Abstract
Background
Toxoplasma gondii is known as the most successful parasite, which can regulate the host immune response through a variety of ways to achieve immune escape. We previously reported that a novel gene wx2 of T. gondii may be a virulence-related molecule. The objective of this study was to explore the mechanism of wx2 regulating host immune response.
Methods
The wx2 knockout strain (RHwx2−/− strain) and complementary strain (RHwx2+/+ strain) were constructed by the CRISPR/Cas9 technique, and the virulence of the wx2 gene was detected and changes in pyroptosis-related molecules were observed.
Results
Compared with the wild RH and RHwx2+/+ strain groups, the survival time for mice infected with the RHwx2−/− strain was prolonged to a certain extent. The mRNA levels of pyroptosis-related molecules of caspase-1, NLRP3, and GSDMD and et al. in mouse lymphocytes in vivo and RAW267.4 cells in vitro infected with RHwx2−/− strain increased to different degrees, compared with infected with wild RH strain and RHwx2+/+ strain. As with the mRNA level, the protein level of caspase-1, caspase-1 p20, IL-1β, NLRP3, GSDMD-FL, GSDMD-N, and phosphorylation level of NF-κB (p65) were also significantly increased. These data suggest that wx2 may regulate the host immune response through the pyroptosis pathway. In infected RAW264.7 cells at 48 h post-infection, the levels of Th1-type cytokines of IFN-γ, Th2-type cytokines such as IL-13, Th17-type cytokine of IL-17 in cells infected with RHwx2−/− were significantly higher than those of RH and RHwx2+/+ strains, suggesting that the wx2 may inhibit the host's immune response.
Conclusion
wx2 is a virulence related gene of T. gondii, and may be involved in host immune regulation by inhibiting the pyroptosis pathway.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-022-05502-5.
Keywords: wx2 gene, Toxoplasma gondii, Virulence, Pyroptosis, Host immune response
Background
Toxoplasma gondii is an obligate intracellular parasite of Apicomplexa, which can infect almost all warm-blooded animals, including humans [1, 2]. Toxoplasma gondii is widely distributed throughout the world, with about one third of the world’s population infected [3]. Although T. gondii has been known for more than 100 years, the mechanism of its invasion is not fully understood. How the host controls the parasite and the mechanisms by which the parasite resists the host’s immune response are unclear. To date, there are no effective drugs against T. gondii bradyzoites. In addition, drug-resistant T. gondii strains have been reported by researchers [4]. Therefore, research is still needed for an in-depth understanding of the pathogenic mechanism of T. gondii to develop effective drugs and vaccines.
The host has a variety of immune mechanisms to resist T. gondii infection. The invading T. gondii are mainly eliminated via helper T type 1 (Th1) cellular immunity [5]. In the early stage of infection, the T. gondii antigen can induce interleukin-12 (IL-12) secretion in dendritic cells [6, 7]. In addition, neutrophils and macrophages can also secrete IL-12 during T. gondii infection [8, 9]. IL-12 plays an important role in T. gondii infection. It can stimulate natural killer (NK) cells, CD4+, and CD8+ T cells to secrete interferon-γ (IFN-γ), which is the most important cytokine for the host to fight against T. gondii [5, 6, 10].
During the long-term parasitic process, to avoid being eradicated by the host immune response, T. gondii has also evolved a series of immune evasion mechanisms to protect itself from being eliminated [11]. After T. gondii invades the cells, it is located in the cytoplasm of the host cells and is wrapped by a parasitophorous vacuole membrane (PVM). The PVM can act as a molecular sieve [12], so that T. gondii can secrete the parasite's specialized secretory proteins, such as the rhoptries (ROP) or dense granules (GRA) [13, 14], which play an important role in the modulation of host signaling pathways to achieve the purpose of immune escape. Activators of transcription (STAT) such as STAT3 and STAT6 of host cells were activated by ROP16 [15, 16], which inhibits lipopolysaccharide (LPS)-induced production of IL-12p40 and tumor necrosis factor (TNF-α) [17]. ROP16 is also a major inhibitor of Toll-like receptors (TLR) and IFN-γ downstream pathways [18]. The inhibitor of STAT1-dependent transcription (TgIST [Toxoplasma inhibitor of STAT1-dependent transcription]) is a dense granule protein, which can also inhibit the expression of STAT1-dependent proinflammatory genes, such as IFN-γ [19]. In addition, T. gondii can also impact the host NF-κB, MAPK and other pathways through molecules such as GRA15 and GRA24 [20] to modulate host signaling pathways. Toxoplasma gondii can also evade host immune response by inhibiting host cell apoptosis, affecting the host cell cycle and cell metabolism [21, 22].
Pyroptosis is a pro-inflammatory form of programmed cell death that is regulated by the cysteine-dependent caspase family of proteases. Different from cell necrosis, apoptosis, and autophagy, it is manifested in the continuous swelling of cells until the cell membrane ruptures, resulting in the release of cell contents and the activation of a strong inflammatory response. Pyroptosis was first discovered in the lysis reaction after Shigella infection of macrophages in 1992 [23], and in 2001, Cookson et al. [24] named it pyroptosis. Studies have confirmed that both pathogenic infection and endogenous damage can induce pyroptosis.
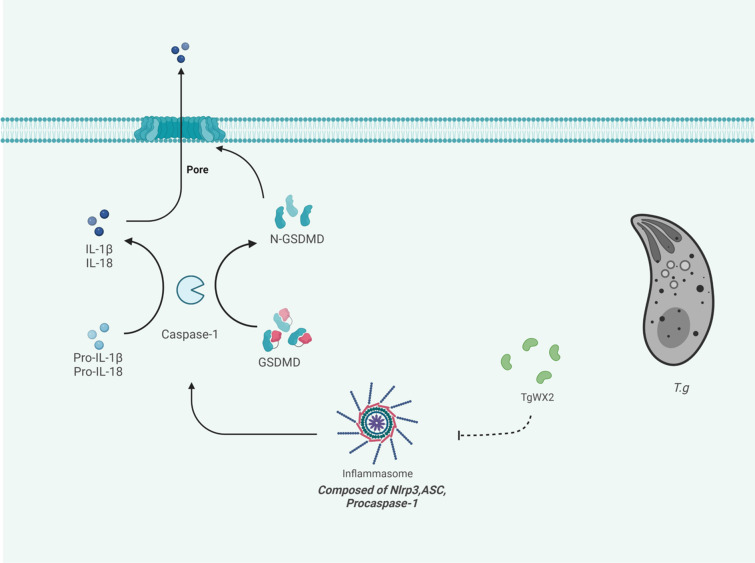
The pathways of pyroptosis can be divided into two categories: the caspase-1-dependent classical pathway and the caspase-1-independent non-canonical pathway, both of which perforate the cell membrane through the regulation of gasdermin D (GSDMD). Inactive caspase-1 exists in the cytoplasm in the form of pro-caspase-1, and its activation depends on the inflammasome action. Nod-like receptor (NLR) is the earliest discovered inflammasome family, including multiple members such as NLRP1, NLRP3, NAIP-NLRC4, NLRP6, and NLRP9b [25]. And NLRP3 is not only the main component of the classical pyroptosis pathway but also an important component of the non-classical pyroptosis pathway [26]. When the host is subjected to exogenous or endogenous damage, stimulated by pathogen-associated molecular patterns/damage-associated molecular patterns/hepcidin antimicrobial peptide (PAMP/DAMP/ HAMP), NLRP3 is activated, recruits apoptosis-associated speck-like protein (ASC) and pro-caspase-1, and assembles into a large cytoplasmic complex, pro-caspase-1 release p20 and p10 subunits undergo autohydrolysis, and further aggregates into active caspase-1. Activated caspase-1 cleaves GSDMD into N-terminal and C-terminal, leading to the formation of active pores in the cell membrane, and further cell membrane perforation occurs until cell lysis and death [27]. At the same time, caspase-1 cleaves the precursors of IL-1β and IL-18 to form mature IL-1β and IL-18; after the cell membrane is perforated, IL-1β and IL-18 are secreted out of the cell via porin, inducing an inflammatory response, and activating the pyroptosis of other cells [28].
Pyroptosis is also one of the ways that the host eliminates various intracellular pathogens, including T. gondii. However, T. gondii has also developed some strategies to inhibit host cell pyroptosis. For example, the C-terminal of GRA9 secreted by T. gondii can bind to NLRP3 and thus block the binding of ASC to NLRP3, thereby destroying the NLRP3 inflammasome [29]. Studies have found that T. gondii could inhibit the activity of caspase-1 in neutrophils and may also affect the activity of GSDMD [30, 31].
Our previous studies have shown that the wx2 gene may be related to T. gondii virulence and may even regulate the host immune response to achieve immune escaping [32]. In this study, we explore whether the wx2 gene of T. gondii affects the host immune response via the pyroptosis pathway in vitro and in vivo. It provides insights into the mechanism of host immune response manipulation by T. gondii that might be useful in the development of anti-toxoplasmosis drugs and vaccines.
Methods
Toxoplasma gondii and cell culture
Tachyzoites of T. gondii RH strain (type I), wx2 knockout (RHwx2−/− strain), and wx2 complementary (RHwx2+/+ strain) were maintained in Kun Ming (KM) mice and were harvested in phosphate-buffered saline (PBS) solution after infection for 4 days.
Tachyzoites of T. gondii RH strain (type I), wx2 knockout (RHwx2−/− strain), and wx2 complementary (RHwx2+/+ strain) were cultured in human foreskin fibroblasts (HFF) using Dulbecco’s modified Eagle medium (DMEM) supplemented with 2% fetal bovine serum (FBS). HFF cells were grown in culture flasks containing DMEM supplemented with 10% FBS, in a 37 °C and 5% CO2 incubator.
Construction of T. gondii wx2 gene complementary strains
Tachyzoites of T. gondii RH strain (type I) and wx2 knockout (RHwx2−/− strain) were stored in our laboratory. The wx2 gene complementary strains were constructed as described in the literature using CRISPR-Cas9 [32, 33]. Ptub::GOI::CAT plasmid with the wx2 fragment and purified wx2-CAT fragment were co-transfected into freshly harvested tachyzoites of RHwx2−/− strain by electroporation. The transgenic parasites were obtained by selection via 25 µg/ml pyrimethamine chloramphenicol (CAT) and 10 µg/ml fluorouracil deoxynucleoside (FUDR). Polymerase chain reaction (PCR) was performed with genomic DNA as a template to confirm the gene of wx2 was complemented. All plasmids and primers used in this study are listed in Table 1.
Table 1.
Information of primers
| Name of primer | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| wx2-CDS | ATGTATATCTGTATAGAAGG | CGGTGTCGCCTGACTTCTGT |
| pTub-Gbison | TACCCGTACGACGTCCCGGACTACGCTGGCTATCCC | TTTGTCGAAAAAGGGAATTCAAGAAAA |
| Com-UPRT-wx2-KZ | TCCTTTTATTCCAAGATCTGTGGCGTCTCGATTGTGAGGAAGTGGAGGACGGGAATTCG | AAACTGCCCGCAAGCCACTTTCCATCGACTCGCCAGCTAATACGACTCACTATAGGGCG |
| JD1-wx2 | TCCGTAAAGCGGTGAGTGTCG | CGGTGTCGCCTGACTTCTGTG |
| JD2-wx2 | AAACATCCGTAAAGCGGTGAG | ACGACGAAGAAGGGAACACG |
| pro-caspase-1 | CACAGCTCTGGAGATGGTGA | CTTTCAAGCTTGGGCACTTC |
| ASC | GACAGTACCAGGCAGTTCGT | AGTCCTTGCAGGTCAGGTTC |
| pro-IL-1β | CAGGCAGGCAGTATCACTCA | AGCTCATATGGGTCCGACAG |
| NF-κB | GAGGAAGGCTGTGAACATGAGG | TTCTGGTGCATTCTGACCTTGC |
| NLRP3 | AGATTACCCGCCCGAGAAAG | TCCCAGCAAACCCATCCACT |
| β-actin | TTCCTTCCTTGGGTATGGAAT | GAGCAATGATCTTGATCTTC |
Virulence assay
Specific-pathogen-free (SPF) inbred female KM mice (8 weeks old) were purchased from the Center of Laboratory of Animals, Central South University, Hunan, China. All mice were handled in strict accordance with the guidelines of the People’s Republic of China and the university’s ethics committee. Each mouse was injected intraperitoneally with 5000 freshly harvested tachyzoites of the RH, RHwx2−/−, and RHwx2+/+ strains (eight mice per strain). All mice were monitored daily until death.
Detection of pyroptosis-related molecules at the mRNA level
The wx2 gene was confirmed to be restored by reverse transcription PCR (RT-PCR) at the mRNA level. Total RNA was extracted from the RH, RHwx2−/− strain, and RHwx2+/+ T. gondii strain using TRIzol (Vazyme Biotech Co., Ltd.) according to the manufacturer’s recommendations. Reverse transcription was performed using a PrimeScript™ 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co.,Ltd.). The mRNA expression levels of Tgwx2 was detected by quantitative RT-PCR. When the BALB/c mice were infected with the RH, RHwx2−/−, and RHwx2+/+ strains respectively, until death. The lymphoid tissues were taken and the total RNA was extracted. RAW264.7 cells were infected with the RH, RHwx2−/−, and RHwx2+/+ strains, respectively, for 24 and 48 h. Total RNA was extracted and reverse transcription was performed. The mRNA expression levels of pro-caspase-1, ASC, NLRP3, and GSDMD were detected by RT-PCR. All primers used in this study are listed in Table 1.
Detection of pyroptosis-related molecules at the protein level
After RH, RHwx2−/− and RHwx2+/+ T. gondii strain infected BALB/c mice, until death. The lymphoid tissue protein was harvested. RAW264.7 cells were infected with RH, RHwx2−/− and RHwx2+/+ strains, respectively, the total protein was harvested 24 h and 48 h later. The protein concentration was measured using a bicinchoninic acid protein assay kit (Beyotime Biotechnology, Inc., Shanghai, China). A total of 20 μg of proteins of RH, RHwx2−/−, and RHwx2+/+ were boiled with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer and added to SDS-PAGE for electrophoresis. The proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane and blocked with 5% non-fat milk for 2 h at room temperature. Subsequently, the membrane was incubated with primary antibodies (GSDMD-N, GSDMD-FL, NLRP3, pro-caspase-1, caspase-1, ASC, IL-1β, and IL-1β p17) at 4 °C overnight. The membrane was subsequently incubated for 1 h at room temperature with the secondary antibody (horseradish peroxidase [HRP]-conjugated rabbit anti-mouse immunoglobulin G [IgG] or HRP-conjugated rabbit anti-rabbit IgG). The membrane was analyzed using an enhanced chemiluminescence (ECL) western blotting system. All antibodies used in this study are listed in Table 2.
Table 2.
Information on antibodies
| Antibody | Company and catalog |
|---|---|
| NLRP3 | Recombinant Anti-NLRP3 antibody: Abcam (ab263899) |
| IL-1β | Recombinant Anti-IL-1 beta antibody: Abcam (ab254360) |
| GSDMD | Recombinant Anti-GSDMD antibody: Abcam (ab219800) |
| GSDMD-N | GSDMDC1 Antibody (64-Y): Santa Cruz (sc-81868) |
| Caspase-1 | Caspase-1 (E2Z1C) Rabbit mAb:CST(#24,232) |
| Caspase-1 p20 | Caspase-1 p20 Antibody (D-4): Santa Cruz (sc-398715) |
| NF-κB(p65) | NF-kB p65 Antibody: SAB catalog no: 48676 |
| p-NF-κB (p-p65) | Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb: CST (#3033) |
Cytokine detection
RAW264.7 cells were infected with RH, RHwx2−/− and RHwx2+/+ strains for 48 h. Total RNA was extracted and reverse transcription was performed. The mRNA expression levels of IFN-γ, IL-4, IL-13, and IL-17 in RAW264.7 cells were detected by qRT-PCR.
Statistical analysis
All experiments were performed at least in triplicate. GraphPad Prism 8 was used for statistical analyses. The differences between groups were analyzed using the t-test and Dunnett's multiple comparisons test, and values of P < 0.05 were considered statistically significant. All results are presented as mean ± SD, which represents a summary of the data from at least three experiments. Statistics symbols used are as follows: ∗ P < 0.05, ∗ ∗ P < 0.01, ∗ ∗ ∗ P < 0.001.
Result
The wx2 gene influences parasite virulence in mice
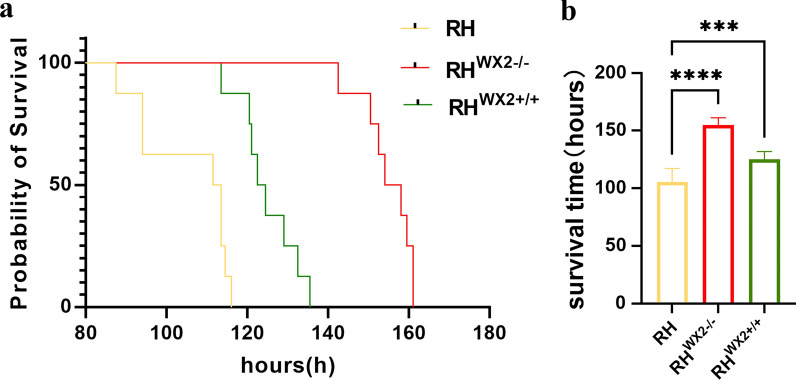
After the wx2 gene in the complementary strain was indeed successfully complemented (Additional file 1: Fig. S1), 5000 fresh wild-type RH tachyzoites and an equal number of RHwx2+/+, RHwx2−/− tachyzoites from mice were used to infect KM mice. The result showed that the average survival time for mice challenged with the RH wild strain, RHwx2−/− strain, and RHwx2+/+ strain was 112.5 h, 156 h, and 123.5 h, respectively (Fig. 1a). The survival time for mice challenged with the RHwx2−/− strain was 32.5 h longer than that of mice challenged with the RHwx2+/+ strain, and 43.5 h longer than that of mice challenged with the RH strain, and these differences are statistically significant (P < 0.05) (Fig. 1b). The above results show that the deletion of the wx2 gene in the RH strain can delay the death of mice, and restoration of wx2 accelerated the death of mice to a certain extent, and the difference was statistically significant(P < 0.05), revealing that the wx2 gene is a virulence-related gene of T. gondii.
Fig. 1.
Mice virulence assay. a The virulence of the RH wild strain, RHwx2−/− strain, and RHwx2+/+ strain was estimated using Kaplan–Meier survival analysis. b The histogram displays the mean survival time of RHwx2−/−, RH and RHwx2+/+ strain, respectively, 112.5 h, 156 h, 123.5 h
wx2 of T. gondii affects host cell pyroptosis in vivo
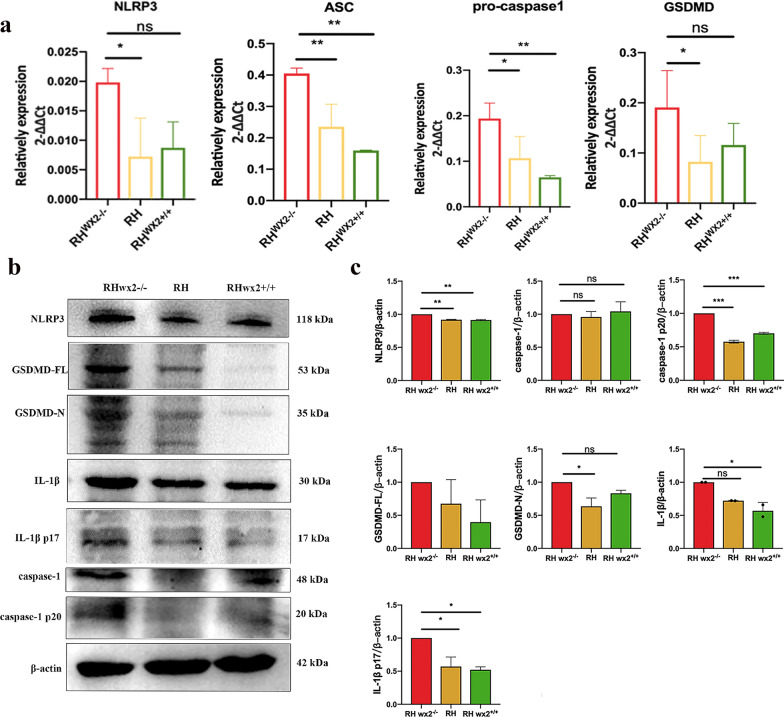
The mRNA expression levels of NLRP3, ASC, pro-caspase-1, and gasdermin D (GSDMD) in mouse lymphocytes, as well as protein expression levels of IL-1β, IL-1β p17, caspase-1 p20, caspase-1, GSDMD-FL, and GSDMD-N were detected. Compared with the RH strain, the mRNA expression levels of NLRP3, ASC, pro-caspase-1, and GSDMD (Fig. 2a) in the lymph nodes of the RHwx2−/− knockout mice were significantly increased, and the difference was statistically significant (P < 0.05), the protein expression levels of NLRP3, caspase-1 p20, GSDMD-F, GSDMD-N, IL-1β, IL-1β p17 (Fig. 2b, c) in the lymph nodes of mice infected with the wx2 knockout strain RHwx2−/− were also significantly higher than those of the RH and RHwx2+/+ strain.
Fig. 2.
Expression of pyroptosis-related molecules in vivo. a Gene expression of pyroptosis-related molecules, NLRP3, ASC, pro-caspase-1, and GSDMD at mRNA levels in the lymph node of infected mice. Mice were infected with RHwx2−/−, RH, RHwx2+/+ strain, respectively, then total RNA was extracted, and RT-qPCR was performed with the indicated primer. b Protein expression of pyroptosis-related molecules of NLRP3, caspase-1, caspase-1p20, GSDMD-FL, GSDMD-N, IL-1β, IL-1β p17 in lymph node of mice infected with the RHwx2−/−, RH, and RHwx2+/+ strains, and western blot was performed with the indicated antibody. c Analysis of grayscale scanning of image B
wx2 of T. gondii affects host cell pyroptosis in vitro
To verify the effect of the wx2 gene on host cell pyroptosis, we performed in vitro experiments. RAW264.7 cells were infected with RH, RHwx2+/+ and RHwx2−/for 24 h and 48 h, respectively, at a multiplicity of infection (MOI) of 5:1.To further verify the effect of the wx2 gene on host cell pyroptosis, we performed in vitro experiments by infecting RAW264.7 cells with RH, RHwx2+/+ and RHwx2−/for 24 h and 48 h, respectively. The mRNA expression levels of pro-caspase-1, pro-IL-1β, GSDMD, and NLRP3, as well as protein levels of caspase-1, caspase-1 p20, GSDMD-N, GSDMD-FL, IL-1β in RAW264.7 cells infected with RH, RHwx2+/+ and RHwx2−/− were detected after 24 h. Compared with the RH strain and RHwx2+/+ group, the mRNA expression levels of NLRP3, GSDMD, and pro-IL-1β (Fig. 3a)in the RAW264.7 cells infected with RHwx2−/− were significantly increased (P < 0.05). As for protein expression levels, the expression levels of NLRP3, GSDMD-FL, GSDMD-N, caspase-1, caspase-1 p20, and IL-1β (Fig. 3b, c) in the RHwx2−/− group were higher than those in the RH strain group and RHwx2+/+ groups. After 48 h post-infection, the mRNA expressions of NLRP3 and GSDMD (Fig. 4a) were higher in the RHwx2−/− group compared with the RH and RHwx2+/+ groups, and the difference was statistically significant (P < 0.05). The protein expression levels of GSDMD-N, NLRP3, caspase-1, caspase-1 p20, and phosphorylation of NF-κB (p65) (Fig. 4 b, c) were also higher in the RHwx2−/− strain than those of the infected RH strain and RHwx2+/+ strain.
Fig. 3.
Expression of pyroptosis-related molecules in infected RAW264.7 cells at 24 h post-infection. a mRNA expression of pyroptosis-related molecules of NLRP3, pro-caspase-1, GSDMD, and pro-IL-1β was detected. RAW264.7 cells were infected with the RHwx2−/−, RH, and RHwx2+/+ strain, respectively, for 24 h, total RNA was extracted, and RT-qPCR was performed with the indicated primer. b Protein expression of pyroptosis-related molecules of NLRP3, pro-caspase-1, GSDMD, and pro-IL-1β was detected. c Analysis of grayscale scanning of image B
Fig. 4.
Expression of pyroptosis-related molecules in infected RAW264.7 cells at 48 h post-infection. a Gene expression of pyroptosis-related molecules of NLRP3, pro-IL-1β, GSDMD, and pro-caspase-1 at the mRNA level. RAW264.7 cells were infected with the RHwx2−/−, RH, and RHwx2+/+ strains, respectively, for 48 h, total RNA was extracted, and qRT-PCR was performed with the indicated primer. b Protein expression of pyroptosis-related molecules. RAW264.7 cells were infected with the RHwx2−/−, RH, and RHwx2+/+ strains, respectively, for 48 h, lysed, and western blot was performed with the indicated antibody, GSDMD-FL, GSDMD-N, NLRP3, p-p65, caspase-1, caspase-1 p20, and IL-1β. c Analysis of grayscale scanning of image B
The wx2 gene influences the host cell immune response in vitro
In infected RAW264.7 cells, the levels of IL-6 (data not shown) and IFN-γ in the cells infected with RHwx2−/− were significantly higher than those of RH and RHwx2+/+ strains at 48 h post-infection (Fig. 5a). Interestingly and consistent with previous findings, the level of IL-4 and IL-13 cytokines were also elevated accordingly in RHwx2−/− strains (Fig. 5b, c), In addition, the Th17-type cytokine IL-17 was also significantly increased in the RHwx2-/- strains (Fig. 5d). Our data showed that wx2 inhibited the immune response and the production of cytokines of the host.
Fig. 5.
wx2 gene influence host cell immune response in vitro. The mRNA expression of Th1-,Th2-, and Th17-related cytokines were measured by qPCR in infected RAW264.7 cells at 48 h post-infection. Total RNA was extracted, and RT-qPCR was performed with the indicated primer. mRNA expression of Th1-, Th2-, and Th17-related cytokines of IFN-γ (a), IL-4 (b), IL-13 (c), and IL-17(d) were detected
Discussion
When T. gondii invades the host cells, the host activates a specific immune response to fight against the parasite, and interferon gamma (IFN-γ) is the most important cytokine in T. gondii elimination. This cytokine is mainly produced by NK cells, macrophages, T cells, and neutrophils [34, 35]. As one of the most successful parasites, T. gondii can parasitize in more than 140 animal species, including humans [20]. After T. gondii invades the host, in order to ensure that it is not cleared by the host’s immune system, it needs to establish a balance with the host, so it has evolved a series of mechanisms to escape the host’s immunity.
Pyroptosis plays an important role during pathogenic infection. It not only has a pro-inflammatory effect, amplifying inflammation through chemotactic reactions, but can also eliminate pathogens by promoting the death of infected cells, and plays a protective role in the infected body. Kuriakose et al. found that the influenza virus induced pyroptosis of infected cells through the NLRP3 pathway and aggravated the inflammatory response in the lungs [36]. After the host is infected with hepatitis B virus (HBV), the virus blocks pyroptosis by inhibiting caspase-1 and avoiding being cleared by hepatic macrophages, thereby achieving immune tolerance [37]. Studies have found that dengue virus infection can induce pyroptosis of human monocytes by activating caspase-1, thus playing a role in pathogen elimination in the body [38]. Carvalho et al. confirmed that parasite infections including Leishmania, Plasmodium, and T. gondii can activate inflammasomes such as NLRP3, induce pyroptosis of infected cells, and remove pathogens [39]. It has been reported that both human and murine NLRP1 can specifically recognize a cytoplasmic serine protease inhibitor of Bacillus anthracis, Shigella, fungi, and T. gondii and activate caspase-1 to initiate the process of pyroptosis [40, 41]. NAIP2 can recognize the rod-shaped protein of T. gondii [42, 43], activating caspase-1 to initiate the classical pyroptosis pathway. Another study found that the P2X7R/NLRP3 pathway plays an important role in IL-1β secretion and inhibition of T. gondii proliferation in infected cells [44]. Under cellular pathophysiological conditions, ATP released from dying cells enhances the activation of P2X7R, thereby upregulating the NLRP3 inflammasome, which promotes the secretion of IL-1β, thereby controlling the proliferation of T. gondii [44], revealing that the NLRP3 inflammasome is involved in the activation mechanism of T. gondii infection and its protective effect on the body.
Based on our previous studies, we found that the wx2 gene is associated with the virulence of T. gondii strains and may be involved in regulating the host immune response [32]. In this study, the virulence assay of mice revealed that the survival time for mice infected with wx2 gene knockout strain RHwx2−/− was significantly longer than that for the mice infected with the wild-type RH strain. The survival time for mice was significantly shortened after the wx2 gene was restored, confirming that the wx2 gene is a virulence-related gene. Studies have found that the production of IL-1β contributes to host control of T. gondii infection [45, 46], and our experiments showed that the mRNA and protein expression levels of IL-1β and IL-1β p17 in the infected RHwx2−/− group were significantly higher than those of the RH wild strain and the RHwx2+/+ strain, and the activation levels of NLRP3, caspase-1, and GSDMD were significantly higher in the RHwx2−/− group than those of the wild RH strain and the RHwx2+/+ strain group. Both in vitro and in vivo results showed that the expression levels of NLRP3 and caspase-1 p20 in the RAW264.7 cells and lymph nodes of mice infected with the RHwx2−/− were higher than those in the infected RH strain and RHwx2+/+ strain. In addition, the phosphorylation level of NF-κB (p65) protein in the RHwx2−/− group was higher than that in wild-type RH and RHwx2+/+ strains after infection for 48 h in vitro.
Therefore, IFN-γ is a critical factor in host protective immunity against T. gondii infection [47, 48], and Th1-related cytokines play a crucial role in the antimicrobial host immune mechanisms [49]. Consistent with previous experimental results, our research showed that wx2 can avoid the host’s immune response by inhibiting the production of IFN-γ and IL-6, accomplishing immune evasion of the parasites to some extent. Interestingly, and consistent with the previous findings, Th2-type cytokines such as IL-4, IL-13, and Th17-type cytokine IL-17 were significantly higher in the RHwx2+/+ strain than in other groups. Our results suggested that wx2 can achieve the immune evasion of T. gondii by inhibiting the host's immune response, thereby ensuring its invasion and reproduction in host cells. Our results confirmed that wx2 gene knockout can promote the occurrence of host cell pyroptosis, thereby inhibiting the parasitism and dissemination of T. gondii in cells and accelerating the elimination by the host. It is suggested that the wx2 gene may promote its proliferation and survival in the host by inhibiting the activation of the NLRP3 inflammasome, caspase-1, and GSDMD, and inhibiting the classical pyroptotic pathway. In addition, we found that the mRNA level of wx2 expression in the complemented strain RHwx2+/+ could not be completely restored to the wild-type strain, which may be related to the CRISP/cas9 technology itself. When a gene is repeatedly knocked out and recovered, it will affect the expression ability of the gene itself, resulting in a difference from the wild type, but the corresponding phenotype can still be observed.
Since macrophages are one of the main cell types infected by T. gondii [50], therefore, macrophage pyroptosis may be a host mechanism to prevent parasite proliferation in the host. In addition, cytokines released by pyroptotic macrophages may attract other immune cells such as dendritic cells to fight infection. Therefore, T. gondii-induced pyroptosis of these cells can also inhibit the spread of T. gondii. Our next experiments also need to detect the level of pyroptosis of other immune cells to further reveal their anti-Toxoplasma mechanisms. But, in general, the wx2 gene is a virulence-related gene of T. gondii, and it may participate in the immune regulation of the host by inhibiting the classical pathway of pyroptosis. In addition, since wx2 was expressed not only in the RH strain but also in the ME49 strain, it suggests that wx2 was expressed in both the acute and chronic phases, and can be used as a potential target for T. gondii detection.
Conclusion
wx2 is a virulence-related gene of T. gondii, and it may be regulate the host immune response by inhibiting the pyroptosis pathway.
Supplementary Information
Additional file 1: Figure S1. Construction of the complementary strain. (a) Frame construction diagram of wx2 complementing strain. Insertion of the wx2 fragment into the Ptub::GOI::CAT plasmid and obtaining a vector contain tub promoter, CDS terminator of the wx2 gene, and CDS region of the CAT gene. (b) Identification of the complementary strain of RHwx2+/+, by the detection of the insertion of wx2 into the RHwx2-/- strain. Lane1, RHwx2-/- strain as a positive control. Lane 2-7, clone 1-6 from pTub::GOI::CAT - wx2 plasmid transformation to RHwx2-/- strain. wx2 fragment was detected in lane 5, namely, clone 4, which meant successful construction and screening of RHwx2+/+ strain. (c) Identification of the wx2 expression at the mRNA level in the RHwx2-/-, RH, and RHwx2+/+ strains. The wx2 expression level of the RHwx2+/+ strain was higher than that of the knockout RHwx2-/- strain but lower than that of the wild-type RH.
Acknowledgements
We appreciate the generous technical support from Dr. Jinlei Wang. We would like to thank Dr, Modeste Judes for English language assistance.
Abbreviations
- T. gondii
Toxoplasma gondii
- Th
Helper T lymphocytes
- IL
Interleukin
- NK
Natural killer
- IFN
Interferon
- PVM
Parasitophorous vacuole membrane
- ROP
Rhoptries
- GRA
Granules
- STAT
Signal transducer and activator of transcription
- LPS
Lipopolysaccharide
- TNF
Tumor necrosis factor
- TLR
Toll-like receptor
- TgIST
Toxoplasma inhibitor of STAT1-dependent transcription;
- GSDMD
Gasdermin D
- NLR
Nod-like receptor
- PAMP
Pathogen-associated molecular patterns
- DAMP
Damage-associated molecular patterns
- HAMP
Hepcidin antimicrobial peptide
- PCR
Polymerase chain reaction
- SPF
Specific-pathogen-free
- qRT-PCR
Quantitative real-time PCR
- HBV
Hepatitis B virus
Author contributions
XW conceived and designed the project. ZL, ZM, and NZ performed the experiments. RJ and KY performed construction of the KO and complementary strains. ND, XL, BL, and YH analyzed the data. XW, ZL, and ZM wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Sciences Foundation of China (82072306).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
All experimental animals were used according to and with the approval of the Local Ethics Committee for the Use of Animals at the Central South University (Changsha, China). The microorganisms included in this study were handled according to the General Biosafety Standard for Microbiological and Biomedical Laboratories of the People’s Republic of China with protocol number WS233-2002.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhenrong Ma, Email: 206501021@csu.edu.cn.
Zhuolin Li, Email: lizhuolin_csu@163.com.
Ruolan Jiang, Email: 727262713@qq.com.
Xuanwu Li, Email: 1049933494@qq.com.
Kang Yan, Email: 973231034@qq.com.
Ni Zhang, Email: zhangni18307439637@163.com.
Bin Lu, Email: lubinxy@csu.edu.cn.
Yehong Huang, Email: huangyehong0907@163.com.
Nouhoum Dibo, Email: nouhoum.dibo@yahoo.fr.
Xiang Wu, Email: wuxiang@csu.edu.cn.
References
- 1.Dubey JP. The history of Toxoplasma gondii–the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. 2017;4:e177–e188. doi: 10.1016/S2352-3018(17)30005-X. [DOI] [PubMed] [Google Scholar]
- 3.Rostami A, Riahi SM, Fakhri Y, Saber V, Hanifehpour H, Valizadeh S, et al. The global seroprevalence of Toxoplasma gondii among wild boars: a systematic review and meta-analysis. Vet Parasitol. 2017;244:12–20. doi: 10.1016/j.vetpar.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol. 2014;14:51–59. doi: 10.1038/nri3561. [DOI] [PubMed] [Google Scholar]
- 5.Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol. 2005;5:162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 6.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, et al. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–4834. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoudzadeh S, Nozad Charoudeh H, Marques CS, Bahadory S, Ahmadpour E. The role of IL-12 in stimulating NK cells against Toxoplasma gondii infection: a mini-review. Parasitol Res. 2021;120:2303–2309. doi: 10.1007/s00436-021-07204-w. [DOI] [PubMed] [Google Scholar]
- 9.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii infection. J Immunol. 2008;180:5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 11.Shen B, Brown KM, Lee TD, Sibley LD. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. mBio. 2014;5:01114–01114. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinai AP. Biogenesis of and activities at the Toxoplasma gondii parasitophorous vacuole membrane. Subcell Biochem. 2008;47:155–164. doi: 10.1007/978-0-387-78267-6_12. [DOI] [PubMed] [Google Scholar]
- 13.Venugopal K, Marion S. Secretory organelle trafficking in Toxoplasma gondii: A long story for a short travel. Int J Med Microbiol. 2018;308:751–760. doi: 10.1016/j.ijmm.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Ihara F, Nishikawa Y. Toxoplasma gondii manipulates host cell signaling pathways via its secreted effector molecules. Parasitol Int. 2021;83:102368. doi: 10.1016/j.parint.2021.102368. [DOI] [PubMed] [Google Scholar]
- 15.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Christian DA, Kochanowsky JA, Phan AT, Clark JT, Wang S, et al. The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J Exp Med. 2020 doi: 10.1084/jem.20181757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, et al. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011;7:e1002236. doi: 10.1371/journal.ppat.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan CE, Sukhumavasi W, Butcher BA, Denkers EY. Functional aspects of Toll-like receptor/MyD88 signalling during protozoan infection: focus on Toxoplasma gondii. Clin Exp Immunol. 2009;156:17–24. doi: 10.1111/j.1365-2249.2009.03876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gay G, Braun L, Brenier-Pinchart MP, Vollaire J, Josserand V, Bertini RL, et al. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-gamma-mediated host defenses. J Exp Med. 2016;213:1779–1798. doi: 10.1084/jem.20160340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapira S, Harb OS, Margarit J, Matrajt M, Han J, Hoffmann A, et al. Initiation and termination of NF-kappaB signaling by the intracellular protozoan parasite Toxoplasma gondii. J Cell Sci. 2005;118:3501–3508. doi: 10.1242/jcs.02428. [DOI] [PubMed] [Google Scholar]
- 21.Mammari N, Halabi MA, Yaacoub S, Chlala H, Darde ML, Courtioux B. Toxoplasma gondii Modulates the host cell responses: an overview of apoptosis pathways. Biomed Res Int. 2019;2019:6152489. doi: 10.1155/2019/6152489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blume M, Seeber F. Metabolic interactions between Toxoplasma gondii and its host. Res. 2018 doi: 10.12688/f1000research.16021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell. 2020;180:941–955e920. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 25.Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- 26.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 28.Itani S, Watanabe T, Nadatani Y, Sugimura N, Shimada S, Takeda S, et al. NLRP3 inflammasome has a protective effect against oxazolone-induced colitis: a possible role in ulcerative colitis. Sci Rep. 2016 doi: 10.1038/srep39075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo Y, Chen L, Gu H, He X, Ye Z, Wang Z, et al. GSDMD-mediated pyroptosis: a critical mechanism of diabetic nephropathy. Expert Rev Mol Med. 2021;23:e23. doi: 10.1017/erm.2021.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai S, Ye B, Zhong L, Chen Y, Hong G, Zhao G, et al. GSDMD mediates LPS-induced septic myocardial dysfunction by regulating ROS-dependent NLRP3 inflammasome activation. Front Cell Dev Biol. 2021;9:779432. doi: 10.3389/fcell.2021.779432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Man SM, Kanneganti TD. Gasdermin D: the long-awaited executioner of pyroptosis. Cell Res. 2015;25:1183–1184. doi: 10.1038/cr.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Z, Yan K, Jiang R, Guan J, Yang L, Huang Y, et al. A novel wx2 gene of Toxoplasma gondii inhibits the parasitic invasion and proliferation in vitro and attenuates virulence in vivo via immune response modulation. Front Microbiol. 2020;11:399. doi: 10.3389/fmicb.2020.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JL, Huang SY, Li TT, Chen K, Ning HR, Zhu XQ. Evaluation of the basic functions of six calcium-dependent protein kinases in Toxoplasma gondii using CRISPR-Cas9 system. Parasitol Res. 2016;115:697–702. doi: 10.1007/s00436-015-4791-6. [DOI] [PubMed] [Google Scholar]
- 34.Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasai M, Pradipta A, Yamamoto M. Host immune responses to Toxoplasma gondii. Int Immunol. 2018;30:113–119. doi: 10.1093/intimm/dxy004. [DOI] [PubMed] [Google Scholar]
- 36.Kuriakose T, Man SM, Malireddi RKS, Karki R, Kesavardhana S, Place DE, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016 doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Dong P, Xu L, Tian Y, Sun H, Shi H, et al. The different expression of caspase-1 in HBV-related liver disease and acts as a biomarker for acute-on-chronic liver failure. BMC Gastroenterol. 2019;19:148. doi: 10.1186/s12876-019-1064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan TY, Chu JJH. Dengue virus-infected human monocytes trigger late activation of caspase-1, which mediates pro-inflammatory IL-1beta secretion and pyroptosis. J Gen Virol. 2013;94:2215–2220. doi: 10.1099/vir.0.055277-0. [DOI] [PubMed] [Google Scholar]
- 39.de Carvalho RVH, Zamboni DS. Inflammasome activation in response to intracellular protozoan parasites. Trends Parasitol. 2020;36:459–472. doi: 10.1016/j.pt.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, Vance RE. Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science. 2019 doi: 10.1126/science.aau1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kongsomboonvech AK, Rodriguez F, Diep AL, Justice BM, Castallanos BE, Camejo A, et al. Naïve CD8 T cell IFNγ responses to a vacuolar antigen are regulated by an inflammasome-independent NLRP3 pathway and Toxoplasma gondii ROP5. PLoS Pathog. 2020;16:e1008327. doi: 10.1371/journal.ppat.1008327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki S, Franchi L, He Y, Munoz-Planillo R, Mimuro H, Suzuki T, et al. Shigella type III secretion protein MxiI is recognized by Naip2 to induce Nlrc4 inflammasome activation independently of Pkc delta. Plos Pathogens. 2014 doi: 10.1371/journal.ppat.1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan JH, Huang R, Wang Z, Huang S, Choi IW, Zhou Y, et al. P2X7 receptor mediates NLRP3-dependent IL-1beta secretion and parasite proliferation in Toxoplasma gondii-infected human small intestinal epithelial cells. Parasit Vectors. 2018;11:1. doi: 10.1186/s13071-017-2573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandori WJ, Lima TS, Mallya S, Kao TH, Gov L, Lodoen MB. Toxoplasma gondii activates a Syk-CARD9-NF-kappaB signaling axis and gasdermin D-independent release of IL-1beta during infection of primary human monocytes. PLoS Pathog. 2019;15:e1007923. doi: 10.1371/journal.ppat.1007923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gov L, Schneider CA, Lima TS, Pandori W, Lodoen MB. NLRP3 and potassium efflux drive rapid IL-1beta release from primary human monocytes during Toxoplasma gondii infection. J Immunol. 2017;199:2855–2864. doi: 10.4049/jimmunol.1700245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohshima J, Lee Y, Sasai M, Saitoh T, Su Ma J, Kamiyama N, et al. Role of mouse and human autophagy proteins in IFN-gamma-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. 2014;192:3328–3335. doi: 10.4049/jimmunol.1302822. [DOI] [PubMed] [Google Scholar]
- 48.Takacs AC, Swierzy IJ, Luder CG. Interferon-gamma restricts Toxoplasma gondii development in murine skeletal muscle cells via nitric oxide production and immunity-related GTPases. PLoS ONE. 2012;7:e45440. doi: 10.1371/journal.pone.0045440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity. 2016;44:659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen KD, Wang Y, Wojno ED, Shastri AJ, Hu K, Cornel L, et al. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe. 2011;9:472–483. doi: 10.1016/j.chom.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Construction of the complementary strain. (a) Frame construction diagram of wx2 complementing strain. Insertion of the wx2 fragment into the Ptub::GOI::CAT plasmid and obtaining a vector contain tub promoter, CDS terminator of the wx2 gene, and CDS region of the CAT gene. (b) Identification of the complementary strain of RHwx2+/+, by the detection of the insertion of wx2 into the RHwx2-/- strain. Lane1, RHwx2-/- strain as a positive control. Lane 2-7, clone 1-6 from pTub::GOI::CAT - wx2 plasmid transformation to RHwx2-/- strain. wx2 fragment was detected in lane 5, namely, clone 4, which meant successful construction and screening of RHwx2+/+ strain. (c) Identification of the wx2 expression at the mRNA level in the RHwx2-/-, RH, and RHwx2+/+ strains. The wx2 expression level of the RHwx2+/+ strain was higher than that of the knockout RHwx2-/- strain but lower than that of the wild-type RH.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.