Abstract
Purpose
Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) can be used to restore organ perfusion in patients with cardiogenic shock until native heart recovery occurs. It may be challenging, however, to determine when patients can be weaned successfully from ECMO—surviving without requiring further mechanical support or heart transplant. We aimed to systematically review the medical literature to determine the biomarkers, hemodynamic and echocardiographic parameters associated with successful weaning of VA-ECMO in adults with cardiogenic shock and to present an evidence-based weaning algorithm incorporating key findings.
Method
We systematically searched PubMed, Embase, ProQuest, Google Scholars, Web of Science and the Grey literature for pertinent original research reports. We excluded studies limited to extracorporeal cardiopulmonary resuscitation (ECPR) as the neurological prognosis may significantly alter the decision-making process surrounding the device removal in this patient population. Studies with a mixed population of VA-ECMO for cardiogenic shock or cardiac arrest were included. We excluded studies limited to patients in which ECMO was only used as a bridge to VAD or heart transplant, as such patients are, by definition, never “successfully weaned.” We used the Risk of Bias Assessment tool for Non-Randomized Studies. The study was registered on the International prospective register of systematic reviews (PROSPERO CRD42020178641).
Results
We screened 14,578 records and included 47 that met our pre-specified criteria. Signs of lower initial severity of shock and myocardial injury, early recovery of systemic perfusion, left and right ventricular recovery, hemodynamic and echocardiographic stability during flow reduction trial and/or pump-controlled retrograde trial off predicted successful weaning. The most widely used parameter was the left ventricular outflow tract velocity time integral, an indicator of stroke volume. Most studies had a moderate or high risk of bias. Heterogeneity in methods, timing, and conditions of measurements precluded any meta-analysis.
Conclusions
In adult patients on VA-ECMO for cardiogenic shock, multiple biomarkers, hemodynamic and echocardiographic parameters may be used to track resolution of systemic hypoperfusion and myocardial recovery in order to identify patients that can be successfully weaned.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04249-w.
Keywords: Cardiogenic shock, Extracorporeal membrane oxygenation, Extracorporeal life support, VA-ECMO, Adults, Biomarkers, Left ventricular function, Right ventricular function, Echocardiography, Hemodynamic parameters
Take-home message
In adult patients on VA-ECMO for cardiogenic shock, the following indices predicted successful weaning from VA-ECMO (survival after removal of ECMO without requirement for further mechanical support or heart transplant):
Lower severity of initial shock (MAP, lactates) and myocardial injury (Troponins).
Early recovery of systemic perfusion (lactate, liver enzymes, microcirculation).
Left ventricular recovery (LVEF > 20–25%, LVOT VTI > 10 cm, Mitral TDSa > 10 cm/s).
Right ventricular recovery (TAPSE ≥ 19 mm, RVEF ≥ 25%, Low RA/PCWP, High PAPi).
Stable MAP, CI, SBP without significant increase in inotropic support and stable or improved LV/RV function during flow reduction trial and PCRTO.
Most studies were observational, unblinded, retrospective and had small sample size and a moderate or high risk of bias.
Introduction
Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) can be used to restore organ perfusion in patients with cardiogenic shock [1, 2]. The device drains deoxygenated blood from a venous inflow cannula, drives it through a membrane lung and returns oxygenated blood in an arterial outflow cannula, providing both respiratory and cardiac support [3]. VA-ECMO can be used as a bridge to recovery, restoring and maintaining systemic perfusion while cardiac recovery occurs. In the absence of myocardial recovery, VA-ECMO may act as a bridge to durable ventricular assist device (VAD) implantation or heart transplant [4]. Successful weaning of VA-ECMO is generally defined as survival after complete removal of the extracorporeal circuit without requirement for further mechanical support or heart transplant [5–8]. Reported success rates range from 30 to 75% [5–9]. The timing of weaning is crucial as premature withdrawal may lead to recurrence of shock and cause secondary injury on barely recuperating organs. Conversely, longer duration of ECMO is associated with higher complications and in-hospital mortality [10, 11]. Identifying patients who are ready to be weaned off VA-ECMO may be challenging. The Extracorporeal Life Support Organization (ELSO) recommends that weaning be attempted in hemodynamically stable patients on low vasoactive support with the use of echocardiography to assess myocardial recovery [12–14]. Echocardiographic indices [15] are widely used to assess readiness to be weaned. Other parameters, such as biomarkers [16] or hemodynamic parameters [17] may also be used. Despite numerous descriptions of weaning protocols based on expert opinion in the literature [7, 18, 19], there has been no systematic reviews to provide more robust guidance. We aimed to systematically review the medical literature to determine the biomarkers, hemodynamic and echocardiographic parameters associated with successful weaning of VA-ECMO in adults with cardiogenic shock. Secondarily, we aimed to present an evidence-based weaning algorithm incorporating key findings to guide clinicians in the weaning process.
Methods
The protocol for this systematic review was registered on the International Prospective Register of Systematic Reviews (CRD42020178641). The results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Guidelines [20].
Study characteristics
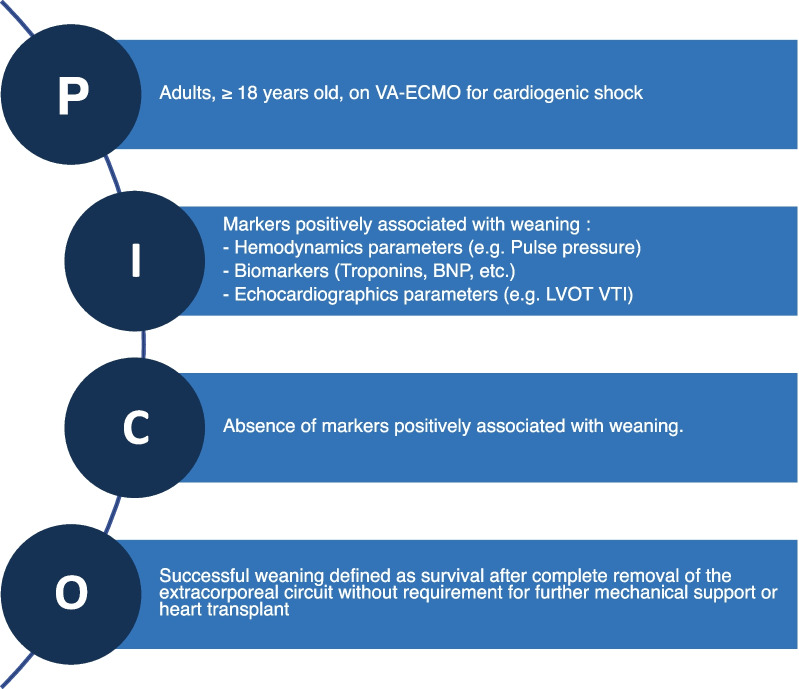
As we did not expect randomized controlled trials to be available on the subject, we elected to include both interventional and observational studies, including cohort studies and case-series ≥ 10 patients. We included records in all languages, both in full text or abstract-only formats, published from database inception to April 10, 2022. Studies had to report on the association between biomarkers, hemodynamic and echocardiographic parameters and VA-ECMO weaning success. Studies evaluating the association between therapies and weaning success were excluded. Studies evaluating exclusively the association of baseline parameters (before ECMO initiation) and weaning success were excluded. The PICO strategy is detailed in Fig. 1.
Fig. 1.
Search strategy. NP brain natriuretic peptide, C comparison, I intervention, LVOT VTI left ventricular outflow tract velocity–time integral, O outcome, P population
Participants
We selected studies that included adults with cardiogenic shock secondary to potentially reversible etiologies treated with VA-ECMO. We excluded studies limited to extracorporeal cardiopulmonary resuscitation (ECPR) as the neurological prognosis may significantly alter the decision-making process surrounding the device removal in this patient population. Studies with a mixed population of VA-ECMO for cardiogenic shock or cardiac arrest were included. We excluded studies limited to patients in which ECMO was only used as a bridge to VAD or heart transplant, as such patients are, by definition, never “successfully weaned.”
Search strategy
The search strategy is detailed in Additional file 1: Table S2. We searched Medline and Embase for studies including the following concepts: VA-ECMO, ECMO, extracorporeal life support (ECLS), ECPR, cardiogenic shock, weaning, success, decannulation. ProQuest, Google Scholar and Grey literature (OpenGrey, GreyLit, GreyNet) were also searched using key search terms. Backward and forward citation tracking was performed using Web of Science. Conference abstracts indexed in Embase and these other sources were eligible for inclusion. The searches were rerun prior to the final analyses (April 10, 2022) and further studies retrieved for inclusion.
Study selection process
Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used for the selection process. Two independent members of the review team (FC and EB or OL or KC) first screened the citations using only titles and abstracts and assessed the full texts for eligibility. Conflicts were resolved by consensus by the corresponding author (YAC).
Data extraction and synthesis
Study design, setting, country, period, sample size, funding source, VA-ECMO indication, weaning protocol, successful weaning definition and reported value, biomarkers, hemodynamic and echocardiographic data as well as effect measures between exposition and outcome were extracted independently by authors on electronic data collection forms (Covidence software). Missing data were presented as not reported in Table 1. The primary outcome was weaning success, defined as survival after complete removal of the extracorporeal circuit without requirement for further mechanical support or heart transplant. We planned to present a narrative synthesis of findings and a meta-analysis of the diagnostic accuracy of parameters to predict a successful weaning if they had been studied by multiple groups under similar conditions.
Table 1.
Characteristics of included studies
| References | Country; study period |
Design; setting; sample size |
Indication for VA-ECMO (%) | Weaning protocol | Parameters measured: (B) biomarkers, (H) hemodynamic, (E) echocardiographic | Successful weaning definition | Weaning success (%) (as reported by authors) |
|---|---|---|---|---|---|---|---|
| Aissaoui et al. 2011 [15] |
France; 2007–2008 |
Prospective cohort; single center; n=38 |
CMP (47%) FM (6%) Post-cardiotomy shock (22%) Post-transplantation (10%) Other (16%) |
66% flow (15 min) 33% flow or 1–1.5 L/min (15 min) Return to 100% if unstable any level If stable minimal flow, VTI > 10 cm, LVEF > 20–25%, circuit clamp and decannulation |
(B) ABG, Lact, Creat; (H) MAP, SBP/DBP, PAP; (E) LVEF, LVOT VTI, TD E, Sa, Ea, E/Ea, RA/RV size |
ECMO removal and no further MCS because of recurring CS over the following 30 days | 20/38 = 53% |
| Aissaoui et al. 2012 [52] |
France; 2007 |
Prospective cohort; single center; n=22 |
CMP (50%) Post-cardiotomy shock (32%) FM (4.5%) Post-transplantation (4.5%) Other (9%) |
See Aissaoui 2011 |
(B) N/A; (H) MAP, SBP, DBP, HR; (E) LVOT VTI, LVEDV, LVEF, TD E VVI: TD Sa, Ea, Sv, strain, strain rate |
ECMO removal and no further MCS because of recurring CS over the following 30 days | 11/22 = 50% |
| Aissaoui et al. 2017 [55] |
France; 2007–2008 |
Prospective study; single center; n=33 |
See Aissaoui 2012 | See Aissaoui 2011 |
(B) Creat, pH, Lact; (H) N/A; (E) LV/RV size, LVEF, LVOT VTI, TD E, Ea, Sa, RVEF, MR, TR |
ECMO removal and no further MCS because of recurring CS over the following 30 days | 16/33 = 48% |
| Akin et al. [45] |
Netherlands; 2014–2016 |
Prospective cohort; single center; n=13 |
PE (38%) Post-cardiotomy shock (23%) CS post-AMI (15%) Myocarditis (15%) Intoxication (8%) |
50% flow If stable, VTI > 10 cm, LVEF > 20–25% at minimal flow, decannulation |
(B) Lact, S/L circulation (TVD, PVD, PPV); (H) MAP; (E) LVOT VTI, LVEF, TAPSE, TDSa |
Successful VA-ECMO explantation within 48 h | 10/13 = 77% |
| Asaumi et al. [29] |
Japan; 1993–2001 |
Retrospective cohort; single center; n=14 |
Fulminant myocarditis (100%) |
LVETc improved to > 200 ms, ECMO flow rate decreased until 1.5 L/min If stable, decannulation |
(B) CK, CK-MB, WBC, CRP, AST/ALT, Creat, BUN; (H) CI, PCWP, RAP; (E) LVETc, LVESD, LVEDD, LVWT, FS, MR, TR |
ECMO removal | 10/14 = 71% |
| Cavarocchi et al. [7] |
USA; 2011–2012 |
Prospective cohort; single center; n=21 |
CMP (48%) Myocarditis (14%) CS post-AMI (14%) Post-cardiotomy (10%) PE (5%) |
50% flow by 0.5 L/min decrement Volume challenge Minimal flow: 1–1.5 L/min Return 100% if distension any level Decannulation if adequate Biventricular function |
(B) N/A; (H) N/A; (E) Qualitative (hTEE): LV/RV function and size, LV FAC |
ECMO removal | 14/21 = 67% |
| Chen et al. [5] |
Taiwan; NR |
Retrospective cohort; single center; n=57 |
NR |
Gradual flow reduction Return to 100% if inotrope increase above predefined dose If stable, decannulation |
(B) CK, CK-MB, Troponins, Bun, Creat, AST, RNI, CBC; (H) N/A; (E) N/A |
Weaning from ECMO and survival beyond 48 h | 38/57 = 67% |
| Chommeloux et al. [44] |
France; NR |
Prospective cohort; single center; n=14 |
CS post-AMI (50%) CMP (28%) Graft failure (14%) FM (7%) |
NR |
(B) Lact, S/L circulation: SVD, PSVD, PPV, MFI, HI; (H) MAP, HR; (E) LVEF, VTI |
ECMO removal | 6/14 = 43% |
| Colombo et al. [22] |
Italy; 2013–2017 |
Retrospective cohort; single center; n=25 |
CPR (71%) CS post-AMI (17%) Myocarditis (7%) PE (4%) Tako-tsubo (2%) Intoxication (2%) |
First weaning trial at 48 h no additional details |
(B) N/A; (H) SV, CO; (E) LV t-IVT, LVEF, LVEDD, MAPSE |
Device removal without requirement for re-cannulation over the following 30 days | 18/25 = 72% |
| Elena Puerto et al. [59] |
Spain; NR |
Retrospective cohort; NR; n=87 |
NR | NR |
(B) N/A; (H) N/A; (E) RV dysfunction, RV basal diameter |
NR | NR |
| Frederiksen et al. [56] |
Denmark; NR |
Cohort; single center; n=15 |
NR | Stable and VTI > 7 cm no additional details |
(B) N/A; (H) N/A; (E) LVOT VTI, LVEF, TD S', TAPSE |
ECMO weaning and being alive 24 h later without hemodynamic MCS | 15/29 = 52% |
| Fried et al. [37] |
USA; 2008–2018 |
Retrospective cohort; Single center; n=126 |
CS post-AMI (100%) | Daily flow reduction to 1L/min once on low-dose inotrope. Hemodynamic and echocardiographic follow up to decide decannulation |
(B) Lactates, Create, peak CK; (H) MAP; (E) LVEF |
Ventricular recovery defined as survival to discharge without durable LVAD or HT | 39/126 = 31% |
| Gambaro et al. [57] |
Italy; NR |
Prospective cohort; single center; n=14 |
NR | NR |
(B) N/A; (H) HR, MAP, CO, SV; (E) CSt/LS (LV, by STE) LVEF, LVOT VTI |
ECMO weaning without adverse outcome within 1 year (MCS, transplant, CV death) | N/A |
| Gonzalez Martin et al. [27] |
Spain; 2013–2020 |
Cohort; Single center; n=85 |
CS (47%) ECPR (9%) Electrical storm (9%) Post-cardiotomy CS (33%) Other (1%) |
NR |
(B) N/A; (H) N/A; (E) LVEDD, LVEF, LVOT VTO, RV basal diameter, RV qualitative function, 1:1 aortic valve aperture |
Survival > 24 h after explant and no mortality from cardiogenic shock/heart failure or cardiac arrest during admission | 52/85 = 61% |
| Hsu et al. [39] |
Taiwan; NR |
Cohort; single center; n=133 |
NR |
Flow reduction trial (< 1.5L/min) If tolerated, ECMO removal |
(B) ABG, Lact, Bic; (H) SBP/DBP, CVP, SVO2; (E) LVEF |
ECMO removal and survival to discharge | 73/133 = 55% |
| Huang et al. [61] |
Taiwan; 2014–2015 |
Retrospective cohort; single center; n=46 |
NR |
Weaning trial when stable Flow reduction to 0.5 L/min (5 min) If tolerated, circuit clamp and decannulation |
(B) N/A; (H) HR, CVP, SV (LV/RV); (E) LVEF, CSt/LS (LV), LV size, MR, RVEF, RV FAC, GLS (RV), RV size, TAPSE, TR |
ECMO removal and no mortality and/or MCS because of recurring CD over the following 48 h | 28/46 = 61% |
| Joseph et al. [49] |
USA; NR |
Retrospective cohort; single center; n=30 |
NR | NR |
(B) N/A; (H) RA/PCWP, TPG, PAPi; (E) LVEF, LVEDD, FS |
NR | NR |
| Kim et al. 2021 (JASE) [58] |
South Korea; 2016–2018 |
Prospective cohort; multicenter; n=92 |
CS post-AMI (48%) Ischemic cardiomyopathy |
30–50% flow (15 min) If unstable back to previous flow |
(B) N/A; (H) N/A; (E) LVEF, Mitral E/A, Mitral TDI (S' e' a'), LVOT VTI, RVFAC, TAPSE, Tricuspid TDI (S') |
ECMO removal and not requiring further MCS over the following 30 days | 64/92 = 70% |
| Kim et al. 2021 (JACC-imaging) [60] |
South Korea; 2016–2019 |
Prospective cohort; single center; n=79 |
Post-MI CMP (52%) Idiopathic dilated CMP (18%) Fulminant myocarditis (4%) Stress-induced CMP (4%) |
If HD stable with low/no vasopressor support, MAP ≥ 65 mmHg, lactate < 2 mmol/L, CVP ≤ 15 mmHg, then gradual weaning |
(B) N/A; (H) N/A; (E) Tricuspid annular S′/RVSP RVFAC/RVSP TAPSE/RVSP [RV FWLS]/RVSP |
Successful removal of VA-ECMO and no further mechanical circulatory support in the following 30 days | 50/79 = 63% |
| Li et al. [16] |
China; 2011–2012 |
Retrospective cohort; single center; n=123 |
Post-cardiotomy shock |
Gradual flow reduction to 1 L/min If stable, decannulation |
(B) Lact, Lact clearance; (H) N/A; (E) N/A |
ECMO removal and no HD deterioration within 48 h after | 69/123 = 56% |
| Lim et al. [48] |
South Korea; 2010–2018 |
Cohort; NR; n=122 |
NR | NR |
(B) N/A; (H) HR, MAP, PP; (E) LVEF, LVOT VTI, TDSa |
NR | 72/122 = 59% |
| Ling et al. [63] |
China; 2010 |
Observational study; single center; n=30 |
Post-cardiotomy shock (57%) Myocarditis (14%) CMP (29%) |
Reduce speed to target retrograde flow of 0.5–1 L/min and Sweep gas off (1 h) If tolerated, decannulation |
(B) N/A; (H) PCRTO; (E) N/A |
N/A | 7/7 decannulated = 100% |
| Luyt et al. [23] |
France; 2009–2010 |
Prospective cohort; single center; n=41 |
CS post-AMI (27%) Myocarditis (17%) Post-cardiotomy (15%) Graft failure (17%) Septic shock (10%) CPR (7%) Rhythm disturbance (7%) |
66% flow (15 min) 33% flow or minimum of 1–1.5 L/min (15 min) If unstable, return to 100% flow If stable minimal flow, LVEF > 20–25%, VTI > 12 cm, Mitral systolic velocity > 6 cm Decannulation |
(B) NT-proBNP, MR-proANP, proADM, Copeptin, TNIc; (H) N/A; (E) N/A |
ECMO removal and survival without MCS for > 30 days | 18/41 = 44% |
| Matsumoto et al. [30] |
Japan; 1995–2014 |
Retrospective cohort; single center; n=37 |
Myocarditis (100%) |
Weaning trial when LVETc > 200 ms Gradual flow reduction to 1.5 L/min If stable, decannulation |
(B) CK, CK-MB, ABG, Lact, Bun, Creat, Bili; (H) HR, MAP; (E) LVEF, LV size, LVPWT |
ECMO removal | 22/37 = 59% |
| Mazet et al. [24] |
France; 2014–2016 |
Cohort; single center; n=31 |
CS (71%) CPR (29%) |
Gradual decrease to < 2 L/min (60 min) If stable, decannulation |
(B) Lact; (H) N/A; (E) LVEF |
NR | NR |
| Mongkolpun et al. [36] |
Belgium; NR |
Cohort; NR; n=22 |
CS post-AMI (64%) Post-cardiotomy (14%) Myocarditis (14%) PE (8%) |
Gradual flow 1 L/min If VTI > 10 cm, decannulation |
(B) Lact, SBF; (H) MAP, CI, SVO2; (E) N/A |
ECMO removal and HD Stabilization without the need to increase the vasopressor dose within 24 h |
12/22 = 55% |
| Morisawa et al. [38] |
Japan; 2006–2008 |
Retrospective cohort; single center; n=29 |
CS post-AMI (100%) | NR |
(B) BE; (H) HR, Peak BP, SVO2; (E) LVEF |
ECMO removal and survival for more than one month | 15/29 = 52% |
| Mork et al. [25] |
Denmark; 2017–2019 |
Prospective cohort; single center; n=38 |
CPR (61%) Heart failure (5%) PE (5%) Post-cardiotomy shock (11%) CS (16%) |
66% flow (5 min) 33% flow (1–2 h) If stable, decannulation If unstable any level, back to full flow |
(B) N/A; (H) N/A; (E) LVEF, LVOT VTI, TAPSE, Mitral S' |
ECMO removal and survival without MCS for > 24 h | 25/38 = 66% |
| Moury et al. [68] |
France; 2018–2019 |
Prospective cohort; single center; n=15 |
Post-cardiotomy shock (60%) AMI (40%) |
Weaning from ECMO was performed if no onset of a new respiratory, neurologic, or cardiovascular failure was clinically assessed |
(B) N/A; (H) N/A; (E) Diaphragm thickening fraction (TF) LVEF |
ECMO weaning failure was defined by the death of the patient while being treated with assistance, the need for heart transplantation, and the need for an LVAD | 9/15 = 60% |
| Naruke et al. 2010 [50] |
Japan; 1996–2008 |
Retrospective cohort; single center; n=25 |
Myocarditis (52%) CS post-AMI (36%) ACHF (12%) |
Gradual flow reduction to 1.0 L/min If stable, decannulation |
(B) CK, BNP, Creat, CRP; (H) HR, MAP, PAP, PCWP, CVP, CI, ETCO2; (E) LVET, LVEF |
ECMO weaning | 18/25 = 72% |
| Naruke et al. 2012 [42] |
Japan; NR |
Cohort; NR; n=30 |
NR | NR |
(B) N/A; (H) SVO2, ETCO2; (E) N/A |
VA-ECMO weaned off without severely deteriorated cardiac output indicated by ETCO2 < 10 mmHg or LVET < 100 ms | 19/30 = 63% |
| North et al. 2018 [46] |
USA; 2012–2017 |
Retrospective cohort; single center; n=60 |
NR | Gradual flow reduction to 0.5–1.5 L/min (10 min). If stable according to precise criteria’s, decannulation |
(B) N/A; (H) MAP, CI, CVP, PA Systolic pressure, PA saturation; (E) LVEF |
Successful wean was defined by the following parameters: MAP > 60 mmHg; cardiac index > 2.2 L/min; CVP ≤ 16 mmHg; and EF ≥ 20% on low doses of inotropes or/and pressors followed by decannulation | 42/60 = 70% |
| North et al. 2022 [31] |
USA; 2012–2019 |
Retrospective cohort; single center; n=62 |
CS post-AMI (100%) | Gradual flow reduction by 0.5–1 L decrement (1–2 min), with echocardiographic evaluation, until 0–0.5 L/min. Decannulation if MAP > 60 mmHg, cardiac index > 2.0, CVP⩽16 mmHg, and LVEF ⩾20% on low-dose inotrope |
(B) Troponin I, Creat, CK, AST, ALT, lact; (H) N/A; (E) LVEF |
ECMO removal without further mechanical circulatory support defined a successful weaning from ECMO | 45/62 = 73% |
| Omar et al. [33] |
USA; 2014–2018 |
Retrospective cohort; Single center; n=238 |
Arrhythmia (37%) MI (24%) HF (45%) PE (33%) Post-cardiotomy (25%) |
NR |
(B) Lact (baseline, 1,3,5, 10 days); (H) N/A; (E) N/A |
NR | 98/238 = 41% |
| Oshima et al. [35] |
Japan; 1997–2004 |
Retrospective cohort; single center; n=32 |
Post-cardiotomy (47%) PE (13%) CS post-AMI (9%) Myocarditis (9%) CMP (3%) |
Gradual flow reduction to 1.5–2 L/min If stable, decannulation |
(B) Lact; (H) N/A; (E) N/A |
ECMO removal and discharged from the ICU | 12/32 = 38% |
| Ouazani et al. [54] |
USA; NR |
Prospective cohort; single center; n=12 |
NR |
Removal considered when LVEF > 25% and VTI > 10 cm If unstable, weaning trial stopped |
(B) N/A; (H) N/A; (E) LVEF, LVOT VTI, TD SaL, SaS, EaL, EaS, LS (LV) |
ECMO removal without requiring any further MCS | 9/12 = 75% |
| Pappalardo et al. [9] |
Italy; 2008–2013 |
Observational study; single center; n=42 |
ECPR (29%) Post-cardiotomy (24%) CS post-AMI (14%) Arrhythmia (13%) PE (4%) Trauma (2%) |
Weaning by 0.5 L/min decrement every 6–24 h to 2 L/min If stable, decannulation |
(B) BNP, Bili, Creat, CRP; (H) MAP,SBP/DBP, HR, CI, PSP, PDP, PCWP, CVP, SVO2; (E) LVEF, LVEDD, LVOT VTI, TR, TD S tricuspid annulus, TAPSE, RVEDD |
ECMO removal | 49/129 = 38% |
| Park et al. [17] |
South Korea; 2009–2011 |
Retrospective cohort; single center; n=69 |
AMI (31.9%) Respiratory failure (18.8%) Sepsis (15.9%) PE (4.3%) Trauma (2.9%) |
Gradual flow reduction to 1 L/min/m2 If stable, decannulation |
(B) ABG, Creat, Hb; (H) SBP, MAP, mean PP; (E) N/A |
Survival for 48 h after weaning with mean systolic blood pressure > 90 mmHg | 27/69 = 39% |
| Sawada et al. [40] |
Japan; 2013–2017 |
Retrospective cohort; single center; n=50 |
CS post-AMI (54%) FM (24%) CMP (10%) other heart disease (12%) |
Weaning trial when stable Flow reduction to 1.5–2 L/min Then 0.5–1 L/min If unstable, return to full flow If stable, decannulation |
(B) pH, Bic, Lact, Bili, AST,ALT, Creat; (H) PAP, PADP, PCWP, RAP, SVO2; (E) LVETc, LVOT VTI, FS, LVEDD/SD |
ECMO removal and survival beyond 30 days without needs for further MCS | 24/50 = 48% |
| Sawamura et al. [43] |
Japan; 2000–2016 |
Retrospective cohort; multicenter; n=99 |
Myocarditis (100%) | NR |
(B) CK, BUN, Creat, AST/ALT, LDH, Bili; (H) N/A; (E) LVEF, LVEDD |
VA-ECMO decannulation and subsequent discharge | 46/99 = 46% |
| Sugiura et al. [32] |
Japan; 2012–2016 |
Retrospective cohort; multicenter; n=55 |
CS post-AMI (100%) |
Weaning trial when stable Flow reduction to 0.5–1.5 L/min If stable, decannulation |
(B) Lact, Creat, Bili; (H) SBP, MAP; (E) LVEF CE-CT LV wall enhancement |
ECMO removal | 28/55 = 51% |
| Suhr et al. [34] |
Germany; 2006–2017 |
Retrospective cohort; single center; n=258 |
NR | NR |
(B) Lactate 1, 6, 12, 24 and 36 h; (H) N/A; (E) N/A |
NR | 136/258 = 53% |
| Vuthoori et al./Heaney et al. [51] |
USA; NR |
Prospective cohort; single center; n=34 |
NR |
Weaning trial with close monitoring Flow reduction by 1 L/min decrements If stable, circuit clamped, decannulation |
(B) macrophage migration inhibitory factor; (H) CI; (E) LVEF, LV size |
ECMO removal and free from pharmacologic and MCS at 30 days post-explant | 8/34 = 24% |
| Wu et al. [47] |
Taiwan; 2003–2008 |
Retrospective cohort; single center; n=72 |
Post-cardiotomy shock (100%) |
Weaning trial when stable Gradual flow reduction to 1 L/min. If stable, decannulation |
(B) Creat; (H) MAP, SVO2, MPAP, MAP:MPAP ratio; (E) N/A |
ECMO removal | 41/72 = 57% |
| Xu et al. [28] |
China; 2019–2021 |
Retrospective cohort; single center; n=20 |
Myocarditis (27%) CMP (23%) Ischemic heart disease (21%) ECPR (19%) Other (10%) |
Weaning trial when HD stable, signs of cardiac and pulmonary recovery. Flow reduction to 1.5 L/min, If HD stable with low-dose inotrope, PCRTO begins, target—0.5–1 L/min for 30 min with arterial gas, HD and respiratory monitoring. Decannulation if successful |
(B) N/A; (H) HR, CVP, PAWP, MAP; (E) LVEDV, LVEF, LVOT VTI, Mitral TD e′ E and lat s′, GLS |
Patients who survived for 48 h after withdrawal and did not require ECMO assistance | 13/20 = 65% |
| Yi et al. [41] |
China; 2018–2020 |
Retrospective cohort; single center; n=24 |
NR | NR |
(B) Create, Lact; (H) MAP; (E) LVEF, LVOT VTI, Mitral TD lat s′, GLS |
NR | 16/24 = 67% |
| Yoshida et al. [26] |
Japan; 2002–2003 |
Cohort; single center; n=15 |
CS post-AMI (80%) CPR (20%) |
NR |
(B) ABG, Lact; (H) HR, MAP, CVP, MPAP, PCWP, SVO2, ETCO2; (E) N/A |
ECMO removal | 6/15 = 40% |
ABG arterial blood gas, ACHF acute on chronic heart failure, AMI acute myocardial infarction, ALT alanine aminotransferase, AST aspartate aminotransferase, BIC bicarbonate, BE base excess, Bili bilirubin, BP blood pressure, BNP brain natriuretic peptide, BUN blood urea nitrogen, CBC complete blood count, CI cardiac index, CK-MB creatine kinase MB, CMP cardiomyopathy, CO cardiac output, CPR cardiopulmonary resuscitation, CREAT creatinine, CRP c-reactive protein, CS cardiogenic shock, CSt circumferential strain, CV cardiovascular, CVP central venous pressure, DBP diastolic blood pressure, ECMO extracorporeal membranous oxygenation ETCO2 end-tidal CO2, e′ early diastolic peak mitral velocities, FAC fractional area change, FM fulminant myocarditis, FS fractional shortening, [FWLS] absolute value of free-wall longitudinal strain, GLS global longitudinal strain, HB hemoglobin, HD hemodynamic, HI heterogeneity index, hTEE hemodynamic transesophageal echocardiography, HR heart rate, INR international normalized ratio, L liter, Lact lactates, LDH lactate dehydrogenase, LS longitudinal strain, LV left ventricle, LVAD left ventricular assist device, LVEDV left ventricular end-diastolic volume, LVEF left ventricular ejection fraction, LVEDD left ventricular end-diastolic dimension, LVESD left ventricular end-systolic dimension, LVETc Left ventricle ejection time corrected, LVOT VTI left ventricular outflow tract velocity time integral, LVPWT left ventricle posterior wall thickness, LVWT left ventricle wall thickness, MAP mean arterial pressure, MAPSE mitral annular plane systolic excursion, MCS mechanical cardiac support, MFI microvascular flow index, MPAP mean pulmonary arterial pressure, MR mitral regurgitation, NR not reported, PA pulmonary artery, PAP pulmonary artery pressure, PAPi pulmonary artery pulsatility index, PCRTO pump-controlled retrograde trial off, PCWP pulmonary capillary wedge pressure, PDB pulmonary diastolic pressure, PE pulmonary embolism, PH pulmonary hypertension, PP pulse pressure, PPV percent perfused vessels, PSP pulmonary systolic pressure, PSVD perfused small vessel density, PVD perfused vessel density, RA right atrial, RAP right atrial pressure, RV right ventricle, RVEDD right ventricle end-diastolic dimension, RVEF right ventricular ejection fraction, RVSP right ventricular systolic pressure, SBF skin blood flow, SBP systolic blood pressure, S/L sublingual, SV stroke volume, SVD small vessel density, SVO2 mixed venous oxygen saturation, S′ systolic peak myocardial velocities, TAPSE tricuspid annular plane systolic excursion, TD tissue Doppler a’ late diastolic atrial contraction velocities E transmitral early peak velocities Ea/e′ lateral mitral annulus early diastolic velocities Sa lateral mitral annulus peak systolic velocities Sv systolic peak velocity, t-IVT total isovolumic time, TNI troponin I, TPG transpulmonary gradient, TR tricuspid regurgitation, VV veno-venous, TVD total vessel density, V-A-V veno-arterio-venous, VTI velocity time integral, VVI velocity vector imaging, WBC white blood count
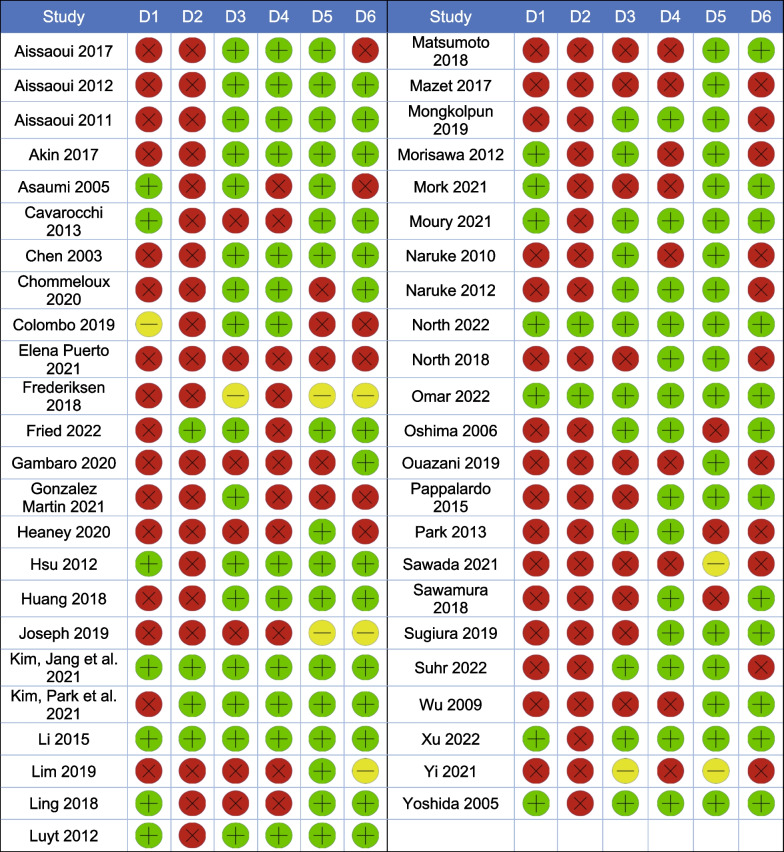
Quality assessment and risk of bias
The quality assessment of all retained articles was performed by two independent reviewers (FC and EB or OL or KC), with conflicts resolved by consensus by the corresponding author (YAC), using the Cochrane’s Risk of Bias Assessment tool for Non-randomized Studies [21].
Results
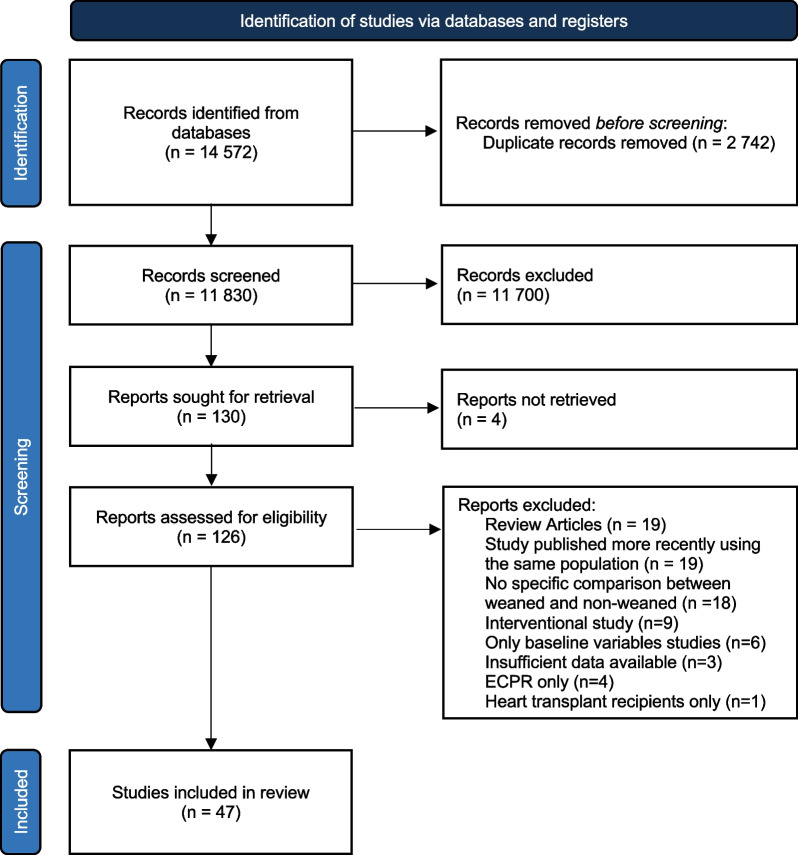
Study selection and characteristics
The search strategy yielded a total of 14,578 records, including 2742 duplicates that were removed. The remaining 11,830 were screened by title and abstract. We reviewed the full text of 130 records to assess eligibility, 47 of which studies were finally selected (Fig. 2 and Table 1). Study sample size ranged from 12 to 258 patients. The main indications for ECMO were cardiogenic shock secondary to acute myocardial infarction (AMI), fulminant myocarditis, other acute cardiomyopathies, post-cardiotomy shock and cardiac arrest. Eight studies included some cardiac arrest patients in their case-mix [9, 22–28]. Specific etiology of cardiac arrest was not specified in most studies. The risk of bias of the included records can be found in Table 2. Fifteen studies were published conference abstracts that did not provide detailed protocols, greatly limiting our methodological assessment. Most studies presented a high risk of bias overall.
Fig. 2.
PRISMA flowchart. ECPR Extracorporeal cardiopulmonary resuscitation
Table 2.
Risk of bias of the included studies
D1 Selection of participants D2 Confounding variables D3 Measurement of exposure D4 Blinding of outcome assessment D5 Incomplete outcome data D6 Selective outcome reporting. Green Low risk of bias Yellow Unclear risk of bias Red High risk of bias
Main findings: parameters to predict weaning success
Selected records reported on the use of various parameters to predict successful weaning, including biomarkers, microcirculation indices, hemodynamic, respiratory and echocardiographic parameters. Individual study findings are detailed in Table 1 and summarized below. Heterogeneity in methods, timing and conditions of measurements precluded any meta-analysis.
Biomarkers
Cardiac injury
Elevation in biomarkers reflecting the severity of myocardial injury was associated with adverse weaning outcomes in most studies. Lower peak CK-MB was associated with higher weaning success and better initial systolic function in patients with myocarditis or cardiac arrest [5, 29, 30]. Peak CK-MB < 183 U/L predicted weaning success with a sensitivity of 86%, a specificity of 71% and an area under the receiving operator characteristic curve (AUROC) of 0.89 [CI95: 0.77–1.00] [30]. Peak troponins were associated with weaning success in AMI patients (p = 0.003) [31], but not in a cohort of patients with refractory CS of mixed etiologies where AMI represented only 27% of the cohort [23]. Several other biomarkers including NT-proBNP failed to demonstrate any predictive value for successful weaning [23].
Oxygen delivery
Early correction of biomarkers reflecting tissue hypoperfusion appeared to predict successful weaning. Blood lactate levels at 24 h of support were independently associated with successful weaning in patients with cardiac arrest caused by AMI cannulated during or after the arrest (OR 0.52, p = 0.018) [32–34]. Lactate clearance in the first 12 h similarly predicted weaning outcomes in post-cardiotomy VA-ECMO (AUROC 0.72; OR 0.3; p = 0.023) [16, 34]. However, initial [5, 16, 26, 30, 32, 33, 35–38] and pre-weaning [23, 39–41] lactate values were inconsistently associated with weaning success. We also found conflicting data concerning the association between mixed venous oxygen saturation (SvO2) and VA-ECMO weaning success. Hsu et al. found a higher pre-weaning SvO2 in patients that survived compared to those that died following weaning [39]. Yoshida et al. found no difference in the mean SvO2 while on VA-ECMO in weaned vs non-weaned patients [26]. Finally, Naruke et al. reported that patients with sustained SvO2 < 75% during VA-ECMO had a higher rate of weaning success (88 vs 47% p < 0.01) compared to patients that experienced periods of SvO2 > 75% [42].
Organ damage
Similarly, signs of persistent organ damage are adversely linked to weaning outcomes. Higher aspartate aminotransferase at 48 h and 72 h after initiation of ECMO was associated with weaning failure [5, 43].
Microcirculation
Multiple studies evaluated the use of microcirculatory parameters in VA-ECMO. During the first 48 h of support, successfully weaned patients showed a significantly higher perfused small vessel density (p = 0.002), small vessel density (p = 0.008) and percent perfused vessels (p = 0.02) [44]. Before first weaning attempt, higher skin blood flow (≥ 34 perfusion units) measured by skin laser Doppler was also found to accurately predict weaning success (AUROC = 0.93 [CI95: 0.81–1]; sensitivity 83%; and specificity 92%) [36]. During an extracorporeal blood flow (ECBF) reduction trial (50% of initial ECBF), total vessel density and perfused vessel density displayed an AUROC of 0.99 and 0.91, respectively, for the prediction of successful weaning. In two of these studies, microcirculatory indices outperformed commonly used echocardiographic parameters such as left ventricular ejection fraction (LVEF) and left ventricular outflow tract (LVOT) velocity time integral (VTI) [36, 45].
Macrocirculation
Better early hemodynamic parameters as well as their maintenance during the weaning phase appear to predict weaning success [46]. Higher mean arterial pressure (MAP) and MAP to pulmonary artery pressure ratio at 24 h were associated with successful weaning [32, 47]. Over the first 6 h of extracorporeal support, a pulse pressure (PP) < 30 mmHg, reflecting reduced residual LV ejection, was found to be independently associated with weaning failure (OR: 0.95, Log rank p < 0.001) [17]. MAP at time of weaning was independently associated with weaning success (OR: 1.05, p = 0.009) [41, 48]. In a cohort of weaned patients, MAP, cardiac index and PP increased significantly from pre-ECMO to weaning despite a reduction in inotropic support. Moreover, compared to patients who died after weaning, patients who survived to ICU discharge had a higher systolic blood pressure (120 [112–140] vs 103 [99–125] mmHg, p = 0.04) despite a lower inotropic score [9] and a lower central venous pressure (CVP) [39]. Successfully weaned patients also presented more favorable right ventricular (RV) hemodynamic parameters at 48 and 72 h: lower right atrial to pulmonary capillary wedge pressure (PCWP), lower transpulmonary gradient and higher pulmonary artery pulsatility index [49].
End-tidal CO2
In patients on VA-ECMO, end-tidal CO2 (EtCO2) is primarily determined by transpulmonary blood flow generated by the native heart. It may thus be used to monitor native cardiac output in this context (Table 3). In a cohort of 37 patients on VA-ECMO, an increase in EtCO2 of 5 mmHg or more above previous mean values during two consecutive 12-h periods occurred in all successfully weaned patients and in none of the patients that could not be successfully weaned [50]. This inflection point in EtCO2 preceded cardiac index increase. Weaned patients were also found to have a higher absolute EtCO2 value at 24 h of extracorporeal support. Their average EtCO2 increased from 9 mmHg immediately post-cannulation to 21 mmHg at 24 h (p = 0.04) [26].
Table 3.
Parameters associated with weaning success
| Parameters | Definition | Reported performance | Advantages (A)/Disadvantages (D) | References |
|---|---|---|---|---|
| EtCO2 |
Partial pressure of CO2 in the gas mixture at the end of exhaled breath. Under constant ventilator settings, its main determinant is transpulmonary flow from the native heart |
Higher values at 24 h in weaned patients; Rapid rise in EtCO2 preceded changes in hemodynamic monitoring and cardiac index; Increase of ≥ 5 mmHg above previous mean values during two consecutive 12-h periods associated with weaning and preceded native cardiac output recovery. |
(A) Noninvasive alternative to thermodilution cardiac catheter; (D) Elimination of CO2 may depend on pulmonary dead-space, which is increased after CPR, thus reducing ETCO2 level; (D) May vary with change in ventilator settings. |
Naruke 2010 [50], Yoshida[26] |
| LVEF | Relation between the amount of blood expelled during each cardiac cycle relative to the size of the ventricle |
Greater increase (> 5%) in the first 48 h and significant improvement from cannulation to weaning associated with weaning; LVEF > 20–25% reported before attempting weaning trial. |
(A) Direct marker of systolic function; (D) Load-dependent; (D) Absolute value at weaning inconsistently predicted weaning success. |
Aissaoui 2011 [15], Aissaoui 2012 [52], Aissaoui 2017 [55], Akin [45], Asaumi [29], Colombo [22], Gambaro [57], Mazet [24], Ouazani [54], Pappalardo [9], Sawamura [43], Sugiura [32], Vuthoori [51],Yi [41] |
| LVOT VTI | Velocity time integral (VTI) of a pulsed wave Doppler in the left ventricular outflow tract (LVOT) is directly proportional to the stroke volume of the native heart |
Improvement from cannulation to weaning, values above 8.5 cm at weaning associated with success; Threshold of 10 cm reported before attempting weaning trials. |
(A) Direct marker of systolic function; (D) Load-dependent. |
Aissaoui 2011 [15], Aissaoui 2012 [52], Aissaoui 2017 [55], Colombo [22], Frederiksen [56], Gambaro [57], Gonzalez Martin [27], Lim [48], Mongkolpun [36], Ouazani [54], Sawada [40], Sawamura [43], Yi [41] |
| TDI | Tissue Doppler velocity imaging (TDI) is a signal which correlates with myocardial motion. TDI is placed on mitral/tricuspid annulus to evaluate longitudinal systolic function |
Mitral systolic velocities (Sa) higher in weaned patients both at maximal and minimal (> 6 cm/s) VA-ECMO flow; Any improvement in lateral mitral e' velocity and/or > 10% improvement in tricuspid annular S′ velocity during flow reduction trial is an independent predictor of weaning success. |
(A) Load-independent, making it useful to guide the weaning process; (A) Better predictive performance than conventional parameters for weaning success; (D) Angle-dependent for valid measurement, interobserver variability. |
Aissaoui 2011 [15], Aissaoui 2012 [52], Frederiksen [56], Mork [25], Ouazani [54], Yi [41] |
| TAPSE | Tricuspid Annular Plane Systolic Excursion (TAPSE) is a M-mode derived marker of longitudinal right ventricular function | Higher at full flow (15 mm) in succesfully weaned patients. |
(D) Limited data; (D) Angle dependency. |
Frederiksen [56], Mork [25] |
| RVEF | Relation between the amount of blood expelled during each cardiac cycle relative to the size of the ventricle |
3D derived RVEF > 24.6% at first intent of decannulation associated with weaning; Higher RVEF in patients without ventricular interdependence during weaning trial. |
(A) Direct marker of systolic function; (D) Load-dependent; (D) 3D Echo is time consuming and requires offline analysis without immediate assessment; (D) Two-dimensional measurement less reliable |
Aissaoui 2017 [55], Huang [61] |
| Ventricular interdependence (VI) | Phenomenon whereby the function of one ventricle is altered by changes in the filling of the other ventricle | Absence of VI (Dep-) on the last day before weaning predicted successful weaning. | (A) Highlight ventricular response to load variation. | Aissaoui 2017 [55] |
Echocardiographic assessment
LV function
Early recovery of LVEF after VA-ECMO initiation has been associated with improved weaning outcomes. LVEF significantly increased after cannulation in weaned patients (from baseline to 24 h: + 8.5%, p = 0.012; from 24 to 48 h, + 9.0%, p = 0.001) [32]. At 48 h of support, both the absolute LVEF (OR 1.11 [1.01–1.22]; p = 0.03) and LVEF change from baseline (OR 1.15 [1.01–1.31]; p = 0.03) were independently associated with weaning success [43]. Multiple studies similarly showed a higher LVEF and fractional shortening (FS) [29, 31, 41] as well as lower LV chamber sizes in successfully weaned patients compared to non-weaned patients [39, 51, 52]. Others found LVEF improvement from cannulation to weaning, without significant absolute differences in LVEF values in weaned vs non-weaned patients [9, 22, 53, 54].
LVOT VTI is the most widely used parameter to track LV recovery in patients on VA-ECMO [15, 22, 27, 36, 40, 41, 48, 52, 54–57]. Successfully weaned patients tend to have a higher VTI at the time of weaning, reflecting a better stroke volume. The most commonly reported threshold to predict successful weaning is > 9.5 cm. The threshold itself and the conditions under which it is measured, especially the timing and the ECBF, vary significantly across studies, making comparisons difficult [15, 22, 36, 40, 48, 52, 54–57]. Two studies reported on the accuracy of the LVOT VTI to predict weaning success. Mongkolpun et al. found an AUROC of 0.85 [CI95: 0.65–1] [36] and Sawada et al. an AUROC of 0.74, with a sensitivity of 75% and a specificity of 72% at an optimal VTI threshold of > 8.6 cm [40]. The ratio of VTI from cannulation to weaning has also shown a strong association with successful weaning (OR, 2.80, p = 0.01) [27, 41, 48]. In two different studies, Aissaoui and colleagues reported a higher VTI in the group of successfully weaned patients compared to those who were not, both at maximal (10.5 vs 6.2 cm, p < 0.001) and minimal ECBF during weaning trials (12.8 vs 9.5 cm, p = 0.010 and 16.4 vs 8.5 cm, p < 0.0001) [15, 52]. The main limitation, Aissaoui found, is the load dependence of most LV function parameters, including LVEF, LVOT VTI, LV systolic velocity, strain and strain rate [52].
Tissue Doppler systolic velocities, which were also found to be higher in successfully weaned patients, are probably less load-dependent [15, 41, 52, 54]. Improvements in lateral e′ velocity and tricuspid annular S′ velocity during a flow reduction trial predicted weaning success with a better performance (AUROC for the presence of both parameters = 0.93 [CI95: 0.863–0.996]; p < 0.001) than conventional parameters, such as LVEF > 20–25%, LVOT VTI ≥ 10 cm and mitral annulus S′ ≥ 6 cm/s [58]. Total isovolumic time (t-IVT, Additional file 1: Table S1) improvement in the first 48 h of support was the strongest predictor of successful VA-ECMO weaning in another study [22]. Measured at an ECBF of 1.5 L/min, a corrected LV ejection time (LVETc) to PCWP ratio > 15.9 s/mmHg was a robust predictor of weaning success (AUROC = 0.82; sensitivity = 88%; specificity = 69%) [40].
RV function
Improvement in RV function during ECMO also appears to be associated with weaning success [59]. Pappalardo and colleagues observed a decrease in the prevalence of RV failure from 52% pre-ECMO to 36% during weaning in successfully weaned patients [9]. Successfully weaned patients exhibited a higher tricuspid plane systolic excursion at full ECBF (16 vs 8 mm, p = 0.02) [25, 56]. Tricuspid lateral annular S′/right ventricular systolic pressure > 0.33 was associated with successful weaning at full and minimal ECMO flow, performing better than conventional LV indexes, reflecting better RV coupling to pulmonary circulation [60]. Huang et al. also found the three-dimensional RV ejection fraction to be the strongest predictor of successful decannulation at first attempt (AUROC 0.90; CI95: 0.80–0.99) [61].
Biventricular function
In an unblinded study, qualitative echocardiographic assessment of biventricular recovery during a weaning trial had a positive predictive value of 100% [CI95: 73–100%] for successful weaning [7]. Interestingly, absence of ventricular interdependence was found to be a robust predictor of successful weaning, with sensitivity of 94% and specificity of 95% [55].
Weaning trial
Weaning protocols varied significantly across studies, as detailed in Table 1. Our main findings concerning weaning protocols reported in the literature are summarized below.
Criteria used to decide to submit patients to a weaning trial were not always explicitly reported and varied significantly. Most commonly used criteria included a MAP ≥ 60 mmHg and systemic arterial pulsatility on minimal inotropic and vasopressor support [5, 9, 15, 16, 23, 25, 32, 35, 40, 45, 47, 52, 54, 58, 61]. Another commonly stated condition was the resolution of shock as indicated, for instance, by lactate normalization [25, 40, 45, 58, 61], SVO2 > 65% [40, 45] or end-organ dysfunction recovery [7, 16, 40, 44]. Most authors used some form of myocardial improvement, globally [47] or a specific parameter such as LV FS [40], LVEF ≥ 20% and S′ > 6 cm/s [25], LVETc > 200 ms [29, 30, 40], LVOT VTI ≥ 10 cm [25, 56, 57] or TAPSE > 10 mm [25]. Other conditions included the absence of ventricular arrhythmia [47], optimized fluid balance [7, 25, 51] with a low CVP [51, 58, 61] and adequate native lung oxygenation capacity [7, 15, 23, 25, 32, 50, 52, 54] with resolution of pulmonary edema and an inspired oxygen fraction < 50% [7, 25].
In patients who met these criteria, a gradual ECBF reduction was usually advocated with close monitoring of hemodynamic, vasopressor needs and biventricular response to load variation. Even with a minimal ECBF, VA-ECMO still provides significant cardiorespiratory support. As such, some centers reported clamping the circuit in patients who remained stable at 0.5–1.5 L/min of ECBF. Others used a pump-controlled retrograde trial off (PCRTO) to challenge the native heart without the increased risk of thrombus formation associated with circuit clamping [62]. Pump speed was reduced in a controlled fashion until the flow became retrograde (− 0.5 to − 1 L/min). This was maintained for up to an hour, after which the test was considered successful if hemodynamic parameters, vasopressors needs and oxygen requirements remained stable. Ling and colleagues compared the outcomes of seven patients weaned after a successful PCRTO, to 23 patients weaned without PCRTO. The reported number of deaths due to cardiac failure in the PCRTO and conventional groups was 0 and 3, respectively (0 vs 13%, p = 0.99) [63]. Lower initial heart rate and PCWP measured by Swan-Ganz catheter at the start of PCRTO were associated with weaning success during this procedure [28].
Discussion
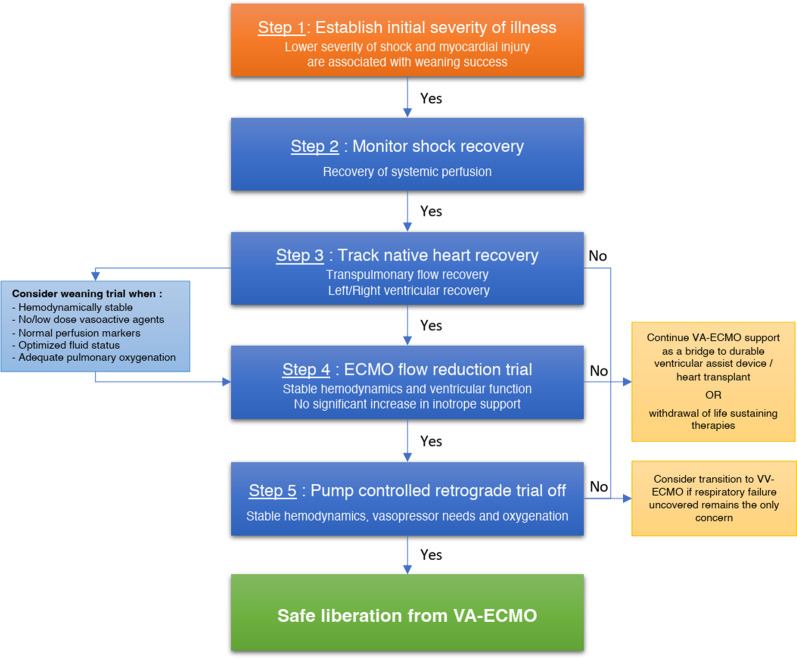
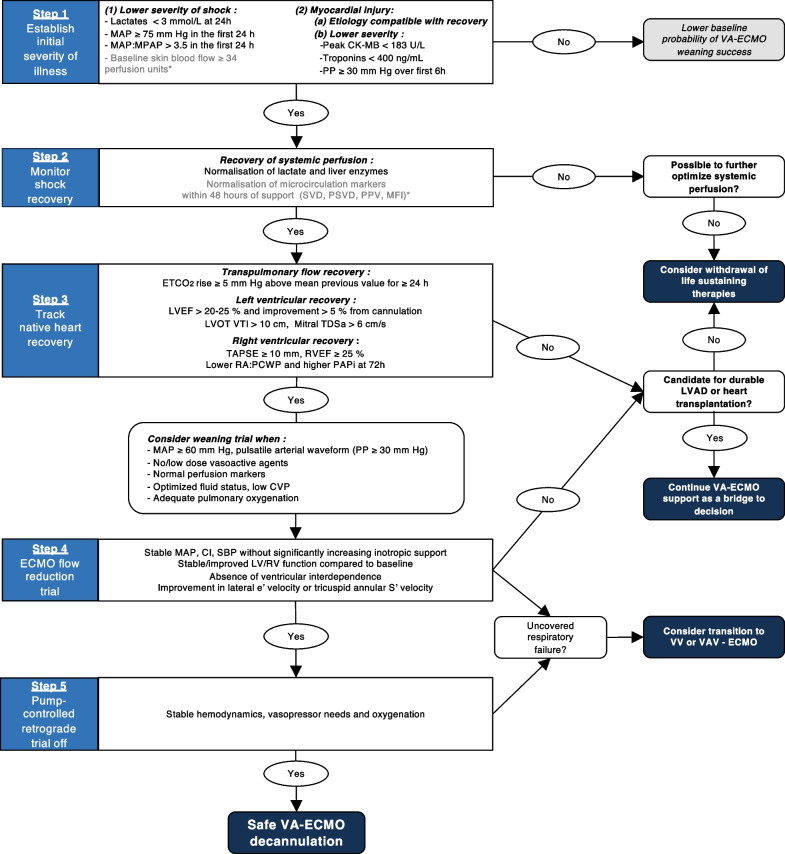
In this systematic review of the literature, we found multiple studies reporting on biomarkers, hemodynamic and echocardiographic parameters associated with successful weaning of VA-ECMO in adults. These were mainly small observational studies with a relatively high risk of bias. They nevertheless allowed to draw some important points to guide the weaning process. First, the initial severity of shock and myocardial injury may help establish baseline prognosis. Second, rapid recovery of systemic perfusion is associated with weaning success. Third, signs of native heart recovery are strongly associated with weaning success. Finally, in patients that show signs of native heart recovery, readiness to be weaned can be further assessed with a flow reduction trial, with or without a PCRTO (Fig. 3).
Fig. 3.
VA-ECMO weaning algorithm. CK-MB creatine kinase MB, CI cardiac index, CVP central venous pressure, ECMO extracorporeal membrane oxygenation, ETCO2 end-tidal CO2, e′ early diastolic peak myocardial velocities, LV left ventricle, LVAD left ventricular assist device, LVEF left ventricular ejection fraction, LVOT VTI left ventricular outflow tract velocity time integral, MAP mean arterial pressure, MPAP mean pulmonary arterial pressure, MFI microvascular flow index, PAPi pulmonary artery pulsatility index, PCWP pulmonary capillary wedge pressure, PH pulmonary hypertension, PP pulse pressure, PPV percent perfused vessels, PSVD perfused small vessel density, RA right atrial, RV right ventricle, RVEF right ventricular ejection fraction, SBP systolic blood pressure, SVD small vessel density, S′ velocity systolic peak myocardial velocities, TAPSE tricuspid annular plane systolic excursion, TDSa systolic tissue Doppler imaging septal mitral annulus, TPG transpulmonary gradient, VV veno-venous, V-A-V veno-arterio-venous, *Investigational markers
Proposed evidenced-based algorithm for VA-ECMO weaning
Step 1: establishing initial illness severity
Initial severity of illness greatly impacts the odds of being weaned successfully from VA-ECMO. Although this may not directly help determine if a patient is ready to be weaned, it may help set expectations. For instance, a patient with very severe initial myocardial injury and limited organ dysfunction may be considered early for durable left ventricular assist device implantation or heart transplantation. Greater initial myocardial injury, reflected by higher CK-MB, troponins and lower initial systolic function are associated with weaning failure in multiple studies [5, 29–31]. At the time of ECMO initiation, parameters of shock severity such as lactate, end-organ perfusion markers, hemodynamics and extent of myocardial injury predict subsequent weaning and survival. In the first 24 h of support, weaned patients have a higher MAP and PP [17, 32, 47]. Microcirculation indices could also help refine prognostication in the future as they appear to strongly predict weaning outcomes early [36, 44] and independently of hemodynamic parameters, lactates and inotropic support [44, 64, 65].
Step 2: monitoring recovery of tissue and organ perfusion
If flow is adequately restored through VA-ECMO, one should expect rapid normalization of markers of hypoperfusion and end-organ damage. Studies showed increased rates of successful weaning with higher lactate clearance and AST normalization in the first 72 h [5, 24, 26, 32–35, 37, 38, 43]. Microcirculatory improvement in the first 48 h has also been associated with successful weaning [36, 44]. Persistence of markers of hypoperfusion should prompt clinicians to try to further improve blood flow by increasing extracorporeal circuit or native heart output, decreasing venous congestion, and seeking local causes of hypoperfusion that could be addressed (such as mesenteric ischemia). In the absence of adequate restoration of tissue perfusion under maximal support, irreversible organ injury usually ensues.
Step 3: assessing recovery of native heart function
Once tissue perfusion is restored, the next step is to assess native heart recovery. Under constant minute ventilation, an increase in EtCO2 reflects an increase in transpulmonary flow, a reliable marker of native cardiac output recovery that may be observed earlier than changes in hemodynamics [26, 50]. Higher LVOT VTI, both at minimal and maximal ECBF, is a widely used parameter of LV recovery with good predictive performance for weaning success [27, 48]. Weaned patients also tend to display higher LVEF, t-IVT, FS, MAPSE and mitral S′ velocity [25, 29, 40, 43, 53]. Echocardiographic (RV ejection fraction and TAPSE) [25, 59, 61] and hemodynamic (RA/PCWP ratio, TPG and PAPi) parameters of RV function are also strongly associated with weaning success [47, 49]. On full extracorporeal support, LV indices tend to underestimate true LV performance, while RV indices tend to overestimate true RV performance. Tissue Doppler systolic velocities are relatively load-independent [15, 52, 54, 58, 66, 67], making them interesting parameters to follow when patients are still supported with high ECBF.
Step 4: performing flow reduction trials
In stable patients with evidence of myocardial recovery, a trial of ECBF reduction is generally attempted prior to decannulation to assess the net hemodynamic effect of reducing the support provided by VA-ECMO. The effect of flow reduction on MAP, CVP and vasopressor-inotropic support is observed. If tolerated, echocardiographic evaluation may be performed. Better indices of LV systolic and diastolic function [7, 15, 51, 52, 55, 57], ventricular interdependence [55] and RV function [58] during flow reduction trials predict favorable weaning outcomes. Importantly, flow reduction may unmask significant underlying hypoxemic respiratory failure that may be associated with increased pulmonary vascular resistance [19].
Step 5: pump-controlled retrograde trial off (PCRTO)
Finally, PCRTO may be performed by reducing the centrifugal pump rotation speed until a retrograde flow occurs through the circuit. This creates a controlled arterio-venous fistula, with the blood being pumped through the ECMO circuit by the native heart, returning in the venous system. This significantly challenges the RV which has to accommodate a substantial preload increase. Thereby, it may simulate VA-ECMO decannulation more accurately than flow reduction and may last longer than circuit clamping, which is limited by the risk of clot formation. During retrograde flow, the sweep gas should be turned off to uncover any residual gas exchange impairment [63]. By reversing the flow, there is a theoretical risk of pulmonary embolism through clot detachment from the venous side of the oxygenator. More studies are needed to validate this method.
Strengths and limitations
This is the first systematic review on VA-ECMO weaning providing an exhaustive and detailed summary of the literature for clinicians and researchers. Our conclusions, however, are limited by the quality of the available literature. We included mostly unblinded observational retrospective studies with small sample sizes and a high risk of bias. Many of the reported parameters, including ETCO2, were studied in small cohorts of patients. This warrants cautious interpretation about their role in clinical practice. Also, the microcirculatory parameters are still in the investigative stage and deserve more validation before drawing conclusions that will influence VA-ECMO weaning guidance. Variability in weaning protocols and in the definition of successful weaning make interpretation and comparison between studies difficult. Most studies did not provide formal assessments of predictive accuracy of the proposed indices and only reported on associations. Selection bias was often present as patients who were not considered for weaning because of complications or futility were often not included. There was often significant residual confounding. These limitations underline the need for high-quality research in this area, more robust data on investigational markers and standardization of weaning protocols and successful VA-ECMO weaning definition.
Conclusion
We found a large number of studies, of generally low quality, reporting on parameters associated with successful weaning of VA-ECMO in adults. We propose a stepwise approach to weaning based on our findings. First, the initial severity of shock and myocardial injury may help establish baseline prognosis. Then, signs of reversal of tissue hypoperfusion and native heart recovery may help determine readiness to be weaned. Finally, careful assessment of the physiological response to extracorporeal blood flow reduction and/or reversal may allow identification of patients who are ready to be safely decannulated.
Supplementary Information
Additional file 1. Supplementary tables.
Acknowledgements
We would like to thank Monique Clar, librarian at Université de Montréal, for her help with the search strategy.
Abbreviations
- ABG
Arterial blood gas
- ACHF
Acute on chronic heart failure
- AMI
Acute myocardial infarction
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AUROC
Area under the receiving operator characteristic curve
- BIC
Bicarbonate
- BE
Base excess
- Bili
Bilirubin
- BP
Blood pressure
- BNP
Brain natriuretic peptide
- BUN
Blood urea nitrogen
- CBC
Complete blood count
- CE-CT
Contrast enhanced computed tomodensitometry
- CI
Cardiac index
- CK-MB
Creatine kinase MB
- CI95
95% confidence interval
- CMP
Cardiomyopathy
- CO
Cardiac output
- CREAT
Creatinine
- CS
Cardiogenic shock
- CSt
Circumferential strain
- CRP
C-reactive protein
- CPR
Cardiopulmonary resuscitation
- CV
Cardiovascular
- CVP
Central venous pressure
- DBP
Diastolic blood pressure
- ECBF
Extracorporeal blood flow
- ECLS
Extracorporeal life support
- ECMO
Extracorporeal membranous oxygenation
- ECPR
Extracorporeal cardiopulmonary resuscitation
- ELSO
Extracorporeal Life Support Organization
- ETCO2
End-tidal CO2
- e′
Early diastolic peak mitral velocities
- FAC
Fractional area change
- FM
Fulminant myocarditis
- FS
Fractional shortening
- [FWLS]
Absolute value of free-wall longitudinal strain
- GLS
Global longitudinal strain
- HB
Hemoglobin
- HD
Hemodynamic
- HI
Heterogeneity index
- hTEE
Hemodynamic transesophageal echocardiography
- HR
Heart rate
- INR
International normalized ratio
- L
Liter
- Lact
Lactates
- LDH
Lactate dehydrogenase
- LS
Longitudinal strain
- LV
Left ventricle
- LVAD
Left ventricular assist device
- LVEDV
Left ventricular end-diastolic volume
- LVEF
Left ventricular ejection fraction
- LVEDD
Left ventricular end-diastolic volume
- LVESD
Left ventricular end systolic volume
- LVETc
Left ventricle ejection time corrected
- LVOT VTI
Left ventricular outflow tract velocity time integral
- LVPWT
Left ventricle posterior wall thickness
- LVWT
Left ventricle wall thickness
- MAP
Mean arterial pressure
- MAPSE
Mitral annular plane systolic excursion
- MCS
Mechanical cardiac support
- MFI
Microvascular flow index
- MPAP
Mean pulmonary arterial pressure
- MR
Mitral regurgitation
- NR
Not reported
- PA
Pulmonary artery
- PAP
Pulmonary artery pressure
- PAPi
Pulmonary artery pulsatility index
- PCRTO
Pump-controlled retrograde trial off
- PCWP
Pulmonary capillary wedge pressure
- PDB
Pulmonary diastolic pressure
- PE
Pulmonary embolism
- PH
Pulmonary hypertension
- PP
Pulse pressure
- PPV
Percent perfused vessels
- PSP
Pulmonary systolic pressure
- PSVD
Perfused small vessel density
- PVD
Perfused vessel density
- RA
Right atrial
- RAP
Right atrial pressure
- RV
Right ventricle
- RVEDD
Right ventricle end-diastolic volume
- RVEF
Right ventricular ejection fraction
- RVSP
Right ventricular systolic pressure
- SBF
Skin blood flow
- SBP
Systolic blood pressure
- S/L
Sublingual
- SV
Stroke volume
- SVD
Small vessel density
- SVO2
Mixed venous oxygen saturation
- S′
Systolic peak myocardial velocities
- TAPSE
Tricuspid annular plane systolic excursion
- TD
Tissue Doppler
- a′
Late diastolic atrial contraction velocities
- E
Transmitral early peak velocities
- Ea/e′
Lateral mitral annulus early diastolic velocities
- Sa
Lateral mitral annulus peak systolic velocities
- Sv
Systolic peak velocity
- t-IVT
Total isovolumic time
- TNI
Troponin I
- TPG
Transpulmonary gradient
- TR
Tricuspid regurgitation
- VV
Veno-venous
- TVD
Total vessel density
- V-A-V
Veno-arterio-venous
- VAD
Ventricular assist device
- VTI
Velocity time integral
- VVI
Velocity vector imaging
- WBC
White blood count
Author contributions
FC and YAC contributed to study design, data analysis and interpretation and quality and risk of bias assessment and drafted the manuscript. FC, KC, EB, OL and YAC were involved in data acquisition and extraction. FC, EL, YL, KS, MA, PEN, AC and YAC contributed to revision of content. All authors read and approved the final manuscript.
Funding
Dr Cavayas’ work is supported by the Fonds de Recherche du Québec – Santé. Dr Cournoyer’s work is supported by the Département de Médecine Familiale et de Médecine d’Urgence de l’Université de Montréal, as well as the Fonds des Urgentistes de l’Hôpital du Sacré-Cœur de Montréal.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
No details, images or videos relating to an individual person were collected. Thus, consent for publication was not sought.
Competing interests
On behalf of all authors, the corresponding authors state that there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abrams D, Garan AR, Abdelbary A, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018;44:717–729. doi: 10.1007/s00134-018-5064-5. [DOI] [PubMed] [Google Scholar]
- 2.Fiser SM, Tribble CG, Kaza AK, et al. When to discontinue extracorporeal membrane oxygenation for postcardiotomy support. Ann Thorac Surg. 2001 doi: 10.1016/S0003-4975(00)02340-7. [DOI] [PubMed] [Google Scholar]
- 3.Mebazaa A, Combes A, van Diepen S, et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 2018;44:760–773. doi: 10.1007/s00134-018-5214-9. [DOI] [PubMed] [Google Scholar]
- 4.Rousse N, Juthier F, Pinçon C, et al. ECMO as a bridge to decision: recovery, VAD, or heart transplantation? Int J Cardiol. 2015;187:620–627. doi: 10.1016/j.ijcard.2015.03.283. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-S, Chao A, Yu H-Y, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41:197–203. doi: 10.1016/S0735-1097(02)02716-X. [DOI] [PubMed] [Google Scholar]
- 6.Chang W-W, Tsai F-C, Tsai T-Y, et al. Predictors of mortality in patients successfully weaned from extracorporeal membrane oxygenation. PLoS ONE. 2012 doi: 10.1371/journal.pone.0042687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavarocchi NC, Pitcher HT, Yang Q, et al. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J Thorac Cardiovasc Surg. 2013;146:1474–1479. doi: 10.1016/j.jtcvs.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care Lond Engl. 2016;20:80–80. doi: 10.1186/s13054-016-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappalardo F, Pieri M, Arnaez Corada B, et al. Timing and strategy for weaning from venoarterial ECMO are complex issues. J Cardiothorac Vasc Anesth. 2015;29:906–911. doi: 10.1053/j.jvca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Zangrillo A. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:7. [PubMed] [Google Scholar]
- 11.Omar HR, Mirsaeidi M, Mangar D, Camporesi EM. Duration of ECMO is an independent predictor of intracranial hemorrhage occurring during ECMO support. ASAIO J. 2016;62:634–636. doi: 10.1097/MAT.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 12.Extracorporal Life Support Organisation (ELSO). Guidelines for adult cardiac failure. https://www.elso.org/Portals/0/IGD/Archive/FileManager/e76ef78eabcusersshyerdocumentselsoguidelinesforadultcardiacfailure1.3.pdf. Accessed 19 Aug 2019
- 13.Extracorporal Life Support Organisation (ELSO). Guidelines for ECPR cases. https://www.elso.org/Portals/0/IGD/Archive/FileManager/6713186745cusersshyerdocumentselsoguidelinesforecprcases1.3.pdf. Accessed 19 Aug 2019
- 14.Lorusso R, Shekar K, MacLaren G, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 2021;67:827–844. doi: 10.1097/MAT.0000000000001510. [DOI] [PubMed] [Google Scholar]
- 15.Aissaoui N, Luyt C-E, Leprince P, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2011;37:1738–1745. doi: 10.1007/s00134-011-2358-2. [DOI] [PubMed] [Google Scholar]
- 16.Li C-L, Wang H, Jia M, et al. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg. 2015;149:1445–1450. doi: 10.1016/j.jtcvs.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 17.Park B-W, Seo D-C, Moon I-K, et al. Pulse pressure as a prognostic marker in patients receiving extracorporeal life support. Resuscitation. 2013;84:1404–1408. doi: 10.1016/j.resuscitation.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Ortuno S, Delmas C, Diehl J-L, et al. Weaning from veno-arterial extra-corporeal membrane oxygenation: which strategy to use? Ann Cardiothorac Surg. 2019;8:E1–E8. doi: 10.21037/acs.2018.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aissaoui N, El-Banayosy A, Combes A. How to wean a patient from veno-arterial extracorporeal membrane oxygenation. Intensive Care Med. 2015;41:902–905. doi: 10.1007/s00134-015-3663-y. [DOI] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risk of Bias Assessment tool for Non-randomized Studies (RoBANS). Development and validation of a new instrument|Colloquium Abstracts. https://abstracts.cochrane.org/2011-madrid/risk-bias-assessment-tool-non-randomized-studies-robans-development-and-validation-new. Accessed 6 Sept 2021
- 22.Colombo CNJ, Dammassa V, Pozzi M, et al. Echocardiographic predictors of VA ECMO weaning in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2019;8(Supplement 1):144–145. doi: 10.1177/2048872619829424. [DOI] [Google Scholar]
- 23.Luyt C-E, Landivier A, Leprince P, et al. Usefulness of cardiac biomarkers to predict cardiac recovery in patients on extracorporeal membrane oxygenation support for refractory cardiogenic shock. J Crit Care. 2012;27:524.e7–524.e14. doi: 10.1016/j.jcrc.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Mazet C, Conil JM, Georges B, et al. How to predict weaning success during ECLS support? Ann Intensive Care. 2017;7(1 Supplement 1):160. doi: 10.1186/s13613-016-0224-7. [DOI] [Google Scholar]
- 25.Mork SR, Frederiksen CA, Nielsen RR, et al. A systematic approach to weaning from extracorporeal membrane oxygenation in patients with refractory cardiac failure. Acta Anaesthesiol Scand. 2021;17:17. doi: 10.1111/aas.13814. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, Watanabe M, Murakami M, et al. End-tidal carbon dioxide monitoring indicates recovery from cardiogenic shock in patients receiving percutaneous cardiopulmonary support. J Artif Organs. 2005;8:63–66. doi: 10.1007/s10047-004-0279-3. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez Martin J, Alonso-Fernandez-Gatta M, Merchan Gomez S, et al. Echocardiographic recovery changes in patients supported with veno-arterial extracorporeal membrane oxygenator and weaning success. Eur Heart J. 2021;42:ehab724 1060. doi: 10.1093/eurheartj/ehab724.1060. [DOI] [Google Scholar]
- 28.Xu Y, Liu N, Dong D, et al. Pulmonary artery flotation catheter (PAFC) combined with pump-controlled retrograde trial off (PCRTO) as a trial for weaning VA-ECMO patients: a retrospective study. Perfusion. 2022 doi: 10.1177/02676591211054976. [DOI] [PubMed] [Google Scholar]
- 29.Asaumi Y, Yasuda S, Morii I, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26:2185–2192. doi: 10.1093/eurheartj/ehi411. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, Asaumi Y, Nakamura Y, et al. Clinical determinants of successful weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis. ESC Heart Fail. 2018;5:675–684. doi: 10.1002/ehf2.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.North M, Eckman P, Samara M, et al. Peak troponin predicts successful weaning from VA ECMO in patients with acute myocardial infarction complicated by cardiogenic shock. Int J Artif Organs. 2022;45:68–74. doi: 10.1177/0391398821991155. [DOI] [PubMed] [Google Scholar]
- 32.Sugiura A, Abe R, Nakayama T, et al. Predictors of successful weaning from veno-arterial extracorporeal membrane oxygenation after coronary revascularization for acute myocardial infarction complicated by cardiac arrest: a retrospective multicenter study. Shock. 2019;51:690–697. doi: 10.1097/SHK.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 33.Omar HR, Handshoe JW, Tribble T, Guglin M. Survival on venoarterial extracorporeal membrane oxygenation in cardiogenic shock: which lactate is most useful? ASAIO J. 2022;68:41–45. doi: 10.1097/MAT.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 34.Suhr L, Djordjevic I, Ivanov B, et al. Concomitant IABP Improves ECMO Weaning: A Single-Center Experience. Thorac Cardiovasc Surg. 2022;70(S 01):S1–S61. doi: 10.1055/s-0042-1742931. [DOI] [Google Scholar]
- 35.Oshima K, Morishita Y, Hinohara H, et al. Factors for weaning from a percutaneous cardiopulmonary support system (PCPS) in patients with severe cardiac failure: a comparative study in weaned and nonweaned patients. Int Heart J. 2006;47:575–584. doi: 10.1536/ihj.47.575. [DOI] [PubMed] [Google Scholar]
- 36.Mongkolpun W, Bakos P, Peluso L, et al. Cutaneous blood flow as a predictor of successful weaning from VA-ECMO. Crit Care. 2019 doi: 10.1186/s13054-019-2358-0. [DOI] [Google Scholar]
- 37.Fried JA, Griffin JM, Masoumi A, et al. Predictors of survival and ventricular recovery following acute myocardial infarction requiring extracorporeal membrane oxygenation therapy. ASAIO J. 2022;68:800–807. doi: 10.1097/MAT.0000000000001570. [DOI] [PubMed] [Google Scholar]
- 38.Morisawa D, Higuchi Y, Iwakura K, et al. Predictive factors for successful weaning from percutaneous cardiopulmonary support in patients with cardiogenic shock complicating acute myocardial infarction. J Cardiol. 2012;60:350–354. doi: 10.1016/j.jjcc.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Hsu J. Clinical parameters for successful weaning from venous-arterial extracorporeal membrane oxygenation after cardiogenic shock. J Am Coll Cardiol. 2012;59:E1022. doi: 10.1016/S0735-1097(12)61023-7. [DOI] [Google Scholar]
- 40.Sawada K, Kawakami S, Murata S, et al. Predicting parameters for successful weaning from veno-arterial extracorporeal membrane oxygenation in cardiogenic shock. ESC Heart Fail. 2021;8:471–480. doi: 10.1002/ehf2.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi R, Guo J, Zhou Q, et al. Effect evaluation of bedside ultrasound monitoring of left ventricular functional parameters combined with clinical indicators on veno-arterial extracorporeal membrane oxygenation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33:329–333. doi: 10.3760/cma.j.cn121430-20201023-00684. [DOI] [PubMed] [Google Scholar]
- 42.Naruke T, Inomata T, Kawaguchi T, et al. Prognostic impact of dramatic alteration of mixed venous oxygen saturation on the hemodynamic recovery from veno-arterial extracorporeal membrane oxygenation. Eur Heart J. 2012;33:69. doi: 10.1093/eurheartj/ehs281. [DOI] [Google Scholar]
- 43.Sawamura A, Okumura T, Hirakawa A, et al. Early prediction model for successful bridge to recovery in patients with fulminant myocarditis supported with percutaneous venoarterial extracorporeal membrane oxygenation—insights from the change pump study. Circ J. 2018;82:699–707. doi: 10.1253/circj.CJ-17-0549. [DOI] [PubMed] [Google Scholar]
- 44.Chommeloux J, Montero S, Franchineau G, et al. Microcirculation evolution in patients on venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2020;48:e9–e17. doi: 10.1097/CCM.0000000000004072. [DOI] [PubMed] [Google Scholar]
- 45.Akin S, dos Reis MD, Caliskan K, et al. Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit Care. 2017;21:265. doi: 10.1186/s13054-017-1855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.North M, Wilson K, Hryniewicz K. Swan-Ganz catheter guided VA ECMO wean leads to successful decannulation. ASAIO J. 2018;64(Supplement 2):44. doi: 10.1097/MAT.0000000000000882. [DOI] [Google Scholar]
- 47.Wu MY, Lin PJ, Tsai FC, et al. (2009) Postcardiotomy extracorporeal life support in adults: the optimal duration of bridging to recovery. ASAIO J Am Soc Artif Intern Organs. 1992;55:608–613. doi: 10.1097/mat.0b013e3181b899c0. [DOI] [PubMed] [Google Scholar]
- 48.Lim JY, Jung JS. Predictors of successful weaning from veno-arterial extracorporeal membrane oxygenation support. Perfus Ger. 2019;34(1 Supplement):88–89. doi: 10.1177/0267659119829686. [DOI] [Google Scholar]
- 49.Joseph A, Venturini J, Blair JEA, et al. Prognostic value of right ventricular function in percutaneous veno-arterial extracorporeal membrane oxygenation. BMC Cardiovasc Disord. 2019;19:1–16. doi: 10.1186/s12872-018-0978-y. [DOI] [Google Scholar]
- 50.Naruke T, Inomata T, Imai H, et al. End-tidal carbon dioxide concentration can estimate the appropriate timing for weaning off from extracorporeal membrane oxygenation for refractory circulatory failure. Int Heart J. 2010;51:116–120. doi: 10.1536/ihj.51.116. [DOI] [PubMed] [Google Scholar]
- 51.Heaney C, Vuthoori R, Lima B, et al. Assessment of cardiac recovery using a formal stepwise weaning protocol in patients supported with venoarterial extracorporeal membrane oxygenation. Artif Organs. 2020;44(3):E97. doi: 10.1111/aor.13651. [DOI] [Google Scholar]
- 52.Aissaoui N, Guerot E, Combes A, et al. Two-dimensional strain rate and doppler tissue myocardial velocities: analysis by echocardiography of hemodynamic and functional changes of the failed left ventricle during different degrees of extracorporeal life support. J Am Soc Echocardiogr. 2012;25:632–640. doi: 10.1016/j.echo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Colombo CNJ, Dammassa V, Pozzi M, et al. Echocardiographic predictors of VA-ECMO weaning in patients with cardiogenic shock. Intensive Care Med Exp. 2019 doi: 10.1186/s40635-019-0265-y. [DOI] [Google Scholar]
- 54.Ouazani N, Shudo Y, Hill C, et al. The interest of tissue doppler imaging in the weaning of venoarterial extracorporeal membrane oxygenation. BMC Cardiovasc Disord. 2019;19:1–16. doi: 10.1186/s12872-018-0978-y. [DOI] [Google Scholar]
- 55.Aissaoui N, Caudron J, Leprince P, et al. Right–left ventricular interdependence: a promising predictor of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2017;43:592–594. doi: 10.1007/s00134-016-4657-0. [DOI] [PubMed] [Google Scholar]
- 56.Frederiksen CA, Nielsen R, Frederiksen AS, et al. Echocardiographic predictors for successful weaning from veno-arterial extracorporeal membrane oxygenation. Eur Heart J. 2018;39(Supplement 1):1200. doi: 10.1093/eurheartj/ehy566.P5689. [DOI] [Google Scholar]
- 57.Gambaro A, Vazir A, Galiatsou E, et al. Can speckle tracking help to guide VA-ECMO weaning? Provisional results of a pilot study. Perfusion. 2020;35(1 SUPPL):110. doi: 10.1177/0267659120909723. [DOI] [Google Scholar]
- 58.Kim D, Jang WJ, Park TK, et al. Echocardiographic predictors of successful extracorporeal membrane oxygenation weaning after refractory cardiogenic shock. J Am Soc Echocardiogr. 2021 doi: 10.1016/j.echo.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Elena Puerto E, Guido Tavazzi G, Alessia Gambaro A, et al. Interaction between veno-arterial extracorporeal membrane oxygenation and the right ventricle. Eur Heart J Acute Cardiovasc Care. 2021;10(zuab020):173. doi: 10.1093/ehjacc/zuab020.173. [DOI] [Google Scholar]
- 60.Kim D, Park Y, Choi KH, et al. Prognostic implication of RV coupling to pulmonary circulation for successful weaning from extracorporeal membrane oxygenation. JACC Cardiovasc Imaging. 2021;14:1523–1531. doi: 10.1016/j.jcmg.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 61.Huang K-C, Lin L-Y, Chen Y-S, et al. Three-dimensional echocardiography-derived right ventricular ejection fraction correlates with success of decannulation and prognosis in patients stabilized by venoarterial extracorporeal life support. J Am Soc Echocardiogr. 2018;31:169–179. doi: 10.1016/j.echo.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Westrope C, Harvey C, Robinson S, et al. Pump controlled retrograde trial off from VA-ECMO. ASAIO J. 2013;59:517–519. doi: 10.1097/MAT.0b013e31829f5e9f. [DOI] [PubMed] [Google Scholar]
- 63.Ling L, Chan KM. Weaning adult patients with cardiogenic shock on veno-arterial extracorporeal membrane oxygenation by pump-controlled retrograde trial off. Perfusion. 2018;33:339–345. doi: 10.1177/0267659118755888. [DOI] [PubMed] [Google Scholar]
- 64.Yeh Y-C, Lee C-T, Wang C-H, et al. Investigation of microcirculation in patients with venoarterial extracorporeal membrane oxygenation life support. Crit Care Lond Engl. 2018;22:200. doi: 10.1186/s13054-018-2081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kara A, Akin S, dos Reis MD, et al. Microcirculatory assessment of patients under VA-ECMO. Crit Care. 2016 doi: 10.1186/s13054-016-1519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abali G, Tokgözoğlu L, Özcebe OI, et al. Which Doppler parameters are load independent? A study in normal volunteers after blood donation. J Am Soc Echocardiogr. 2005;18:1260–1265. doi: 10.1016/j.echo.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 67.Yalçin F, Kaftan A, Muderrisoğlu H, et al. Is Doppler tissue velocity during early left ventricular filling preload independent? Heart. 2002;87:336–339. doi: 10.1136/heart.87.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moury PH, Zunarelli R, Bailly S, et al. Diaphragm Thickening during venoarterial extracorporeal membrane oxygenation weaning: an observational prospective study. J Cardiothorac Vasc Anesth. 2021;35:1981–1988. doi: 10.1053/j.jvca.2020.10.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary tables.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information file.