Abstract
Aims
The effect of the COVID-19 pandemic on care and outcomes across non-COVID-19 cardiovascular (CV) diseases is unknown. A systematic review and meta-analysis was performed to quantify the effect and investigate for variation by CV disease, geographic region, country income classification and the time course of the pandemic.
Methods and results
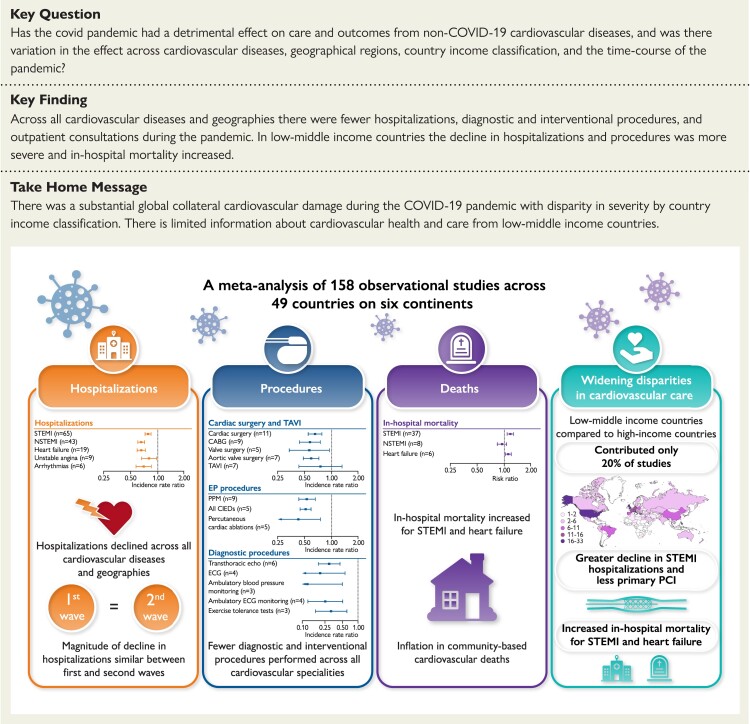
From January 2019 to December 2021, Medline and Embase databases were searched for observational studies comparing a pandemic and pre-pandemic period with relation to CV disease hospitalisations, diagnostic and interventional procedures, outpatient consultations, and mortality. Observational data were synthesised by incidence rate ratios (IRR) and risk ratios (RR) for binary outcomes and weighted mean differences for continuous outcomes with 95% confidence intervals. The study was registered with PROSPERO (CRD42021265930). A total of 158 studies, covering 49 countries and 6 continents, were used for quantitative synthesis. Most studies (80%) reported information for high-income countries (HICs). Across all CV disease and geographies there were fewer hospitalisations, diagnostic and interventional procedures, and outpatient consultations during the pandemic. By meta-regression, in low-middle income countries (LMICs) compared to HICs the decline in ST-segment elevation myocardial infarction (STEMI) hospitalisations (RR 0.79, 95% confidence interval [CI] 0.66–0.94) and revascularisation (RR 0.73, 95% CI 0.62–0.87) was more severe. In LMICs, but not HICs, in-hospital mortality increased for STEMI (RR 1.22, 95% CI 1.10–1.37) and heart failure (RR 1.08, 95% CI 1.04–1.12). The magnitude of decline in hospitalisations for CV diseases did not differ between the first and second wave.
Conclusions
There was substantial global collateral CV damage during the COVID-19 pandemic with disparity in severity by country income classification.
Keywords: Cardiovascular, COVID-19, Hospitalization, Mortality, Treatment
Structured Graphical Abstract
Structured Graphical Abstract.
Major findings of the collateral damage of the COVID-19 pandemic on cardiovascular services. Abbreviations in text.
Listen to the audio abstract of this contribution.
Introduction
During the coronavirus disease 2019 (COVID-19) pandemic, reports described fewer hospitalizations, procedures, and consultations for non-COVID-19 cardiovascular (CV) diseases.1–3 After a short period of ‘recovery’, the emergence and rapid spread of the Omicron variant triggered the re-introduction of ‘lockdown’ restrictions,4,5 portending a future of preparing for and coping with waves of the contagion.
Previous systematic reviews of the impact of the COVID-19 pandemic on CV services have provided an incomplete overview. Some studies focused on hospitalizations,6,7 others were restricted to specific conditions,8–16 and one investigated only a specific outcome.17 Only one report has considered the impact of the pandemic across different geographic territories, and was limited to one CV care pathway.9 None has considered whether the effect of the pandemic on CV services has varied over time. A quantitative understanding of the global impact of the COVID-19 pandemic on the breadth of CV services and health of individuals with CV disease could facilitate better preparation for future waves.
We therefore provide a systematic review of the literature with a meta-analysis to quantify the effects of the pandemic on CV services in terms of access, treatment, and outcomes. We investigate the occurrence of variation across CV conditions, geographic region, country income classification, and the time course of the pandemic. Finally, we consider how to better manage CV services to minimize collateral CV damage.
Methods
We searched the Medline and Embase databases through the Ovid platform from 1 January 2019 through 15 December 2021 (because the earliest case was diagnosed in Wuhan, China in November 2019) for studies that reported a comparison of hospitalizations, diagnostic and interventional procedures, outpatient and community consultations, and mortality. The full search strategy is available in Supplementary material online, S1. We defined CV services as healthcare services provided by any CV practitioner (cardiologist, cardiac surgeon, cardiac physiologist, cardiac nurse, or trainee) relating to CV diseases specified in the ESC Textbook of Cardiovascular Medicine.18 We excluded CV diseases where care would primarily be overseen by other medical and surgical specialities—venous thrombo-embolism and peripheral vascular diseases (including aortic, peripheral arterial, and cerebrovascular disease)—which have been summarized elsewhere.6,19 This review was registered on PROSPERO (CRD42021265930) and informed by the PRISMA statement (see Supplementary material online, Table S63).20 The risk of bias for each report for each outcome was assessed using the ROBINS-I tool.21 Reports with critical risk of bias were excluded.
We undertook quantitative syntheses of cohort studies that compared the COVID-19 pandemic period and a pre-pandemic period (all definitions in Supplementary material online, S1). A meta-analysis was performed to synthesize observational data for binary and continuous outcomes. Incidence rate ratios (IRRs, a comparison of incidence rates during each period) and risk ratios (RRs, a ratio of the probability of an event occurring in the intervention compared with the probability of the event occurring in the control, where each event is independent) were used for binary outcomes and counts data; weighted mean differences (WMDs) were used for continuous outcomes measured with the same scale. The DerSimonian and Laird random effects models were fitted in all analyses because of the variation amongst studies in population, intervention, comparator, timing, and setting.22 Funnel plots and Egger’s test were used to assess publication bias.23 Heterogeneity scores were measured by the I² statistic and Cochran’s Q test, with 40% or P < 0.10, respectively, indicative of substantial heterogeneity.24 Where quantitative synthesis could not be undertaken, we have provided a narrative synthesis.
To explore for differences in effect of the pandemic across geographic boundaries, country wealth, and time course, we performed meta-regression by geographic region, country-level income, and wave of pandemic covered by each report. Geographic regions were defined as Europe, North America, and other countries, and country-level income as high income (HIC) vs. low–middle income (LMIC) using the World Bank classification of income.25 We also investigated for sources of heterogeneity by meta-regression of a range of study characteristics: sample size, data source, duration of study period during the pandemic, presence or absence of matched comparator periods, study definition of pandemic period, and whether or not patients with co-existent COVID-19 diagnosis were included. Detailed methods are available in Supplementary material online, S2.
Results
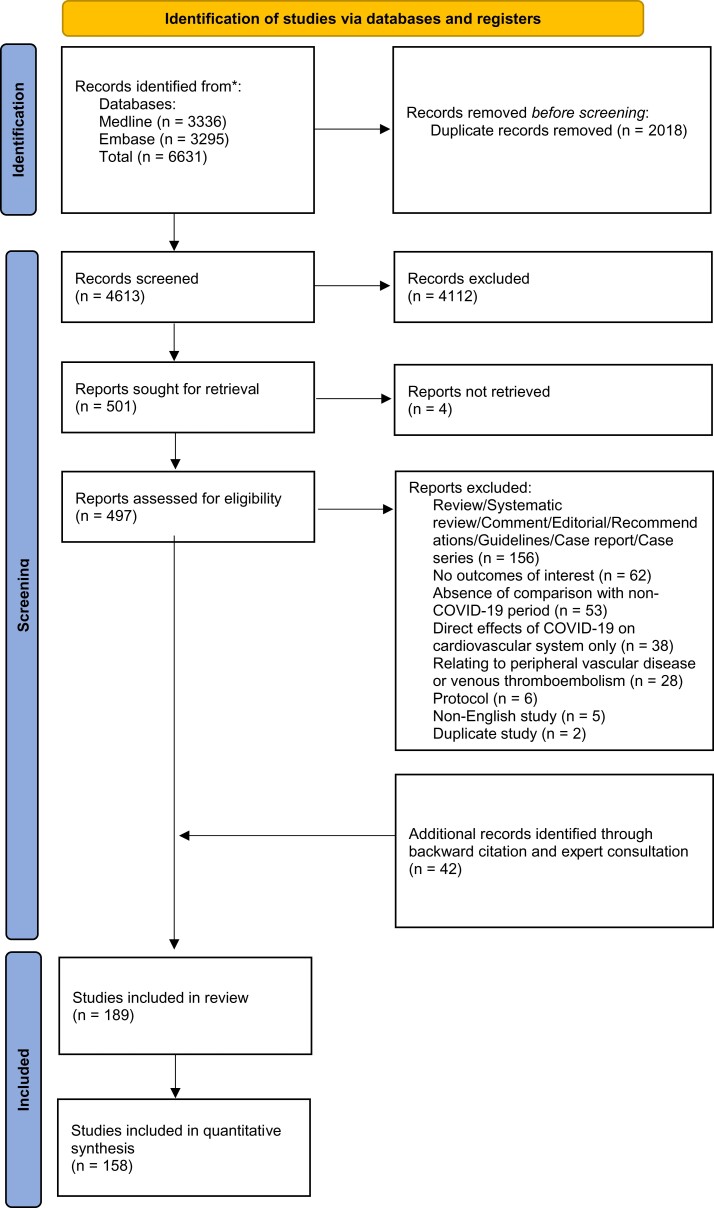
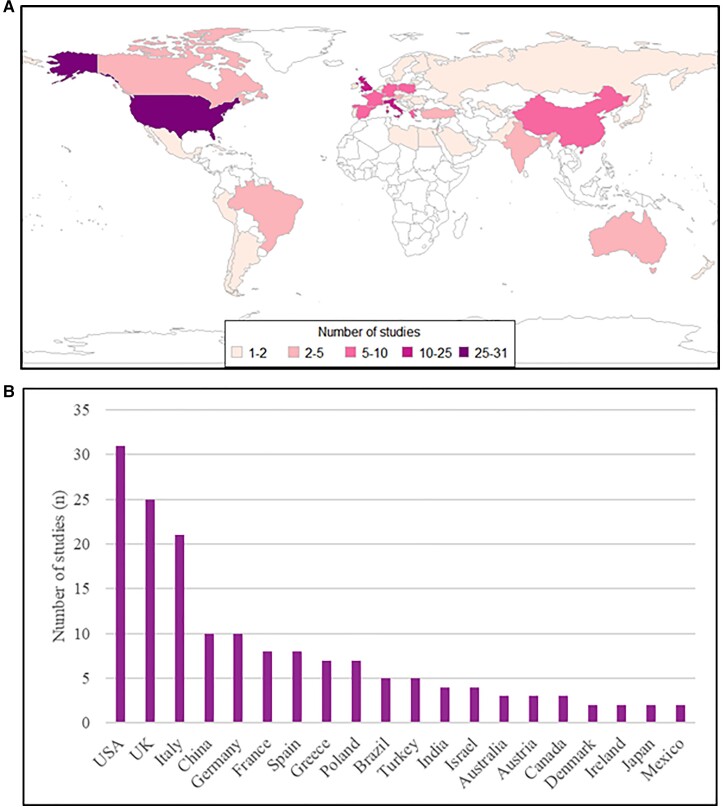
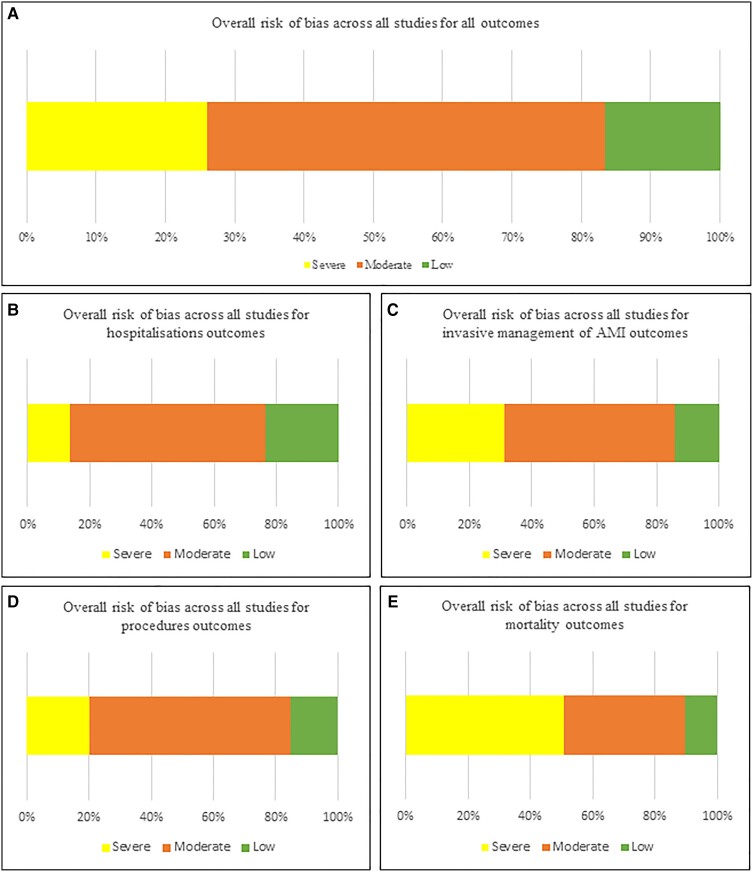
We identified 4613 unique records, reviewed 497 full-text reports, and included 189 studies,158 of which were used in quantitative synthesis (Supplementary material online, S4 Tables S38–S61). Figure 1 shows the PRISMA flow diagram. In total, 49 countries were covered across six continents. There was geographic and economic disparity in the number of available studies; the majority were from Europe (n = 111, 59%; of which the UK n = 25, 13%, and Italy n = 21, 11%) and North America (n = 34, 18%) (Figure 2). Most studies provided information exclusively relating to HICs (n = 151, 80%). Over half of studies described acute coronary syndromes (ACS) (n = 96, 51%), followed by heart failure (HF) (n = 16, 8%) and arrhythmias (n = 15, 8%). The vast majority of studies reported data from the first wave of the pandemic (n = 152, 80%). A minority of studies (n = 19, 10%) excluded patients diagnosed with concurrent SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection. We classified 26% of studies across all outcomes as being at severe risk of bias, with 57% at moderate risk of bias (Figure 3; Supplementary material online, S3 Tables S1–S37). Confounding was the most common source of elevated risk of bias (26% severe, 56% moderate). Studies reporting mortality outcomes were the most likely to be classified as being at severe risk of bias (51%), partly due to incomplete reporting of concurrent SARS-CoV-2 infection. Egger’s test did not identify any significant publication bias (Supplementary material online, S6 Figures S19–S22; all P-values were non-significant).
Figure 1.
Flowchart of selected studies. Flowchart based on the Preferred Reported Items for Systematic Review and Meta-Analysis (PRISMA) statement.
Figure 2.
The origin of included studies demonstrated on a global choropleth (A), and a chart including the number of studies per country for the 20 most commonly represented countries (B).
Figure 3.
Summary of overall risk of bias scores assessed using the ROBINS-I tool for all studies across all outcomes (A) and subdivided by categories of outcomes (B–E). AMI, acute myocardial infarction.
Acute cardiovascular disease hospitalizations
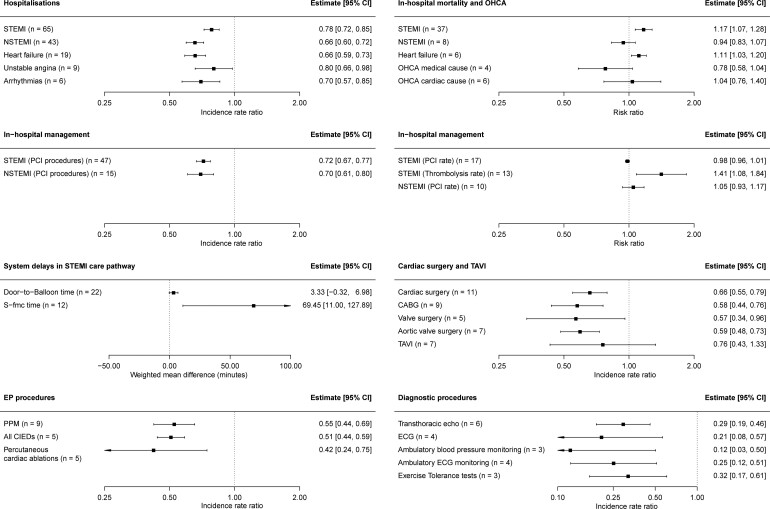
Hospitalizations declined across the breadth of CV disease during the pandemic. Hospitalization rates for each subtype of ACS declined; ST-elevation myocardial infarction (STEMI) [IRR 0.78, 95% confidence interval (CI) 0.72–0.85, I2 = 97.4%], non-STEMI (NSTEMI) (IRR 0.66, 95% CI 0.60–0.72, I2 = 98.3%), and unstable angina (IRR 0.80, 95% CI 0.66–0.98, I2 = 85.8%) (Figure 4; Supplementary material online, S1–S3). Hospitalizations for HF declined during the pandemic (IRR 0.66, 95% CI 0.59–0.73, I2 = 99.9%) (Supplementary material online, Figure S4), reflective of a decline in admissions with both decompensated chronic HF and de novo presentations.26
Figure 4.
Summary estimates for analyses across hospitalizations, in-hospital management, diagnostic and interventional procedures, and mortality. The full forest plots for each analysis are available in Supplementary material online, Figures S1–S18. EP, electrophysiology.
The total number of hospitalizations for arrhythmias also declined (IRR 0.70, 95% CI 0.57–0.85, I2 = 95.2%) (Supplementary material online, Figure S5), an effect consistently reported for each of bradyarrhythmias,27–29 atrial fibrillation/flutter,30–32 and ventricular arrhythmias (VAs).28 However, studies reporting arrhythmias detected by remote monitoring of cardiac implantable electronic devices (CIEDs) painted a different picture of arrhythmia incidence in the community in individuals with CV disease. Three studies reported increases in episodes of atrial fibrillation during the pandemic, which correlated with areas of high COVID-19 prevalence.33–35 During the peak COVID-19 incidence in New York City, New Orleans, and Boston, an increase in implantable cardioverter defibrillator (ICD) shock burden was observed,36 whilst two large studies found a reduction in VA incidence amongst individuals with ICDs after major public health restrictions.37,38
On meta-regression, we found that the decline in hospitalizations for CV disease was consistent across different geographical regions (Supplementary material online, Table S62). However, there was a greater decline in STEMI hospitalizations during the pandemic in LMICs (RR = 0.79, 95% CI 0.66–0.94). Notably, between the first and second wave, we found no difference in decline of hospitalizations for STEMI, NSTEMI, and HF. However, studies that reported data pertaining to a longer time span during the pandemic demonstrated a less extreme effect size for decline in hospitalizations for STEMI and NSTEMI compared with studies that reported a shorter time span (STEMI hospitalizations RR 1.17, 95% CI 1.00–1.38; NSTEMI hospitalizations RR 1.30, 95% CI 1.09–1.57).
For other acute CV presentations, there is limited evidence for the impact of the pandemic. A single-centre study reported that the number of hospitalizations with pericarditis and hypertensive crisis did not increase during the pandemic.39 A Danish nationwide study of infective endocarditis (IE) hospitalizations found no difference during the pandemic, whereas a Mexican single-centre study showed a 93% reduction.40,41 One single-centre study reported a decline in hospitalizations with adult congenital heart disease (ACHD) during the pandemic,42 and two studies demonstrated a significant increase in the incidence of stress cardiomyopathy.43,44
Invasive management of acute myocardial infarction
The number of percutaneous coronary intervention (PCI) procedures for STEMI and NSTEMI declined during the pandemic to a similar extent to the decline in hospitalizations (PCI for STEMI, IRR 0.72, 95% CI 0.67–0.77, I2 = 92.5%; PCI for NSTEMI, IRR 0.70, 95% CI 0.61–0.80, I2 = 88.1%) (Figure 4; Supplementary material online, S6 and S7). However, amongst patients hospitalized for STEMI and NSTEMI, the proportion who received revascularization did not change during the pandemic (PCI for STEMI hospitalizations, RR 0.98, 95% CI 0.96–1.01, I2 = 82.3%; PCI for NSTEMI hospitalizations, RR 1.05, 95% CI 0.93–1.17, I2 = 88.3%) (Supplementary material online, Figures S8 and S9).
The detrimental effect of the pandemic is evident in system delays related to the STEMI care pathway. Whilst door-to-balloon times (D2B) did not increase significantly during the pandemic (WMD 3.33 min, 95% CI −0.32 to 6.98 min, I2 = 94.2%) we estimated that there was over an hour greater delay between symptoms to first medical contact (S-FMC) during the pandemic (WMD 69.45 min, 95% CI 11.00–127.89 min, I2 = 99.4%) (Supplementary material online, Figure S10).
There was divergence by geographic region and country-level income in the management of acute myocardial infarction during the pandemic. Meta-regression demonstrated that the decline in revascularization was greater in LMICs compared with HICs (PCI for STEMI, RR 0.73, 95% CI 0.62–0.87; PCI for NSTEMI, RR 0.69, 95% CI 0.48–0.99) (Supplementary material online, Table S62). Increases in D2B and S-FMC time were only found to be significant in countries outside of Europe and North America (Table 1). Finally, the proportion of patients treated for STEMI with thrombolysis increased during the pandemic (RR 1.41, 95% CI 1.08–1.84, I2 = 55.3%) (Supplementary material online, Figure S8), driven by increased use of thrombolysis in LMICs and countries outside of Europe and North America (Table 1).
Table 1.
Summary estimates for all outcomes by subgroups of geographical region and country-level income classification
| Geographic region | Country-level income | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All data | Europe | North America | Other countries | High-income countries | Low–middle income countries | |||||||
| Study | Estimate (95% CI) | Study | Estimate (95% CI) | Study | Estimate (95% CI) | Study | Estimate (95% CI) | Study | Estimate (95% CI) | Study | Estimate (95% CI) | |
| Hospitalization (IRR) | ||||||||||||
| STEMI | 65 | 0.78 (0.72–0.85) | 39 | 0.80 (0.74–0.87) | 7 | 0.83 (0.73–0.94) | 19 | 0.72 (0.58–0.89) | 48 | 0.82 (0.77–0.88) | 17 | 0.68 (0.54–0.85) |
| NSTEMI | 43 | 0.66 (0.60–0.72) | 29 | 0.68 (0.60–0.76) | 3 | 0.71 (0.66–0.75) | 11 | 0.60 (0.51–0.71) | 32 | 0.68 (0.61–0.76) | 11 | 0.58 (0.49–0.69) |
| Unstable angina | 9 | 0.80 (0.66–0.98) | 6 | 0.77 (0.63–0.95) | 0 | 3 | 0.88 (0.53–1.49) | 7 | 0.78 (0.60–1.02) | 2 | 0.85 (0.69–1.03) | |
| Heart failure | 19 | 0.66 (0.59–0.73) | 12 | 0.70 (0.65–0.74) | 5 | 0.58 (0.41–0.84) | 2 | 0.65 (0.39–1.08) | 17 | 0.66 (0.58–0.74) | 2 | 0.65 (0.39–1.08) |
| Arrhythmias | 6 | 0.70 (0.57–0.85) | 5 | 0.73 (0.59–0.90) | 0 | 1 | 0.51 (0.36–0.72) | 4 | 0.75 (0.58–0.99) | 2 | 0.61 (0.49–0.77) | |
| AMI management (IRR or RR) | ||||||||||||
| STEMI (PCI procedures, IRR) | 47 | 0.72 (0.67–0.77) | 28 | 0.75 (0.70–0.80) | 3 | 0.75 (0.58–0.97) | 16 | 0.66 (0.54–0.79) | 37 | 0.76 (0.71–0.81) | 10 | 0.60 (0.49–0.72) |
| NSTEMI (PCI procedures, IRR) | 15 | 0.70 (0.61–0.80) | 10 | 0.72 (0.66–0.78) | 2 | 0.75 (0.67–0.83) | 3 | 0.59 (0.31–1.13) | 12 | 0.72 (0.67–0.77) | 3 | 0.59 (0.31–1.13) |
| STEMI (thrombolysis rate, RR) | 13 | 1.41 (1.08–1.84) | 6 | 1.02 (0.80–1.29) | 0 | 7 | 2.18 (1.10–4.31) | 7 | 1.07 (0.87–1.33) | 6 | 2.70 (1.07–6.86) | |
| STEMI (PCI rate, RR) | 17 | 0.98 (0.96–1.01) | 9 | 0.99 (0.96–1.02) | 1 | 1.04 (1.00–1.09) | 7 | 0.96 (0.93–1.00) | 12 | 0.99 (0.97–1.02) | 5 | 0.89 (0.72–1.10) |
| NSTEMI (PCI rate, RR) | 10 | 1.05 (0.93–1.17) | 4 | 1.06 (0.88–1.29) | 2 | 1.15 (0.91–1.46) | 4 | 0.99 (0.80–1.22) | 6 | 1.02 (0.95–1.10) | 4 | 1.12 (0.83–1.52) |
| Delays in STEMI care (WMD, minutes) | ||||||||||||
| Symptom to first medical contact time | 12 | 69.5 (11.0 to 127.9) | 5 | 14.6 (–11.1 to 40.3) | 2 | 225.1 (–23.1–473.2) | 5 | 48.0 (7.1– 88.9) | 7 | 85.4 (14.5–185.3) | 5 | 48.0 (7.1– 88.9) |
| Door-to-balloon time | 22 | 3.3 (–0.3 to 7.0) | 11 | 0.9 (–2.8 to 4.8) | 2 | –1.7 (–4.5 to 1.2) | 9 | 8.4 (0.6– 16.2) | 17 | 0.9 (–2.2 to 4.0) | 5 | 9.5 (–0.5 to 19.4) |
| Cardiac surgery and TAVI (IRR) | ||||||||||||
| Cardiac surgery | 11 | 0.66 (0.55–0.79) | 5 | 0.59 (0.41–0.84) | 2 | 0.66 (0.61–0.72) | 4 | 0.87 (0.85–0.88) | 9 | 0.64 (0.53–0.79) | 2 | 0.76 (0.49–1.18) |
| CABG | 9 | 0.58 (0.44–0.76) | 4 | 0.45 (0.33–0.61) | 2 | 0.78 (0.39–1.55) | 3 | 0.62 (0.39–1.00) | 7 | 0.59 (0.42–0.82) | 2 | 0.54 (0.29–1.03) |
| Valve surgery | 5 | 0.57 (0.34–0.96) | 3 | 0.53 (0.29–0.96) | 2 | 0.59 (0.17–2.02) | 0 | 5 | 0.57 (0.34–0.96) | 0 | ||
| Aortic valve surgery | 7 | 0.59 (0.48–0.73) | 6 | 0.56 (0.47–0.67) | 0 | 1 | 1.13 (0.57–2.27) | 7 | 0.59 (0.48–0.73) | 0 | ||
| TAVI | 7 | 0.76 (0.43–1.33) | 6 | 0.65 (0.37–1.14) | 1 | 1.83 (1.67–2.00) | 0 | 7 | 0.76 (0.43–1.33) | 0 | ||
| EP procedures (IRR) | ||||||||||||
| PPM | 8 | 0.55 (0.44–0.69) | 6 | 0.54 (0.45–0.64) | 0 | 2 | 0.58 (0.23–1.44) | 6 | 0.54 (0.45–0.64) | 2 | 0.58 (0.23–1.44) | |
| All CIED | 5 | 0.51 (0.44–0.59) | 5 | 0.51 (0.44–0.59) | 0 | 0 | 5 | 0.51 (0.44–0.59) | 0 | |||
| Percutaneous catheter ablation | 5 | 0.42 (0.24–0.75) | 3 | 0.47 (0.22–0.97) | 1 | 0.20 (0.17–0.24) | 1 | 0.68 (0.46–1.03) | 5 | 0.42 (0.24–0.75) | 0 | |
| Diagnostic procedures (IRR) | ||||||||||||
| Transthoracic echo | 6 | 0.29 (0.19–0.46) | 4 | 0.28 (0.16–0.47) | 0 | 2 | 0.33 (0.11–1.00) | 4 | 0.28 (0.16–0.47) | 2 | 0.33 (0.11–1.00) | |
| ECG | 4 | 0.21 (0.08–0.57) | 2 | 0.22 (0.08–0.60) | 0 | 2 | 0.19 (0.02–1.82) | 2 | 0.22 (0.08–0.60) | 2 | 0.19 (0.02–1.82) | |
| ABPM | 3 | 0.12 (0.03–0.50) | 1 | 0.22 (0.14–0.33) | 0 | 2 | 0.08 (0.01–0.93) | 1 | 0.22 (0.14–0.33) | 2 | 0.08 (0.01–0.93) | |
| Ambulatory ECG monitoring | 4 | 0.25 (0.12–0.51) | 2 | 0.28 (0.23–0.34) | 0 | 2 | 0.19 (0.03–1.39) | 2 | 0.28 (0.23–0.34) | 2 | 0.19 (0.03–1.39) | |
| Exercise tolerance tests | 3 | 0.32 (0.17–0.61) | 1 | 0.47 (0.32–0.69) | 0 | 2 | 0.26 (0.10–0.66) | 1 | 0.47 (0.32–0.69) | 2 | 0.26 (0.10–0.66) | |
| Mortality (RR) | ||||||||||||
| STEMI | 37 | 1.17 (1.07–1.28) | 18 | 1.20 (1.04–1.38) | 3 | 0.97 (0.56–1.69) | 16 | 1.14 (1.04–1.26) | 23 | 1.11 (0.97–1.28) | 14 | 1.22 (1.10–1.37) |
| NSTEMI | 8 | 0.94 (0.83–1.07) | 5 | 0.94 (0.82–1.07) | 0 | 3 | 1.12 (0.44–2.86) | 4 | 0.94 (0.82–1.07) | 4 | 1.06 (0.55–2.05) | |
| Heart failure | 6 | 1.11 (1.03–1.20) | 4 | 1.13 (0.99–1.29) | 0 | 2 | 1.08 (1.04–1.12) | 4 | 1.13 (0.99–1.29) | 2 | 1.08 (1.04–1.12) | |
| OHCA medical cause | 4 | 0.78 (0.58–1.04) | 3 | 0.70 (0.52–0.95) | 1 | 1.03 (0.92–1.15) | 0 | 4 | 0.78 (0.58–1.04) | 0 | ||
| OHCA cardiac cause | 6 | 1.04 (0.76–1.40) | 2 | 0.91 (0.36–2.27) | 2 | 1.27 (0.79–2.03) | 2 | 0.95 (0.78–1.17) | 6 | 1.04 (0.76–1.40) | 0 | |
ABPM, ambulatory blood pressure monitoring; AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CI, confidence interval; CIED, cardiac implantable electronic device; ECG, electrocardiogram; EP, electrophysiology; IRR; incidence rate ratio; NSTEMI, non-ST-elevation myocardial infarction; OHCA, out-of-hospital cardiac arrest; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; RR, relative risk; STEMI, ST-elevation myocardial infarction, TAVI, transcatheter aortic valve implantation; WMD, weighted mean difference.
Interventional procedures
Nationwide data from the UK and the USA found that elective PCI decreased by >50% during the pandemic,45,46 and disproportionately affected older ages and Black, Asian, and minority ethnic (BAME) groups.45 During the pandemic, we observed a reduction in implantations of permanent pacemakers (IRR 0.55, 95% CI 0.44–0.69, I2 = 98.3%), implantations of all CIEDs (IRR 0.51, 95% CI 0.44–0.59, I2 = 86.0%), and the overall number of percutaneous catheter ablations performed (IRR 0.42, 95% CI 0.24–0.75, I2 = 99.4%) (Figure 4; Supplementary material online, Figure S11). In contrast, we found conflicting reports for rates of transcatheter aortic valve implantations (TAVIs) during the pandemic compared with pre-pandemic (IRR 0.76, 95% CI 0.43–1.33, I2 = 99.2%) (Supplementary material online, Figure S12). Whilst reports from most of Europe showed a decline in TAVI rates,1,47–50 there was an increase in the number of TAVI procedures performed during the pandemic in Poland and Ontario, Canada.51,52
The total number of cardiac surgical operations fell during the pandemic (IRR 0.66; 95% CI 0.55–0.79, I2 = 99.6%) (Supplementary material online, Figure S12). There were clear declines in coronary artery bypass graft (CABG) operations (IRR 0.58, 95% CI 0.44–0.76, I2 = 99.0%) and surgical interventions for the aortic valve (IRR 0.59, 95% CI 0.48–0.73, I2 = 85.6%).
Diagnostic procedures
Observational studies reporting a comparison of the number of diagnostic CV procedures during and pre-pandemic were infrequent. Available studies reported declines in exercise tolerance tests (IRR 0.32, 95% CI 0.17–0.61, I2 = 92.9%), ambulatory ECG monitoring (IRR 0.25, 95% CI 0.12–0.51, I2 = 96.6%), ambulatory blood pressure monitoring (IRR 0.12, 95% CI 0.03–0.50, I2 = 97.1%), 12-lead ECGs (IRR 0.21, 95% CI 0.08–0.57, I2 = 99.3%), and transthoracic echocardiograms (IRR 0.29, 95% CI 0.19–0.46, I2 = 98.1%) during the pandemic (Figure 4; Supplementary material online, S13). The use of diagnostic invasive coronary angiography has been reported to fall by as much as 74%.53 Single-centre studies demonstrated that transoesophageal echocardiograms, computed tomography coronary angiograms, and myocardial perfusion scans either ceased or sharply declined.27,54,55
Outpatient and community consultations
During the pandemic, we found a marked decline in in-person outpatient consultations (IRR 0.27, 95% CI 0.09–0.75, I2 = 100%) (see Supplementary material online, Figure S14). Five studies reported an increase in telemedicine cardiology outpatient appointments in both HICs and LMICs during the pandemic.54,56–59 However, multicentre reports from the USA and Germany suggested overall deficits of 61%, 33%, and 5% in outpatient CV consultations even after including telemedicine appointments.56,58,60 Surveys showed that almost half of all exercise-based cardiac rehabilitation programmes closed during the pandemic,61–63 and of programmes that continued many used technology to provide virtual consultations.62–64
Mortality
In-hospital all-cause mortality
For patients hospitalized with acute CV disease, in-hospital all-cause mortality was reported frequently and 30-day all-cause mortality rarely. For both STEMI and HF, in-hospital mortality increased during the pandemic (STEMI, RR 1.17, 95% CI 1.07–1.28, I2 = 23.3%; HF, RR 1.11, 95% CI 1.03–1.20, I2 = 63.9%) and did not differ for NSTEMI (RR 0.94, 95% CI 0.83–1.07, I2 = 0.0%) (Figure 4; Supplementary material online, S15 and S16). For both STEMI and HF, in-hospital mortality increased during the pandemic in LMICs but not in HICs (Table 1).
30-day all-cause mortality
Only six studies reported 30-day all-cause mortality for NSTEMI, STEMI, or HF.65–70 Three studies showed that 30-day mortality increased during the pandemic for NSTEMI but not STEMI.65–67 In one report, higher 30-day mortality for NSTEMI was correlated with concurrent SARS-CoV-2 infection.67 For the other two studies, infection status was not reported but primary PCI (PPCI) was ‘protected’ during the pandemic whilst patients admitted for NSTEMI received lower rates of and a greater delay to angiography.65,66 An analysis of nationwide health records described increased odds of 30-day mortality following admission with HF.70 Notably, studies of mortality in the mid- to long term suggest that these trends may continue. One-year cardiac-related mortality for patients admitted for STEMI during the pandemic was reported to be no different from a historical control group, in spite of worse in-hospital outcomes.71 Patients admitted for NSTEMI during the pandemic, who on average waited longer for revascularization, have been reported to have over twice as high a risk of all-cause mortality and a 20-fold increased risk of hospitalization with HF at 6 months compared with historical controls.72 Patients surviving hospitalization for HF during the pandemic also have higher all-cause mortality at 1 year compared with patients hospitalized in 2019, correlated with fewer receiving their inpatient care on specialist cardiology wards.73
Out-of-hospital cardiac arrest
We found no evidence for an increase during the pandemic period of out-of-hospital cardiac arrest (OHCA) of presumed medical or cardiac cause—as defined by attending emergency medical service personnel (OHCA medical cause, IRR 0.78, 95% CI 0.58–1.04, I2 = 95.1%; OHCA cardiac cause, IRR 1.04, 95% CI 0.76–1.40, I2 = 98.6%) (Figure 4; Supplementary material online, S17 and S18).
Population-level cardiovascular mortality
Four studies using UK nationwide data reported increased non-COVID-19 acute CV mortality compared with the historical average in the early months of the pandemic,74–77 with a ‘displacement of death’ occurring in homes (30.9% vs. 23.5%) and care homes (15.7% vs. 13.5%).77 In the USA, two studies demonstrated increased deaths from heart disease during the pandemic compared with previous years,78,79 with a greater excess in areas of higher density of COVID-19 infection.78 This pattern was also noted in LMICs, with the greatest excess CV mortality reported in the most deprived cities.80,81
Discussion
This systematic review and meta-analysis of the effect of the COVID-19 pandemic on CV services has identified a number of important points. First, the COVID-19 pandemic witnessed a substantial global decline in hospitalizations with acute CV disease, fewer diagnostic and interventional procedures, and fewer outpatient and community consultations. Second, we found no difference in the decline in hospitalizations for STEMI, NSTEMI, and HF during the second wave compared with the first wave. Third, there is disparity in the severity of collateral CV damage across geographic and economic boundaries. Across LMICs and countries outside of Europe and North America, we observed a more severe decline in hospitalizations and revascularization for STEMI, greater delays in STEMI care pathways with more frequent use of thrombolysis, and elevated in-hospital mortality for both STEMI and HF (Structured Graphical Abstract).
Previous reviews have observed a decline in hospitalizations for ACS during the pandemic,8–10 but here we extend the quantitative analysis of hospitalization rates to HF and arrhythmias, and demonstrate similar patterns. Other authors have shown that in-hospital mortality rose during the pandemic when studies reporting different CV diseases are combined,17 and specifically in patients who underwent PPCI for STEMI.9 In this analysis, we are able to demonstrate elevated in-hospital mortality during the pandemic for both STEMI and HF, and demonstrate variation across geographic regions and by country economic development. Finally, we provide the first estimates of the detrimental effect of the pandemic on interventional procedures, diagnostic procedures, and outpatient consultations.
We found that the decline in hospitalization for acute CV disease occurred across the breadth of CV diseases, and reports suggest that reductions occurred irrespective of formal restrictions on movement,65,82,83 or the extent of COVID-19 diagnoses within the local population.84 We observed delays to seeking help and receiving medical attention, independent reports of increased CV deaths in homes and care homes, and reports of increased case severity amongst those who did reach hospital.3,42,85–87 One may infer that fear of the contagion, ‘stay at home campaigns’, and overwhelmed emergency medical services prevented and delayed hospitalization of unwell patients. The scale of disruption to public interaction with CV services was not fully anticipated before the pandemic. In response, information campaigns, such as ‘You can’t pause a heart’ by the European Society of Cardiology (ESC),88 aimed to equilibrate public health messaging by accentuating the importance of expediently seeking medical attention for symptoms of acute CV disease. Whilst some studies reported that information campaigns quickened recovery in rates of hospitalization for acute myocardial infarction,82,83,89,90 we did not find a significant difference in the decline of hospitalization rates between the first and second wave across STEMI, NSTEMI, and HF. However, we did observe that studies reporting a longer time span of the pandemic period, and thus better reflecting both ‘decline’ and ‘recovery’ phases of hospitalization rates related to public health restrictions,65 evidenced a less extreme decline in hospitalizations for acute CV disease. Initial evidence on the Omicron variant suggests that it is more easily spread, but generally causes less severe disease, than previous SARS-CoV-2 variants.91 As the public and healthcare services become more familiar with ‘living with’ COVID-19 and widespread vaccination in HICs limits morbidity and mortality directly related to SARS-CoV-2 infection,92 it remains to be seen if hospitalization rates for acute CV disease will be robust to future waves.
There were comparatively few available data for the effect of the pandemic on CV services in LMICs. Only for hospitalizations, STEMI care pathways, and in-hospital mortality were we able to investigate for disparities compared with HICs, and we consistently found more severe collateral CV damage. The 143 LMICs constitute 80% of the world’s population—approximately 6 billion people—and the World Health Organization (WHO) estimates that 80% of all CV deaths now occur in LMICs.93 Whilst guideline-based therapy for STEMI has dramatically improved outcomes in HICs, regional systems of care for STEMI in LMICs are sparse. There are few emergency medical services, catheterization labs tend to be clustered in urban centres, and poor insurance coverage for the majority of the population limits the applicability of expensive procedures, leaving fibrinolysis as the most common treatment of STEMI.94 Historically, inpatients with acute HF in North America and Europe have had lower mortality rates than patients in South America and Asia,95 and 6-month mortality rates of almost 20% after HF hospitalization have been reported in sub-Saharan Africa.96 Access to diagnostic and interventional cardiac procedures is limited in LMICs,97 as is the ability to be able to provide guideline-directed management for other CV diseases.98 The pandemic exacerbated established challenges to the delivery of STEMI and HF care in LMICs. We are concerned that the gap in CV care and outcomes between HICs and LMICs may have widened during the pandemic across the breadth of CV diseases and services, yet data are not available to evidence this notion.
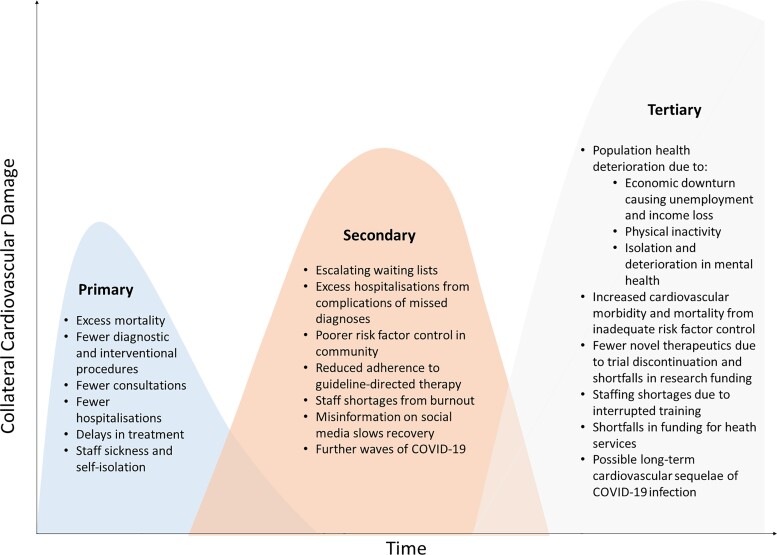
Collateral CV damage from missed diagnoses and delayed treatments will continue to accrue unless mitigation strategies are speedily implemented (Figure 5). The deferral of interventional procedures, especially for structural heart disease, leaves many patients at high risk of adverse outcomes.99 Risk stratification and prioritization will be needed to avert substantial excess mortality,100,101 and the pragmatic use of percutaneous over surgical options should be considered.102–104 A digital transformation in the healthcare model could cut the deficit in outpatient care and improve risk factor control. During the pandemic, there have been fewer contacts for CV diagnoses and risk factor monitoring,105,106 and lockdowns led to a significant decline in physical activity, weight gain, and worsening psychological health.107,108 Virtual consultations and tele-rehabilitation can provide better patient engagement with similar outcomes to in-person interactions, and patients can be empowered to manage their CV health by integrating home health equipment into routine clinical practice.59,109,110 Nonetheless, inequitable access to telemedicine and digital technology has been described for female, non-English-speaking, older, and poorer patients, and we must guard against reinforcing such inequities in healthcare.111
Figure 5.
Potential collateral damage of the COVID-19 pandemic to cardiovascular services. The height and time scale of the three peaks depicted are not certain or to scale. We do expect the disruption to cardiovascular services to accumulate over time unless mitigation strategies are utilized.
As this review reveals, there is limited information about CV health and care from LMICs (data gaps exist in the African, South American, and Western Pacific regions). There are a few nationwide initiatives to systematically collect and report data on CV health in LMICs,112 and the WHO is engaging with member states and technology partners to strengthen their local health information systems.113 The ESC Atlas of Cardiology provides an enviable resource for data relating to population health in Europe.114 A global living collaborative network focusing on CV care during the pandemic at an institutional level could be established,115 and internationally harmonized CV data available in a responsive fashion could enable a ‘global barometer’ of the consequences of the pandemic as well as the opportunity to prepare for future major health crises.116
There are limitations to our analysis. The evidence base is skewed to HICs in Europe and North America, the earlier part of the pandemic, certain CV diseases, and short-term outcome measures, which limit quantitative insights. We classified most studies as being at severe or moderate risk of bias across all outcomes, which is in agreement with previous reports of the methodological quality of publications during the COVID-19 pandemic.17,117 Many studies did not report the number or proportion of included patients that had co-existent COVID-19 infection, which introduces bias and prohibits detailed analysis of what contribution the direct effect of COVID-19 on the CV system may have had on our estimates for in-hospital mortality and hospitalizations. Nonetheless, a meta-analysis including >27 000 patients demonstrated that in-hospital mortality in CV disease was increased during the pandemic independent of co-infection with COVID-19, and the direction of effect was consistent between studies at moderate and severe risk of bias.17 Furthermore, the direct CV consequences of COVID-19 include myocarditis, HF, arrhythmias, and acute myocardial injury,118 so the number of hospitalizations for acute CV disease would probably increase if direct COVID-19 pathology was the predominant factor, in contrast to our findings.
Heterogeneity was high in most analyses, which we investigated through meta-regression for a range of factors in outcomes of hospitalizations, invasive management of acute myocardial infarction, and in-hospital mortality. We found that geographic region, income classification, and whether the first or second wave was reported introduced variability in effect size, as did study characteristics such as the data source, presence of a matched comparator period, the length of the pandemic study period, and the time point at which data collection started during the pandemic period (Supplementary material online, Table S62). Significance was often not reached for individual factors due to the small number of studies. The smaller number of studies reporting procedures and outpatient consultations precluded meta-regression to investigate heterogeneity. Nevertheless, the direction of association is consistent across outcomes (Supplementary material online, Figures S1–S18), suggesting that the conclusions we draw for trends during the pandemic are reliable.
Conclusions
This systematic review with a meta-analysis provides, to date, the most comprehensive summary of the effect of the COVID-19 pandemic on CV services and individuals with CV disease. From 189 articles, we show evidence of fewer hospitalizations, procedures, and consultations with increased mortality amongst in-hospital and community populations. We identified disparity by geographical region and country income classification in the availability of data and the severity of the detrimental effect of the pandemic on CV services, and presently there are insufficient data to fully characterize the effects to CV services in LMICs. Notwithstanding this, we provide synthesized evidence that the COVID-19 pandemic resulted in substantial global collateral CV damage.
Author contributions
C.P.G. conceived the idea of the study. R.N. and B.H. screened the studies and reviewed the selected articles. R.N. and B.H. undertook data extraction. J.W. carried out the statistical analysis. R.N., J.W., and C.P.G. interpreted the findings, and R.N. drafted the manuscript. J.W., B.H., S.A., D.L.B., G.B.Z., L.S.M., C.V.S.R., A.P.L.R., H.G.C.V.S., J.E.D., T.F.L., M.M., and C.P.G. critically reviewed the manuscript, and R.N. revised the manuscript for final submission. All authors have approved the final draft of the manuscript. R.N. is the guarantor. R.N. accepts full responsibility for the work and the conduct of the review, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We acknowledge the tremendous help of Katerina Davidson in formatting the manuscript, organizing records, and designing tables, Keerthenan Raveendra for screening articles and discussing data extraction strategies, and Karen Abel, library research support and data advisor at the University of Leeds, in developing the initial search terms and strategy.
Conflict of interest: none declared.
Glossary
Abbreviations
- ACHD
adult congenital heart disease
- ACS
acute coronary syndrome
- CABG
coronary artery bypass graft
- CIED
cardiac implantable electronic device
- COVID-19
coronavirus disease 2019
- CV
cardiovascular
- D2B
door-to-balloon time
- ECG
electrocardiogram
- ESC
European Society of Cardiology
- HF
heart failure
- HIC
high-income country
- ICD
implantable cardioverter defibrillator
- IE
infective endocarditis
- IRR
incidence rate ratio
- LMIC
low–middle income country
- NSTEMI
non-ST-elevation myocardial infarction
- OHCA
out-of-hospital cardiac arrest
- PCI
percutaneous coronary intervention
- PPCI
primary PCI
- RR
risk ratio
- S-FMC
symptom to first medical contact
- STEMI
ST-elevation myocardial infarction
- TAVI
transcatheter aortic valve implantation
- VA
ventricular arrhythmia
- WHO
World Health Organization
- WMD
weighted mean difference
Contributor Information
Ramesh Nadarajah, Leeds Institute for Cardiovascular and Metabolic Medicine, University of Leeds, 6 Clarendon Way, Leeds LS2 9DA, UK; Leeds Institute of Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Jianhua Wu, Leeds Institute of Data Analytics, University of Leeds, Leeds, UK; School of Dentistry, University of Leeds, Leeds, UK.
Ben Hurdus, Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Samira Asma, Division of Data, Analytics and Delivery for Impact, World Health Organization, Geneva, Switzerland.
Deepak L. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Giuseppe Biondi-Zoccai, Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Latina, Italy; Mediterranea Cardiocentro, Napoli, Italy.
Laxmi S. Mehta, Division of Cardiology, The Ohio State University Wexner Medical Center, Columbus, OH, USA
C. Venkata S. Ram, Apollo Hospitals and Medical College, Hyderabad, Telangana, India University of Texas Southwestern Medical School, Dallas, TX, USA; Faculty of Medical and Health Sciences, Macquarie University, Sydney, Australia.
Antonio Luiz P. Ribeiro, Cardiology Service and Telehealth Center, Hospital das Clínicas, and Department of Internal Medicine, Faculdade de Medicina, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
Harriette G.C. Van Spall, Department of Medicine and Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Canada Population Health Research Institute, Hamilton, Canada.
John E. Deanfield, National Institute for Cardiovascular Outcomes Research, Barts Health NHS Trust, London, UK Institute of Cardiovascular Sciences, University College, London, UK.
Thomas F. Lüscher, Imperial College, National Heart and Lung Institute, London, UK Royal Brompton & Harefield Hospital, Imperial College, London, UK.
Mamas Mamas, Keele Cardiovascular Research Group, Institute for Prognosis Research, University of Keele, Keele, UK.
Chris P. Gale, Leeds Institute for Cardiovascular and Metabolic Medicine, University of Leeds, 6 Clarendon Way, Leeds LS2 9DA, UK Leeds Institute of Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Ethical approval
Ethical approval was not required.
Data sharing
Data are available on reasonable request. The technical appendix, statistical code, and dataset are available from the corresponding author at r.nadarajah@leeds.ac.uk.
References
- 1. Leyva F, Zegard A, Okafor O, Stegemann B, Ludman P, Qiu T. Cardiac operations and interventions during the COVID-19 pandemic: a nationwide perspective. Europace 2021;23:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Einstein AJ, Shaw LJ, Hirschfeld C, Williams MC, Villines TC, Better N, et al. International impact of COVID-19 on the diagnosis of heart disease. J Am Coll Cardiol 2021;77:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol 2020;76:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torjesen I. Covid restrictions tighten as omicron cases double every two to three days. BMJ 2021;375:n3051. [DOI] [PubMed] [Google Scholar]
- 5. Callaway E, Ledford H. How bad is Omicron? What scientists know so far. Nature 2021;600:197–199. [DOI] [PubMed] [Google Scholar]
- 6. Kiss P, Carcel C, Hockham C, Peters SA. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes 2021;7:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seidu S, Kunutsor SK, Cos X, Khunti K. Indirect impact of the COVID-19 pandemic on hospitalisations for cardiometabolic conditions and their management: a systematic review. Prim Care Diabetes 2021;15:653–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helal A, Shahin L, Abdelsalam M, Ibrahim M. Global effect of COVID-19 pandemic on the rate of acute coronary syndrome admissions: a comprehensive review of published literature. Open Heart 2021;8:e001645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chew NW, Ow ZGW, Teo VXY, Heng RRY, Ng CH, Lee CH, et al. The global impact of the COVID-19 pandemic on STEMI care: a systematic review and meta-analysis. Can J Cardiol 2021;37:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rattka M, Dreyhaupt J, Winsauer C, Stuhler L, Baumhardt M, Thiessen K, et al. Effect of the COVID-19 pandemic on mortality of patients with STEMI: a systematic review and meta-analysis. Heart 2021;107:482–487. [DOI] [PubMed] [Google Scholar]
- 11. Baumhardt M, Dreyhaupt J, Winsauer C, Stuhler L, Thiessen K, Stephan T, et al. The effect of the lockdown on patients with myocardial infarction during the COVID-19 pandemic: a systematic review and meta-analysis. Dtsch Arztebl Int 2021;118:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh S, Fong HK, Desai R, Zwinderman AH. Impact of COVID-19 on acute coronary syndrome-related hospitalizations: a pooled analysis. Int J Cardiol Heart Vasc 2021;32:100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borkowska MJ, Jaguszewski MJ, Koda M, Gasecka A, Szarpak A, Gilis-Malinowska N, et al. Impact of coronavirus disease 2019 on out-of-hospital cardiac arrest survival rate: a systematic review with meta-analysis. J Clin Med 2021;10:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim ZJ, Reddy MP, Afroz A, Billah B, Shekar K, Subramaniam A. Incidence and outcome of out-of-hospital cardiac arrests in the COVID-19 era: a systematic review and meta-analysis. Resuscitation 2020;157:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh S, Fong HK, Mercedes BR, Serwat A, Malik FA, Desai R. COVID-19 and out-of-hospital cardiac arrest: a systematic review and meta-analysis. Resuscitation 2020;156:164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teoh SE, Masuda Y, Tan DJH, Liu N, Morrison LJ, Ong MEH, et al. Impact of the COVID-19 pandemic on the epidemiology of out-of-hospital cardiac arrest: a systematic review and meta-analysis. Ann Intensive Care 2021;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cannata A, Watson SA, Daniel A, Giacca M, Shah AM, McDonagh TA, et al. Impact of the COVID-19 pandemic on in-hospital mortality in cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 2021:zwab119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camm AJ, Lüscher TF, Serruys PW. The ESC Textbook of Cardiovascular Medicine. Oxford: Oxford University Press; 2009. [Google Scholar]
- 19. Porfidia A, Valeriani E, Pola R, Porreca E, Rutjes AW, Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res 2020;196:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 23. Sterne JA, Egger M. Regression methods to detect publication and other bias in meta-analysis. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta-Analysis: Prevention. Assessment and Adjustments. Chichester: Wiley; 2005;99:110. [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003:327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The World Bank . World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 1 July 2021.
- 26. Andersson C, Gerds T, Fosbøl E, Phelps M, Andersen J, Lamberts M, et al. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail 2020;13:e007274. [DOI] [PubMed] [Google Scholar]
- 27. Caamaño MN, Flores JP, Gómez CM. Impact of COVID-19 pandemic in cardiology admissions. Med Clin 2020;155:179–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sokolski M, Gajewski P, Zymliński R, Biegus J, Ten Berg JM, Bor W, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on acute admissions at the emergency and cardiology departments across Europe. Am J Med 2021;134:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toniolo M, Negri F, Antonutti M, Mase M, Facchin D. Unpredictable fall of severe emergent cardiovascular diseases hospital admissions during the covid-19 pandemic: experience of a single large center in northern Italy. J Am Heart Assoc 2020;9:e017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holt A, Gislason GH, Schou M, Zareini B, Biering-Sørensen T, Phelps M, et al. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur Heart J 2020;41:3072–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ueberham L, König S, Pellissier V, Hohenstein S, Meier-Hellmann A, Kuhlen R, et al. Admission rates and care pathways in patients with atrial fibrillation during the COVID-19 pandemic—insights from the German-wide Helios hospital network. Eur Heart J Qual Care Clin Outcomes 2021;7:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Christensen DM, Butt JH, Fosbøl E, Køber L, Torp-Pedersen C, Gislason G, et al. Nationwide cardiovascular disease admission rates during a second COVID-19 lockdown. Am Heart J 2021;241:35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harding I, Khan P, Alves K, Weerasinghe N, Daily T, Arumugam P, et al. Remote monitoring of arrhythmias in the COVID lockdown era: a multicentre experience. Circ Arrhythm Electrophysiol 2021;14:e008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Shea CJ, Middeldorp ME, Thomas G, Harper C, Elliott AD, Ray N, et al. Atrial fibrillation burden during the coronavirus disease 2019 pandemic. Europace 2021;23:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y-J, Jin Q-Q, Zheng C, Lin J-X, Lin Y-F, Xu Q, et al. One-year recording of cardiac arrhythmias in a non-infected population with cardiac implantable devices during the COVID-19 pandemic. Int J Gen Med 2021;14:7337–7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adabag S, Zimmerman P, Black A, Madjid M, Safavi-Naeini P, Cheng A. Implantable cardioverter-defibrillator shocks during COVID-19 outbreak. J Am Heart Assoc 2021;10:e019708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galand V, Hwang E, Gandjbakhch E, Sebag F, Marijon E, Boveda S, et al. Impact of COVID-19 on the incidence of cardiac arrhythmias in implantable cardioverter defibrillator recipients followed by remote monitoring. Arch Cardiovasc Dis 2021;114:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Shea CJ, Thomas G, Middeldorp ME, Harper C, Elliott AD, Ray N, et al. Ventricular arrhythmia burden during the coronavirus disease 2019 (COVID-19) pandemic. Eur Heart J 2021;42:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oikonomou E, Aznaouridis K, Barbetseas J, Charalambous G, Gastouniotis I, Fotopoulos V, et al. Hospital attendance and admission trends for cardiac diseases during the COVID-19 outbreak and lockdown in Greece. Public Health 2020;187:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gaspar-Hernández J, Araiza-Garaygordobil D, Gopar-Nieto R, Martínez-Amezcua P, Arias-Mendoza A. Impact of the coronavirus disease-19 pandemic on acute cardiovascular emergencies in a third level cardiology hospital: a call for action. Rev Invest Clin 2020;72:280–282. [DOI] [PubMed] [Google Scholar]
- 41. Havers-Borgersen E, Fosbøl EL, Butt JH, Petersen JK, Dalsgaard A, Kyhl F, et al. Incidence of infective endocarditis during the coronavirus disease 2019 pandemic: a nationwide study. Int J Cardiol Heart Vasc 2020;31:100675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scognamiglio G, Fusco F, Merola A, Palma M, Correra A, Sarubbi B. Caring for adults with CHD in the era of coronavirus disease 2019 pandemic: early experience in an Italian tertiary centre. Cardiol Young 2020;30:1405–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open 2020;3:e2014780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guan X, Zhang J, Li Y, Ma N. Safety measures for COVID-19 do not compromise the outcomes of patients undergoing primary percutaneous coronary intervention: a single center study. Sci Rep 2020;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kwok CS, Gale CP, Curzen N, De Belder MA, Ludman P, Lüscher TF, et al. Impact of the COVID-19 pandemic on percutaneous coronary intervention in England: insights from the British Cardiovascular Intervention Society PCI database cohort. Circ Cardiovasc Interv 2020;13:e009654. [DOI] [PubMed] [Google Scholar]
- 46. Waldo SW, Plomondon ME, O’Donnell CI, Heidenreich PA, Riatt MH, Ballard-Hernandez J, et al. Trends in cardiovascular procedural volumes in the setting of COVID-19: insights from the VA clinical assessment, reporting, and tracking program. Catheter Cardiovasc Interv 2021;98:E326–E328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tan J, Teoh TK, Ivanova J, Jadhav S, Varcoe R, Baig K, et al. 17 The impact of the COVID-19 pandemic on transcatheter aortic valve implantation (TAVI) services in the United Kingdom: a tertiary centre experience [abstract]. Heart 2021;107:A13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin GP, Curzen N, Goodwin AT, Nolan J, Balacumaraswami L, Ludman PF, et al. Indirect impact of the COVID-19 pandemic on activity and outcomes of transcatheter and surgical treatment of aortic stenosis in England. Circ Cardiovasc Interv 2021;14:e010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quadri G, Rognoni A, Cerrato E, Baralis G, Boccuzzi G, Brscic E, et al. Catheterization laboratory activity before and during COVID-19 spread: a comparative analysis in Piedmont, Italy, by the Italian Society of Interventional Cardiology (GISE). Int J Cardiol 2021;323:288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Albani S, Vinhas H, Ferre GF, Basavarajaiah S, Khattak S, Tzanis G, et al. Epidemiological findings on interventional cardiology procedures during the COVID-19 pandemic: a multi-center study. Indian Heart J 2021;73:647–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perek B, Olasinska-Wisniewska A, Misterski M, Puslecki M, Grygier M, Buczkowski P, et al. How the COVID-19 pandemic changed treatment of severe aortic stenosis: a single cardiac center experience. J Thorac Dis 2021;13:906–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tam DY, Qiu F, Manoragavan R, Fremes SE, Hassan A, Ko DT, et al. The impact of the COVID-19 pandemic on cardiac procedure wait list mortality in Ontario, Canada. Can J Cardiol 2021;37:1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siudak Z, Grygier M, Wojakowski W, Malinowski KP, Witkowski A, Gąsior M, et al. Clinical and procedural characteristics of COVID-19 patients treated with percutaneous coronary interventions. Catheter Cardiovasc Interv 2020;96:E568–E575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fersia O, Bryant S, Nicholson R, McMeeken K, Brown C, Donaldson B, et al. The impact of the COVID-19 pandemic on cardiology services. Open Heart 2020;7:e001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nappi C, Megna R, Acampa W, Assante R, Zampella E, Gaudieri V, et al. Effects of the COVID-19 pandemic on myocardial perfusion imaging for ischemic heart disease. Eur J Nucl Med Mol Imaging 2021;48:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mehrotra A, Chernew M, Linetsky D, Hatch H, Cutler D. The Impact of the COVID-19 Pandemic on Outpatient Visits: A Rebound Emerges. https://www.commonwealthfund.org/publications/2020/apr/impact-covid-19-outpatient-visits 7 July 2021.
- 57. Paruchuri K, Bhattacharya R, Pagliaro J, Bhatt A. Virtual care: empowering patients and providers [abstract]. Circulation 2020;142:A15616. [Google Scholar]
- 58. Wosik J, Clowse ME, Overton R, Adagarla B, Economou-Zavlanos N, Cavalier J, et al. Impact of the COVID-19 pandemic on patterns of outpatient cardiovascular care. Am Heart J 2021;231:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sammour Y, Shatla I, Miller L, Dean E, Nassif M, Magalski A, et al. Outcomes in the outpatient management of heart failure patients during the COVID-19 pandemic after robust adoption of a telehealth model [abstract]. J Am Coll Cardiol 2021;77:580. [Google Scholar]
- 60. Bollmann A, Hohenstein S, Pellissier V, Stengler K, Reichardt P, Ritz J-P, et al. Utilization of in- and outpatient hospital care in Germany during the Covid-19 pandemic insights from the German-wide Helios hospital network. PLoS One 2021;16:e0249251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marzolini S, de Melo Ghisi GL, Hébert A-A, Ahden S, Oh P. Cardiac rehabilitation in Canada during COVID-19. CJC Open 2021;3:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O’Doherty AF, Humphreys H, Dawkes S, Cowie A, Hinton S, Brubaker PH, et al. How has technology been used to deliver cardiac rehabilitation during the COVID-19 pandemic? An international cross-sectional survey of healthcare professionals conducted by the BACPR. BMJ Open 2021;11:e046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Melo Ghisi GL, Xu Z, Liu X, Mola A, Gallagher R, Babu AS, et al. Impacts of the COVID-19 pandemic on cardiac rehabilitation delivery around the world. Global Heart 2021;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Scherrenberg M, Frederix I, De Sutter J, Dendale P. Use of cardiac telerehabilitation during COVID-19 pandemic in Belgium. Acta Cardiol 2021;76:773–776. [DOI] [PubMed] [Google Scholar]
- 65. Wu J, Mamas M, Rashid M, Weston C, Hains J, Luescher T, et al. Patient response, treatments, and mortality for acute myocardial infarction during the COVID-19 pandemic. Eur Heart J Qual Care Clin Outcomes 2021;7:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arai R, Fukamachi D, Ebuchi Y, Migita S, Morikawa T, Monden M, et al. Impact of the COVID-19 outbreak on hospitalizations and outcomes in patients with acute myocardial infarction in a Japanese single center. Heart Vessels 2021;36:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Salinas P, Travieso A, Vergara-Uzcategui C, Tirado-Conte G, Macaya F, Mejía-Rentería H, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J 2021;62:274–281. [DOI] [PubMed] [Google Scholar]
- 68. Kundi H, Birinci S, Surel AA, Ulgu MM, Balci MM, Coskun N, et al. Trends in acute myocardial infarction volume and related outcomes during the coronavirus disease 2019 pandemic in Turkey. Coron Artery Dis 2021;32:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Doolub G, Wong C, Hewitson L, Mohamed A, Todd F, Gogola L, et al. Impact of COVID-19 on inpatient referral of acute heart failure: a single-centre experience from the south-west of the UK. ESC Heart Fail 2021;8:1691–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shoaib A, Van Spall HG, Wu J, Cleland JG, McDonagh TA, Rashid M, et al. Substantial decline in hospital admissions for heart failure accompanied by increased community mortality during COVID-19 pandemic. Eur Heart J Qual Care Clin Outcomes 2021;7:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Phua K, Chew NW, Sim V, Zhang AA, Rastogi S, Kojodjojo P, et al. One-year outcomes of patients with ST-segment elevation myocardial infarction during the COVID-19 pandemic. J Thromb Thrombolysis 2022;53:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aldujeli A, Hamadeh A, Tecson KM, Krivickas Z, Maciulevicius L, Stiklioraitis S, et al. Six-month outcomes for COVID-19 negative patients with acute myocardial infarction before versus during the COVID-19 pandemic. Am J Cardiol 2021;147:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anyu TA, Badawy L, Cannata A, Bromage DI, Rind IA, Albarjas M, et al. Long-term outcomes after heart failure hospitalization during the COVID-19 pandemic: a multisite report from heart failure referral centers in London. ESC Heart Fail 2021;8:4701–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kontopantelis E, Mamas MA, Webb RT, Castro A, Rutter MK, Gale CP, et al. Excess deaths from COVID-19 and other causes by region, neighbourhood deprivation level and place of death during the first 30 weeks of the pandemic in England and Wales: a retrospective registry study. Lancet Reg Health Eur 2021;7:100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang J, Zhu J, Yang H, Hu Y, Sun Y, Ying Z, et al. Cardiovascular-related deaths at the beginning of the COVID-19 outbreak: a prospective analysis based on the UK Biobank. BMJ Open 2021;11:e046931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu J, Mafham M, Mamas MA, Rashid M, Kontopantelis E, Deanfield JE, et al. Place and underlying cause of death during the COVID-19 pandemic: retrospective cohort study of 3.5 million deaths in England and Wales, 2014 to 2020. Mayo Clin Proc 2021;96:952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu J, Mamas MA, Mohamed MO, Kwok CS, Roebuck C, Humberstone B, et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart 2021;107:113–119. [DOI] [PubMed] [Google Scholar]
- 78. Wadhera RK, Shen C, Gondi S, Chen S, Kazi DS, Yeh RW. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol 2021;77:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes. JAMA 2020;324:510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brant LCC, Nascimento BR, Teixeira RA, Lopes MACQ, Malta DC, Oliveira GMM, et al. Excess of cardiovascular deaths during the COVID-19 pandemic in Brazilian capital cities. Heart 2020;106:1898–1905. [DOI] [PubMed] [Google Scholar]
- 81. Liu J, Zhang L, Yan Y, Zhou Y, Yin P, Qi J, et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ 2021;372:n415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020;396:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mohammad MA, Koul S, Olivecrona GK, Götberg M, Tydén P, Rydberg E, et al. Incidence and outcome of myocardial infarction treated with percutaneous coronary intervention during COVID-19 pandemic. Heart 2020;106:1812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mesnier J, Cottin Y, Coste P, Ferrari E, Schiele F, Lemesle G, et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health 2020;5:e536–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cannatà A, Bromage DI, Rind IA, Gregorio C, Bannister C, Albarjas M, et al. Temporal trends in decompensated heart failure and outcomes during COVID-19: a multisite report from heart failure referral centres in London. Eur J Heart Fail 2020;22:2219–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bromage DI, Cannatà A, Rind IA, Gregorio C, Piper S, Shah AM, et al. The impact of COVID-19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail 2020;22:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. König S, Hohenstein S, Meier-Hellmann A, Kuhlen R, Hindricks G, Bollmann A, et al. In-hospital care in acute heart failure during the COVID-19 pandemic: insights from the German-wide Helios hospital network. Eur J Heart Fail 2020;22:2190–2201. [DOI] [PubMed] [Google Scholar]
- 88. European Society of Cardiology . You can’t pause a heart. https://www.cantpauseaheart.org/.
- 89. Van Belle E, Manigold T, Piérache A, Furber A, Debry N, Luycx-Bore A, et al. Myocardial infarction incidence during national lockdown in two French provinces unevenly affected by COVID-19 outbreak: an observational study. Lancet Reg Health Eur 2021;2:100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gluckman TJ, Wilson MA, Chiu S-T, Penny BW, Chepuri VB, Waggoner JW, et al. Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol 2020;5:1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Centres for Disease Control and Prevention . Omicron Variant: What You Need to Know. https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html 2 February 2022.
- 92. Jabłońska K, Aballéa S, Toumi M. The real-life impact of vaccination on COVID-19 mortality in Europe and Israel. Public Health 2021;198:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mendis S, Puska P, Norrving B, World Heath Organization . Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization; 2011. [Google Scholar]
- 94. Chandrashekhar Y, Alexander T, Mullasari A, Kumbhani DJ, Alam S, Alexanderson E, et al. Resource and infrastructure-appropriate management of ST-segment elevation myocardial infarction in low-and middle-income countries. Circulation 2020;141:2004–2025. [DOI] [PubMed] [Google Scholar]
- 95. Greene SJ, Fonarow GC, Solomon SD, Subacius H, Maggioni AP, Böhm M, et al. Global variation in clinical profile, management, and post-discharge outcomes among patients hospitalized for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail 2015;17:591–600. [DOI] [PubMed] [Google Scholar]
- 96. Sliwa K, Davison BA, Mayosi BM, Damasceno A, Sani M, Ogah OS, et al. Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. Eur Heart J 2013;34:3151–3159. [DOI] [PubMed] [Google Scholar]
- 97. Michelis KC, Narotsky DL, Choi BG. Cardiovascular imaging in global health radiology. In: Mollura DJ, Culp MP, Lungren MP, eds. Radiology in Global Health: Strategies, Implementation, and Applications. Cham: Springer; 2019: 207–224. [Google Scholar]
- 98. Mkoko P, Bahiru E, Ajijola OA, Bonny A, Chin A. Cardiac arrhythmias in low- and middle-income countries. Cardiovasc Diagn Ther 2020;10:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Moreno R, Díez JL, Diarte JA, Macaya F, de la Torrre Hernández JM, Rodríguez-Leor O, et al. Consequences of canceling elective invasive cardiac procedures during Covid-19 outbreak. Catheter Cardiovasc Interv 2021;97:927–937. [DOI] [PubMed] [Google Scholar]
- 100. Minamino-Muta E, Kato T, Morimoto T, Taniguchi T, Ando K, Kanamori N, et al. A risk prediction model in asymptomatic patients with severe aortic stenosis: CURRENT-AS risk score. Eur Heart J Qual Care Clin Outcomes 2020;6:166–174. [DOI] [PubMed] [Google Scholar]
- 101. Prachand VN, Milner R, Angelos P, Posner MC, Fung JJ, Agrawal N, et al. Medically necessary, time-sensitive procedures: scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg 2020;231:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kite TA, Ladwiniec A, Owens CG, Chase A, Shaukat A, Mozid AM, et al. Outcomes following PCI in CABG candidates during the COVID-19 pandemic: the prospective multicentre UK-ReVasc registry. Catheter Cardiovasc Interv 2022;99:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Adlam D, Chan N, Baron J, Kovac J. Aortic stenosis in the time of COVID-19: development and outcomes of a rapid turnaround TAVI service. Catheter Cardiovasc Interv 2021;98:E478–E482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shafi AM, Awad WI. Transcatheter aortic valve implantation versus surgical aortic valve replacement during the COVID-19 pandemic—current practice and concerns. J Card Surg 2021;36:260–264. [DOI] [PubMed] [Google Scholar]
- 105. Mansfield KE, Mathur R, Tazare J, Henderson AD, Mulick AR, Carreira H, et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digital Health 2021;3:e217–e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rachamin Y, Senn O, Streit S, Dubois J, Deml M, Jungo KT. Impact of the COVID-19 pandemic on the intensity of health services use in general practice: a retrospective cohort study. Int J Public Health 2021;66:635508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Duffy E, Chilazi M, Cainzos-Achirica M, Michos ED. Cardiovascular disease prevention during the COVID-19 pandemic: lessons learned and future opportunities. Methodist Debakey Cardiovasc J 2021; 17:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ćosić K, Popović S, Šarlija M, Kesedžić I. Impact of human disasters and COVID-19 pandemic on mental health: potential of digital psychiatry. Psychiatr Danub 2020;32:25–31. [DOI] [PubMed] [Google Scholar]
- 109. Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299:2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dalal HM, Doherty P, McDonagh ST, Paul K, Taylor RS. Virtual and in-person cardiac rehabilitation. BMJ 2021;373:n1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Eberly LA, Khatana SAM, Nathan AS, Snider C, Julien HM, Deleener ME, et al. Telemedicine outpatient cardiovascular care during the COVID-19 pandemic: bridging or opening the digital divide? Circulation 2020;142:510–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. de Oliveira GMM, Brant LCC, Polanczyk CA, Biolo A, Nascimento BR, Malta DC, et al. Cardiovascular statistics–Brazil. Arq Bras Cardiol 2020;2020:308–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. World Health Organisation . The true death toll of COVID-19: estimating global excess mortality. https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality 21 July 2021.
- 114. Vardas P, Maniadakis N, Bardinet I, Pinto F. The European Society of Cardiology atlas of cardiology: rational, objectives, and methods. Eur Heart J Qual Care Clin Outcomes 2016;2:6–15. [DOI] [PubMed] [Google Scholar]
- 115. Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S. The prospective urban rural epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J 2009;158:1–7.e1. [DOI] [PubMed] [Google Scholar]
- 116. Jia Q, Guo Y, Wang G, Barnes SJ. Big data analytics in the fight against major public health incidents (including COVID-19): a conceptual framework. Int J Environ Res Public Health 2020;17:6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jung RG, Di Santo P, Clifford C, Prosperi-Porta G, Skanes S, Hung A, et al. Methodological quality of COVID-19 clinical research. Nat Commun 2021;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Azevedo RB, Botelho BG, de Hollanda JVG, Ferreira LVL, de Andrade LZJ, Oei SSML, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens 2021;35:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.