Abstract
Metal-catalyzed C–H functionalizations on the aryl ring of anilines usually need cumbersome N-protection–deprotection strategies to ensure chemoselectivity. We describe here the Pd-catalyzed direct C–H arylation of unprotected anilines with no competition of the N-arylation product. The ligand [2,2′-bipyridin]-6(1H)-one drives the chemoselectivity by kinetic differentiation in the product-forming step, while playing a cooperating role in the C–H cleavage step. The latter is favored in an anionic intermediate where the NH moiety is deprotonated, driving the regioselectivity of the reaction toward ortho substitution.
Keywords: C−H activation, unprotected anilines, direct arylation, palladium, metal−ligand cooperation, pyridones
Anilines are attractive substrates for C–H functionalization. Many biologically relevant compounds have the aniline motif in their structure; thus, there is a great deal of interest in developing efficient derivatization methods of the parent aniline that can be used in the synthesis of more complex molecules. The functionalization by direct transformation of the C–H bonds of the aryl ring of aniline into C–C bonds allows the synthesis of useful derivatives in a lower number of steps, taking into account that there is no need to prepare the intermediate reagents required for conventional coupling reactions. Most metal-catalyzed processes of this type use N-protected anilines as substrates.1 Tertiary anilines, anilides, or anilines bearing N-bound directing groups such as 2-pyridyl have been functionalized in a number of ways. For example, the alkenylation of protected anilines using the Fujiwara–Moritani or oxidative Heck reaction of arenes has been reported,2,3 as well as arylation (Scheme 1a),4−7 alkylation,8 alkynylation,9 and acylation reactions.10 The presence of the protecting group directs the ortho selectivity observed in most cases, via chelate-assisted C–H activation. Also, the careful design of the protecting group allows the selective synthesis of other regioisomers.4e,11
Scheme 1. C–H Arylation of Protected Secondary and Tertiary Anilines (a) and Unprotected Anilines (b).
The use of unprotected primary anilines in C–H activation reactions is rare. Fernández-Ibáñez reported the para-selective palladium-catalyzed alkenylation of anilines, and a few examples of the functionalization of ortho-disubstituted primary anilines were included.3Ortho-substituted aryl anilines have been used as substrates in Pd-catalyzed C–H functionalization, but in these cases the −NH2 group directs the functionalization to the aryl substituent rather than the aniline ring.12 To our knowledge, no examples of palladium-catalyzed direct arylation of unprotected anilines have been reported. This is not surprising, since the combination of an aryl halide and ArNH2 could easily produce the Buchwald–Hartwig amination product, leading to N–H functionalization and the corresponding secondary aniline. Therefore, N-protection is the common practice. It would be very interesting to develop a chemoselective catalytic system that could functionalize the aryl ring with no interference of the amino group, so that the additional protection–deprotection steps could be avoided.
A few methods for the arylation of primary anilines have been reported, based on radical reactions of aryl diazo derivatives or arylhydrazines, both being rather hazardous reagents.13Ortho arylation of anilines has been achieved via the in situ generation of benzyne intermediates.14 Daugulis et al. described such a reaction using ArCl as a benzyne precursor in the presence of a strong lithium base, which was applied to a large number of anilines.15
We describe here a palladium catalytic system that brings about the selective ortho arylation of unprotected anilines (Scheme 1, b). The use of the ligand [2,2′-bipyridin]-6(1H)-one (bipy-6-OH) is crucial. It is responsible for the activity of the catalyst by playing a cooperating role in the C–H cleavage.16,17 It is also important in determining the selectivity of the process by favoring the C–C vs the C–N coupling (chemoselectivity) and also the ortho regioselectivity.
The well-defined complex [Pd(bipy-6-OH)Br(C6F5)] (1) was tested in the reaction of aniline with p-CF3C6H4I, an aryl halide that allows the easy monitoring of the reaction by 19F NMR. Following our previous work,18 we used pinacolone as the solvent and a moderate excess of aniline (10-fold). The reaction goes to completion in 24 h, and good yields of the ortho-arylated aniline were obtained. Similar results can be achieved in a much shorter time (6 h) using DMA (entries 1 and 2, Table 1); therefore, this solvent was selected for our experiments. The reaction is equally effective when an equimolar mixture of palladium acetate and bipy-6-OH was used as the precatalyst (cf. entries 2 and 3, Table 1). The presence of the cooperating ligand is necessary, and negligible conversion was observed when no ligand was added, when the pyridone moiety was not present in the ligand, or when it was in a position far from the metal so that it was not able to play a cooperating role (entries 4–6, Table 1). In contrast, the ligand phen-2-OH is also effective, although the reaction is slower. The amount of aniline reactant can be reduced to almost the stoichiometric amount at the expense of a moderate reduction of the yield and a much longer reaction time (entry 8, Table 1).
Table 1. Arylation of Aniline with p-CF3C6H4I Using Different Catalysts According to eq 1a.
| crude yield, % (conversn, %) |
|||
|---|---|---|---|
| entry | [Pd] | 6 hb | 24 hb |
| 1c | 1 | 46 (51) | 92 (100) |
| 2 | 1 | 83 (100) | |
| 3 | [Pd(OAc)2] + bipy-6-OH | 86 (100) | |
| 4 | [Pd(OAc)2] | 0 (3) | 0 (9) |
| 5 | 2 | 0 (4) | 1 (10) |
| 6 | 3 | 0 (0) | 0 (6) |
| 7 | 4 | 31 (40) | 91 (100) |
| 8d | 1 | 13 (20) | 74 (100) |
Reaction conditions unless specified otherwise: p-CF3C6H4I (0.34 mmol), aniline (3.4 mmol), [Pd] (5 mol %), Cs2CO3 (0.68 mmol), DMA (2.7 mL); 130 °C.
Crude yields determined by 19F NMR of the reaction mixture. Mixture of regioisomers o:m:p = 25:1:1. The reduction of the aryl iodide (ArH) and homocoupling (Ar–Ar) are the observed byproducts.
Pinacolone as solvent.
Aniline (0.37 mmol).
The ortho-arylated product was the major one and only 5% of the Buchwald-Hartwig amination product (N-arylation) was detected in the crude mixture (Figure S21 in the Supporting Information). In fact, the amination product was obtained cleanly when the same base (Cs2CO3) and solvent (DMF) similar to those in eq 1 were used, but a different Pd catalyst: a mixture of a Pd(0) precursor and XPhos (eq 2). Thus, the Pd-bipy-6-OH catalyst system can be used in combination with other catalysts for the orthogonal functionalization of aniline by C–C and C–N coupling.
 |
1 |
 |
2 |
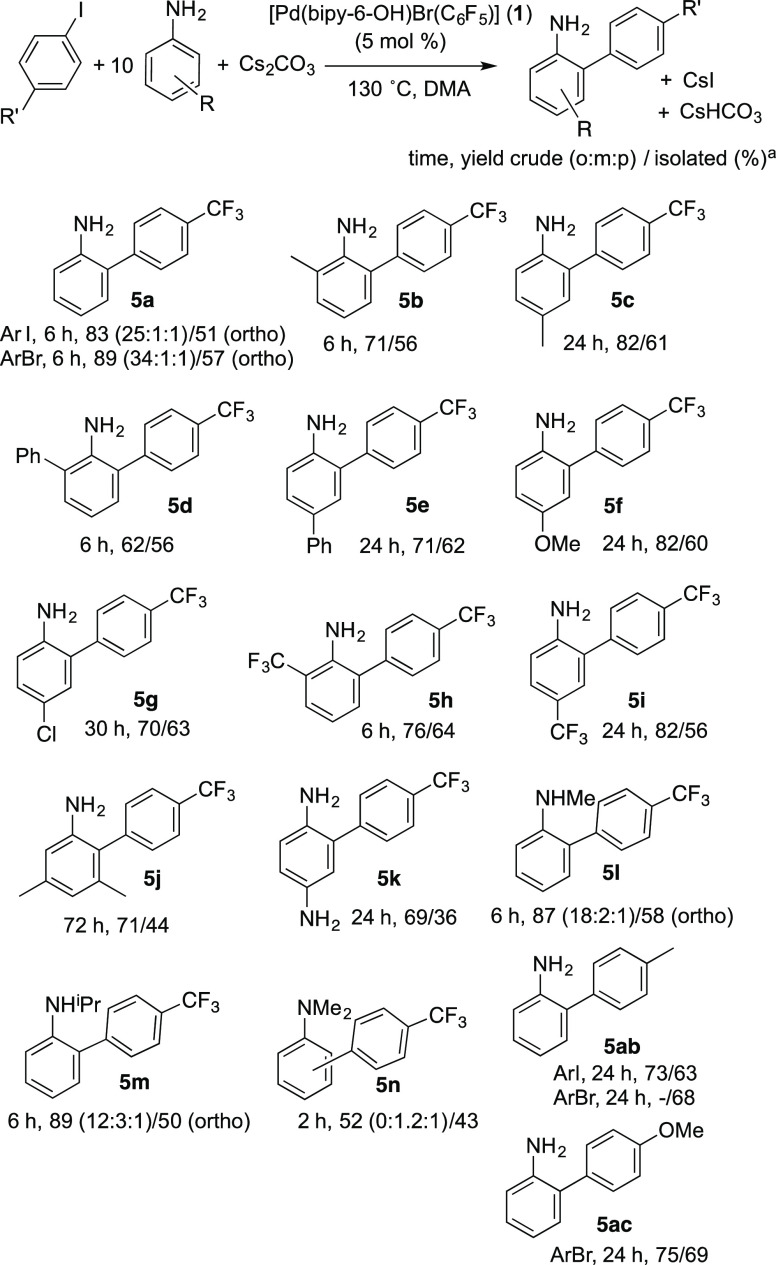
The selective ortho arylation can be extended to primary anilines with different substitution patterns in the aromatic ring (ortho-, meta- and para-substituted). Electron-withdrawing and electron-donating groups are tolerated in the aniline and in the aryl halide (Scheme 2). Only monoarylation was observed in all cases, and the ortho-arylated anilines were obtained in good to moderate yields, with the only exception being the tertiary dimethylaniline (5n). Some of the derivatives shown can be interesting precursors of biologically active compounds as, for example, in the synthesis of biphenylbenzamide microbiocidal agents (i.e., 5a–c,f,g),19 carbazole alkaloids,20 and dyes (5k).21 Again, in the few cases that it was observed, the C–N coupling product only accounts for about 2–5% of the crude yield (see the Supporting Information). Note that when o-phenylaniline is used as substrate only the functionalization of the aniline ring occurs and the aryl substituent remains unaltered, in contrast to other reactions in the literature that use 2-anilino as a directing group.12
Scheme 2. Arylation of Anilines with Complex 1.
Scheme 2 also shows the arylation products of several secondary and tertiary anilines. We observed that the regioselectivity is eroded as the N-substitution increases. However, whereas the ortho isomer is still the major isomer for secondary anilines, the arylation of N,N-dimethylaniline only affords a mixture of the meta and para isomers.
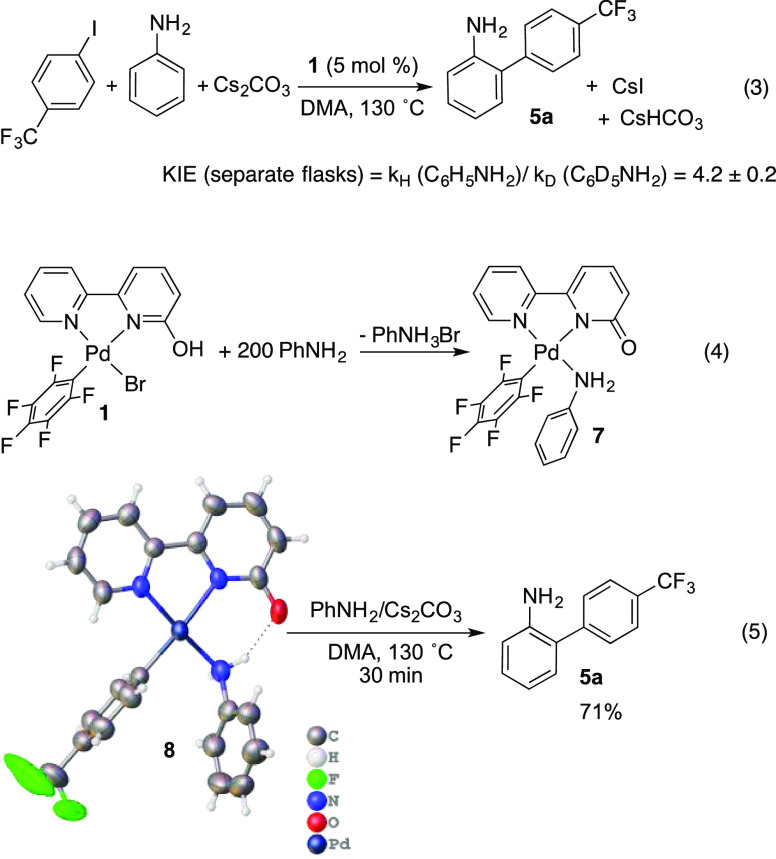
Mechanistic experiments were carried out to gather information on the catalytic cycle and the origin of the selectivity observed. The reaction shown in eq 3 was used as a model. Complex 1 is transformed under catalytic conditions (DMA, excess of aniline) into the amino derivative 7, which was independently synthesized and characterized (eq 4; see molecular structure in Figure S10 in the Supporting Information). The analogous complex [Pd(bipy-6-O)(p-CF3C6H4)(PhNH2)] (8) was also prepared, and it is catalytically competent for the reaction in eq 3 (90% yield in 6 h). It also decomposes under catalytic conditions to the ortho-arylated aniline (eq 5); thus, the presence of 8 is plausible in the catalytic reaction.
 |
3 |
Kinetic experiments show that the rate of the reaction exhibits a first-order dependence on the catalyst (complex 1) and is independent of the concentration of the aryl halide. The reaction rate in also insensitive to the concentration of aniline in the excess range used for this reactant in the catalysis (about 10-fold). As can be seen in eq 4, under these conditions the coordination equilibrium is completely shifted to the aniline-coordinated species (Figure S1 in the Supporting Information). A large kinetic isotope effect was found (KIE = 4.2 ± 0.4), pointing to the C–H activation as the turnover-limiting step. The reaction in eq 3 was also carried out in the presence of D2O. Since the H/D exchange in the protonated ligand is facile (Figure S4 in the Supporting Information), a reversible C–H activation should lead to the incorporation of deuterium in the final substituted aniline. No deuterium incorporation was observed, supporting an irreversible C–H cleavage.
The reaction mechanism has been investigated by computational methods. A thorough exploration of several routes, with the locations of intermediates and transition states, was carried out using the M06 functional with basis set BS1 and including solvation in the optimizations through the SMD implicit solvent method. However, to obtain accurate energies, additional single-point calculations were performed on all optimized structures employing the domain-based local pair natural orbital coupled cluster approach (DLPNO-CCSD(T)) and an extended basis set (def2-TZVP) (see computational details in the Supporting Information). This method can be considered the state of the art for providing energies of systems of this size, and it has proved to be very effective in obtaining accurate reaction thermodynamics and barrier heights,22 including palladium-catalyzed cross-coupling reactions.23 All of the Gibbs energies collected in the text have been obtained, adding to the DLPNO-CCSD(T)/def2-TZVp electronic energies thermal and entropic corrections as well as solvation energies (ΔG(solv)) obtained at the M06/BS1 level.
We found that the choice of model is crucial to reproduce the basic features of the reaction: C–C coupling vs C–N chemoselectivity, ortho regioselectivity, and a turnover-limiting C–H cleavage. The simplest model, consisting of just the palladium, the (bipy-6-OH) ligand, the aryl group, and aniline, fails to account for the experimental results (see Figure S89 in the Supporting Information). To improve it, we enlarged the model, adding to the computational model other species present in the reaction medium, as carbonate and cesium ions. We found the smallest model able to reproduce the prevalence of C–C coupling over C–N coupling must involve, in addition to the cesium carbonate and the continuum representation of the solvent, several explicit DMA solvent molecules (model 4 in Figure S90; see the Supporting Information for inconsistent results with other models). This has been the model employed in all of the calculations.
Figure 1 shows a complete profile for the reaction yielding the ortho-arylated product. The rearrangement of the aniline from a N- to a C-bound mode transforms complex 8 into c1ortho. At this point, the deprotonation of the aniline is facile to give c1NHortho, which undergoes C–H cleavage (via TS-c1NHortho-c2NHortho) with a lower energy barrier than that from c1ortho (TS-c1ortho-c2ortho; Gibbs energy barriers of 12.1 vs 16 kcal mol–1, respectively). Therefore, an anionic route on an amido-type intermediate is preferred. A series of proton transfer steps occur on biaryl intermediate c2NHortho with the involvement of the pair HCO3–/CO32–, which eventually leads to c2bortho. In this way the ortho CH proton ends up in the carbonate, with a notable stabilization of the system. From c2bortho a reductive elimination follows through transition state TS-c2bortho-c3, leading to the arylation product and the Pd(0) intermediate c4. In the presence of aniline oxidative addition occurs, leading to c1ortho that closes the cycle. The conversion of c1ortho into 8 has a lower energy barrier than the C–H cleavage, and therefore 8 is the plausible resting state of the reaction, outside the catalytic cycle. The equilibrium between 8 and c1ortho controls the actual concentration of palladium in the catalytic cycle and leads to an energetic span of 29.9 kcal mol–1 for the reaction, consistent with the reaction conditions needed. The computed catalytic cycle is represented in Scheme 3.24 A microkinetic simulation of this pathway is also consistent with the conversions observed experimentally (see the Supporting Information for details).
Figure 1.

Gibbs energy profile for the Pd-catalyzed arylation of aniline in the ortho position, assisted by the ligand bipy-6-O. Energies are given in kcal mol–1.
Scheme 3. Plausible Catalytic Cycle for the ortho Arylation of Aniline.
The energy barriers for the other two regioisomers were also calculated. Both the neutral (implying NH2 in the aniline) and the anionic (with NH) pathways were computed. The C–H cleavage via the neutral pathway (c1 to c2) is preferred to the anionic pathway (c1NH to c2NH) for the meta CH activation, with Gibbs energy barriers of 19.0 and. 25.7 kcal mol–1, respectively, whereas the anionic route is slightly preferred for the para CH activation (19.4 vs 19.7 kcal mol–1 Gibbs energy barriers; see Figure S92 in the Supporting Information). The lowest energy pathway for each regioisomer is less favored than the ortho arylation by 6.9 kcal mol–1 (meta) and 7.3 kcal mol–1 (para). Therefore, the facile deprotonation of the aniline by the carbonate anion, forming an amido type intermediate in these reactions, is important to drive the regioselectivity toward ortho arylation. This is possible for primary and secondary anilines but not for the tertiary N,N-dimethylaniline where the ortho isomer was not observed.25
As commented below, the C–N coupling route to give the Buchwald–Hartwig amination product was also calculated. The deprotonation of the aniline in complex 8, is facile and the amido version of complex 8 is found 0.6 kcal mol–1 below the neutral form, pointing out the existence of and amino–amido equilibrium in the presence of carbonate in the reaction medium (Figure S93 in the Supporting Information). The aryl-amido reductive elimination barrier is 14.1 kcal mol–1, as shown in Figure 1. This value is 2 kcal mol–1 higher than the barrier for the C–H ortho cleavage in the aniline (12.1 kcal mol–1, Figure 1), which makes the ortho C–H functionalization preferred, in good agreement with the experimental chemoselectivity. In contrast to bulky phosphine ligands, the ligand bipy-6-OH does not favor the reductive elimination step, which, eventually, is an advantage for chemoselectivity (cf. eqs 1 and 2). Using this enlarged model, the barrier for the CH ortho activation is found to be 2.0 kcal mol–1 below that of the C–N coupling,
In conclusion, we have shown that unprotected anilines can be selectively arylated in the ortho position using the Pd/bipy-6-OH catalyst system. The cooperating role of bipy-6-OH in the C–H cleavage step along with a high discriminating barrier for reductive elimination eliminates the competition of the C–N coupling product (amination).
Acknowledgments
We acknowledge the financial support of the Spanish MICINN (PID2019-111406GB-I00 and PID-2020-116861GB-I00) and the Junta de Castilla y León-FEDER (VA224P20 and VA087-18 fellowship to C.P.). This project has been carried out using CSUC high-performance computing resources.
Glossary
Abbreviations
- CMD
concerted metalation–deprotonation
Supporting Information Available
(PDF). . The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.2c05206.
Experimental details, characterization, kinetic and X-ray structure determination data, and selected spectra (PDF)
Computational details, extended description of the computational results, and Cartesian coordinates and absolute energies of all the optimized structures (PDF)
rystallographic data for complex 7 (CIF)
Crystallographic data for complex 8 (CIF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Tischler M. O.; Tóth M. B.; Novák Z. Mild Palladium Catalyzed Ortho C–H Bond Functionalizations of Aniline Derivatives. Chem. Rec. 2017, 17, 184–199. 10.1002/tcr.201600059. [DOI] [PubMed] [Google Scholar]; b Leitch J. A.; Frost C. G. Regioselective Transition-Metal-Catalyzed C–H Functionalization of Anilines. Synth. 2018, 50, 2693–2706. 10.1055/s-0037-1610142. [DOI] [Google Scholar]
- Boele M. D. K.; Van Strijdonck G. P. F.; De Vries A. H. M.; Kamer P. C. J.; De Vries J. G.; Van Leeuwen P. W. N. M. Selective Pd-Catalyzed Oxidative Coupling of Anilides with Olefins through C–H Bond Activation at Room Temperature. J. Am. Chem. Soc. 2002, 124, 1586–1587. 10.1021/ja0176907. [DOI] [PubMed] [Google Scholar]; b Mizuta Y.; Obora Y.; Shimizu Y.; Ishii Y. Para-Selective Aerobic Oxidative C–H Olefination of Aminobenzenes Catalyzed by Palladium/Molybdovanadophosphoric Acid/2,4,6-Trimethylbenzoic Acid System. ChemCatChem. 2012, 4, 187–191. 10.1002/cctc.201100375. [DOI] [Google Scholar]; c Yao Q. J.; Xie P. P.; Wu Y. J.; Feng Y. L.; Teng M. Y.; Hong X.; Shi B. F. Enantioselective Synthesis of Atropisomeric Anilides via Pd(II)-Catalyzed Asymmetric C–H Olefination. J. Am. Chem. Soc. 2020, 142, 18266–18276. 10.1021/jacs.0c09400. [DOI] [PubMed] [Google Scholar]
- Naksomboon K.; Poater J.; Bickelhaupt F. M.; Fernández-Ibáñez M. A. Para-Selective C–H Olefination of Aniline Derivatives via Pd/S,O-Ligand Catalysis. J. Am. Chem. Soc. 2019, 141, 6719–6725. 10.1021/jacs.9b01908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direct arylation using ArX:; a Daugulis O.; Zaitsev V. G. Anilide Ortho-Arylation by Using C–H Activation Methodology. Angew. Chem., Int. Ed. 2005, 44, 4046–4048. 10.1002/anie.200500589. [DOI] [PubMed] [Google Scholar]; b Scarborough C. C.; McDonald R. I.; Hartmann C.; Sazama G. T.; Bergant A.; Stahl S. S. Steric Modulation of Chiral Biaryl Diamines via Pd-Catalyzed Directed C–H Arylation. J. Org. Chem. 2009, 74, 2613–2615. 10.1021/jo802632v. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wan C.; Zhao J.; Xu M.; Huang J. Palladium-Catalyzed C(sp2)-H Arylation Using Formamide as a Transformable Directing Group. J. Org. Chem. 2014, 79, 4751–4756. 10.1021/jo5007043. [DOI] [PubMed] [Google Scholar]; d Kwak S. H.; Gulia N.; Daugulis O. Synthesis of Unsymmetrical 2,6-Diarylanilines by Palladium-Catalyzed C–H Bond Functionalization Methodology. J. Org. Chem. 2018, 83, 5844–5850. 10.1021/acs.joc.8b00659. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Lichte D.; Pirkl N.; Heinrich G.; Dutta S.; Goebel J. F.; Koley D.; Goossen L. J. Palladium-Catalyzed para-C–H Arylation of Anilines with Aromatic Halides. Angew, Chem, Int. Ed. 2022, 10.1002/anie.202210009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arylation using diaryliodonium salts:; a Kalyani D.; Deprez N. R.; Desai L. V.; Sanford M. S. Oxidative C–H Activation/C-C Bond Forming Reactions: Synthetic Scope and Mechanistic Insights. J. Am. Chem. Soc. 2005, 127, 7330–7331. 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]; b Phipps R. J.; Gaunt M. J. A Meta-Selective Copper-Catalyzed C–H Bond Arylation. Science 2009, 323, 1593–1597. 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]; c Ciana C. L.; Phipps R. J.; Brandt J. R.; Meyer F. M.; Gaunt M. J. A Highly Para-Selective Copper(II)-Catalyzed Direct Arylation of Aniline and Phenol Derivatives. Angew. Chem., Int. Ed. 2011, 50, 458–462. 10.1002/anie.201004703. [DOI] [PubMed] [Google Scholar]
- Oxidative arylation with arenes:; a Li B. J.; Tian S. L.; Fang Z.; Shi Z. J. Multiple C–H Activations to Construct Biologically Active Molecules in a Process Completely Free of Organohalogen and Organometallic Components. Angew. Chem., Int. Ed. 2008, 47 (6), 1115–1118. 10.1002/anie.200704092. [DOI] [PubMed] [Google Scholar]; b Yeung C. S.; Zhao X.; Borduas N.; Dong V. M. Pd-Catalyzed Ortho-Arylation of Phenylacetamides, Benzamides, and Anilides with Simple Arenes Using Sodium Persulfate. Chem. Sci. 2010, 1, 331–336. 10.1039/c0sc00231c. [DOI] [Google Scholar]; c Brasche G.; García-Fortanet J.; Buchwald S. L. Twofold C–H Functionalization: Palladium-Catalyzed Ortho Arylation of Anilides. Org. Lett. 2008, 10, 2207–2210. 10.1021/ol800619c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxidative arylation with arylboronic derivatives:; a Nishikata T.; Abela A. R.; Huang S.; Lipshutz B. H. Cationic Palladium(II) Catalysis: C–H Activation/Suzuki–Miyaura Couplings at Room Temperature. J. Am. Chem. Soc. 2010, 132, 4978–4979. 10.1021/ja910973a. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Koley M.; Dastbaravardeh N.; Schnürch M.; Mihovilovic M. D. Palladium(II)-Catalyzed Regioselective Ortho Arylation of sp2 C–H Bonds of N-Aryl-2-Amino Pyridine Derivatives. ChemCatChem. 2012, 4 (9), 1345–1352. 10.1002/cctc.201200155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorai D.; Finger L. H.; Zanoni G.; Ackermann L. Bimetallic Nickel Complexes for Aniline C–H Alkylations. ACS Catal. 2018, 8, 11657–11662. 10.1021/acscatal.8b03770. [DOI] [Google Scholar]
- Deng K. Z.; Jia W. L.; Fernández-Ibáñez M. A. Selective para-C–H Alkynylation of Aniline Derivatives by Pd/S,O-Ligand Catalysis. Chem. - Eur. J. 2022, 28, e202104107 10.1002/chem.202104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. W.; Zhou Z.; Yu W. Y. Palladium(II)-Catalyzed Direct Ortho-C–H Acylation of Anilides by Oxidative Cross-Coupling with Aldehydes Using Tert-Butyl Hydroperoxide as Oxidant. Adv. Synth. Catal. 2011, 353, 2999–3006. 10.1002/adsc.201100472. [DOI] [Google Scholar]
- Tang R.-Y.; Li G.; Yu J.-Q. Conformation-Induced Remote Meta-C–H Activation of Amines. Nature 2014, 507, 215–220. 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju S.; Hsiao H. C.; Thirupathi S.; Chen P. L.; Chuang S. C. Palladium-Catalyzed Benzofulvenation of o-Arylanilines through C–H Bond Activation by Using Two Diarylacetylenes as an Implicit Benzofulvene. Adv. Synth. Catal. 2019, 361, 683–689. 10.1002/adsc.201801352. [DOI] [Google Scholar]
- a Wetzel A.; Ehrhardt V.; Heinrich M. R. Synthesis of Amino- and Hydroxybiphenyls by Radical Chain Reaction of Arenediazonium Salts. Angew. Chem., Int. Ed. 2008, 47, 9130–9133. 10.1002/anie.200803785. [DOI] [PubMed] [Google Scholar]; b Jiang T.; Chen S. Y.; Zhuang H.; Zeng R. S.; Zou J. P. Air-Promoted Direct Radical Arylation of Anilines with Arylhydrazines. Tetrahedron Lett. 2014, 55 (33), 4549–4552. 10.1016/j.tetlet.2014.06.099. [DOI] [Google Scholar]
- Pirali T.; Zhang F.; Miller A. H.; Head J. L.; McAusland D.; Greaney M. F. Transition-Metal-Free Direct Arylation of Anilines. Angew. Chem., Int. Ed. 2012, 51, 1006–1009. 10.1002/anie.201106150. [DOI] [PubMed] [Google Scholar]
- Truong T.; Daugulis O. Direct Intermolecular Aniline Ortho- Arylation via Benzyne Intermediates. Org. Lett. 2012, 14, 5964–5967. 10.1021/ol302875x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca V.; Toledo A.; Albéniz A. C. [2,2′-Bipyridin]-6(1H)-One, a Truly Cooperating Ligand in the Palladium-Mediated C–H Activation Step: Experimental Evidence in the Direct C-3 Arylation of Pyridine. J. Am. Chem. Soc. 2018, 140, 17851–17856. 10.1021/jacs.8b10680. [DOI] [PubMed] [Google Scholar]
- Pyridones are useful cooperating ligands in many C–H activation processes:; a Wang P.; Verma P.; Xia G.; Shi J.; Qiao J. X.; Tao S.; Cheng P. T. W.; Poss M. A.; Farmer M. E.; Yeung K.-S.; Yu J.-Q. Ligand-accelerated non-directed C–H functionalization of arenes. Nature 2017, 551, 489–494. 10.1038/nature24632. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fan Z.; Bay K. L.; Chen X.; Zhuang Z.; Park H. S.; Yeung K.-S.; Houk K. N.; Yu J.-Q. Rational Development of Remote C–H Functionalization of Biphenyl: Experimental and Computational Studies. Angew. Chem., Int. Ed. 2020, 59, 4770–4777. 10.1002/anie.201915624. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang Z.; Hu L.; Chekshin N.; Zhuang Z.; Qian S.; Qiao J. X.; Yu J. – Q. Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation. Science 2021, 374, 1281–1285. 10.1126/science.abl3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca V.; Albéniz A. C. Faster Palladium-Catalyzed Arylation of Simple Arenes in the Presence of a Methylketone: Beneficial Effect of an a Priori Interfering Solvent in C–H Activation. Org. Chem. Front. 2021, 8, 1941–1951. 10.1039/D1QO00236H. [DOI] [Google Scholar]
- Rieck H.; Dunkel R.; Elbe H. L.; Wachendorff-Neumann U.; Mauleer-Machnik A.; Kuck K. H.. Microbiocidal Agents on the Basis of Biphenylbenzamide Derivatives. US 7186862 B2, 2007.
- a Dhanak D.; Knight S. D.; Moore M. L.; Newlander K. A.. Methods and Compositions. WO 2006/005063 A2, 2006.; b Schmidt A. W.; Reddy K. R.; Knölker H. J. Occurrence, Biogenesis, and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2012, 112, 3193–3328. 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]; c Suzuki C.; Hirano K.; Satoh T.; Miura M. Direct Synthesis of N-H Carbazoles via Iridium(III)-catalyzed Intramolecular C–H Amination. Org. Lett. 2015, 17, 1597–1600. 10.1021/acs.orglett.5b00502. [DOI] [PubMed] [Google Scholar]
- Braun H. J.; Chassot L.. Oxidizing Hair Coloring Agents Containing 2,5-Diamino-1-phenylbenzene Derivatives and Novel 2,5-Diamino-1-phenylbenzene Derivatives. US 6500213 B1, 2002.
- Sparta M.; Riplinger C.; Neese F. Mechanism of Olefin Asymmetric Hydrogenation Catalyzed by Iridium Phosphino-Oxazoline: A Pair Natural Orbital Coupled Cluster Study. J. Chem. Theory Comput. 2014, 10, 1099–1108. 10.1021/ct400917j. [DOI] [PubMed] [Google Scholar]
- Cusumano A. Q.; Stoltz B. M.; Goddard W. A. III Reaction Mechanism, Origins of Enantioselectivity, and Reactivity. J. Am. Chem. Soc. 2020, 142, 13917–13933. 10.1021/jacs.0c06243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Other alternatives such as a Pd(II)/Pd(IV) mechanism where the C–H activation occurs first on a Pd(II) complex followed by an oxidative addition of ArX can be thought of. This route is less favorable because the aryl halide is not a strong enough oxidant to oxidize a Pd(II) aryl complex to a Pd(IV) derivative. DFT calculations on this step shows that the energy barrier for this step would be about 46 kcal mol–1. Experimentally, when either complex 7 or a benzylamine palladacycle was heated with p-CF3C6H4I in DMA at 130 °C, we did not observe any C–C cross-coupling product (see sections 1.4.5 and 4.5 in the Supporting Information for further details).
- The coordination abilities of PhNH2 and PhNMe2 are very different, but the κ1-N coordination is not responsible for directing the position of the C–H cleavage. We rated the coordination ability of the N-substituted anilines and aniline by measuring the equilibrium constants for their coordination to the model complex (NBu4)2[Pd2(μ-Br)2(C6F5)4] (see section 1.4.4 in the Supporting Information). Although significant differences were found (Keq: PhNH2 > PhNHMe > PhNHiPr > PhNMe2), no correlation between Keq and the selectivity was observed for any of the anilines (Figure S7 in the Supporting Information).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.