Abstract
To investigate the role of allelic variants of streptokinase in the pathogenesis of acute poststreptococcal glomerulonephritis (APSGN), site-specific integration plasmids were constructed, which contained either the non-nephritis-associated streptokinase gene (skc5) from the group C streptococcal strain Streptococcus equisimilis H46A or the nephritis-associated streptokinase gene (ska1) from the group A streptococcal nephritogenic strain NZ131. The plasmids were introduced by electroporation and homologous recombination into the chromosome of an isogenic derivative of strain NZ131, in which the streptokinase gene had been deleted and which had thereby lost its nephritogenic capacity in a mouse model of APSGN. The introduction of a non-nephritis-associated allelic variant of streptokinase did not rescue the nephritogenic capacity of the strain. The mutant and the wild-type strains produced equivalent amounts of streptokinase. Complementation of the ska deletion derivative with the original ska allele reconstituted the nephritogenicity of wild-type NZ131. The findings support the hypothesis that the role of streptokinase in the pathogenesis of APSGN is related to the allelic variant of the protein.
Acute poststreptococcal glomerulonephritis (APSGN) is considered to be immune mediated since C3 and immunoglobulin G (IgG) are found deposited in glomeruli of patients with the disease. The symptoms of kidney injury typically appear 7 to 21 days after infection with group A streptococci (GAS). Occasionally, APSGN also occurs following infections with streptococci of groups C and G (GCS and GGS) (1, 7, 27, 32). Since C3 deposition precedes that of IgG in the disease process (17, 25), the initial activation of complement does not appear to be due to IgG deposition or the presence of immune complexes within the glomeruli. Thus, a prevalent hypothesis is that glomerular deposition of streptococcal antigen may precede the tissue damage and lead to non-immune-mediated local activation of the complement system, with subsequent deposition of C3 (8). Several streptococcal products have been suggested to be the so-called nephritogenic factor (5, 22, 33–36), and some of these factors have been demonstrated in glomeruli of APSGN patients (34). Of the implicated factors, streptokinase has received especial attention. Streptokinase is considered a spreading factor for GAS, GCS, and GGS, forming a tight 1:1 stoichometric complex with either plasminogen or plasmin (2). The complex can activate plasminogen to the broad specific serine protease plasmin, which has the potential to activate the complement cascade as well as to degrade fibrin clots and extracellular matrix. The enzymatic activity of the complex cannot be inhibited by the inhibitors normally acting to inhibit plasmin in plasma (3). The streptokinase gene is highly conserved, except for two polymorphic regions, designated variable regions 1 (V1) and 2 (V2) (10). By PCR amplification and restriction enzyme analysis of the V1 region of GAS isolated from patients with different disease manifestations, Johnston and coworkers demonstrated nine different allelic variants of the streptokinase gene (ska), where ska1, ska2, ska6, and ska9 were associated with APSGN isolates (12, 32). It was speculated that the variants of the protein may have different affinities to glomerular structures and thereby may affect the nephritogenic potential of a strain (9, 20). The role of streptokinase may be to initiate the nephritis process by activation of plasminogen to plasmin, which locally would activate the complement cascade and lead to C3 deposition in the glomeruli (8).
In a mouse model of APSGN, infection experiments were performed with the GAS nephritis isolate NZ131, which harbors a streptokinase gene of a nephritis-associated allele (ska1), and an isogenic derivative of the strain, with ska1 deleted. The results showed that streptokinase production was a prerequisite for the capacity of the strain to induce nephritis (19). In addition, deposition of streptokinase was demonstrated in the kidneys of mice infected with nephritogenic strains which harbored streptokinase genes of nephritis-associated alleles but not after infection with a nonnephritogenic strain, which produced streptokinase of a non-nephritis-associated allelic variant (19). Hence, whereas the results showed the importance of streptokinase production for the nephritogenic potential, the association of the disease with specific variants of the protein was neither contradicted nor established. It could not be excluded that the genetic context of a nephritis isolate such as NZ131 would also allow non-nephritis-associated streptokinase variants to become deposited and initiate the nephritis process.
Shuttle-suicide plasmid vectors capable of site-specific integration via phage integrases into the genome of streptococci have recently been constructed (15, 16). In the present study, such a vector was used to introduce a streptokinase gene of a non-nephritis-associated allele into the chromosome of a non-streptokinase-producing derivative of strain NZ131 (24). Furthermore, the NZ131 mutant derivative was complemented with the original nephritis-associated streptokinase gene of strain NZ131. The effect of the change of streptokinase alleles on the nephritogenic potential of the strain was evaluated in the mouse model (18).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The GAS strain used was NZ131 Δska::Emr, an isogenic derivative of strain NZ131, with the streptokinase gene deleted through allelic replacement (24). The Escherichia coli DH5α strain was used for vector construction and was cultivated in Luria broth and agar. The antibiotics for selection of resistance in E. coli were erythromycin (300 μg/ml), kanamycin (50 μg/ml), and ampicillin (300 μg/ml for broth, 100 μg/ml for plates). Streptococci were grown in Todd-Hewitt broth supplemented with yeast extract (THY), on blood agar, or on THY plates at 37°C with 5% CO2. When erythromycin or kanamycin was added to the THY medium, the concentrations were 3 and 400 μg/ml, respectively.
Enzymes and primers.
Restriction endonucleases and T4 DNA ligase were purchased from Bethesda Research Laboratories or Boehringer Mannheim (Indianapolis, Ind., or Bromma, Sweden) and were used as recommended by the suppliers. The Klenow fragment of DNA polymerase I was obtained from Bethesda Research Laboratories.
Plasmid and strain construction.
The pUC18-derived streptococcal integration vector p7INT (15) was used for construction of pAN103 and pAN104. Removal of erm by ScaI and HincII digestion was followed by a Klenow fill-in reaction. Blunt-end ligation proceeded with an agarose gel-purified kan-containing fragment obtained by BamHI digestion of plasmid pG3K (6). The resulting plasmid was designated pAN100. A 2.5-kb fragment containing the complete streptokinase gene from the non-nephritis-associated GCS strain S. equisimilis H46A, with a skc5 allele, was obtained by PstI digestion of plasmid pMF5 (13). After purification of the fragment from an agarose gel, ligation proceeded with PstI-digested pBluescript to provide flanking restriction sites appropriate for further cloning of skc into the pAN100 vector. The resulting construct was named pAN101. A 2.5-kb fragment containing the streptokinase gene from the nephritis-associated GAS strain NZ131, with the ska1 allele, was obtained by XbaI and SalI digestion of plasmid pSF88 (11). After gel purification, the fragment was ligated into XbaI- and SalI-digested pBluescript. The resulting construct, pAN102, and pAN101 were digested with XhoI and XbaI, and then the fragments, containing ska1 and skc5, respectively, were ligated into the lacZ multiple-cloning site of pAN100, digested with the same enzymes. The presence of inserts was confirmed by SstI digestion. The identity of the inserts was analyzed on plasmids by sequencing of the inserts and by PCR amplification of the V1 regions with subsequent restriction enzyme analysis. The strains were tested for streptokinase activity by a caseinolytic assay (see below). Strain NZ131 Δska::Emr was made competent for transformation by electroporation and was transformed with pAN100, pAN103, and pAN104 by the method described by McLaughlin and Ferretti (14). The transformants were designated NZAN0, NZAN5, and NZAN1, respectively, and analyzed by dot blot and Southern hybridization, PCR, caseinolytic assay, and Western blot analysis (see below).
DNA sequencing.
Sequence analysis of the inserts was done on plasmid DNA with universal primers by using primer extension dideoxy terminating reactions (23).
Dot blot hybridization.
Chromosomal DNA was isolated from streptococci by the method of Pitcher et al. (21). The plasmid p7ERM (a gift from R. E. McLaughlin) was labeled by using the Boehringer Mannheim Genius digoxigenin-dUTP labeling kit as specified by the manufacturer. Dot blot analysis was performed on nylon membranes, following the protocol of the Genius System, with hybridization of p7ERM at 65°C to dots of chromosomal DNA from kanamycin-resistant colonies.
Southern analysis.
The DNA probes for attB of bacteriophage T12, cloned into pWM130 (16), and for the streptokinase gene (11) were prepared using the Genius DIG-dUTP labeling kit as specified by the manufacturer. Agarose gel electrophoresis, Southern transfer of HindIII-digested streptococcal chromosomal DNA to nylon membranes, and hybridization at 65°C were done as specified in the Genius user's guide protocols (Boehringer Mannheim).
PCR.
Plasmids and streptococcal chromosomal DNA were analyzed for the presence and identity of the alleles of the streptokinase gene by PCR amplification and restriction enzyme analysis of the V1 region (12, 26). The primers used were 5′-AACCTTGCCGACCCAACCTGT-3′ and 3′-GGCATCGTAAAATGCTTACCT-5′ (accession no. M19346) (11). The reaction mixture contained 10 mM Tris HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 0.1 mg of gelatin per ml, 200 μM each dATP, dCTP, dGTP, and dTTP, 170 μg of bovine serum albumin per ml, 2.5 μM each primer, and 2.5 U of Ampli-Taq (Boehringer Mannheim, Bromma, Sweden). The settings for amplification were 1 cycle of 94°C for 1 min; 25 cycles of 94°C for 1 min, 45°C for 1 min, and 72°C for 2 min; and 1 cycle of 72°C for 3 min. The amplified V1 region was characterized by digestion with MluI, PvuII, DraI, and DdeI, as specified by Johnston et al. (12).
Caseinolytic assay and Western analysis.
A soft-agar overlay containing 1.5 ml of skim milk, 8.5 ml of 0.8% agar, and 100 μg of human plasminogen was used on THY plates onto which colonies of streptococcal or E. coli strains had been patched or wells to which supernatants had been added. Positive streptokinase activity was indicated by the appearance of a clear zone around the colony or well, after incubation at 37°C for 2 to 8 h (13). Supernatants of 10-ml in vitro cultures of the NZ131 derivatives were precipitated overnight with 95% ethanol at −20°C and resuspended in 1 ml of H2O, after which 2-, 4-, and 8-μl samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide) followed by electrotransfer to nitrocellulose filters (28). Streptokinase was demonstrated with a monoclonal antibody to the protein (18); alkaline phosphatase-conjugated, affinity-purified goat anti-mouse IgG (Cappel, West Chester, Pa.) was used as the secondary antiserum.
Infection model.
A tissue cage model for APSGN was used as described previously (18). Male BALB/c mice (Bomholtgård Breeding and Research Centre A/S, Ry, Denmark), 2 to 3 months old at the time of infection, were used. The streptococcal strains used for infection were passaged once in heparinized mouse blood prior to injection into the tissue cage fluid (TCF) of the subcutaneous cages. The bacteria were injected at 108 CFU/ml. Of mice infected with NZAN0, 15 were treated with benzylpenicillin from day 7 postinfection (p.i.) and 14 were treated from day 16 p.i. Of mice infected with NZAN5, the antibiotic treatment was initiated on day 7 p.i. for 25 and on day 16 p.i. for 16. In addition, 23 uninfected mice were included. In a subsequent experiment, 36 mice were infected with NZAN1 and treated with antibiotics from day 16 p.i. In this series, 10 uninfected mice were used as controls. Samples of TCF (0.1 ml) and urine were taken on days 0, 3, 7, 14, and 21 p.i. Blood was collected on days 0 and 21 p.i. Bacterial growth in TCF was analyzed on blood agar plates. All animals were sacrificed on day 21 p.i. by exsanguination under anesthesia (Hypnorm [Jansen Cilag Ltd., Saunderton, United Kingdom] and Dormicum [F. Hoffman-La Roche AG, Basel, Switzerland]). Kidneys were perfused in situ with phosphate-buffered saline via the left heart ventricle until macroscopically free of blood, whereafter renal samples were removed and prepared for immunohistological and morphological analysis (see below).
Demonstration of streptokinase production in vivo.
Semiquantitative Western blot analysis of streptokinase in TCF from selected mice was done as described previously (18). Mouse serum samples were analyzed for the presence of antibodies to streptokinase by an immunoblot procedure, as described previously (18). Streptokinase used for the detection of antibodies was a gift from KABI Vitrum (Stockholm, Sweden) and was derived from strain H46A.
Assessment of glomerular injury.
Urine samples were analyzed for protein and hematuria, where proteinuria was defined as a protein concentration of at least 1.0 g/liter and hematuria was defined as a hemoglobin concentration corresponding to at least 10 erythrocytes/μl (N-Labstix; Bayer Sverige AB, Gothenburg, Sweden). Formalin-fixed, paraffin-embedded kidney tissue was sectioned to a 5-μm thickness and stained with hematoxylin and periodic acid-Schiff. Ten glomeruli per mouse were evaluated for morphological changes such as thickening of the basement membrane, capillary walls, and capsule epithelium, as well as lobulation of the glomerular tuft, occlusion of capillaries, and hypercellularity. Quantitative analysis of hypercellularity was performed by calculating the number of glomeruli touching the intersections of an ocular inserted square pattern as described previously (18, 19, 30). The recorded cell numbers were calculated to reflect glomeruli of identical area. Three different percentiles were used as cutoff limits to assess the occurrence and severity of hypercellularity in the mouse groups. The number of cells corresponding to the 60th percentile of the cell numbers of the uninfected mice of the same experimental series was chosen as the lowest limit for definition of hypercellularity of a glomerulus (18, 19). A kidney was defined as hypercellular when more than 50% of the evaluated glomeruli were hypercellular. A Leitz Dialux 20 light microscope was used for the histopathological analyses. Frozen kidney tissue was sectioned to 5-μm-thick sections and analyzed for C3 deposition in an Aristoplan microscope. Fluorescein isothiocyanate-conjugated goat F(ab′)2 fragment anti-mouse C3 (Cappel) and a nonfading mounting medium (Vectashield; Vector, Burlingame, Calif.) were used. All analyses of kidney tissue and urine were performed blinded; i.e., the observer did not know the origin of the samples.
Ethics.
The study was approved by the local ethics committee at Umeå University.
Statistical analysis.
The statistical method for comparing proportions involved a normal approximation of binominal distribution (4). The criterion for significant differences throughout the study was that the probability of random occurrence was less than 0.05.
RESULTS
Vector construction.
For the aim of introducing a streptokinase gene into the genome of a GAS strain, vector plasmid pAN100 was constructed from the streptococcal phage-derived integration vector p7INT (15). Plasmids pAN103 and pAN104 were generated by introduction of a fragment containing a streptokinase gene of a non-nephritis-associated allele and of a nephritis-associated allele, respectively, into pAN100 (for details, see Materials and Methods). E. coli colonies transformed with pAN100, pAN103, or pAN104 were kanamycin resistant and erythromycin sensitive. SstI digestion verified inserts of the sizes corresponding to those of the skc5 and ska1 fragments in pAN103 and pAN104, whereas no insert was demonstrated in pAN100. The identity of the ska1 and skc5 alleles was verified by sequencing, as well as by PCR amplification of the V1 regions with subsequent restriction enzyme analysis. The caseinolytic assay showed streptokinase activity for the E. coli colonies with pAN103 and pAN104, whereas no activity was detected with a colony carrying the pAN100 vector (data not shown).
Allelic complementation in GAS.
The streptococcal integration vector pAN103 was used to introduce a streptokinase gene of a non-nephritis-associated allele into the genome of a derivative of strain NZ131 with the streptokinase gene of a nephritis-associated allele deleted, NZ131 Δska::Emr (24). To verify that the observed influence on the nephritogenic capacity was related to the identity of the streptokinase gene, as opposed to the integration, pAN104, which carried the original nephritis-associated ska1 allele, was also included. The predicted orientation of the vector after integration is shown in Fig. 1. To obtain a fully isogenic streptococcal strain, which would differ only with regard to the presence of the streptokinase gene, pAN100 was transformed into NZ131 Δska::Emr. Kanamycin-resistant colonies were obtained after electroporation with pAN100, pAN103, and pAN104. Plasmid integration was demonstrated by dot blot analysis of chromosomal DNA hybridized to labeled plasmid as well as by Southern blotting of HindIII-digested chromosomal DNA, hybridized with a probe to attB. A second hybridization band was seen in the chromosome of the transformants, indicating the duplication of the integration sequence, which is known to appear after integration of the T12 phage into the streptococcal genome (Fig. 2) (16). Hybridization with a probe directed to the streptokinase gene and PCR amplification of the V1 region of streptokinase verified the presence of the streptokinase gene in the pAN103 and pAN104 transformants. Furthermore, restriction enzyme digestion of the PCR products confirmed the identities of the streptokinase alleles (data not shown). No amplification product was obtained by PCR of the pAN100-transformed colony. The strain transformed with pAN100 was named NZAN0, since it lacked the streptokinase gene, the strain transformed with pAN103 was designated NZAN5, and the strain transformed with pAN104 was designated NZAN1, to reflect the identity of the introduced streptokinase allele.
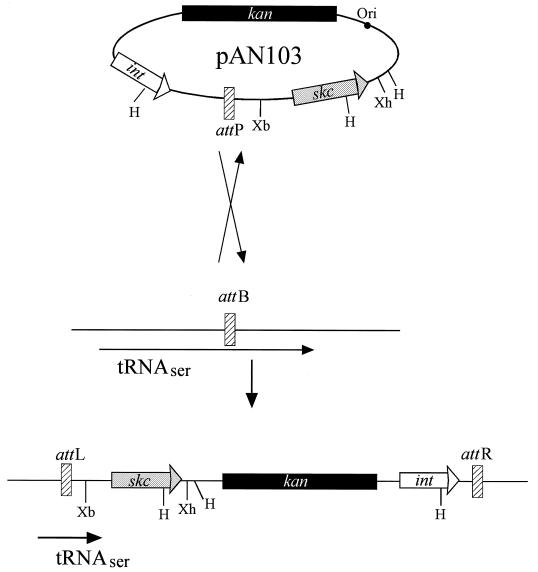
FIG. 1.
Predicted recombination event for the introduction of pAN103 into the genome of strain NZ131 Δska::Emr. pAN103 (9,435 bp) contains genes for the phage T12 integrase (int), streptokinase (skc) from S. equisimilis H46A, and kanamycin resistance (aphA-3). The plasmid is integrated via a single crossover event between the attachment sites attP and attB. By a 96-bp duplication sequence in attP of the 3′ end of the serine-tRNA gene, which serves as the attachment site on the chromosome, integration leaves the gene intact (16). H, Xb, and Xh represent cleavage sites for restriction enzymes HindIII, XbaI, and XhoI, respectively.
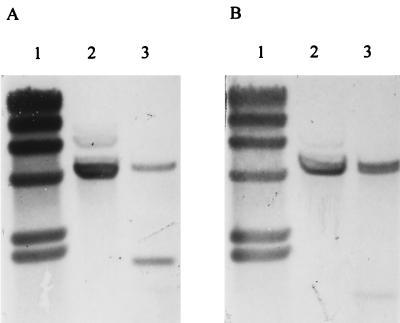
FIG. 2.
Integration of pAN103 into the phage T12 attB site. (A) Insertion of pAN103 into attB of strain NZ131 Δska::Emr was detected by hybridization of HindIII-digested chromosomal DNA to a probe specific for attB. The site is contained on one fragment when unoccupied by the integrative plasmid (lane 2). In NZAN5, the restriction endonuclease sites associated with the integrated pAN103 generate two hybridization fragments (lane 3). (B) The new genetic material is identified by hybridization of a duplicate blot to a probe for a conserved region shared by ska and skc (11). In NZ131 Δska::Emr, one hybridizing band is observed from the remaining sequence of the inactivated ska gene (lane 2). In NZAN5, the additional site for HindIII cleavage in skc generates a smaller (958-bp) hybridizing fragment, and a higher-molecular-weight doublet exists from the presence of both genes (lane 3). HindIII-digested bacteriophage lambda DNA was used as a molecular weight standard (lanes 1).
In vitro and in vivo expression of streptokinase by GAS.
To ascertain that the streptokinase gene was transcribed after the introduction of the gene into the streptococcal genome and that the extracellular release of the gene product was comparable to that of the wild-type NZ131 strain, streptokinase production was examined. Caseinolytic assay of patched colonies and culture supernatants, as well as Western blot analysis of in vitro- and in vivo-produced extracellular products, showed that strains NZAN5 and NZAN1 produced streptokinase whereas strain NZAN0 did not. By the methods used for analysis of streptokinase production, no differences in the amounts produced could be detected between the wild-type strain NZ131 and strain NZAN5. Both strains gave clearance zones of 6 mm at the 1:100 culture supernatant dilution (Fig. 3). Strain NZAN1 gave clearance zones approximately 70% of the size of those of strain NZAN5 (data not shown). Immunoblot analysis of sera from mice infected with strain NZAN5 showed that 25% of the mice (3 of 12) treated from day 7 p.i. with penicillin and 62.5% of those (10 of 16) treated from day 16 p.i. had developed antibodies to streptokinase during the infection. Of strain NZAN1-infected mice, which were all treated from day 16 p.i., 61.1% (22 of 36) revealed serum antibodies to streptokinase, compared to 0 of 7 analyzed mice infected with strain NZAN0 and 0 of 11 analyzed uninfected animals. No apparent differences in bacterial numbers in TCF during the infectious process could be demonstrated between the strains of this study and the wild-type strain (19).
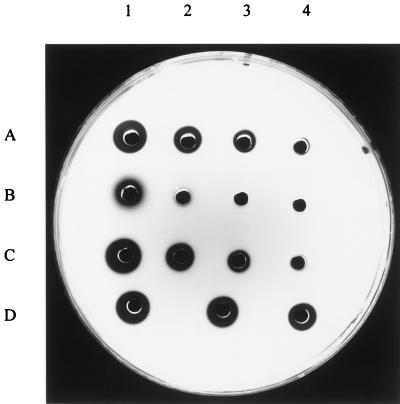
FIG. 3.
Semiquantitative analysis of streptokinase production. By casein agar plate analysis (11), streptokinase production was assayed in culture supernatants of strains NZ131 (row A), NZ131 Δska::Emr (row B), and NZAN5 (row C), which corresponds to NZ131 Δska::Emr containing the skc5 gene from S. equisimilis H46A. Wells 1 contain undiluted supernatants, and the following wells contain three 10-fold supernatant dilutions. Row D shows three wells with streptokinase at 1, 0.5, and 0.1 U (in numerical order of the wells). The clearance in B1, of NZ131 Δska::Emr, is due to background protease activity. Streptokinase is produced in equal amounts by NZ131 and NZAN5 (A3 and C3) but is not produced by NZ131 Δska::Emr (B3).
Effect on nephritogenicity by streptokinase allele substitution in strain NZ131.
Apart from deposition of C3 in the mouse group infected with strain NZAN5 and treated with penicillin from day 7 p.i., no urinary or renal signs of nephritis were present in NZAN5- or NZAN0-infected mice of this study compared to the occurrences of the different parameters in the uninfected mouse group (P > 0.05) (Tables 1 and 2). After infection with strain NZAN1, hypercellularity was induced at both the 60th and 70th percentiles, as well as C3 deposition and proteinuria. Statistical comparison between mouse groups infected with strains NZAN5 and NZAN1 showed that the latter strain induced occluded capillaries, C3 deposition, and proteinuria to a greater extent than did strain NZAN5 after 16 days of infection (Table 2).
TABLE 1.
Number of mice with diffuse hypercellularity
| Strain | Phenotype | Time (day p.i.) when penicillin treatment was startedb | No. of animals with diffuse hypercellularity at following percentilea:
|
||
|---|---|---|---|---|---|
| 60th | 70th | 80th | |||
| NZAN0 | Ska1− Emr Kanr | 7 | 6/15 | 3/15 | 1/15 |
| NZAN5 | Ska1− Emr Kanr Skc5+ | 7 | 6/25 | 2/25 | 0/25 |
| NZAN0 | Ska1− Emr Kanr | 16 | 2/14 | 2/14 | 0/14 |
| NZAN5 | Ska1− Emr Kanr Skc5+ | 16 | 5/16 | 2/16 | 0/16 |
| NZAN1 | Ska1− Emr Kanr Ska1+ | 16 | 18/36*** | 9/36*** | 2/36 |
| Uninfected mice | |||||
| NZAN0 and NZAN5 controlsc | 7/23 | 3/23 | 0/23 | ||
| NZAN1 controlsd | 0/10 | 0/10 | 0/10 | ||
Corresponds to a value below which the designated percentage of the total calculated glomeruli of the uninfected control group is found. We defined diffuse hypercellularity when more than 50% of the glomeruli analyzed in a mouse displayed cell numbers above that of the percentile of the uninfected control mice in the same experiment. Data are given as the number with diffuse hypercellularity/total number tested. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (degree of significance for the occurrence of diffuse hypercellularity, compared to the uninfected controls in the same experiment).
All animals were sacrificed on day 21 p.i.
Uninfected mice belonging to the same experiment as NZAN0- and NZAN5-infected mice.
Uninfected mice belonging to the same experiment as NZAN1-infected mice.
TABLE 2.
Morphological, immunohistopathological, and urinary findings
| Strain | Phenotype | Time (day p.i.) when penicillin treatment was startedb | No. of mice/total no. witha:
|
||||
|---|---|---|---|---|---|---|---|
| Glomeruli
|
Urine
|
||||||
| Occl | Lob | C3 | Protein | Hem | |||
| NZAN0 | Ska1− Emr Kanr | 7 | 1/15 | 0/15 | 1/15 | 2/15 | 0/15 |
| NZAN5 | Ska1− Emr Kanr Skc5+ | 7 | 1/25 | 0/25 | 4/25* | 7/25 | 0/25 |
| NZAN0 | Ska1− Emr Kanr | 16 | 0/14 | 0/14 | 0/14 | 1/14 | 0/14 |
| NZAN5 | Ska1− Emr Kanr Skc5+ | 16 | 0/16 | 0/16 | 1/16 | 3/16 | 0/16 |
| NZAN1 | Ska1− Emr Kanr Ska1+ | 16 | 11/36c | 0/36 | 13/36*c | 16/36**c | 0/36 |
| Uninfected mice | |||||||
| NZAN0 and NZAN5 controlsd | 0/23 | 0/23 | 0/23 | 2/23 | 1/23 | ||
| NZAN1 controlse | 1/10 | 0/10 | 1/10 | 1/10 | 0/10 | ||
For proteinuria, the minimum concentration limit was set at 1.0 g/liter; for hematuria, a hemoglobin concentration corresponding to 10 erythrocytes/μl was measured. ∗, P < 0.005; ∗∗, P < 0.01 (degree of significance for occurrence of analyzed findings, compared to the uninfected control mice in the same experiment). Abbreviations: Occl, occlusion of capillaries; Lob, lobulation; Protein, proteinuria; Hem, hematuria.
All animals were sacrificed on day 21 p.i.
Mice infected with NZAN1 had a higher occurrence of occluded capillaries (P < 0.001), C3 deposition (P < 0.01), and proteinuria (P < 0.05) than did those infected with NZAN5, treated correspondingly with penicillin.
Uninfected mice belonging to the same experiment as NZAN0- and NZAN5-infected mice.
Uninfected mice belonging to the same experiment as NZAN1-infected mice.
DISCUSSION
In this study, we show that site-specific integration vectors can be used to introduce novel genes into the chromosome of GAS. The streptokinase genes from S. equisimilis H46A (skc5) and from the GAS strain NZ131 (ska1) were introduced into the genome of a streptokinase-defective isogenic derivative of strain NZ131. In addition, we show that the change of streptokinase allelic variant in strain NZ131 resulted in almost complete loss of the capacity to induce signs of nephritis in a mouse model of APSGN. The findings indicate that the capacity of a strain to induce nephritis is largely dependent on the streptokinase allele present.
Shuttle-suicide plasmid vectors capable of site-specific integration have recently been constructed for GAS (15, 16). In the present study, skc5, a non-nephritis-associated streptokinase allele from the GCS strain S. equisimilis H46A, and ska1, a nephritis-associated streptokinase allele from the GAS strain NZ131, were independently cloned into such a vector, p7INT. The vector contains the phage T12-derived integrase gene (int) and phage attachment site (attP), as well as a replication origin from E. coli, plasmid pUC18, but no origin active in streptococci (15, 16). Thus, the plasmid can exist in the streptococcus only if integrated in the chromosome. Furthermore, since it does not contain the gene for excisionase, integration is irreversible. The integration reaction is highly specific (16, 31). attP contains a 96-bp duplication of the 3′ end of a serine-tRNA gene, which, after integration, leaves the gene intact (16). This suggests the possibility of using the vector to introduce a gene into the genome of a strain without interrupting the genetic background. Since the streptokinase-defective NZ131 strain carried a gene for erythromycin resistance, the erm of p7INT was replaced by a gene encoding kanamycin resistance to provide a selection marker for chromosomal integration. The resulting plasmid was designated pAN100. By subsequent cloning of skc5 and ska1 into the vector, pAN103 and pAN104, respectively, were obtained.
In the mouse tissue cage model of APSGN, the nephritogenic capacity of strain NZ131 was observed to be almost completely abolished if the streptokinase gene had been deleted (19). In the present study, the same streptokinase-defective strain, NZ131 Δska::Emr, was transformed with pAN103 and pAN104 to create strains NZAN5 and NZAN1, respectively. The strains were named according to the allele number of the introduced streptokinase gene. NZAN5 and NZAN1 excreted streptokinase with native substrate specificity. Transformation was also done with pAN100, to provide a negative control strain for nephritogenicity, NZAN0, which contained the integration vector but not the streptokinase gene. Infection in the mouse tissue cage model for 7 days with strain NZ131 has in a previous study resulted in proteinuria, C3 deposition, and diffuse hypercellularity, all at P < 0.05. Infection for 16 days with the same strain resulted in proteinuria, C3 deposition, and occluded capillaries, all at P < 0.05, as well as diffuse hypercellularity, at P < 0.001. The P values for different parameters were determined by comparing the proportions obtained among the infected mice with those obtained with the uninfected control mice of that study (19). The results with the NZ131 wild-type strain are reproducible in the animal model (18, 19). In the present study, we show that the original nephritogenic phenotype of NZ131 could be restored after complementation of NZ131 Δska::Emr with the original ska1 allele, since infection with strain NZAN1 for 16 days resulted in proteinuria, C3 deposition, and diffuse hypercellularity, all at a significant level. In contrast, infection for 7 and 16 days with strain NZAN5, which contained the non-nephritis-associated skc5 allele, did not result in any signs of nephritis, except for C3 deposition after 7 days of infection (P < 0.05). Comparison between the two complemented strains confirmed that the induction of occluded capillaries (P < 0.001), C3 deposition (P < 0.01), and proteinuria (P < 0.05) were higher after 16 days of infection with NZAN1 than after the same period of infection with NZAN5. Deposition of IgG was not investigated since the mouse model reflects the early stage of APSGN and IgG deposition has previously been shown not to occur in the model (18, 19). The fact that glomerular C3 deposition is known to precede that of IgG in humans indicates that complement activation occurs before IgG is deposited in the glomeruli (25). IgG deposition may be due to autoantibodies to glomerular epitopes, deposition of circulating immune complexes, and/or antibodies to deposited streptococcal antigen(s). As observed by different detection methods, strains NZ131 and NZAN5 produced equal amounts of streptokinase in vitro as well as in the tissue cages during infection (18). Strain NZAN1 produced a somewhat smaller amount of streptokinase than did NZAN5 and the wild-type strain. No differences were noted in growth in the tissue cages during infection with any of the strains used. Hence, the observed differences in nephritogenic potential between strains NZAN5 and NZAN1 should not be attributed to variations in growth or the amounts of streptokinase produced. The inability of streptokinase of the skc5 allele to compensate NZ131 Δska::Emr for the loss of the nephritogenic potential, which was observed after deletion of ska1 (19), shows that the identity of the streptokinase allele is critical to the nephritogenic capacity of a strain.
Johnston and coworkers demonstrated an association of certain allelic variants of streptokinase with streptococcal isolates from APSGN patients (12, 32). The non-nephritis-associated streptokinase variant from the GCS strain S. equisimilis H46A, the same as was analyzed in the present study, has been shown to have lower affinity for isolated glomeruli than does streptokinase of a nephritis-associated allelic variant (ska2) (20). Conformational studies of these two variants of streptokinase show that the nephritis-associated variant has three energetic folding units whereas the non-nephritis-associated variant has two, as well as a C-terminal region with higher sensitivity to trypsin degradation (29). Since the major differences between the proteins are located in the V1 region, it appears probable that these differences have influences on the observed differences in tertiary structure of these proteins. In the mouse model of APSGN, streptokinase was more often detected in kidney tissue of mice with severe hypercellularity (19). Thus, the severity of the pathological process may reflect the degree of streptokinase deposition. Analysis of the symptoms of kidney damage indicated that the GAS strain EF514 may have a stronger nephritogenic potential than does NZ131. In addition, there was a tendency for higher streptokinase deposition in mice infected with strain EF514 (ska2) than in those infected with NZ131 (ska1). It has been proposed that the nephritogenic potential of a strain may reflect the identity of the ska allele, where, e.g., streptokinase of the ska3 allelic variant would have the lowest affinity, that of the ska1 variant would have a higher affinity, and that of the ska2 variant would have the highest affinity for glomeruli (19). The findings of the present study, that exchange of the ska1 allele with the non-nephritis-associated skc5 allele resulted in almost complete loss of nephritogenic capacity of strain NZ131, support the findings of Peake et al. (20) that this non-nephritis-associated streptokinase variant has lower affinity for glomeruli than does ska2 and also indicates that the affinity of the non-nephritis-associated protein is lower than that of ska1, the original streptokinase allelic variant of NZ131.
C3 deposition was demonstrated to be the only sign of kidney damage in the mice after infection with strain NZAN5. This finding further supports the theory that C3 deposition is an early event in the pathogenesis of APSGN and that other signs, such as hypercellularity, appear at a later stage in the disease process. Furthermore, although the affinity of this type of streptokinase for glomeruli is low, the presence of C3 indicates that some deposition of the protein had occurred. Deposition of C3 did not occur to a significant extent in mice infected with the same strain, when the infection was prolonged to 16 days. This observation might be explained by the fact that there were fewer animals in this group (16 animals) than in the 7-day-infection group (25 animals). It is unlikely that our findings are related to differences in growth characteristics, since these were the same for the two groups between days 3 and 7. Rather, they may indicate that the extent of deposition was so minute that a larger number of mice had to be included in order to demonstrate its occurrence. In conclusion, the so-called non-nephritis-associated variants of streptokinase may have the potential to induce APSGN, but their affinity to glomeruli is so weak that this event is rather rare. In an infection experiment with four GAS strains which harbored nephritis-associated ska alleles, one of the strains did not induce nephritis (18). Thus, the mere production of streptokinase of a nephritis-associated variant is not enough to cause the disease in mice. The results indicate that apart from a critical influence of certain allelic variants of streptokinase on the nephritogenic capacity of a strain, additional factors are important in the pathogenesis of APSGN.
ACKNOWLEDGMENTS
The study was supported by grants from the Medical Research Council (08675 and 729/96) and Wibergs and Bergvalls Foundation to M.N.; Umeå University, Medical Faculty, and Västerbottens Läns Landsting to S.E.H. and M.N.; and the Kempe Foundation to A.N.
We thank Charles Primaux for performing the streptokinase caseinolytic assays.
REFERENCES
- 1.Barnham M, Thornton T J, Lange K. Nephritis caused by Streptococcus zooepidemicus (Lancefield group C) Lancet. 1983;i:945–948. doi: 10.1016/s0140-6736(83)92078-0. [DOI] [PubMed] [Google Scholar]
- 2.Castellino F J, Bajaj S P. Activation of human plasminogen by equimolar levels of streptokinase. J Biol Chem. 1977;252:492–498. [PubMed] [Google Scholar]
- 3.Cederholm-Williams S A, De Cock F, Lijnen H R, Collen D. Kinetics of the reactions between streptokinase, plasmin and α2-antiplasmin. Eur J Biochem. 1979;100:125–132. doi: 10.1111/j.1432-1033.1979.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 4.Colton T. Inference on proportions. In: Colton T, editor. Statistics in medicine. Boston, Mass: Little Brown & Co.; 1974. pp. 151–188. [Google Scholar]
- 5.Cronin W, Deol H, Azadegan A, Lange K. Endostreptosin: isolation of the probable immunogen of acute post streptococcal glomerulonephritis (PSGN) Clin Exp Immunol. 1989;76:198–203. [PMC free article] [PubMed] [Google Scholar]
- 6.Geist R T, Okada N, Caparon M G. Analysis of Streptococcus pyogenes promoters by using novel Tn916-based shuttle vectors for the construction of transcriptional fusions to chloramphenicol acetyltransferase. J Bacteriol. 1993;175:7561–7570. doi: 10.1128/jb.175.23.7561-7570.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grann J W, Gray B M, Griffin F M, Dismuke W E. Acute glomerulonephritis following group G streptococcal infection. J Infect Dis. 1987;156:411–412. doi: 10.1093/infdis/156.2.411. [DOI] [PubMed] [Google Scholar]
- 8.Holm S E. The pathogenesis of acute post-streptococcal glomerulonephritis in new lights. APMIS. 1988;96:189–193. doi: 10.1111/j.1699-0463.1988.tb05289.x. [DOI] [PubMed] [Google Scholar]
- 9.Holm S E. Hypothesis on the pathogenesis of post-streptococcal glomerulonephritis based on recent clinical and experimental research. Zentbl Bakteriol Mikrobiol Hyg. 1990;274:325–332. doi: 10.1016/s0934-8840(11)80689-4. [DOI] [PubMed] [Google Scholar]
- 10.Huang T T, Malke H, Ferretti J J. Heterogeneity of the streptokinase gene in group A streptococci. Infect Immun. 1989;57:502–506. doi: 10.1128/iai.57.2.502-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang T T, Malke H, Ferretti J J. The streptokinase gene of group A streptococci: cloning, expression in Escherichia coli, and sequence analysis. Mol Microbiol. 1989;3:197–205. doi: 10.1111/j.1365-2958.1989.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnston K H, Chaiban J E, Wheeler R C. Analysis of the variable domain of the streptokinase gene from streptococci associated with post streptococcal glomerulonephritis. In: Orefici G, editor. New perspectives on streptococci and streptococcal infections. Stuttgart, Germany: Gustav Fischer Verlag; 1992. pp. 339–341. [Google Scholar]
- 13.Malke H, Ferretti J J. Streptokinase: cloning, expression, and excretion by Escherichia coli. Proc Natl Acad Sci USA. 1984;81:3557–3561. doi: 10.1073/pnas.81.11.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin R E, Ferretti J J. Electrotransformation of streptococci. In: Nickoloff A, editor. Electroporation protocols for microorganisms. Vol. 47. Totowa, N.J: Humana Press Inc.; 1995. pp. 185–193. [Google Scholar]
- 15.McShan W M, McLaughlin R E, Nordstrand A, Ferretti J J. Vectors containing streptococcal bacteriophage integrases for site-specific gene insertion. Methods Cell Sci. 1998;20:51–57. [Google Scholar]
- 16.McShan W M, Tang Y-F, Ferretti J J. Bacteriophage T12 of Streptococcus pyogenes integrates into the gene encoding a serine tRNA. Mol Microbiol. 1997;23:719–728. doi: 10.1046/j.1365-2958.1997.2591616.x. [DOI] [PubMed] [Google Scholar]
- 17.Michael A F, Hoyer J R, Westberg N G, Fish A J. Experimental models for the pathogenesis of acute poststreptococcal glomerulonephritis. In: Wannamaker L W, Matsen L J, editors. Streptococci and streptococcal diseases. Recognition, understanding, and management. Academic Press, Inc., New York, N.Y. 1972. pp. 481–496. [Google Scholar]
- 18.Nordstrand A, Norgren M, Holm S E. An experimental model for acute poststreptococcal glomerulonephritis in mice. APMIS. 1996;104:805–816. doi: 10.1111/j.1699-0463.1996.tb04946.x. [DOI] [PubMed] [Google Scholar]
- 19.Nordstrand A, Norgren M, Ferretti J J, Holm S E. Streptokinase as a mediator of acute post-streptococcal glomerulonephritis in an experimental mouse model. Infect Immun. 1998;66:315–321. doi: 10.1128/iai.66.1.315-321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peake P W, Pussell B A, Karplus T E, Riley E H, Charlesworth J A. Post-streptococcal glomerulonephritis: studies on the interaction between nephritis strain-associated protein (NSAP), complement and the glomerulus. APMIS. 1991;99:460–466. doi: 10.1111/j.1699-0463.1991.tb05176.x. [DOI] [PubMed] [Google Scholar]
- 21.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 22.Poon-King R, Bannan J, Viteri A, Cu G, Zabriskie J B. Identification of an extracellular plasmin binding protein from nephritogenic streptococci. J Exp Med. 1993;178:759–763. doi: 10.1084/jem.178.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon D, Ferretti J J. Electrotransformation of Streptococcus pyogenes with plasmid and linear DNA. FEMS Microbiol Lett. 1991;82:219–224. doi: 10.1016/0378-1097(91)90336-9. [DOI] [PubMed] [Google Scholar]
- 25.Sorger K. Postinfectious glomerulonephritis. Subtypes, clinicopathological correlations, and follow-up studies. New York, N.Y: Gustav Fischer Verlag; 1986. pp. 15–19. [PubMed] [Google Scholar]
- 26.Tewodros W, Nordstrand A, Kronvall G, Holm S E, Norgren M. Streptokinase gene polymorphism in group A streptococci isolated from Ethiopian children with various disease manifestations. Microb Pathog. 1993;15:303–311. doi: 10.1006/mpat.1993.1080. [DOI] [PubMed] [Google Scholar]
- 27.Tewodros W, Muhe L, Daniel E, Schalén C, Kronvall G. A one-year study of streptococcal infections and their complications among Ethiopian children. Epidemiol Infect. 1992;109:211–225. doi: 10.1017/s0950268800050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wefle K, Misselwitz R, Schaup A, Gerlach D, Wefle H. Conformation and stability of streptokinases from nephritogenic and nonnephritogenic strains of streptococci. Proteins Struct Funct Genet. 1997;27:26–35. [PubMed] [Google Scholar]
- 30.Weibel E R. Practical methods for biological morphometry. In: Weibel E R, editor. Stereological methods. Vol. 1. London, United Kingdom: Academic Press, Ltd.; 1979. [Google Scholar]
- 31.Weisberg R A, Landy A. Site-specific recombination in phage lambda. In: Roger J W R, Hendrix W, Stahl F W, Weisberg R A, editors. Lambda II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1983. pp. 211–250. [Google Scholar]
- 32.Wheeler R C, Chaiban J E, Johnston K H. Analysis of the streptokinase gene from group C streptococci S. equisimilis and S. Zooepidemicus by the polymerase chain reaction and possible relation to poststreptococcal glomerulonephritis. In: Orefici G, editor. New perspectives on streptococci and streptococcal infections. Stuttgart, Germany: Gustav Fischer Verlag; 1992. pp. 343–345. [Google Scholar]
- 33.Villareal H, Jr, Fischetti V A, Van de Rijn I, Zabriskie J B. The occurrence of a protein in the extracellular products of streptococci isolated from patients with acute glomerulonephritis. J Exp Med. 1979;149:459–472. doi: 10.1084/jem.149.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt A, Mertz A, Batsford S, Rodriguez-Iturbe B. Cationic extracellular streptococcal antigens; affinity for the renal glomerulus. In: Kimura Y, Kotami S, Shiokawa Y, editors. Recent advances in streptococci and streptococcal diseases. Proceedings of the IXth international symposium on streptococci and streptococcal diseases. Windsor, England: Reedbooks Ltd.; 1985. pp. 170–171. [Google Scholar]
- 35.Yoshizawa N, Treser G, Iwasaki M, Takahashi K. Further characterization of a streptococcal antigen in acute glomerulonephritis. In: Holm S E, Christensen P, editors. Basic concepts of streptococci and streptococcal diseases. Windsor, England: Reedbooks Ltd.; 1982. pp. 257–259. [Google Scholar]
- 36.Yoshizawa N, Oshima S, Sagel I, Shimizu J, Treser G. Role of a streptococcal antigen in the pathogenesis of acute poststreptococcal glomerulonephritis. Characterization of the antigen and a proposed mechanism for the disease. J Immunol. 1992;148:3110–3116. [PubMed] [Google Scholar]