam, The Netherlands; final concentration, 1:1,000 each), or the superantigen staphylococcal enterotoxin B (SEB) (Sigma, St. Louis, Mo.; 1 μg/ml). After incubation, the supernatant was collected after centrifugation and stored at −20°C until assays were performed.
In a separate series of experiments, serum samples obtained from the seven volunteers before (normal serum) and 3 h after in vivo exposure to LPS (post-LPS serum) were pooled and subsequently incubated for 24 h at 37°C with whole blood obtained from six other healthy donors (who were not exposed to LPS) in the presence or absence of αCD3/αCD28. Whole blood was collected aseptically using a sterile collecting system consisting of a butterfly needle connected to a syringe (Becton Dickinson & Co, Rutherford, N.J.). Anticoagulation was obtained using endotoxin-free heparin (Leo Pharmaceutical Products B.V., Weesp, The Netherlands; final concentration, 10 U/ml). In these experiments, whole blood was diluted 1:1 in pyrogen-free RPMI 1640 containing different dilutions of pooled normal or post-LPS serum (final concentrations, 1 to 20%). After incubation, plasma was prepared by centrifugation and stored at −20°C until assays were performed.
FACScan analysis.
PBMCs were isolated by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation at room temperature for 20 min at 1,000 × g. PBMCs were collected in the interphase, washed twice with phosphate-buffered saline, and resuspended in FACS buffer (phosphate-buffered saline supplemented with 0.01% [wt/vol] NaN3, 0.5% [wt/vol] bovine serum albumin and 0.3 mM EDTA). For staining, 0.5 × 106 cells/tube were incubated with the following mouse monoclonal antibodies: Cy-Chrome5-labeled anti-CD3 (Immunotech, Marseille, France), phycoerythrin-labeled anti-CD4 (Immunotech), or isotype controls (Immunotech). Lymphocytes were gated by forward and side scatter, and 5,000 cells were counted.
Assays.
IFN-γ and IL-4 (both from CLB; detection limits, 4 and 1.2 pg/ml, respectively), IL-2 (R&D Systems, Abingdon, United Kingdom; detection limit, 8 pg/ml), and IL-5 (Medgenix, Fleurus, Belgium; detection limit, 8.2 pg/ml) were measured by enzyme-linked immunosorbent assays according to the instructions of the manufacturers. Leukocyte counts and differential counts were determined with K3-EDTA-anticoagulated blood by flow cytometry.
Statistical analysis.
All values are given as means ± standard errors (SE). Comparisons were done using the Wilcoxon test. A P value of <0.05 was considered to represent a significant difference.
RESULTS
Clinical response to LPS.
Intravenous injection of LPS was associated with transient influenza-like symptoms, including headache, nausea, myalgia, and chills, starting 1 to 2 h after LPS administration and lasting no longer than 3 to 4 h. In addition, a rise in body temperature was recorded, peaking at 3 to 4 h after LPS administration (38.8 ± 0.2°C; P, <0.05).
Effects of LPS on lymphocyte counts.
Effects of LPS on leukocyte counts and differential counts at time points at which whole blood was collected for in vitro stimulation are listed in Table 1. After an initial decline, leukocyte counts strongly increased after LPS administration and remained high until 24 h. Lymphocyte counts strongly decreased after LPS administration, with the lowest cell counts occurring after 6 h and returning to baseline after 24 h. This decrease in lymphocyte counts was associated with a decrease in the number of CD3+ CD4+ cells, with the lowest cell counts occurring at 6 h.
TABLE 1.
Effects of LPS administration in vivo on cell counts and differential countsa
| Time after LPS injection (h) | No.
(109/liter) of:
|
% of CD3+ CD4+ lymphocytes | ||
|---|---|---|---|---|
| Leukocytes | Lymphocytes
|
|||
| Total | CD3+ CD4+ | |||
| 0 | 5.00 ± 0.50 | 1.67 ± 0.12 | 0.79 ± 0.09 | 46.40 ± 2.80 |
| 3 | 3.99 ± 0.57* | 0.36 ± 0.03* | 0.14 ± 0.03* | 36.29 ± 4.79* |
| 6 | 10.77 ± 0.91* | 0.28 ± 0.02* | 0.07 ± 0.02* | 23.73 ± 3.31* |
| 24 | 11.73 ± 0.46* | 1.62 ± 0.11 | 0.77 ± 0.07 | 47.67 ± 3.36 |
Values are means ± SE for seven health subjects. LPS (4 ng/kg) was given as an intravenous bolus injection at 0 h. Analysis was performed by flow cytometry and FACScan analysis. *, P < 0.05 in comparison with the baseline, as determined by the Wilcoxon test.
In vitro cytokine production by whole blood after in vivo LPS injection.
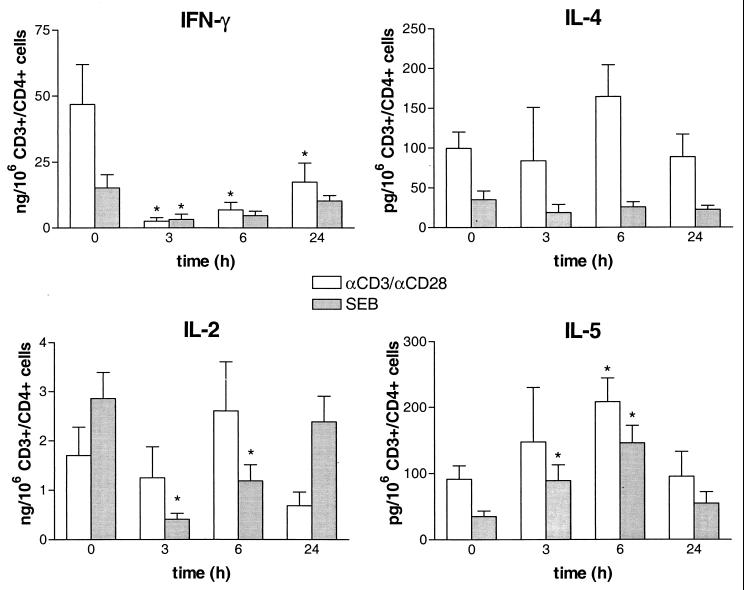
Since the number of peripheral blood T-helper cells changed after LPS injection, cytokine production was corrected for the number of CD3+ CD4+ lymphocytes present at the selected time points and expressed per 106 CD3+ CD4+ cells. IFN-γ and IL-2 were measured as Th1 cytokines, while IL-4 and IL-5 were measured as Th2 cytokines. Incubation of whole blood without a stimulus did not result in detectable cytokine levels. After stimulation with αCD3/αCD28 or SEB, high levels of IFN-γ and IL-2 and low levels of IL-4 and IL-5 were found (Fig. 1). The capacity of whole blood to produce INF-γ after stimulation with αCD3/αCD28 or SEB was markedly reduced at 3 and 6 h after in vivo exposure to LPS. Also, SEB-induced IL-2 production was strongly decreased. In contrast, αCD3/αCD28-stimulated IL-4 production was slightly increased, although this difference was not significant. The capacity to produce IL-5 after stimulation with αCD3/αCD28 or SEB increased after LPS injection, peaking at 6 h.
FIG. 1.
Effect of LPS exposure on Th1 (IFN-γ and IL-2) and Th2 (IL-4 and IL-5) cytokine production. Whole blood, obtained from seven healthy subjects before and at different time points after LPS injection (4 ng/kg), was stimulated in vitro for 24 h at 37°C with αCD3/αCD28 (1:1,000) or SEB (1 μg/ml). Data are means ± SE and are expressed as cytokine production per 106 CD3+CD4+ lymphocytes. ∗, P < 0.05 in comparison with the baseline, as determined by the Wilcoxon test.
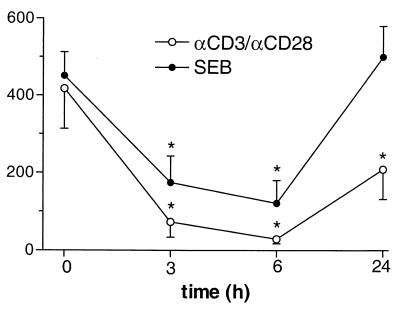
To determine whether LPS exposure induced a shift in the Th1/Th2 balance, we calculated the ration between the production of IFN-γ, the prototypic Th1 cytokine, and the production of IL-4, the prototypic Th2 cytokine, at different time points. The IFN-γ/IL-4 ratio strongly decreased after restimulation in vitro with either αCD3/αCD28 or SEB, with the lowest ratio occurring at 6 h after LPS injection (Fig. 2).
FIG. 2.
Effect of LPS exposure on the IFN-γ/IL-4 ratio (per CD3+CD4+ cells) during in vitro stimulation. The ratio between IFN-γ, as the prototypic Th1 cytokine, and IL-4, as the prototypic Th2 cytokine, was calculated after in vitro whole-blood stimulation with αCD3/αCD28 or SEB after LPS exposure in vivo. Data are means ± SE. ∗, P < 0.05 in comparison with the baseline, as determined by the Wilcoxon test.
Effect of serum obtained after in vivo exposure to LPS on cytokine production by normal whole blood.
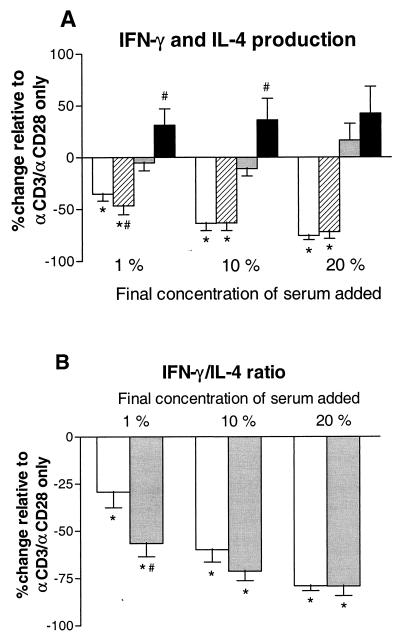
To study whether soluble factors present in serum play a role in the effects on Th1 and Th2 cytokine production, serum was collected before and after LPS exposure and added during stimulation with αCD3/αCD28 of whole blood drawn from subjects not previously exposed to LPS in vivo. Normal serum inhibited the production of IFN-γ induced by αCD3/αCD28 in a dose-dependent manner (P, < 0.05), while IL-4 production was not changed (Fig. 3). Compared with normal serum at similar concentrations, serum obtained 3 h after in vivo exposure to LPS slightly reduced αCD3/αCD28-induced IFN-γ production when added at a concentration of 1% (P, <0.05). In contrast, post-LPS serum at 1 and 10% increased αCD3/αCD28-induced IL-4 production compared to normal serum (P for both, <0.05). The effect of post-LPS serum on IFN-γ and IL-4 production did not differ from that of normal serum when added at 20%. The IFN-γ/IL-4 ratio after whole-blood stimulation with αCD3/αCD28 was 370.5 ± 95.9 when no serum was added. The addition of normal serum caused a dose-dependent decrease in the IFN-γ/IL-4 ration (P, <0.05) (Fig. 3). Serum obtained at 3 h after LPS injection caused a further decrease in the IFN-γ/IL-4 ratio compared to that observed with normal serum (P, <0.05, when serum was added at 1%).
FIG. 3.
Effect of serum obtained after LPS exposure on IFN-γ and IL-4 production (A) and IFN-γ/IL-4 ratio (B) compared to the effect of normal serum. Serum samples obtained from seven healthy subjects directly before (normal serum) and 3 h after (post-LPS serum) an intravenous injection of LPS (4 ng/kg) were pooled and added to whole blood obtained from six subjects not previously exposed to LPS, which was then stimulated with αCD3/αCD28 for 24 h at 37°C. Data (means ± SE) are expressed as the percent change relative to that observed for incubation of whole blood with αCD3/αCD28 only (i.e., without serum added). IFN-γ concentrations after whole-blood stimulation with αCD3/αCD28 were 17.2 ± 4.3 ng/ml; IL-4 levels were 49.4 ± 10.0 pg/ml. ∗, P < 0.05 in comparison with no serum; #, P < 0.05 in comparison with normal serum. Symbols: panel A—□, INF-γ normal serum; ▨, INF-γ post-LPS serum; ░⃞, IL-4 normal serum; ■, IL-4 post-LPS serum; panel B—□, normal serum; ░⃞, post-LPS serum.
The IL-2 concentration after whole-blood stimulation with αCD3/αCD28 was 330.7 ± 120.2 pg/ml. IL-2 production was not changed by the addition of normal serum or post-LPS serum. The IL-5 level after αCD3/αCD28 stimulation was 78.8 ± 9.8 pg/ml. The addition of normal serum caused a dose-dependent increase in IL-5 production (percent increase; 54.4 ± 10.1, versus that observed with αCD3/αCD28 only, when serum was added at 20%; P, <0.05). Similar to the alterations in IL-4 production, post-LPS serum at 1 and 10% further enhanced IL-5 release compared to similar concentrations of normal serum, the difference being most pronounced at 10% (percent increase, 43.7 ± 8.5, versus that observed with αCD3/αCD28 only; P, <0.05 [versus normal serum]). Post-LPS serum did not differ from normal serum when added at 20%.
DISCUSSION
LPS tolerance is characterized by a reduced responsiveness of monocytes isolated from healthy subjects after LPS exposure or from sepsis patients upon restimulation in vitro. In the present study, we sought to determine whether LPS exposure in vivo induces a change in the capacity of lymphocytes to produce cytokines and whether this result in a shift in the Th1/Th2 balance. We found that after injection of LPS into healthy subjects, stimulation of whole blood in vitro with specific T-cell stimuli resulted in a markedly decreased production of the Th1 cytokines IFN-γ and, partly, IL-2, while the production of the Th2 cytokines IL-4 and IL-5 was not influenced or was slightly increased. Consequently, the Th1/Th2 balance was shifted towards a Th2 cytokine response. The addition of serum obtained after LPS exposure to normal blood could in part mimic the LPS-tolerant state found after direct αCD3/αCD28 stimulation of blood obtained post-LPS exposure.
CD4+ T-helper cells can be divided into Th1 and Th2 cells, which can be distinguished by the pattern of cytokine production upon ex vivo stimulation (22). Th1 cells produce cytokines such as IFN-γ, IL-2, and TNF, while Th2 cells secreted IL-4, IL-5, and IL-10. In the present study, we chose to measure IFN-γ, IL-2, IL-4, and IL-5 since, unlike TNF and IL-10, they are produced predominantly or exclusively by lymphocytes (22). LPS administration induced a strong decrease in the number of CD3+ CD4+ lymphocytes. Therefore, cytokine production by whole blood was expressed a nanograms or picograms per 106 CD3+ CD4+ lymphocytes present in blood at the selected time points. Hence, the decrease in cell numbers cannot explain the observed changes in cytokine production. We used whole-blood stimulation rather than stimulation of isolated cells, since the former system is considered to mimic in vivo conditions best, with hormones, cytokines, and other soluble factors that are able to influence cytokine production being present (27).
The results of our study are consistent with data reported on lymphocyte function after major surgery. Surgical stress results in decreased T-cell effector function, associated with a reduced capacity to produce Th1 cytokines and a severe defect in T-cell proliferation (8, 14). In addition, major trauma has been reported to result in a shift toward a Th2 cytokine response (26). Until recently, it was thought that LPS does not have an effect on T-lymphocyte function. However, a study with mice demonstrated that LPS administration results in activation of CD4+ T cells, as measured by the expression of T-cell activation markers (7). Also, LPS injection was associated with a diminished capacity of splenocytes to accumulate mRNAs encoding IL-2, IL-4, and IFN-γ upon in vitro stimulation with concanavalin A. In the present study, we found that LPS exposure in vivo results in a decreased capacity of lymphocytes to produce Th1 cytokines, with a shift toward a Th2 cytokine response. The Th1/Th2 balance plays a critical role in the outcome of several infectious and autoimmune diseases (24). A Th1-mediated response is known to enhance cell-mediated immunity, while a Th2-mediated response is associated with humoral immunity (1). Our data suggests that during systemic infection, a shift toward a Th2 cytokine response occurs and may result in a defect in cell-mediated immunity. In addition, since IFN-γ is a major activator of monocyte functions (4), our data suggest that the reduced capacity of T cells to produce IFN-γ may contribute to the diminished monocyte responsiveness during endotoxemia and sepsis. Indeed, treatment of sepsis patients with IFN-γ has been found to restore monocyte LPS-induced TNF production ex vivo (9).
Some differences were seen between αCD3/αCD28- and SEB-stimulated cytokine production, in particular, IL-2 production. It is conceivable that differences in the mechanisms by which αCD3/αCD28 and SEB activate T cells contribute to this discrepancy. Indeed, cross-linking of CD3 and CD28 results in direct T-cell activation, which is independent of the presence of antigen-presenting cells. SEB, a product of Staphylococcus aureus, is a superantigen which requires binding to both an antigen-presenting cell and a T cell to induce T-cell stimulation. By binding to the major histocompatibility complex class II peptide of the APC, SEB can bind to the Vβ region of the T-cell receptor, resulting in polyclonal T-cell activation (18).
Previous studies have tried to elucidate the mechanisms which contribute to the development of an LPS-refractory state of monocytes. After injection of LPS into healthy subjects, the capacity of whole blood to produce monocyte-derived proinflammatory cytokines is strongly diminished (32). This effect is partly mediated by soluble mediators produced within 2 h after LPS administration, since stimulation of normal whole blood with serum obtained after LPS exposure could in part mimic the LPS-tolerant state (32). It has been reported that serum from sepsis patients inhibits TNF production by whole blood stimulated with E. coli (28). Also, plasma obtained from patients with meningococcal septic shock strongly inhibits LPS-induced activation of normal human monocytes, with an important role of IL-10 in the decreased monocyte responsiveness (6). Given these data and considering that LPS cannot influence lymphocyte function directly, we found it of interest to evaluate the role of soluble factors induced by LPS in the observed alterations in lymphocyte cytokine secretion. The addition of serum obtained after LPS exposure in vivo could qualitatively mimic the effects on the production of lymphocyte cytokines found after direct stimulation of post-LPS exposure blood with T-cell stimuli. Hence, these data suggest that these changes likely occur in an indirect way, possibly via soluble factors produced after LPS exposure. Recently, it has been reported that increased expression of the p50 subunit of NF-κB is involved in the suppression of TNF production in LPS-tolerant monocytes and macrophages (5). Which intracellular effect mediates the change in lymphocyte cytokine production remains to be established.
Interestingly, normal serum dose dependently inhibited αCD3/αCD28-induced IFN-γ production while not influencing IL-4 production, thus resulting in a decrease in the IFN-γ/IL-4 ration. These data suggest that normal serum contains soluble factors which direct the immune response toward a Th2 cytokine response that LPS exposure increases the concentrations and/or activities of these or other factors in serum. However, since the effects of 20% normal serum and post-LPS serum on lymphocyte cytokine release were similar, the LPS effect apparently is overruled when serum is added in larger amounts, i.e., when the physiologically present “Th1 inhibitory factors” are added in relatively high concentrations. At present it remains speculative which serum factors may be involved in the inhibition of a Th1 cytokine response, although the IL-12 p40 homodimer and the recently identified IL-18-binding protein seem to be conceivable candidates (11, 19, 25). IL-12 p40 homodimers function as IL-12 receptor antagonists, thereby inhibiting the Th1-driving cytokine IL-12, and IL-12 p40 homodimer levels increase after an LPS challenge (13). IL-18-binding protein can be considered a soluble IL-18 decoy receptor which reduces the biological availability of IL-18, an important cofactor for IFN-γ production and IL-121-driven Th1 development (21, 25, 29).
In addition, glucocorticoids and stress hormones are known to influence cytokine production. After LPS injection, levels of cortisol and epinephrine in plasma show a transient increase, peaking after 2 to 4 h (3, 33). Both cortisol and epinephrine have been reported to inhibit the release of TNF and IL-6 during human endotoxemia (3, 31) while upregulating the release of IL-10 (30, 31). Therefore, cortisol and epinephrine may have influenced lymphocyte cytokine production in the present study, In vitro, supraphysiologic levels of cortisol where shown to inhibit the production of IFN-γ from stimulated PBMCs while not altering the release of IL-4 (2). Also, epinephrine dose dependently inhibits IFN-γ production in whole blood in vitro (unpublished data). After minimally invasive surgery, concentrations of cortisol and epinephrine in plasma are slightly increased 2 h postoperatively but show no correlation with changes in IFN-γ production during whole-blood stimulation in vitro, while IL-4 release remains unaltered (16). In contrast to these data, in our study IL-4 production was increased both after LPS exposure in vivo and after the addition of post-LPS serum. To our knowledge, the effects of cortisol and epinephrine on the production of IL-2 and IL-5 have not been reported. Although there is little evidence that cortisol and epinephrine can influence IFN-γ, IL-2, IL-4, and IL-5 production after LPS exposure in vivo, the possibility that these factors play a role in the changes found in lymphocyte cytokine production cannot be excluded.
Injection of LPS into normal humans alters the profile of cytokines released by activated T cells, which is associated with a shift toward a Th2 cytokine response. Serum obtained after LPS exposure could qualitatively reproduce these changes during stimulation of normal blood, suggesting that soluble factors in serum contribute to this effect. Further studies are required to identify which soluble factors and which intracellular pathways are involved in LPS-induced changes in lymphocyte cytokine production.
ACKNOWLEDGMENT
This study was financially supported by a grant from The Royal Netherlands Academy of Arts and Science to T. van der Poll.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S K, Marshall G D., Jr Glucocorticoid-induced type 1/type 2 cytokine alterations in humans: a model for stress-related immune dysfunction. J Interferon Cytokine Res. 1998;18:1059–1068. doi: 10.1089/jir.1998.18.1059. [DOI] [PubMed] [Google Scholar]
- 3.Barber A E, Coyle S M, Marano M A, Fischer E, Calvano S E, Fong Y, Moldawer L L, Lowry S F. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- 4.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 5.Bohuslav J, Kravchenko V V, Parry G C, Erlich J H, Gerondakis S, Mackman N, Ulevitch R J. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Investig. 1998;102:1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg P, Osnes L, Ovstebo R, Britt Joo G, Westvik A B, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med. 1996;184:51–60. doi: 10.1084/jem.184.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro A, Bemer V, Nobrega A, Coutinho A, Truffa-Bachi P. Administration to mice of endotoxin from gram-negative bacteria leads to activation and apoptosis of T lymphocytes. Eur J Immunol. 1998;28:488–495. doi: 10.1002/(SICI)1521-4141(199802)28:02<488::AID-IMMU488>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Decker D, Schöndorf M, Bidlingmaier F, Hirner A, von Ruecker A A. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery. 1996;119:316–325. doi: 10.1016/s0039-6060(96)80118-8. [DOI] [PubMed] [Google Scholar]
- 9.Docke W D, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk H D, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 10.Ertel W, Kremer J P, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg F W. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–1347. [PubMed] [Google Scholar]
- 11.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 12.Granowitz E V, Porat R, Mier J W, Orencole S F, Kaplanski G, Lynch E A, Ye K, Vannier E, Wolff S M, Dinarello C A. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol. 1993;151:1637–1645. [PubMed] [Google Scholar]
- 13.Heinzel F P, Hujer A M, Ahmed F N, Rerko R M. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- 14.Hensler T, Hecker H, Heeg K, Heidecke C D, Bartels H, Barthlen W, Wagner H, Siewert J R, Holzmann B. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–2291. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keuter M, Dharmana E, Gasem M H, van der Ven-Jongekrijg J, Djokomoeljanto R, Dolmans W M, Demacker P, Sauerwein R, Gallati H, van der Meer J W. Patterns of proinflammatory cytokines and inhibitors during typhoid fever. J Infect Dis. 1994;169:1306–1311. doi: 10.1093/infdis/169.6.1306. [DOI] [PubMed] [Google Scholar]
- 16.Lemaire L C J M, van der Poll T, van Lanschot J J B, Endert E, Buurman W A, van Deventer S J H, Gouma D J. Minimally invasive surgery induces endotoxin-tolerance in the absence of detectable endotoxemia. J Clin Immunol. 1998;18:414–420. doi: 10.1023/a:1023282706945. [DOI] [PubMed] [Google Scholar]
- 17.Marie C, Muret J, Fitting C, Losser M R, Payen D, Cavaillon J M. Reduced ex vivo interleukin-8 production by neutrophils in septic and nonseptic systemic inflammatory response syndrome. Blood. 1998;91:3439–3446. [PubMed] [Google Scholar]
- 18.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 19.Mattner F, Ozmen L, Podlaski F J, Wilkinson V L, Presky D H, Gately M K, Alber G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCall C E, Grosso-Wilmoth L M, LaRue K, Guzman R N, Cousart S L. Tolerance to endotoxin-induced expression of the interleukin-1β gene in blood neutrophils of humans with the sepsis syndrome. J Clin Investig. 1993;91:853–861. doi: 10.1172/JCI116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micallef M J, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 22.Mossman T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 23.Munoz C, Carlet J, Fitting C, Misset B, Bleriot J P, Bleriot J P, Cavaillon J M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Investig. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mureille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1–9. doi: 10.1111/j.1365-3083.1998-47-1.00383.x. [DOI] [PubMed] [Google Scholar]
- 25.Novick D, Kim S H, Fantuzzi G, Reznikov L L, Dinarello C A, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 26.O'Sullivan S T, Lederer J A, Horgan A F, Chin D H, Mannick J A, Rodrick M L. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–492. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrovsky N, Harrison L C. Diurnal rhythmicity of human cytokine production. A dynamic disequilibrium in T helper cell type 1/T helper cell type 2 balance? J Immunol. 1997;158:5163–5168. [PubMed] [Google Scholar]
- 28.Prins J M, Kuijper E J, Mevissen M L, Speelman P, van Deventer S J H. Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coliin whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect Immun. 1995;63:2236–2242. doi: 10.1128/iai.63.6.2236-2242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFκ B. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 30.van der Poll T, Barber A E, Coyle S M, Lowry S F. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3604–3606. doi: 10.1210/jcem.81.10.8855809. [DOI] [PubMed] [Google Scholar]
- 31.van der Poll T, Coyle S M, Barbosa K, Braxton C C, Lowry S F. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Investig. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Poll T, Coyle S M, Moldawer L L, Lowry S F. Changes in endotoxin-induced cytokine production by whole blood after in vivo exposure of normal humans to endotoxin. J Infect Dis. 1996;174:1356–1360. doi: 10.1093/infdis/174.6.1356. [DOI] [PubMed] [Google Scholar]
- 33.van der Poll T, van Deventer S J H. Endotoxemia in healthy subjects as a human model of inflammation. In: Cohen J, Marshall J, editors. The immune response in the critically ill. New York, N.Y: Springer-Verlag; 1999. pp. 335–357. [Google Scholar]
- 34.van Deuren M, van der Ven-Jongekrijg J, Demacker P N, Bartelink A K, van Dalen R, Sauerwein R W, Gallati H, Vannice J L, van der Meer J W. Differential expression of proinflammatory cytokines and their inhibitors during the course of meningococcal infections. J Infect Dis. 1994;169:157–161. doi: 10.1093/infdis/169.1.157. [DOI] [PubMed] [Google Scholar]