Abstract
A phase 1 clinical trial was conducted among 35 healthy adult volunteers to evaluate the safety, immunogenicity, and shedding of different doses of CVD 1207, a live attenuated Shigella flexneri 2a vaccine candidate with specific deletion mutations in virG, sen, set, and guaBA. CVD 1207 retains the ability to invade epithelial cells but cannot effectively spread intercellularly after invasion (ΔvirG), does not produce enterotoxin (Δsen and Δset), and has limited proliferation in vivo (ΔguaBA). In a consecutive fashion, groups of three to seven subjects ingested a single oral dose of CVD 1207 at an inoculum of either 106, 107, 108, 109, or 1010 CFU. CVD 1207 was remarkably well-tolerated at inocula as high as 108 CFU. In comparison, one of 12 subjects who received 109 CFU experienced mild diarrhea and another experienced a single episode of emesis. One of five subjects who received 1010 CFU experienced watery diarrhea and emesis. All subjects who ingested doses of 108 to 1010 CFU excreted the vaccine; in 23 of 25, the duration of excretion was ≤3 days. A dose-related, immunoglobulin A antibody-secreting cell (ASC) response to S. flexneri 2a O-specific lipopolysaccharide was seen, with geometric mean peak values of 6.1 to 35.2 ASCs/106 peripheral blood mononuclear cells (PBMC) among recipients of 107 to 1010 CFU. The cytokine response to Shigella-specific antigens observed in volunteers' PBMC following vaccination suggested a Th1 pattern with stimulation of gamma interferon and absence of interleukin 4 (IL-4) or IL-5. CVD 1207 represents a Shigella live oral vaccine strain prepared from wild-type S. flexneri 2a by rational use of recombinant DNA technology that achieves a remarkable degree of attenuation compared with earlier recombinant strains, even when administered at high dosage.
The number of Shigella episodes that occur throughout the world each year is estimated to be 165 million, with more than 1 million of these illnesses resulting in death (19). In some areas of the world, mortality among children 1 to 4 years old attributed to dysentery exceeds mortality from watery diarrhea, and Shigella is the most common etiologic agent associated with dysentery (1). The increasing burden of shigellosis reflects an inability of existing medical and public health measures to diminish transmission adequately or to curtail the clinical consequences of infection. Alternatively, prevention by the use of a safe and effective vaccine offers great potential as a means of diminishing the global disease burden from shigellosis.
One approach to the development of Shigella vaccines is to attenuate wild-type strains by mutating genes that regulate specific virulence properties. The aim is to construct a vaccine that expresses critical antigens in their native form but cannot induce undesirable pathological processes. To date, the generation of a live attenuated vaccine that elicits protective immunity yet is safe and genetically stable has been problematic (6, 13, 18).
Here we report the safety, immunogenicity, and dose response of CVD 1207, a ΔvirG Δsen Δset ΔguaBA Shigella flexneri 2a live attenuated vaccine candidate. CVD 1207, derived from the wild-type strain 2457T (7), contains precise mutations in the genes encoding either virulence factors or essential metabolic enzymes: (i) the plasmid gene virG (also known as icsA), which encodes a protein responsible for cell-to-cell spread of Shigella in the intestinal epithelium (2, 20); (ii) the chromosomal gene set encoding Shigella enterotoxin 1 (ShET1), which is present almost exclusively in S. flexneri 2a (11); (iii) the plasmid gene sen, encoding Shigella enterotoxin 2 (ShET2), which is present in virtually all serotypes of Shigella (29); and (iv) the guaBA chromosomal operon that regulates synthesis of IMP dehydrogenase (encoded by guaB) and GMP synthetase (encoded by guaA), two enzymes employed in the distal de novo purine biosynthesis pathway (32). CVD 1207 thus expresses type-specific O-polysaccharide and invades epithelial cells (albeit less competently than the wild type) but undergoes only limited intracellular proliferation and intercellular spread and has no detectable enterotoxic activity.
MATERIALS AND METHODS
Construction of CVD 1207.
CVD 1207 was constructed from wild-type S. flexneri 2a strain 2457T by a series of double homologous recombinations using suicide plasmid deletion cassette technology as described in detail elsewhere (31). In brief, a specific, in-frame deletion mutation in the guaBA operon was first introduced, followed by a second in-frame deletion mutation in the plasmid virulence gene virG (32). The chromosomal mutation Δset was accomplished with deletion of 85% of subunit A of set (31). Finally, a Δsen cassette was constructed by fusing two 700-bp segments that include the N and C termini of sen minus 300 bp corresponding to the putative active site in the N-terminal region. The ars operon, conferring resistance to arsenite, was cloned into the Δsen locus to allow facile transfer of the double-deletion mutation (ΔvirG and Δsen) virulence plasmid to candidate Shigella vaccine strains and as a marker to distinguish CVD 1207 in the field (31).
As previously described, CVD 1207 does not grow in minimum medium unless supplemented with guanine (31). The lack of enterotoxic activity has been confirmed in Ussing chambers (31). CVD 1207 is significantly less invasive for HeLa cells than its wild-type parent strain 2457T (approximately 1 log unit fewer intracellular CFU detected) but does not differ from its single-mutant strain progenitor ΔguaBA CVD 1204 (unpublished observations). CVD 1207 undergoes fewer intracellular generations in HeLa cells (7.5-fold; 3 doublings in 4 h) than either CVD 1204 (10-fold; 4.5 doublings in 4 h) or 2457T (30-fold; 5 doublings in 4 h) (unpublished observations). In the guinea pig purulent keratoconjunctivitis (Serény) model, CVD 1207 is fully attenuated (evokes no inflammatory response) and confers 85% protection against challenge with the wild-type parent strain (31).
Preparation of challenge inoculum.
The vaccine inocula were prepared from frozen master seed stocks (to guarantee clonal continuity), which were plated onto Trypticase soy agar (TSA; Becton Dickinson, Cockeysville, Md.) containing 0.01% Congo red dye (Sigma Chemical Co., St. Louis, Mo.) and 0.005% guanine (Sigma). After incubation at 35°C for 18 to 24 h, single, isolated Congo red-positive colonies that exhibited characteristic Shigella morphology were confirmed as S. flexneri 2a by using specific antisera (Difco Laboratories, Detroit, Mich.). Several well-isolated Congo red-positive colonies were picked and suspended in sterile saline. The saline suspension was then used to inoculate (for heavy growth) guanine-supplemented TSA plates, which were incubated overnight at 35°C. Overnight growth from the TSA plates was then harvested into sterile phosphate-buffered saline, pH 7.4, and washed three times. The heavy bacterial suspension was diluted with additional sterile phosphate-buffered saline to produce a suspension with an optical density at 660 nm corresponding to the desired bacterial count per milliliter. Replicate colony counts performed before and after vaccination were averaged to estimate the actual inoculum of vaccine ingested.
Subject selection.
Adult volunteers 18 to 55 years of age, recruited from the University of Maryland Baltimore campus, underwent a battery of clinical and laboratory screening tests to ensure that they were healthy and comprehended the study protocol. Subjects were excluded if they were employed as food handlers, shared a household with an immunocompromised person or a child younger than 5 years of age, had previous exposure to Shigella (in the form of a vaccine or a known history of wild-type infection), or had received antibiotic therapy during the 7 days before inoculation. As a precaution, volunteers who tested positive for HLA B27 were excluded from participating because this haplotype is rarely associated with reactive arthritis following infection with certain strains of Shigella which bear a 2-MDa plasmid not contained in CVD 1207 (14, 43). Informed, written consent was obtained according to the guidelines of the Institutional Review Board of the University of Maryland, Baltimore.
Study design.
Groups of 3 to 7 outpatient volunteers were assigned, in an incremental fashion, to receive a single oral dose of CVD 1207 at a desired inoculum (the actual inocula administered are in parentheses) of either 106 (1.2 × 106), 107 (1.9 × 107), 108 (1.7 × 108), 109 (8.9 × 108, 2.1 × 109, or 4.1 × 109), or 1010 (7.8 × 109 or 2.1 × 1010) CFU (Table 1). Fasting volunteers swallowed the vaccine suspended in a solution of NaHCO3 buffer, as previously described (15).
TABLE 1.
Clinical and immunological responses to live attenuated vaccines derived from S. flexneri 2a strain 2457T and to the wild-type parent in trials conducted at the Center for Vaccine Development
| Immunogen | Dose (CFU)a | No. of subjects | No. (%) of subjects with:
|
Anti-LPS IgA ASC
|
|||
|---|---|---|---|---|---|---|---|
| Diarrhea | Fever | Dysentery | No. (%) respondersb | Geometric mean | |||
| CVD 1207 | 106 | 7 | 0 | 0 | 0 | 0 | 0.1 |
| 107 | 7 | 0 | 0 | 0 | 6 (100)c | 6.1 | |
| 108 | 3 | 0 | 0 | 0 | 2 (67) | 5.3 | |
| 109 | 12d | 1 (8) | 0 | 0 | 7 (64) | 8.7 | |
| 1010 | 6e | 1 (20)f | 0 | 0 | 5 (100)e | 35.2 | |
| CVD 1203 (18) | 106 | 10 | 0 | 0 | 0 | 6 (60) | 13.0 |
| 108 | 11 | 2 (18) | 1 (9) | 1 (9) | 10 (91) | 43.0 | |
| 109 | 11 | 3 (27) | 7 (64) | 3 (27) | 11 (100) | 175.0 | |
| Wild-type 2457T (17)g | 102 | 7 | 3 (43) | 2 (29) | 3 (43) | 5 (71) | 18.4 |
| 103 | 12 | 10 (83) | 10 (83) | 10 (83) | 11 (92) | 239.0 | |
Volunteers were assigned to a dose group, for the purpose of assessing dose response, by rounding the inoculum received in 0.5-log-unit increments to the nearest log value.
Response was defined as a geometric mean postvaccination count per 106 PBMC of >2 standard deviations above the mean prevaccination count.
Two subjects were excluded from the analysis of IgA anti-LPS responses. One recipient of 107 CFU had a prevaccination IgA anti-LPS ASC level of 20, and one recipient of 1010 CFU had no blood sample for measurement of IgA anti-LPS ASC on day 10.
One recipient of this inoculum vomited once.
One subject was excluded from analysis of clinical symptoms because she developed an acute febrile respiratory influenza A illness.
This subject also experienced three episodes of vomiting.
Protective efficacy against shigellosis following rechallenge was 70%; P = 0.003.
For 14 days after vaccination, the volunteers telephoned or visited the center to report the occurrence of symptoms, their evening oral temperatures, the total number of formed and loose stools passed, and the presence of blood in the stool. Adverse clinical responses occurring within 7 days of inoculation were considered possibly vaccine related and are reported here. Volunteers who developed clinical symptoms or prolonged shedding could receive ciprofloxacin (500 mg) by mouth twice daily for 5 days at the investigator's discretion.
Bacteriology.
Fecal excretion of the vaccine strain in the volunteer's stools was measured on days 1, 2, 3, 7, 10, 14, and 21 after ingestion of the vaccine. To obtain a stool specimen, volunteers swabbed the perirectal area immediately after defecation and placed the swab in a vial containing buffered glycerol saline (47). The swab was maintained in an insulated bag cooled with an ice pack until delivery to the laboratory within 24 h of passage (47). Volunteers provided a rectal swab (inoculated into gram-negative broth [Becton Dickinson]) at the study site if a perirectal specimen was not collected at home. Swabs were cultivated on Salmonella-Shigella and MacConkey's enteric media (Becton Dickinson). Lactose-negative colonies identified after 24 or 48 h of incubation at 35°C on solid medium were inoculated onto triple sugar iron slants (Difco Laboratories). Those producing an alkaline slant and an acid butt with no gas were verified as S. flexneri by demonstrating agglutination with group B polyclonal antiserum (Difco Laboratories). All media were supplemented with 0.01% guanine (Sigma).
ASC responses.
Peripheral blood mononuclear cells (PBMC) were collected before and on days 7 and 10 after vaccination to measure circulating immunoglobulin A (IgA) antibody-secreting cells (ASC) recognizing O-specific lipopolysaccharide (LPS) antigen of S. flexneri 2a (45), as an indication of intestinal priming induced by vaccination. A positive ELISPOT response was defined as a postvaccination count per 106 PBMC of ≥3 standard deviations above the mean prevaccination count (in the log metric); one volunteer who had a prevaccination ASC count of 20 was excluded from this analysis.
Serum antibody responses.
Sera were collected before and 14, 21, 28, and 42 days after immunization and evaluated by enzyme-linked immunosorbent assay (ELISA) for IgA and IgG antibodies to the O-specific LPS antigen of S. flexneri 2a (3) and the invasiveness plasmid antigens using published methods (35, 46). Seroconversion was defined as a fourfold or greater rise in titer.
Cell-mediated immune responses. (i) Isolation of PBMC.
PBMC from volunteers drawn before and 28 to 45 days after immunization were isolated and either cryopreserved in 10% dimethyl sulfoxide (Sigma) in liquid N2 as described previously (44) or used immediately. No significant differences were observed in the proliferative responses of freshly obtained or cryopreserved PBMC in response to either tetanus toxoid (TT) or phytohemagglutinin (PHA) stimulation.
(ii) Bacterial antigen preparations.
A ΔaroA mutant strain of S. flexneri 2a (CVD 1201) (33) was used as a source of homogenate and particulate Shigella antigens. Particulate Shigella consisted of a heat-phenolized preparation (46). Bacterial homogenate was prepared as described previously (39), except that a whole-bacterial pellet was used rather than a periplasmic extraction and the 100,000 × g ultracentrifugation step was not performed. The Bacillus subtilis control strain (Ehrenberg) Cohn, ATCC 7067, was obtained from the American Type Culture Collection (Manassas, Va.). Purified recombinant S. flexneri IpaC and IpaD invasins were prepared as previously described (23, 37).
(iii) Incubation of PBMC with antigens.
Frozen PBMC were quickly thawed, resuspended in complete medium, washed (44), and resuspended in AIM-V medium (Gibco BRL, Grand Island, N.Y.) at a density of 1.5 × 106/ml. The antigens were prepared in AIM-V medium and then added to achieve the following final concentrations: S. flexneri 2a homogenate, 1, 2, 10, or 25 μg/ml; S. flexneri 2a particulate, 5 × 104, 2 × 105, or 8 × 105 particles (CFU of heat-phenolized whole-cell bacteria)/well; IpaC, 2, 6, or 12 μg/ml; IpaD, 2, 6, or 12 μg/ml; B. subtilis homogenate, 1, 2, 10, or 25 μg/ml; B. subtilis particulate, 5 × 104, 2 × 105, or 8 × 105 particles/well; TT (Connaught Laboratories, Toronto, Canada), 2 μg/ml; PHA, 1.8 μg/ml; and bovine serum albumin (Sigma), 10 or 25 μg/ml. For cytokine and proliferation assays, 7.5 × 105 cells and 1.5 × 105 PBMC were added to 24-well (in duplicate) and 96-well (in triplicate) plates (Corning, Corning, N.Y.), respectively. The plates were incubated at 37°C and 5% CO2 in humidified chambers. The supernatants were collected after 3 days for determination of cytokine levels. Proliferation plates were pulsed with [3H]thymidine on day 6 and harvested 18 h later.
(iv) Proliferation assays and cytokine analysis by chemiluminescence ELISA.
[3H]thymidine incorporation and measurement of cytokines by chemiluminescence ELISA were performed as described previously (36, 39, 43). The sensitivities of the ELISAs were as follows: interleukin 2 (IL-2), 13 pg/ml; IL-4, 23 pg/ml; IL-5, 24 pg/ml; gamma interferon (IFN-γ), 18 pg/ml; IL-10, 10 pg/ml; IL-12, 44 pg/ml; IL-15, 45 pg/ml; and transforming growth factor β (TGF-β), 11 pg/ml. No cytokine production or proliferative response to control antigens was observed in any volunteer.
Analytic methods.
The frequency of adverse clinical responses during days 0 to 6 following vaccination was determined for each group of subjects, with day 0 beginning with vaccination. The primary outcome measures were fever (oral temperature, ≥100.0°F), diarrhea (three or more loose stools within a 24-h period), and dysentery (gross blood in a loose stool). The relationship between dose and ASC count was analyzed by linear regression, using log-transformed data. Volunteers were assigned to a dose group, for the purpose of assessing dose response, by rounding the inoculum received in 0.5-log-unit increments to the nearest log value.
For lymphocyte proliferative responses, the “net” (antigen-specific response minus the PBMC in medium alone) counts per minute in triplicate wells of PBMC from each subject before and after immunization, incubated with the same concentration of the same antigen, were compared by paired two-tailed t tests. For cytokine responses, duplicate chemiluminescence units before and after immunization following PBMC exposure to the same concentration of the same antigen were compared for each subject by paired two-tailed t tests. Responses were defined as occurring when statistical tests yielded a probability that was ≤5%.
RESULTS
Clinical tolerance.
CVD 1207 was well tolerated in doses ranging from 106 to 108 CFU (Table 1). One subject, who received 109 CFU, reported a single episode of emesis on day 2. A second subject, who received 109 CFU, experienced diarrhea, with passage of eight loose stools over 24 h. The diarrhea began 24 h after inoculation, required no medical intervention, and resolved spontaneously. One recipient of 1010 CFU passed five loose stools and vomited three times during the first 24 to 36 h after inoculation. She was treated with ciprofloxacin and recovered rapidly. No subject experienced fever or dysentery.
Bacteriologic response.
The pattern of excretion was dose related, such that all recipients of 108 or more CFU had positive stool cultures (Table 2). The duration of excretion was 1 to 3 days except for two recipients of ca. 109 CFU, who each had one additional positive stool culture 2 weeks after vaccination. Both subjects were treated with ciprofloxacin and had three subsequent negative stool cultures.
TABLE 2.
Fecal excretion of Shigella CVD 1207 by dose group
| Dose group (CFU)a | No. (%) of subjects with positive stool culture/total | Mean no. of days with positive stool culture (range)b |
|---|---|---|
| 106 | 1/7 (14) | 1.0 (NAc) |
| 107 | 2/7 (28) | 1.5 (1–2) |
| 108 | 3/3 (100) | 2.0 (1–3) |
| 109 | 12/12 (100) | 5.9 (1–14) |
| 1010 | 10/10 (100) | 1.7 (1–3) |
Volunteers were assigned to a dose group, for the purpose of assessing dose response, by rounding the inoculum received in 0.5-log-unit increments to the nearest log value.
This represents the number of days after vaccination that the last positive stool culture was detected and includes only subjects found to be excreting the vaccine for at least one day.
NA, not applicable.
ASC response.
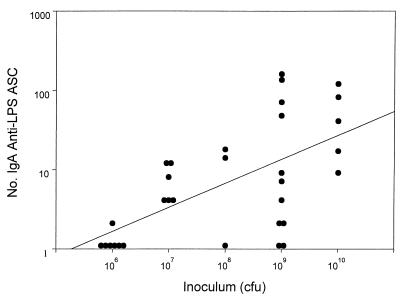
An anti-LPS IgA-producing ASC response (>2 ASC per 106 PBMC) was detected in 64 to 100% of subjects who ingested an inoculum of 107 CFU or higher (Table 1). The geometric mean of the peak postvaccination response ranged from 5.3 to 35.2 ASC count per 106 PBMC among recipients of at least 107 CFU. As shown in Fig. 1, there was a significant positive relationship between dose and number of IgA-producing anti-LPS ASC (linear regression of log-transformed data; r = 0.61; P = 0.001).
FIG. 1.
Anti-LPS IgA ASC responses to oral inoculation with ΔguaBA ΔvirG Δsen Δset S. flexneri 2a vaccine CVD 1207, by dose. The peak IgA anti-LPS ASC response measured 7 or 10 days after vaccination for each volunteer is indicated by a circle. There was a significant positive relationship between dose and peak number of ASC enumerated (linear regression of log-transformed data; r = 0.61; P = 0.001).
Humoral immune response.
The serologic response to vaccination was modest and was seen primarily at the higher doses. Among 18 recipients of 109 or 1010 CFU, 3 (17%) mounted a fourfold rise in IgG and 2 (11%) produced a fourfold rise in IgA anti-LPS antibody. Vaccination elicited a fourfold rise in anti-invasiveness plasmid antigen IgG antibody in two subjects (11%) and IgA antibody in one subject (6%).
Cytokine production and proliferation responses by PBMC.
Among subjects in the 109-CFU dose group with evaluable assays (i.e., strong proliferation in response to PHA and TT), one had a proliferative response both to the S. flexneri 2a homogenate and to IpaD (Table 3). This subject also produced an IFN-γ and IL-10 response to IpaD, both TGF-β and IL-10 responses to homogenate, and a TGF-β response IpaC. A second volunteer mounted an IL-10 response to S. flexneri particulate. A third recipient of 109 CFU produced strong IFN-γ responses to S. flexneri homogenate and particulate preparations and an IL-10 response to IpaC.
TABLE 3.
Peak proliferation and peak cytokine production by PBMC in response to various antigens after immunization with CVD 1207, by dose
| Dose group | Antigena | Response (no. responding/total)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proliferation | IL-2 | IL-4 | IL-5 | IFN-γ | IL-10 | IL-12 | IL-15 | TGF-β | ||
| 109 CFU | SF homogenate | 1/7 | 0/8 | 0/4 | 0/4 | 1/8 | 1/8 | 0/3 | 0/3 | 1/1 |
| SF particulate | 0/7 | 0/8 | 0/4 | 0/4 | 1/8 | 1/8 | 0/3 | 0/3 | 0/1 | |
| IpaC | 0/6 | 0/7 | 0/4 | 0/4 | 0/8 | 1/8 | 0/3 | 0/3 | 1/1 | |
| IpaD | 1/6 | 0/7 | 0/4 | 0/4 | 1/8 | 1/8 | 0/4 | 0/4 | 0/1 | |
| 1010 CFU | SF homogenate | 0/3 | NDb | 0/3 | ND | 0/3 | 0/3 | ND | ND | 0/3 |
| SF particulate | 1/3 | ND | 0/3 | ND | 0/3 | 0/3 | ND | ND | 0/3 | |
| IpaC | 0/3 | ND | 0/3 | ND | 0/3 | 0/3 | ND | ND | 0/3 | |
| IpaD | 1/3 | ND | 0/3 | ND | 0/3 | 0/3 | ND | ND | 2/3 | |
SF, S. flexneri.
ND, not done.
One of the three individuals with evaluable assays in the 1010-CFU dose group had a strong proliferative response to S. flexneri 2a particulate antigens and also proliferated in response to IpaD (Table 3). This same volunteer, in addition to one other subject, produced TGF-β in response to IpaD. No increases in IL-4 or IL-5 levels produced by PBMC were noted following immunization in either cohort.
DISCUSSION
Shigella is notorious for its ability to cause clinical disease after ingestion of as few as 10 organisms (10). Indeed, when the wild-type parent of CVD 1207, S. flexneri 2a strain 2457T, is administered with bicarbonate buffer to volunteers, as little as 103 CFU consistently causes diarrhea and dysentery in 80 to 90% of the subjects (17). Previous attempts to attenuate virulent S. flexneri 2a strain 2457T by engineering precise deletions of genes encoding virulence attributes met with only limited success; at doses producing immune responses (108 or more CFU), shigellosis-like adverse reactions (diarrhea, fever, and dysentery) were observed in many vaccinees (18, 22). The findings in this paper are noteworthy because they demonstrate that the introduction of multiple rational mutations has attenuated strain 2457T to a degree not previously achieved. This generates optimism that it may indeed be possible to prepare a safe and well-tolerated live, invasive vaccine strain from a fully virulent strain of S. flexneri 2a using recombinant techniques. Among the 35 subjects who ingested 106 to 1010 CFU of CVD 1207, adverse reactions were mild and occurred only at the highest (≥109 CFU) inocula. Furthermore, no subject experienced fever or dysentery, generally the most uncomfortable symptoms of shigellosis. Since a safety margin of approximately 2 log units may be acceptable for a live vaccine candidate, an inoculum of 107 CFU of CVD 1207 would be appropriate for further testing. Since the 108-CFU dose group contained only three subjects, additional evaluation of this inoculum would further ensure that there is no significant reactogenicity at this level.
CVD 1207 represents an important step in the considerable clinical experience that has accrued with vaccines derived from the virulent parent, S. flexneri 2a strain 2457T. Recently, practical progress has been made toward moving into clinical trial another S. flexneri 2a candidate vaccine, SC602, a ΔvirG (icsA) Δiuc live oral vaccine derived from S. flexneri 2a strain 454. While SC602 and CVD 1207 share a mutation in virG (icsA) as an attenuating lesion, one cannot draw conclusions about the attenuating effects of this mutation, since the two strains were derived from different wild-type backgrounds and each vaccine candidate possesses distinct additional mutations. Notably, CVD 1207 is constructed from strain 2457T, whose virulence has been repeatedly demonstrated in volunteers (15–17); to our knowledge, the degree of virulence of the wild-type parent of SC602 (strain 454) has not been assessed in volunteer studies.
By examining the clinical response to vaccine candidates derived from S. flexneri 2a strain 2457T, one can begin to elucidate the attenuating effect in humans of specific genetic mutations, a process that is not possible when evaluating Shigella vaccines that are constructed from different parent strains or from parent strains with uncharacterized pathogenicities. Lindberg and colleagues constructed SFL 1070 from S. flexneri 2a strain 2457T using a chromosomal mutation in aroD, which encodes an enzyme in the aromatic amino acid biosynthesis pathway that is necessary to sustain bacterial replication within human cells (22). In volunteer trials, SFL 1070 was measurably attenuated compared to the wild type but still induced residual dose-related reactogenicity (gastrointestinal symptoms and fever) in 10 to 44% of the subjects who received three doses of 105 to 109 CFU (13). In an attempt to further attenuate S. flexneri 2a strain 2457T, Noriega et al. constructed CVD 1203 by introducing a deletion in the plasmid gene virG (34), in addition to a deletion in the chromosomal gene aroA (34). CVD 1203 was highly immunogenic, with geometric mean IgA anti-LPS ASC counts of 13, 43, and 175 per 106 PBMC following administration of inocula of 106, 108, and 109 CFU, respectively (Table 1). However, whereas no adverse reactions (diarrhea, dysentery, or fever) were observed in recipients of 106 CFU, unacceptable reactions were seen in 18% of subjects who ingested 108 CFU and in 78% of those who ingested 109 CFU (Table 1) (18).
Noriega et al. next explored the attenuating effects of chromosomal mutations affecting enzymes employed in the distal purine biosynthesis pathway. First, they constructed CVD 1204 by introducing specific deletions in the guaBA operon (32). When directly compared with a ΔaroA mutant strain, CVD 1204 was significantly less invasive in HeLa cells and induced significantly less conjunctival inflammation in the guinea pig keratoconjunctivitis (Serény) test (32). A double ΔguaBA ΔvirG mutant, CVD 1205, elicited fewer inflamed eyes than did CVD 1204 (5 out of 16 versus 11 out of 16), although this difference was not statistically significant (32). It was hoped that the diminished virulence in preclinical studies of S. flexneri 2a attenuated with ΔguaBA and ΔvirG would eliminate the residual constitutional symptoms associated with ΔaroA ΔvirG CVD 1203 in volunteers. Next, in an effort to minimize diarrheal reactogenicity, CVD 1207 was constructed, harboring deletion mutations in the S. flexneri 2a enterotoxin genes sen (29) and set (11), in addition to guaBA and virG. As expected, CVD 1207 was markedly attenuated compared with previous constructs (15, 18); it was completely devoid of reactions at 108 CFU and induced only mild gastrointestinal complaints, without fever or dysentery, in 3 of 18 subjects (17%) inoculated with 109 or 1010 CFU. The etiology of residual diarrhea at higher doses remains elusive, since in vivo studies suggest that enterotoxic activity is absent; one possible explanation is that the vaccine strain elicited a local intestinal inflammatory response, including the release of proinflammatory cytokines, which manifested at the high inocula.
The significance of the modest serum antibody responses to CVD 1207 (and other attenuated Shigella vaccine candidates) is uncertain. Anti-LPS serum IgG is a response that has been correlated (3, 4, 5) (albeit inconsistently [9; D. Cohen, M. S. Green, C. Block, R. Slepon, and Y. Lerman, Letter, J. Infect. Dis. 165:785–787, 1992]) with immunity to shigellosis. Although the failure of CVD 1207 to induce a vigorous serologic response is disappointing, there is ample precedent for inoculations which elicit protective immunity evoking relatively weak serum antibody responses in clinical trials. For example, streptomycin-dependent S. flexneri 2a vaccine (also derived from wild-type strain 2457T) (28) induced a fourfold rise in anti-LPS hemagglutinating antibody in only 38% of subjects who received five doses of 1010 CFU (8), a dosage level which confers 49% protective immunity against experimental challenge (9), and >85% protection against natural infection in field trials (24, 27). Similarly, experimental wild-type S. flexneri 2a challenge induces serum anti-LPS IgG antibody responses in approximately 50% of subjects (16) yet confers 70% protection against illness following rechallenge (17).
Anti-Shigella LPS ASC responses have also been correlated with protective immunity in clinical trials (16). The geometric mean IgA anti-LPS ASC response is approximately 239 per 106 PBMC following virulent infection (Table 1) (17) and 18 per 106 PBMC following vaccination with SC602, a ΔvirG Δiuc live oral vaccine derived from S. flexneri 2a strain 454 that conferred protection against illness following experimental challenge (6). The magnitude of the IgA anti-LPS ASC response that was elicited by CVD 1207 at well-tolerated dose levels (5.3 to 6.1 per 106 PBMC) falls short of these values. It is necessary to conduct challenge studies to determine whether CVD 1207 can provide protective immunity.
Significant IFN-γ, IL-10, and/or TGF-β responses to Shigella-specific antigens were detected in five volunteers immunized with CVD 1207. A similar (albeit more pronounced) cytokine response was found in a recently completed study using PBMC from volunteers exposed to a ΔstxA (i.e., non-Shiga toxin-producing) wild-type Shigella dysenteriae type 1 strain (39). This cytokine pattern, characteristic of a Th1 response—as indicated by the production of IFN-γ and the absence of IL-4 or IL-5—is likely to play an important role in protection from Shigella, an intracellular pathogen. IFN-γ is produced by T cells following antigen-specific stimulation, as well as by natural killer cells, and has the ability to stimulate macrophages to kill phagocytosed microbes and to prevent Shigella invasion of epithelial cells (30). While both TGF-β and IL-10 may be involved in stimulating an antibody response, IL-10 in particular may serve as a counterregulatory cytokine to the IFN-γ responses induced by Shigella. Furthermore, IL-10 may inhibit IL-1, a critical trigger of the strong intestinal inflammatory response that occurs with shigellosis (41).
The relatively meager proliferative responses in this trial (observed in 2 of 10 subjects who received 109 or 1010 CFU of CVD 1207) and in the previous trial using virulent nontoxigenic S. dysenteriae type 1 (39), is consistent with the lack of production of IL-2, IL-12, and IL-15, all of which promote T-cell proliferation. However, these limited specific proliferative responses cannot necessarily be construed as an indication that this attenuated Shigella vaccine strain is a poor immunogen. Bacterial species may inhibit proliferation by triggering IFN-γ, IL-10, and TGF-β production and by using a variety of independent mechanisms (38, 40). Some investigators have suggested that Shigella subverts host defenses by inhibiting macrophage presentation of Shigella antigens and possibly even by inducing apoptosis of macrophages (12, 42).
It is noteworthy that specific cytokine and/or proliferation responses to IpaC and IpaD were observed in 4 of 11 volunteers immunized with CVD 1207, suggesting that these proteins are potentially important epitopes in the human host immune response to shigellosis. These responses were specific, since neither TT nor bovine serum albumin induced cytokine responses nor were the responses attributable to contamination with LPS (data not shown). To our knowledge, this is the first demonstration in humans that S. flexneri 2a vaccination elicits cell-mediated immune responses to Shigella invasins. Since these proteins are conserved among Shigella species, they offer the potential for inducing cross-serotype protection.
In sum, we have demonstrated that CVD 1207 is highly attenuated and well tolerated at dosage levels that were markedly reactogenic with earlier invasive S. flexneri 2a vaccine candidates. Moreover, when the occasional adverse reactions did occur at very high dosage levels, neither fever nor dysentery was encountered. The clinical response to CVD 1207 appears to be at least as acceptable as the responses to other S. flexneri 2a vaccines that progressed to phase 2 evaluations, such as the streptomycin-dependent strains (21, 25, 26), the Escherichia coli-S. flexneri 2a hybrid EcSf2a-2 (15), and SC602 (6). Nonetheless, it is conceivable that at well-tolerated dosage levels, CVD 1207 may be insufficiently immunogenic after a single dose. Therefore, a strategy of administering two spaced doses at inoculum levels that are clinically well tolerated may be advisable to achieve an acceptable balance between reactogenicity and immunogenicity. It is possible that lyophilization may temper the post-vaccination reactions without compromising immunogenicity, as was the case with streptomycin-dependent vaccines (24). Another consideration for future trials is to evaluate other engineered S. flexneri 2a 2457T strains, such as CVD 1204 (ΔguaBA) (32) and CVD 1208 (ΔguaBA Δsen Δset), to assess their immunogenicities and reactogenicities in relation to those of CVD 1207.
ACKNOWLEDGMENTS
We are grateful to the volunteers who participated in this trial. We thank Kathleen Palmer, Brenda Berger, Cathy Black, Ron Grochowski, and Theresa Mowrey for assistance in the recruitment and care of volunteers, and Mardi Reymann and Sofie Livio for technical support.
These studies were supported in part by a grant from the World Health Organization. Funding came from Public Health Service grants from the National Institute of Allergy and Infectious Diseases as follows: R01-AI-29471 and R01-AI-40297 (to M.M.L.) and R21-AI-40261 (to F.R.N.) for strain construction and R29-AI-34428 (to W.D.P.) for the production of purified recombinant IpaC and IpaD.
REFERENCES
- 1.Bennish M L, Wojtyniak B J. Mortality due to shigellosis: community and hospital data. Rev Infect Dis. 1991;13(Suppl. 4):S245–S251. doi: 10.1093/clinids/13.supplement_4.s245. [DOI] [PubMed] [Google Scholar]
- 2.Bernardini M L, Mounier J, D'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella-flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black R E, Levine M M, Clements M L, Losonsky G, Herrington D, Berman S, Formal S B. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155:1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D, Ashkenazi S, Green M S, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor D N, Hale T L, Sadoff J C, Pavliakova D, Schneerson R, Robbins J B. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Green M S, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coster T S, Hoge C W, Van de Verg L L, Hartman A B, Oaks E V, Venkatesan M M, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti P J, Hale T L. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuPont H L, Hornick R B, Dawkins A T, Snyder M J, Formal S B. The response of man to virulent Shigella flexneri 2a. J Infect Dis. 1969;119:296–299. doi: 10.1093/infdis/119.3.296. [DOI] [PubMed] [Google Scholar]
- 8.DuPont H L, Hornick R B, Snyder M J, Libonati J P, Formal S B, Gangarosa E J. Immunity in shigellosis. I. Response of man to attenuated strains of Shigella. J Infect Dis. 1972;125:5–11. doi: 10.1093/infdis/125.1.5. [DOI] [PubMed] [Google Scholar]
- 9.DuPont H L, Hornick R B, Snyder M J, Libonati J P, Formal S B, Gangarosa E J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972;125:12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- 10.DuPont H L, Levine M M, Hornick R B, Formal S B. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- 11.Fasano A, Noriega F R, Maneval D R, Jr, Chanasongcram S, Russell R, Guandalini S, Levine M M. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Investig. 1995;95:2853–2861. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell R A, Yuan J, Sansonetti P J, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 13.Karnell A, Li A, Zhao C R, Karlsson K, Nguyen B M, Lindberg A A. Safety and immunogenicity study of the auxotrophic Shigella flexneri 2a vaccine SFL1070 with a deleted aroD gene in adult Swedish volunteers. Vaccine. 1995;13:88–99. doi: 10.1016/0264-410x(95)80017-8. [DOI] [PubMed] [Google Scholar]
- 14.Keat A. Reiter's syndrome and reactive arthritis in perspective. N Engl J Med. 1983;309:1606–1615. doi: 10.1056/NEJM198312293092604. [DOI] [PubMed] [Google Scholar]
- 15.Kotloff K L, Herrington D A, Hale T L, Newland J W, Van de Verg L, Cogan J P, Snoy P J, Sadoff J C, Formal S B, Levine M M. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1992;60:2218–2224. doi: 10.1128/iai.60.6.2218-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotloff K L, Losonsky G A, Nataro J P, Wasserman S S, Hale T L, Taylor D N, Newland J W, Sadoff J C, Formal S B, Levine M M. Evaluation of the safety, immunogenicity and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13:495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 17.Kotloff K L, Nataro J P, Losonsky G A, Wasserman S S, Hale T L, Taylor D N, Sadoff J C, Levine M M. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995;13:1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 18.Kotloff K L, Noriega F, Losonsky G A, Sztein M B, Wasserman S S, Nataro J P, Levine M M. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect Immun. 1996;64:4542–4548. doi: 10.1128/iai.64.11.4542-4548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotloff K L, Winickoff J P, Ivanoff B, Clemens J D, Swerdlow D L, Sansonetti P J, Adak G K, Levine M M. Global burden of Shigella infections: implications for vaccine development and implementation. Bull W H O. 1999;77:651–656. [PMC free article] [PubMed] [Google Scholar]
- 20.Lett M, Sasakawa C, Okada N, Sakai T, Makino S, Yamada M, Komatsu K, Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the VirG protein and determination of the complete coding sequence. J Bacteriol. 1989;171:353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine M M, DuPont H L, Gangarosa E J, Hornick R B, Snyder M J, Libonati J P, Glaser K, Formal S B. Shigellosis in custodial institutions. II. Clinical, immunologic and bacteriologic response of institutionalized children to oral attenuated Shigella vaccines. Am J Epidemiol. 1972;96:40–49. doi: 10.1093/oxfordjournals.aje.a121431. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg A A, Karnell A, Pal T, Sweiha H, Hultenby K, Stocker B A. Construction of an auxotrophic Shigella flexneri strain for use as a live vaccine. Microb Pathog. 1990;8:433–440. doi: 10.1016/0882-4010(90)90030-t. [DOI] [PubMed] [Google Scholar]
- 23.Marquart M E, Picking W L, Picking W D. Structural analysis of invasion plasmid antigen D (IpaD) from Shigella flexneri. Biochem Biophys Res Commun. 1995;214:963–970. doi: 10.1006/bbrc.1995.2380. [DOI] [PubMed] [Google Scholar]
- 24.Mel D M, Arsic B L, Nikolic B D, Radovanovic M L. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull W H O. 1968;39:375–380. [PMC free article] [PubMed] [Google Scholar]
- 25.Mel D M, Gangarosa E J, Radovanovic M L, Arsic B L, Litvinjenko S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull W H O. 1971;45:457–464. [PMC free article] [PubMed] [Google Scholar]
- 26.Mel D M, Papo R G, Terzin A L, Vuksic L. Studies on vaccination against bacillary dysentery. 2. Safety tests and reactogenicity studies on a live dysentery vaccine intended for use in field trials. Bull W H O. 1965;32:637–645. [PMC free article] [PubMed] [Google Scholar]
- 27.Mel D M, Terzin A L, Vuksic L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull W H O. 1965;32:647–655. [PMC free article] [PubMed] [Google Scholar]
- 28.Mel D M, Terzin A L, Vuksic L. Studies on vaccination against bacillary dysentery. 1. Immunization of mice against experimental Shigella infection. Bull W H O. 1965;32:633–636. [PMC free article] [PubMed] [Google Scholar]
- 29.Nataro J P, Seriwatana J, Fasano A, Maneval D R, Guers L D, Noriega F, Dubovsky F, Levine M M, Morris J G., Jr Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun. 1995;63:4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niesel D W, Hess C B, Cho Y J, Klimpel K D, Klimpel G R. Natural and recombinant interferons inhibit epithelial cell invasion by Shigella spp. Infect Immun. 1986;52:828–833. doi: 10.1128/iai.52.3.828-833.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noriega F R, Liao F M, Maneval D R, Ren S, Formal S B, Levine M M. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noriega F R, Losonsky G, Lauderbaugh C, Liao F M, Wang J Y, Levine M M. Engineered ΔguaB-A ΔvirG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity, and potential efficacy as a mucosal vaccine. Infect Immun. 1996;64:3055–3061. doi: 10.1128/iai.64.8.3055-3061.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noriega F R, Wang J Y, Losonsky G, Maneval D R, Hone D M, Levine M M. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1994;62:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noriega F R, Wang J Y, Losonsky G, Maneval D R, Hone D M, Levine M M. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1995;65:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oaks E V, Hale T L, Formal S B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53:57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasetti M, Anderson R J, Noriega F R, Levine M M, Sztein M B. Attenuated ΔguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin Immunol. 1999;92:76–89. doi: 10.1006/clim.1999.4733. [DOI] [PubMed] [Google Scholar]
- 37.Picking W L, Mertz J A, Marquart M E, Picking W D. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr Purif. 1996;8:401–408. doi: 10.1006/prep.1996.0117. [DOI] [PubMed] [Google Scholar]
- 38.Pryjma J, Baran J, Ernst M, Woloszyn M, Flad H D. Altered antigen-presenting capacity of human monocytes after phagocytosis of bacteria. Infect Immun. 1994;62:1961–1967. doi: 10.1128/iai.62.5.1961-1967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samandari, T., K. L. Kotloff, G. A. Losonsky, W. D. Picking, P. J. Sansonetti, M. M. Levine, and M. B. Sztein. Production of interferon-γ and IL-10 to Shigella invasins by mononuclear cells form volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J. Immunol., in press. [DOI] [PubMed]
- 40.Samandari, T., M. M. Levine, and M. B. Sztein. Mechanisms for establishing persistence: immune modulation. In J. P. Nataro, M. J. Blaser, and S. Cunningham-Rundles (ed.), Persistent bacterial infections, in press. ASM Press, Washington, D.C.
- 41.Sansonetti P J, Arondel J, Cavaillon J M, Huerre M. Role of interleukin-1 in the pathogenesis of experimental shigellosis. J Clin Investig. 1995;96:884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwan W R, Kopecko D J. Uptake of pathogenic intracellular bacteria into human and murine macrophages downregulates the eukaryotic 26S protease complex ATPase gene. Infect Immun. 1997;65:4754–4760. doi: 10.1128/iai.65.11.4754-4760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stieglitz H, Fosmire S, Lipsky P. Identification of a 2-Md plasmid from Shigella flexneri associated with reactive arthritis. Arthritis Rheum. 1989;32:937–946. doi: 10.1002/anr.1780320802. [DOI] [PubMed] [Google Scholar]
- 44.Sztein M B, Wasserman S S, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 45.Van de Verg L, Herrington D A, Murphy J R, Wasserman S S, Formal S B, Levine M M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990;58:2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van de Verg L L, Herrington D A, Boslego J, Lindberg A A, Levine M M. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis. 1992;166:158–161. doi: 10.1093/infdis/166.1.158. [DOI] [PubMed] [Google Scholar]
- 47.Wells J G, Morris G K. Evaluation of transport methods for isolating Shigella spp. J Clin Microbiol. 1981;13:789–790. doi: 10.1128/jcm.13.4.789-790.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]