Abstract
Background
Despite the availability of several clinical guidelines, not all health professionals use their recommendations to manage patients with Pompe disease, a rare genetic disorder involving high-impact therapy. Through several discussion meetings and a survey, the present study aimed to learn about the management of Pompe disease in routine clinical practice in Spain, to improve clinical care in a real-life situation.
Results
The survey was sent to 42 healthcare professionals who manage patients with Pompe disease in their clinical practice. Although most respondents followed the clinical guidelines, clinical practice differed from the expert recommendations in many cases. Approximately 7% did not request a genetic study to confirm the diagnosis before starting treatment, and 21% considered that only two dried blood spot determinations suffice to establish the diagnosis. About 76% requested anti-GAA antibodies when there is a suspicion of lack of treatment efficacy, though a significant percentage of respondents have never requested such antibodies. According to 31% of the respondents, significant impairment of motor function and/or respiratory insufficiency is a requirement for authorizing medication at their hospital. Up to 26% waited for improvements over the clinical follow-up to maintain treatment and withdrew it in the absence of improvement since they did not consider disease stabilization to be a satisfactory outcome.
Conclusions
The results highlight the lack of experience and/or knowledge of some professionals caring for patients with Pompe disease. It is necessary to develop and disseminate simple guidelines that help to apply the expert recommendations better or centralize patient follow-up in highly specialized centers.
Keywords: Antibodies, Diagnosis, Follow-up, Guidelines, Pompe disease, Treatment
Background
Pompe disease (PD) is an autosomal recessive multisystemic disorder characterized by acid α-glucosidase (GAA) deficiency that leads to lysosomal glycogen storage. The PD phenotype encompasses a continuous clinical spectrum with variable age of onset, progression rate, and severity. The classification of PD differs among authors. According to the age of onset, we can classify it into three groups: 1) Classic infantile-onset PD (IOPD), which includes patients with GAA activity close to 0%, the onset of symptoms during the first year of life, and hypertrophic cardiomyopathy; 2) Childhood or juvenile-onset PD (JOPD), that includes patients diagnosed before age 18; and 3) Adult-onset or late-onset PD (LOPD), that can present after early to late adulthood. Patients in the last two groups usually have residual GAA activity (1–40%) and do not have cardiomyopathy [1–4]. Respiratory failure is the leading cause of morbidity and mortality in JOPD and LOPD. One-third of all patients require ventilatory support before losing the ability to walk independently [3, 5–8].
In 2006, the treatment of PD with recombinant human alglucosidase alfa enzyme (rhGAA) was authorized in Europe. The efficacy and safety of this treatment have been demonstrated in many clinical studies, affording significant improvement of motor function, at least stabilization of lung function, and a prolongation of survival [9–19]. It should be emphasized that patients with less muscle damage at the start of treatment may obtain a more significant benefit, hence the importance of starting treatment as soon as possible [20, 21]. Several clinical studies suggest that starting enzyme replacement therapy (ERT) in a better functional situation indicates a good prognosis; however, the possible benefit of treating presymptomatic patients has not been demonstrated [11, 15, 22, 23]. Considering that almost one-third of all PD patients do not respond to treatment, it is essential to monitor the disease properly, since treatment withdrawal should be considered if no response is observed [24].
Numerous clinical guidelines have been published offering recommendations for diagnosing, treating, and following up on patients with PD [24–28]. For this work, we have taken as a reference the recommendations of the European group of experts on Pompe disease, as it is the most complete and up-to-date guide [24].
It is important to highlight that rhGAA is a high-cost treatment that can be prescribed by any physician in any health center in Spain. Furthermore, the clinical management of PD in Spain is not centralized in reference centers as in other European countries and with other therapies in Spain. For all these reasons, we have conducted a study to know the diagnosis, treatment with rhGAA, and follow-up of patients with PD in real clinical practice in Spain through a 5-question survey. The information obtained will make it possible to determine whether the treatment and follow-up of patients with PD in Spain align with international recommendations, identify inequalities in access to high-impact medications, and identify areas for improvement to optimize their clinical management in the country.
Results
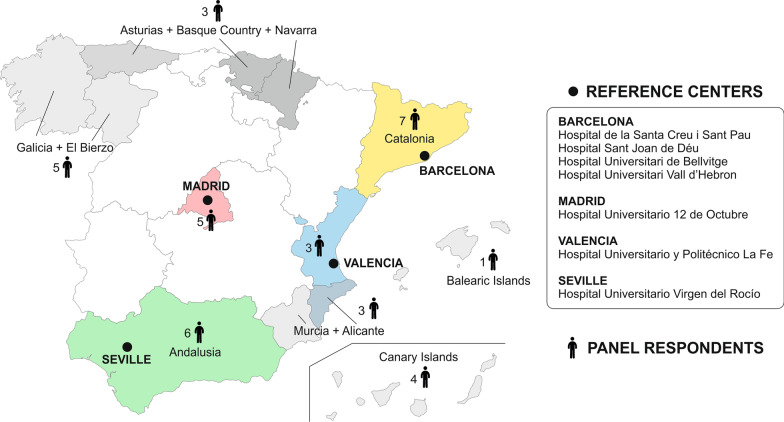
The survey was sent to 42 healthcare professionals: 5 treated patients with JOPD (5 pediatricians), and the remaining 37 (8 internal medicine physicians and 29 neurologists) treated patients with adult-onset PD (55% attended more than one). The latter were geographically distributed as follows: 3 in Alicante and Murcia, 6 in Andalusia, 1 in the Balearic Islands, 4 in the Canary Islands, 7 in Catalonia, 5 in Galicia and El Bierzo, 5 in Madrid, 3 in Asturias, the Basque Country, and Navarra, and 3 in Valencia (Fig. 1). The remaining 5 treat JOPD at the National level.
Fig. 1.
Spanish reference centers across regions in Spain and the origin of the panel respondents that treated patients with adult-onset Pompe disease
When do you consider a definitive diagnosis of PD?
Most respondents (97.6%) considered one pathological confirmation in dried blood spot (DBS) testing and the identification of biallelic pathogenic mutations in the genetic study as a confirmatory test for PD (Table 1). Opinions were widely divergent as to whether the presence of reduced enzyme activity in two tissues allows for a diagnosis of PD despite a negative genetic result. Three participants in the survey (7.1%) did not request a genetic analysis to confirm the diagnosis before initiating treatment, and 9 participants (21.4%) indicated that only two DBS determinations were sufficient to diagnose PD.
Table 1.
Questions proposed in the survey
| N = 42 N (%) |
|
|---|---|
| When do you consider a definitive diagnosis of Pompe disease? | |
| When two DBS determinations show pathological values of enzyme activity | 9 (21.4) |
| When a determination of enzyme activity in DBS is pathological and in addition the muscle biopsy shows PAS + vacuoles | 17 (40.5) |
| When DBS is pathological and biallelic pathogenic mutations have been identified in the genetic study | 41 (97.6) |
| When the clinical phenotype is compatible and reduced lymphocyte activity has been demonstrated, even though only one pathogenic mutation has been identified in the genetic study | 35 (83.3) |
| When reduced enzyme activity has been demonstrated in two tissues, even though the genetic study is negative | 21 (50.0) |
| When Pompe disease is suspected, I always request a confirmatory genetic study | 39 (92.9) |
| Regarding anti-GAA antibodies: | |
| I have never requested the determination of anti-GAA antibodies | 13 (31.0) |
| After the initiation of treatment, I request anti-GAA antibody determination on a regular basis | 18 (42.9) |
| If antibodies remain at high titers during follow-up, I consider discontinuing treatment | 1 (2.4) |
| I request antibodies when there is a suspicion of lack of efficacy of the treatment (objective clinical worsening) | 32 (76.2) |
| What tests do you perform during clinical follow-up to evaluate the response to treatment? | |
| MRC scale | 39 (92.9) |
| 6MWT | 42 (100) |
| Other timed tests | 24 (57.1) |
| Fatigue scale | 19 (45.2) |
| Activity or quality of life scales | 30 (71.4) |
| Muscle MRI scan | 22 (52.4) |
| Pulmonary function tests | 42 (100) |
| Do you have to do follow-up reports to keep the medication authorized? | |
| No | 23 (54.8) |
| Yes, only occasionally | 5 (11.9) |
| Yes, every 6 months | 8 (19.0) |
| Yes, annually | 10 (23.8) |
| The existence of significant impairment of motor function and/or the presence of respiratory failure is a requirement for the authorization of medication in my hospital | 13 (31.0) |
| Improvement of follow-up parameters is a prerequisite for the maintenance of treatment | 11 (26.2) |
| In what situations would you consider interrupting or stopping treatment? | |
| Never | 2 (4.8) |
| If there are no objective data of stabilization or improvement of motor and/or respiratory function during follow-up | 8 (19.0) |
| If there is evidence of progressive clinical worsening | 32 (76.2) |
6MWT six-minute walk test; DBS dried blood spots; GAA α-glucosidase, acid; MRC Medical Research Council; MRI magnetic resonance imaging; PAS periodic acid-Schiff
Regarding anti-GAA antibodies
31% of the respondents had never requested antibodies against GAA, and 42.9% asked for anti-GAA antibody determination regularly after the initiation of treatment. A total of 76.2% requested antibodies when there was a suspicion of a lack of therapy efficacy (objective clinical worsening) (Table 1). Regardless of when these antibodies are requested, almost all the respondents agreed that high antibody titers do not mean treatment should be discontinued (only 2.4% considered high antibody titers as a criterion for discontinuing treatment).
What tests do you perform during clinical follow-up to assess treatment response?
Most of the respondents chose the Medical Research Council (MRC) scale (92.9%), the six-minute walk test (6MWT) (100%), and pulmonary function tests (100%) (Table 1). The least common tests were activity or quality of life scales (71.4%), other timed tests (57.1%), muscle MRI (52.4%), and fatigue scales (45.2%). Among those using the MRC scale, 64.1% evaluated 10–20 muscles, while 35.9% only explored 5–10 muscles.
Do you have to do follow-up reports to keep the medication authorized?
54.8% of the respondents answered no, and 54.7% answered yes (23.8% report annually, 19% every six months, and 11.9% only occasionally) (Table 1). The results by region were highly diverse. In Catalonia, all respondents made follow-up reports, versus only one in Alicante and Murcia or Basque Country and Navarra, and none in the Balearic Islands.
According to 31% of the respondents, significant impairment of motor function and/or respiratory insufficiency is a requirement for authorizing medication at their hospital. This affirmation was particularly frequent in Andalusia (50%), Catalonia (57.1%), and in Asturias, Navarre, and the Basque Country (100%). On the other hand, 26.2% considered the improvement of follow-up parameters a prerequisite for maintaining treatment. This statement was particularly frequent in the Canary Islands (75%).
In what situations would you consider interrupting or stopping treatment?
Most respondents (76.2%) interrupted or stopped treatment when there was evidence of progressive clinical worsening. Only two respondents chose never to interrupt treatment and 19% did so when there was no objective evidence of stabilization or improvement of motor and/or respiratory function during follow-up (Table 1).
Discussion
The present survey on the management of patients with PD in routine clinical practice yielded highly variable results, with occasional deviation from the recommendations established in the clinical guidelines. The data obtained also reveal the inequality in access to treatment throughout Spain, the absence of homogeneous criteria in accordance with the experts’ recommendations, and the lack of optimization of a treatment with a high economic cost. All of this was noted despite the existence of a robust network of reference centers for rare neuromuscular diseases, which in this case, fail to centralize the management of these low-prevalence, high-complexity patients.
Based on the survey results, several discrepancies have been identified between the usual practice of physicians who care for patients with PD in Spain and the latest published expert recommendations [24–28].
According to the European guidelines, the diagnosis of the disease must be made by a certified laboratory and should be confirmed through enzyme analysis in leukocytes, fibroblasts, or skeletal muscle and/or genetically via mutation analysis (preferably by both methods) [24]. Moreover, although DBS has recently become available and is a good PD screening test, it always requires diagnostic confirmation [29]. However, according to the survey results, some clinicians do not use the criterion of reduced enzyme activity in two tissues to establish the diagnosis of PD in patients with inconclusive genetic testing. Others do not confirm the DBS results with genetic analysis, which can influence the accuracy of diagnosis.
The European consensus recommends discontinuing treatment if high antibody titers are detected that significantly counteract the effect of ERT, which, although rare, is also possible in adults [24]. For this reason, most guidelines recommend determining these antibodies in patients receiving ERT every three months for two years and then annually [25, 28]. In the survey, although many respondents request anti-GAA antibodies when there is a suspicion of lack of treatment efficacy, a significant percentage of those surveyed have never requested them due to the complexity of their determination in real clinical practice. It is interesting to note that recent publications have described that anti-GAA antibody titers decrease as patients are exposed to treatment, and only a few studies have correlated antibody titers to clinical outcomes on a prospective basis [30–33]. Thus, most PD experts agree that the determination of antibodies over time is not very helpful in LOPD patients, and they will rarely interfere with the response to treatment. This justifies the need to update the current recommendations, published in 2017.
During the follow-up of patients, most respondents chose to use pulmonary function tests, the 6MWT, and the MRC scale to evaluate the response to treatment. This is in accordance with the recommendations of several clinical guidelines [26–28]. Other assessments recommended by these guidelines and less frequently employed in clinical practice include the evaluation of nutritional status, the Rasch-built Pompe-specific Activity (R-PAct) scale, and fatigue scales [26–28]. To promote the use of scales for evaluating treatment response, it is necessary to make them more widely known and available.
The European consensus, based on the available evidence and discussion among experts, recommends starting treatment when the patient has symptoms (i.e. the patient should have skeletal muscle weakness or respiratory muscle involvement as observed using clinical assessments) and not starting treatment in pre-symptomatic patients due to the lack of sufficient evidence to support it. Therefore, any patient with symptoms should start treatment [24]. However, a considerable part of those surveyed consider that there must be a significant deterioration in motor function and/or the presence of respiratory failure as a requirement for rhGAA to be authorized in their hospital or that an improvement in monitored parameters is required to maintain treatment.
To maintain authorization of the treatment, the European guidelines state that both an improvement and stabilization in motor and/or respiratory function suggests that the treatment is beneficial and should be continued [24]. They recommend stopping therapy in cases of serious adverse events that cannot be controlled, severe comorbidity that limits the patient’s life expectancy, or if the patient should decide [26]. In addition, they recommend stopping treatment if the patient shows a substantial deterioration in motor and respiratory functions but restarting it if the progression of the disease accelerates after stopping the ERT [24]. A large majority of respondents in our survey agreed with these recommendations. However, 26.2% of those surveyed in this study do not consider stabilization a beneficial effect of the ERT and recommend its suspension in these cases.
The variety in the responses suggests that there is no standardized diagnosis or care protocol in the country and that published guidelines are not always followed. Consequently, patient care is based on the expertise of each physician. On the other hand, some answers seem to depend more on the center than on the health care region involved and, in many cases, the authorization to start and stop ERT does not rely only on the doctor but also on the pharmacy committee of the center, which may not follow the clinical guideline recommendations.
The discrepancies observed in our study with the published evidence highlight the need to establish clear and straightforward diagnostic and therapeutic guidelines for this disease or to restrict the indication and follow-up of high-impact treatments to reference centers for PD. In Spain, there are seven major reference centers for rare neuromuscular diseases designated by the Spanish Ministry of Health (Fig. 1) [34]. These centers can also be essential in avoiding inequalities in access to medicines depending on the area of residence.
The main limitations of this study are those inherent to studies based on online surveys, i.e., differences in understanding and interpretation of the questions, closed-ended questions, non-response bias, and lack of personalization, among others.
Conclusions
The present survey results on the management of PD in Spain (a rare disease with a high-impact treatment) reveal some professionals’ lack of experience or knowledge. Developing and disseminating simple guidelines that help understand and follow the recommendations is necessary. It might even be interesting to suggest that the follow-up of these patients be carried out in the existing reference centers. The inequity detected in access to rhGAA and the discrepancies with the current evidence justify the need to involve the Administration in the homogenization and optimization of the clinical management of patients with PD.
Methods
Study design
The present study involved a survey to gather opinions on the management of PD in real clinical practice in Spain. The study was conducted in several phases: 1) project definition and creation of the scientific committee; 2) generation of materials to be presented at meetings based on the current state of the art in the diagnosis and treatment of PD; 3) a total of 10 online meetings conducted by an expert to discuss the diagnosis, treatment indication, clinical management, and follow-up of patients with PD and to identify controversies; 4) creation and distribution of an online survey to collect the opinion of the respondents on the management of PD, and 5) a final meeting with the scientific committee to discuss the results.
Participants
The study involved three types of professionals: a scientific committee, a technical team, and a panel of respondents. The scientific committee consisted of 10 experts in the management of PD, whose role was to lead several discussion meetings and to develop a survey to know the usual clinical practice in the management of PD. The technical team, which directed and supervised the entire process, was responsible for the instrumental implementation of the method (literature search, survey distribution, analysis of the responses, and statistical interpretation of the survey). The scientific committee chose the panel of respondents among health professionals treating patients with PD. This panel consisted of 42 professionals (8 internal medicine physicians, 29 neurologists, and five pediatricians) with experience in the management of PD from all over Spain (Fig. 1).
Discussion meetings
Through ten meetings with healthcare professionals who attend to patients with PD, the scientific committee presented the most recent scientific evidence about this disease, showing the main recommendations of clinical guidelines on the diagnosis, treatment, and follow-up of patients. During these meetings, the management of the disorder was discussed, and the most relevant controversies were identified and collected in a worksheet. Each session was held online, lasted 2 h, was moderated by a scientific committee member, and was attended by 4–6 physicians.
Survey
Based on the controversies identified in the discussion meetings, a 5-question survey of diagnostic and treatment practices in patients with PD was developed. Each question included several answers, and the respondents could choose any. The purpose of the survey was to ascertain the disparity of opinions about the management of the disease and to identify opportunities for improvement in the diagnosis, treatment, and follow-up of patients with PD. The results were analyzed anonymously, without differentiating between the professionals’ responses belonging to reference centers and the rest.
Acknowledgements
The authors wish to thank the Research Unit at Luzán 5 (Madrid) for design and coordination assistance and Fernando Sánchez Barbero, PhD for support in the preparation of this manuscript. Cristina Domínguez-González and Jordi Díaz-Manera are members of the European Reference Network for Rare Neuromuscular Diseases (EURO-NMD).
Abbreviations
- 6MWT
Six-minute walk test
- DBS
Dried blood spot
- ERT
Enzyme replacement therapy
- GAA
Acid α-glucosidase
- IOPD
Infantile-onset PD
- JOPD
Juvenile-onset PD
- LOPD
Late-onset PD
- MRC
Medical Research Council
- MRI
Magnetic resonance imaging
- PD
Pompe disease
- R-PAct
Rasch-built Pompe-specific Activity
- rhGAA
Recombinant human alglucosidase alfa enzyme
Author contributions
CDG conceived and planned the project, coordinated the scientific committee, and validated the materials produced in all the project phases. JDM, CDM, RJM, ANO, and ARG participated in the project development, created the materials to be presented at meetings, designed the questionnaire, evaluated and discussed the survey results, and contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Funding
Sanofi has sponsored this project without participating in the article’s design, data analysis, or writing.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
CDG has received honoraria from Sanofi for speaking activities and participating in Advisory Boards. JDM has received honoraria from Sanofi Genzyme as an external reviewer of the company's educational material and has presented at congresses on behalf of the company. He has received research funding. CDM has received fees from Sanofi-Genzyme for participating in the Tracking on Pompe program. RJM has received honoraria for speaking activities and congresses from Sanofi. ANO has received speaker and consultancy fees from Sanofi, and is an investigator in clinical trials sponsored by Sanofi. ARG has received fees from Sanofi Genzyme for lectures and travel and from Takeda for lectures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cristina Domínguez-González, Email: cdgonzalez@salud.madrid.org.
Carmina Díaz-Marín, Email: carmina.diaz.marin@gva.es.
Raúl Juntas-Morales, Email: rjuntas@vhebron.net.
Andrés Nascimiento-Osorio, Email: anascimento@hsjdbcn.es.
Alberto Rivera-Gallego, Email: alberto.jose.rivera.gallego@sergas.es.
Jordi Díaz-Manera, Email: jordi.diaz-manera@newcastle.ac.uk.
References
- 1.van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet. 2008;372(9646):1342–1353. doi: 10.1016/S0140-6736(08)61555-X. [DOI] [PubMed] [Google Scholar]
- 2.Wokke JH, Escolar DM, Pestronk A, Jaffe KM, Carter GT, van den Berg LH, et al. Clinical features of late-onset Pompe disease: a prospective cohort study. Muscle Nerve. 2008;38(4):1236–1245. doi: 10.1002/mus.21025. [DOI] [PubMed] [Google Scholar]
- 3.Hagemans ML, Winkel LP, Van Doorn PA, Hop WJ, Loonen MC, Reuser AJ, et al. Clinical manifestation and natural course of late-onset Pompe's disease in 54 Dutch patients. Brain. 2005;128(Pt 3):671–677. doi: 10.1093/brain/awh384. [DOI] [PubMed] [Google Scholar]
- 4.Güngör D, Reuser AJ. How to describe the clinical spectrum in Pompe disease? Am J Med Genet A. 2013;161A(2):399–400. doi: 10.1002/ajmg.a.35662. [DOI] [PubMed] [Google Scholar]
- 5.Boentert M, Prigent H, Vardi K, Jones HN, Mellies U, Simonds AK, et al. Practical recommendations for diagnosis and management of respiratory muscle weakness in late-onset pompe disease. Int J Mol Sci. 2016;17(10):1735. doi: 10.3390/ijms17101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellies U, Lofaso F. Pompe disease: a neuromuscular disease with respiratory muscle involvement. Respir Med. 2009;103(4):477–484. doi: 10.1016/j.rmed.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Byrne BJ, Kishnani PS, Case LE, Merlini L, Muller-Felber W, Prasad S, et al. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab. 2011;103(1):1–11. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Mellies U, Ragette R, Schwake C, Baethmann M, Voit T, Teschler H. Sleep-disordered breathing and respiratory failure in acid maltase deficiency. Neurology. 2001;57(7):1290–1295. doi: 10.1212/WNL.57.7.1290. [DOI] [PubMed] [Google Scholar]
- 9.van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, et al. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med. 2010;362(15):1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 10.van der Ploeg AT, Barohn R, Carlson L, Charrow J, Clemens PR, Hopkin RJ, et al. Open-label extension study following the Late-Onset Treatment Study (LOTS) of alglucosidase alfa. Mol Genet Metab. 2012;107(3):456–461. doi: 10.1016/j.ymgme.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 11.de Vries JM, van der Beek NA, Hop WC, Karstens FP, Wokke JH, de Visser M, et al. Effect of enzyme therapy and prognostic factors in 69 adults with Pompe disease: an open-label single-center study. Orphanet J Rare Dis. 2012;7:73. doi: 10.1186/1750-1172-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Güngör D, Kruijshaar ME, Plug I, D'Agostino RB, Hagemans ML, van Doorn PA, et al. Impact of enzyme replacement therapy on survival in adults with Pompe disease: results from a prospective international observational study. Orphanet J Rare Dis. 2013;8:49. doi: 10.1186/1750-1172-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toscano A, Schoser B. Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J Neurol. 2013;260(4):951–959. doi: 10.1007/s00415-012-6636-x. [DOI] [PubMed] [Google Scholar]
- 14.Schoser B, Stewart A, Kanters S, Hamed A, Jansen J, Chan K, et al. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis. J Neurol. 2017;264(4):621–630. doi: 10.1007/s00415-016-8219-8. [DOI] [PubMed] [Google Scholar]
- 15.Stepien KM, Hendriksz CJ, Roberts M, Sharma R. Observational clinical study of 22 adult-onset Pompe disease patients undergoing enzyme replacement therapy over 5years. Mol Genet Metab. 2016;117(4):413–418. doi: 10.1016/j.ymgme.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 16.van der Meijden JC, Kruijshaar ME, Rizopoulos D, van Doorn PA, van der Beek N, van der Ploeg AT. Enzyme replacement therapy reduces the risk for wheelchair dependency in adult Pompe patients. Orphanet J Rare Dis. 2018;13(1):82. doi: 10.1186/s13023-018-0824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Meijden JC, Kruijshaar ME, Harlaar L, Rizopoulos D, van der Beek N, van der Ploeg AT. Long-term follow-up of 17 patients with childhood Pompe disease treated with enzyme replacement therapy. J Inherit Metab Dis. 2018;41(6):1205–1214. doi: 10.1007/s10545-018-0166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlaar L, Hogrel JY, Perniconi B, Kruijshaar ME, Rizopoulos D, Taouagh N, et al. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology. 2019;93(19):e1756–e1767. doi: 10.1212/WNL.0000000000008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semplicini C, De Antonio M, Taouagh N, Behin A, Bouhour F, Echaniz-Laguna A, et al. Long-term benefit of enzyme replacement therapy with alglucosidase alfa in adults with Pompe disease: Prospective analysis from the French Pompe Registry. J Inherit Metab Dis. 2020;43(6):1219–1231. doi: 10.1002/jimd.12272. [DOI] [PubMed] [Google Scholar]
- 20.Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC, Bossen E, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Investig. 2006;86(12):1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- 21.van der Ploeg A, Carlier PG, Carlier RY, Kissel JT, Schoser B, Wenninger S, et al. Prospective exploratory muscle biopsy, imaging, and functional assessment in patients with late-onset Pompe disease treated with alglucosidase alfa: The EMBASSY Study. Mol Genet Metab. 2016;119(1–2):115–123. doi: 10.1016/j.ymgme.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Winkel LP, Van den Hout JM, Kamphoven JH, Disseldorp JA, Remmerswaal M, Arts WF, et al. Enzyme replacement therapy in late-onset Pompe's disease: a three-year follow-up. Ann Neurol. 2004;55(4):495–502. doi: 10.1002/ana.20019. [DOI] [PubMed] [Google Scholar]
- 23.Stockton DW, Kishnani P, van der Ploeg A, Llerena J, Jr, Boentert M, Roberts M, et al. Respiratory function during enzyme replacement therapy in late-onset Pompe disease: longitudinal course, prognostic factors, and the impact of time from diagnosis to treatment start. J Neurol. 2020;267(10):3038–3053. doi: 10.1007/s00415-020-09936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Ploeg AT, Kruijshaar ME, Toscano A, Laforet P, Angelini C, Lachmann RH, et al. European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: a 10-year experience. Eur J Neurol. 2017;24(6):768–e31. doi: 10.1111/ene.13285. [DOI] [PubMed] [Google Scholar]
- 25.Cupler EJ, Berger KI, Leshner RT, Wolfe GI, Han JJ, Barohn RJ, et al. Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve. 2012;45(3):319–33. doi: 10.1002/mus.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barba-Romero MA, Barrot E, Bautista-Lorite J, Gutiérrez-Rivas E, Illa I, Jiménez LM, et al. Clinical guidelines for late-onset Pompe disease. Rev Neurol. 2012;54(8):497–507. [PubMed] [Google Scholar]
- 27.Al Jasmi F, Al Jumah M, Alqarni F, Al-Sanna'a N, Al-Sharif F, Bohlega S, et al. Diagnosis and treatment of late-onset Pompe disease in the Middle East and North Africa region: consensus recommendations from an expert group. BMC Neurol. 2015;15:205. doi: 10.1186/s12883-015-0412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutiérrez-Rivas E, Illa I, Pascual-Pascual SI, Pérez-López J, Vílchez-Padilla JJ, Bautista-Lorite J, et al. Guidelines for monitoring late-onset Pompe disease. Rev Neurol. 2015;60(7):321–8. [PubMed] [Google Scholar]
- 29.Lukacs Z, Nieves Cobos P, Wenninger S, Willis TA, Guglieri M, Roberts M, et al. Prevalence of Pompe disease in 3,076 patients with hyperCKemia and limb-girdle muscular weakness. Neurology. 2016;87(3):295–8. doi: 10.1212/WNL.0000000000002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel TT, Banugaria SG, Case LE, Wenninger S, Schoser B, Kishnani PS. The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol Genet Metab. 2012;106(3):301–9. doi: 10.1016/j.ymgme.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Simón E, Carrasco-Rozas A, Gallardo E, González-Quereda L, Alonso-Pérez J, Belmonte I, et al. Study of the effect of anti-rhGAA antibodies at low and intermediate titers in late onset Pompe patients treated with ERT. Mol Genet Metab. 2019;128(1–2):129–36. doi: 10.1016/j.ymgme.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Filosto M, Cotti Piccinelli S, Ravaglia S, Servidei S, Moggio M, Musumeci O, et al. Assessing the role of anti rh-GAA in modulating response to ERT in a late-onset Pompe disease cohort from the Italian GSDII Study Group. Adv Ther. 2019;36(5):1177–89. doi: 10.1007/s12325-019-00926-5. [DOI] [PubMed] [Google Scholar]
- 33.de Vries JM, Kuperus E, Hoogeveen-Westerveld M, Kroos MA, Wens SC, Stok M, et al. Pompe disease in adulthood: effects of antibody formation on enzyme replacement therapy. Genet Med. 2017;19(1):90–7. doi: 10.1038/gim.2016.70. [DOI] [PubMed] [Google Scholar]
- 34.Domínguez-González C, Madruga-Garrido M, Hirano M, Martí I, Martín MA, Munell F, et al. Collaborative model for diagnosis and treatment of very rare diseases: experience in Spain with thymidine kinase 2 deficiency. Orphanet J Rare Dis. 2021;16(1):407. doi: 10.1186/s13023-021-02030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.