Abstract
Purpose
Most of the existing studies focus on the early inflammation of rosacea, with few interventions on the later development of fibrosis and the relationship between thalidomide and rosacea. The purpose of this study was to construct a long-term induction model and explore the effects of thalidomide on the later stage of inflammation and early stage of fibrosis in rosacea.
Patients and Methods
BALB/c male mice were randomly divided into four groups: control group, control plus thalidomide group, LL-37 group and LL-37 plus thalidomide group, Intradermal and intraperitoneal injections were given. After repeated induction, skin changes were recorded by taking photos. The animals were sacrificed, the back skin was used for HE staining and VG staining to detect histomorphological characteristics. Immunofluorescence staining and Western blot were used to detect the expression of inflammatory and fibrosis-related factors.
Results
The results were compared with the early stage of the model, wherein the skin inflammation of the 20-day mice was more obvious with a trend of fibrosis. Compared with the control group, histopathological examination showed that the inflammatory cell infiltration in the LL-37 group was significantly increased, and the skin was thickened with collagen deposition. LL-37 induction significantly increased the expression of inflammatory markers (eg, TNF-α and IL-1β) and fibrotic markers (eg, COL1, α-SMA, vimentin and N-Cadherin). Intervention with thalidomide significantly reduced erythema, inflammatory cell infiltration, collagen deposition, and down-regulate the expression of inflammation and fibrosis related factors in rosacea mice.
Conclusion
The long-term continuous induction of LL-37 in mice could simulate the occurrence and development of rosacea, and thalidomide could ameliorate the rosacea induced by long-term exposure to LL-37 by regulating inflammatory infiltration, collagen deposition and fibrosis-related processes.
Keywords: rosacea, LL-37, thalidomide, inflammation, skin fibrosis
Introduction
Rosacea is a chronic inflammatory skin disease that tends to occur on the face, near the nose, forehead, cheeks, and jaw. In the early stage, patients are mainly affected by inflammation with persistent erythema, papules, and pustules. In the late stage, due to repeated stimulation of inflammation and overgeneration of blood vessels, patients often suffer from connective tissue hyperplasia, sebaceous gland hypertrophy, skin fibrosis, and, eventually, the formation of nasal vegetation.1 According to its clinical manifestations, rosacea is divided into four subtypes, namely, erythematotelengiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PHR), and ocular rosacea (OR).2 The disease tends to occur in women between age groups 35 and 50, but mostly rhinophyma occurs in males.3 The pathophysiological mechanism of this disease involves factors such as genetic background, presence of microorganisms, and dysfunction of immune neurovascular and skin barriers. However, the exact pathogenesis is still not completely clear.4 Rosacea can be locally treated with azelaic acid and metronidazole or systematically treated with tetracycline, ivermectin, macrolide antibiotics, and isotretinoin. It can also be treated by laser, radiofrequency ablation, and surgery.5 In the case of recurrent rosacea, long-term administration of these drugs prone to a series of adverse reactions. Therefore, its pathogenesis and effective treatment methods need to be further explored.
LL-37, the only known human cathelicidin-like antimicrobial peptide, is an active component of the precursor protein, hCAP-18.6 The peptide consists of 37 amino acids from the N-terminal of cathelicidin protein, starting from fragment L-L, thus named LL-37.7 LL-37 has anti-inflammatory, antibacterial, angiogenic, chemotactic, and immunomodulatory effects.8 Existing research has shown that the rosacea inflammation model can be obtained by inducing LL-37 and simulating the onset of ETR. However, there are currently no animal models for other subtypes of rosacea.9 The late clinical manifestations of rosacea are mostly caused by disease course migration and repeated stimulation of inflammation. The previous inflammatory model did not fully simulate the pathological changes of rosacea due to the short induction time of LL-37, which developed in a short time and healed quickly. Therefore, we induced the pre-fibrotic model of rosacea by prolonging the modeling time and changing the induction cycle.
Thalidomide is a synthetic glutamic acid derivative once used as a hypnotic sedative and antiemetic drug.10 However, it was withdrawn from the market due to its teratogenicity and peripheral neurotoxicity. Recent literature shows that it has anti-inflammatory, anti-angiogenic, and immunomodulatory effects and thus can be applied to a variety of dermatological diseases related to inflammation and autoimmunity.11 In the clinical treatment of skin diseases, thalidomide is already used in some inflammatory skin conditions such as pyoderma gangrenosum12 or cutaneous lupus erythematosus.13 At the same time, it also has a regulatory effect on pulmonary fibrosis,14 intestinal fibrosis,15 and other fibrotic diseases. It can also inhibit the inflammatory phenotype of rosacea,16 however, its association with the fibrotic phenotype of rosacea remains to be studied.
Therefore, the purpose of this study was to investigate whether thalidomide has a therapeutic effect on LL-37-induced rosacea pre-fibrotic mice and to explore the possible mechanism.
Materials and Methods
Animal Experiments
All animal experiments in this study were approved and reviewed by the Ethics Committee of North China University of Science and Technology (LX2021178) and complied with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Four-week-old male BALB/c mice weighing 20 grams were purchased from Vital River Laboratory Animal Technology. All mice were adaptively fed for two weeks.
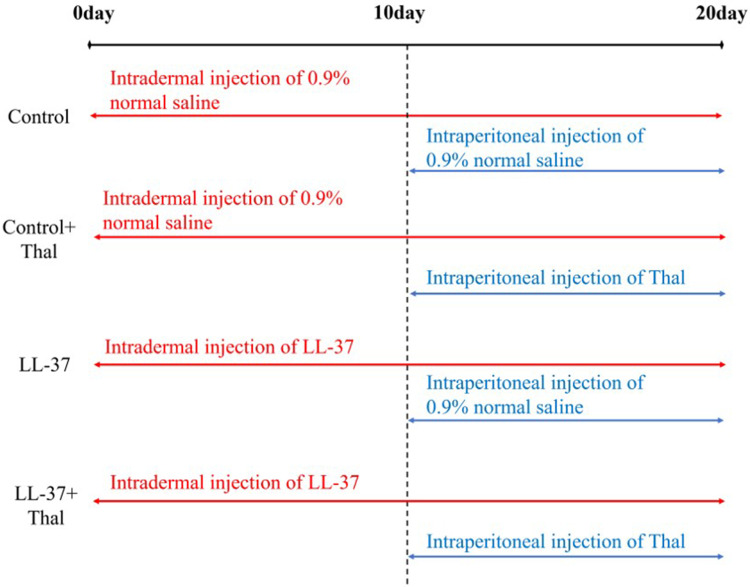
The back hair of all mice was shaved 24 hours before treatment. As shown in Figure 1, the mice were randomly divided into four groups (n=5 for each group). The control group was Intradermally injected with 40 μL normal saline (0.9%) once a day for 20 days, with simultaneous intraperitoneal injection of 100 μL normal saline (0.9%) started from day 10, once a day for the next ten days. The 2nd Control plus thalidomide group was Intradermally injected with 40 μL normal saline (0.9%) was given once a day for 20 days, with a simultaneous intraperitoneal injection of 100 μL thalidomide (50 mg/kg) (H32028128, Changzhou Pharmaceutical Factory Co. LTD, Changzhou, China) started from day 10, once a day for next 10 days. Group 3, ie, LL-37, was injected with 40 μL LL-37 peptide (320 μM) (24,461, Cayman Chemical Company, Ann Arbor, MI, USA) once a day for 20 days, with a simultaneous intraperitoneal injection of 100 μL normal saline (0.9%) started from day 10, once a day for 10 days. Lastly, group 4 (LL-37+ thalidomide group) was injected with 40 μL LL-37 peptide (320μM) once a day for 20 days, with simultaneous intraperitoneal injection of 100 μL thalidomide (50mg/kg) starting from day 10, once a day for 10 days. The dorsal skin was photographed before each injection. Mice were sacrificed by anesthesia 24 hours after the last injection. Part of the back skin tissue was fixed, dehydrated, and embedded in paraffin for hematoxylin-eosin (H&E), Van Gieson’s (V.G.), and immunofluorescence staining. The remaining tissues were stored at −80° for Western blot analysis.
Figure 1.
Administration of rosacea in long-term mice model. According to the different methods of administration, there were four groups. Intradermal injection of LL-37 or 0.9% normal saline was given from day 1. The continuous intradermal injection was given, along with an intraperitoneal injection of thalidomide or 0.9% normal saline from day 10. Each operation was done once a day. All Samples were sacrificed 24 hours after the last injection.
Hematoxylin–Eosin Staining
Skin tissue sections were dewaxed and rehydrated and –and stained with H&E dye (BA4025, Baso Diagnostics Inc., Zhuhai, China) to observe the morphology of the tissue. The epidermis and dermis thickness after H&E staining was analyzed by ImageJ software (1.52a, National Institutes of Health, Bethesda, MD, USA).
Van Gieson’s Staining
The dewaxed and rehydrated skin tissue sections were added with the mixture of hematoxylin A and hematoxylin B, followed by V.G. dye (BA4084, Baso Diagnostics Inc., Zhuhai, China) to observe the content of collagen content. The collagen area after V.G. staining was analyzed by ImageJ software.
Immunofluorescence Staining (IF)
Paraffin sections were dewaxed and rehydrated. After high-pressure repair, tissues were treated with primary antibodies of tumor necrosis factor (TNF-α, 1:100 dilution, GTX110520, GeneTex, San Antonio, TX, USA), smooth muscle alpha-actin (α -SMA, 1:100 dilution, ab5694, Abcam, Cambridge, UK), and vimentin (1:200 dilution, ab92547, Abcam, Cambridge, UK) at 4°C, overnight. They were then combined with goat anti-rabbit secondary antibodies (130,154, SeraCare, Milford, MA, USA) at 37°C for an hour after washing thrice with phosphate buffer. Finally, sections were sealed with 4′,6-diamidino-2′-phenylindole (DAPI, 8961s, Cell Signaling Technology, Inc., Danvers, MA, USA). The expressions of TNF-α, α -SMA, and vimentin were observed under an Olympus DP80 microscope (Olympus, Hamburg, Germany). The TNF-α, α -SMA, and vimentin were marked by green fluorescence, and the nucleus was marked by blue fluorescence.
Western Blot
Radioimmunoprecipitation assay (RIPA) buffer was used to split skin tissue proteins. The primary antibodies included TNF-α (1:1000 dilution), Interleukin 1 beta (IL-1β, 1:1000 dilution, DF6251, Affinity Biosciences, Cincinnati, OH, USA), Collagen 1 (COL1, 1:4000 dilution, ab34710, Abcam, Cambridge, UK), vimentin (1:4000 dilution), N-Cadherin (1:1000 Dilution, ARG23870, Arigo, Shanghai, China), and GAPDH (1:10,000 dilution, ab181602, Abcam, Cambridge, UK). They were then incubated with goat anti-rabbit or anti-mouse secondary antibodies (074–1506/074-1806, Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA). The target bands were visualized using ECL Prime Western Blotting Detection Reagent (ZD310A, ZomanBio, Beijing, China). GAPDH was used as an internal reference.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 20.0 software (IBM Corp., Armonk, NY, USA) and the GraphPad Prism 8.4.3 (686) statistical packages. The data were expressed as means ± standard deviations. Multiple comparisons were performed using a one-way analysis of variance followed by Tukey’s post-hoc test. Statistical significance was achieved when p < 0.05 at a 95% confidence interval.
Results
Thalidomide Attenuates Skin Lesions in LL-37- Induced Rosacea Mice
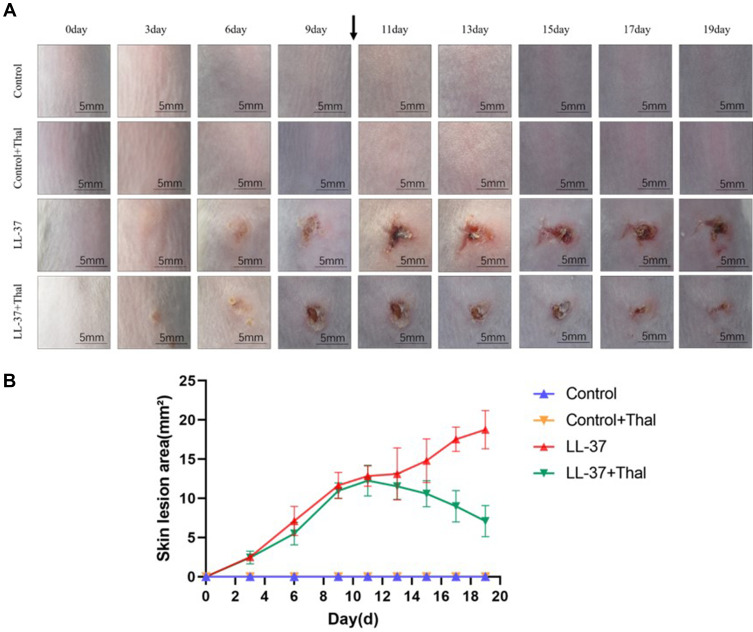
Previous studies have shown that LL-37-induced rosacea-like inflammation has been recognized as an established approach in animal models.9 The original short-term model used an intradermal injection of LL-37 twice a day for two consecutive days. We extended the administration time and cycle of LL-37 to induce the pre-fibrosis mice model of rosacea. The long-term simulated the pathological process of skin fibrosis caused by rosacea inflammation and repeated stimulation of angiogenesis. To investigate whether thalidomide has a therapeutic effect on rosacea inflammation and fibrosis, we intraperitoneally injected thalidomide and normal saline into mice and compared the differences between the two early- and late-term groups. The gross view of the skin recorded showed that LL-37 could induce erythema and capillary angiogenesis. By comparing the skin manifestations of mice in early and late term, we found that the skin erythema on the back of mice in the early-term model was not as serious as that in the late-term model. With the extension of induction time, the area of erythema gradually increased while papules, pustules, and crusts were formed. After thalidomide administration, erythema and edema were alleviated, and the skin lesions gradually repaired (Figure 2A). As shown in Figure 2B, the line chart reflects the change in skin lesion area with the administration time. After the intraperitoneal injection of thalidomide from day 10, the skin lesion area of the LL-37 group continued to increase, while the skin lesion area of mice in the LL-37 plus thalidomide group began to decrease.
Figure 2.
Gross view of skin lesions from rosacea-like mice model. (A) The back skin performance of mice injected intradermally with LL-37 or 0.9% normal saline and injected intraperitoneally with thalidomide or 0.9% normal saline. Images were captured before each injection (Scale Bar=5mm). (B) The severity of the rosacea-like phenotype was assessed based on the area of lesions (n=5 for each group). With the extension of LL-37 induction time, the area of dorsal skin lesions gradually increased, while that with the intraperitoneal injection of thalidomide was significantly reduced compared with 0.9% normal saline.
Thalidomide Reduces LL-37-Induced Rosacea-Like Inflammation
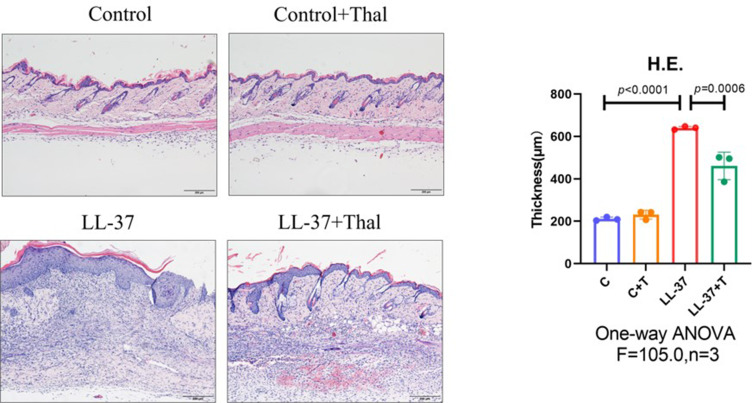
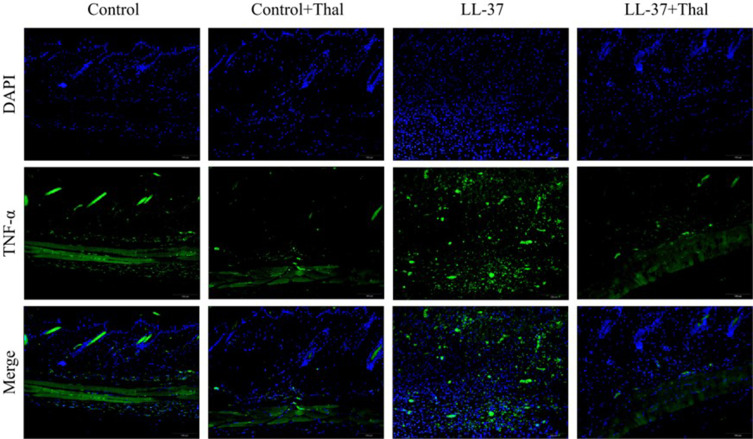
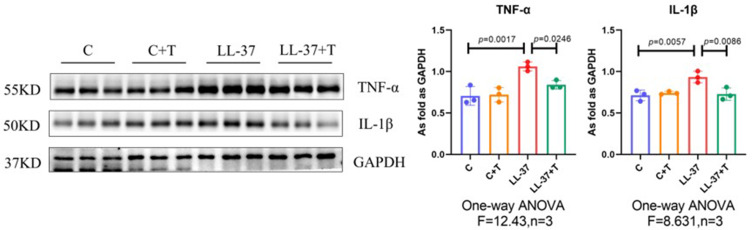
Previous studies have detected that thalidomide inhibits inflammatory expression in a two-day model of rosacea mice.16 Compared with previous models, our results showed that in the pre-fibrotic model, mice had more severe skin lesions, thickening of the dermal epidermis, increased infiltration of inflammatory cells in the dermal cortex, the fat vacuoles and muscle structure were destructed, thalidomide significantly reduced the infiltration of inflammatory cells and the thickness of skin (Figures 3). The expression of inflammatory factors, TNF-α and IL-1β, have been shown to upregulated in the skin of rosacea patients and increase in rosacea-like mice inflammation model.17 In this model, immunofluorescence staining showed that thalidomide reduced the expression of inflammatory cytokine TNF-α in LL-37-induced skin (Figure 4). The expression of TNF-α and IL-1β in skin protein induced by LL-37 were also upregulated, while thalidomide decreased the expression (Figures 5). These results thus suggested that thalidomide can inhibit the inflammatory response and inflammatory factors in rosacea.
Figure 3.
H&E staining of skin lesions from mice. The dermal epidermis of LL-37-induced rosacea mice was thickened, and the infiltration of dermal inflammatory cells increased, which were relieved after treatment with thalidomide. (Scale Bar=200 μm).
Figure 4.
Immunofluorescence staining of TNF-α in skin lesions from mice treated with 0.9% normal saline, 0.9% normal saline plus thalidomide, LL-37 plus 0.9% normal saline, and LL-37 plus thalidomide. (Scale Bar=100 μm).
Figure 5.
Western blot of TNF-α and IL-1β protein expression in dorsal skin. Data are presented as the mean ± SD, n=3 for each group.
Thalidomide Reduces LL-37- Induced Rosacea-Like Fibrosis
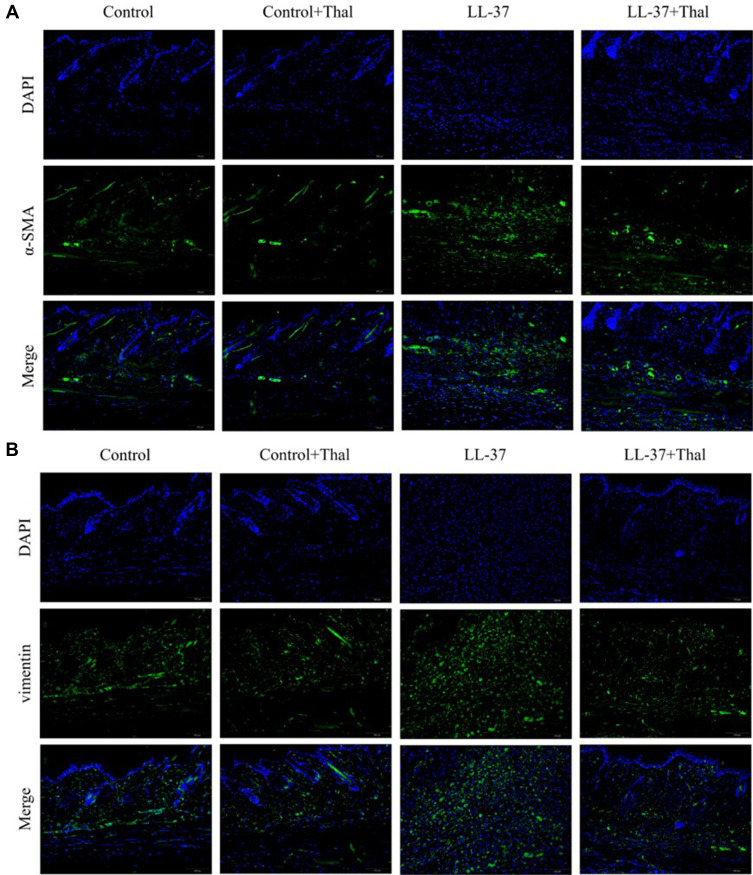
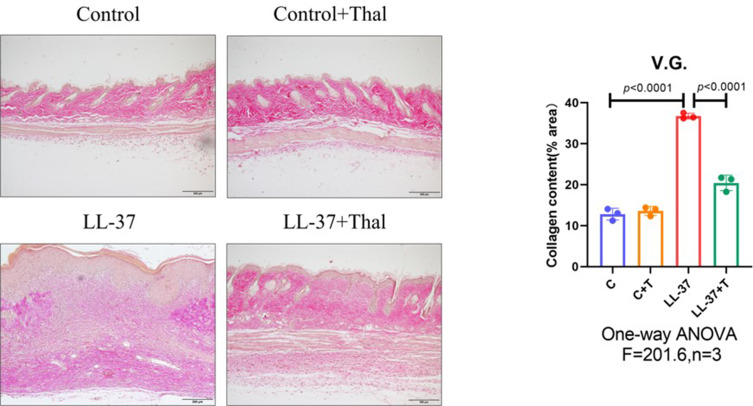
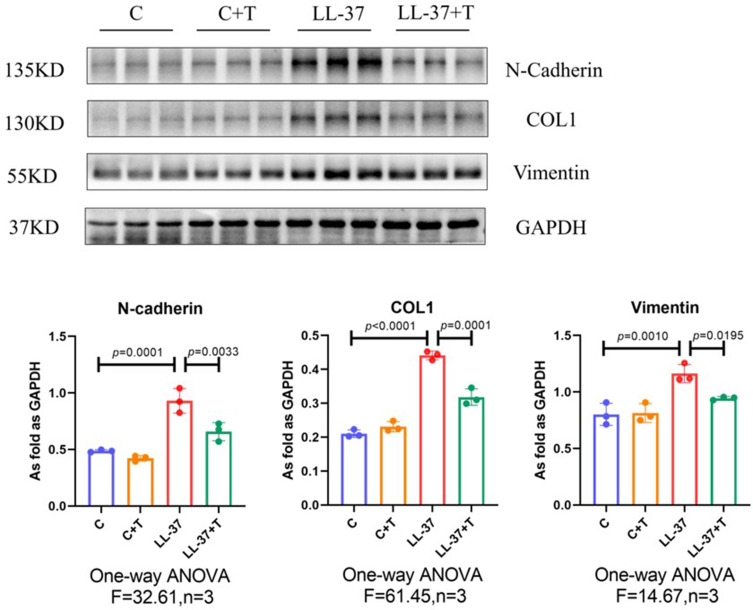
The process of skin fibrosis involves chronic inflammation, alteration of epithelial-mesenchymal interactions, fibroblast proliferation, differentiation into myofibroblasts, and extracellular matrix deposition (ECM).18 With the prolonged administration of LL-37, the release of inflammatory factors in the skin was continuously stimulated, and capillary dilatation occurred, which together led to repeated infections and congestion. It was found that α-SMA, a marker of myofibroblasts, was positively expressed in vascular smooth muscle cells and myofibroblasts in the model group, while its expression was decreased after thalidomide treatment, thus confirming that the process of rosacea fibrosis was activated (Figure 6A). At the same time, the mesenchymal cell marker vimentin was positively expressed in hair follicles, sebaceous glands, blood vessels, and mesenchymal cells. Its expression level was enhanced after LL-37 induction, and thalidomide administration inhibited its expression (Figure 6B). V.G. staining was used to determine the histological changes of skin fibrosis. The results showed that the content of dermal collagen in LL-37-induced skin tissue was higher than that in the control group, while the skin collagen infiltration showed a downward trend after thalidomide treatment (Figures 7). Western blot results indicated that COL1, vimentin, and N-cadherin in mouse skin tissue protein were up-regulated by LL-37, and this trend was inhibited by thalidomide (Figures 8). These findings thus suggested that thalidomide can inhibit the process of rosacea fibrosis.
Figure 6.
(A) Immunofluorescence staining of α-SMA in skin lesions from mice treated with 0.9% normal saline, 0.9% normal saline plus thalidomide, LL-37 plus 0.9% normal saline, and LL-37 plus thalidomide (Scale Bar=100 μm). (B) Immunofluorescence staining of vimentin in skin lesions from control mice and LL37-induced mice treated with 0.9% normal saline or thalidomide (Scale Bar=100 μm).
Figure 7.
V.G. staining of skin lesions from mice. Compared with the control group, the collagen deposition in the dermis of LL-37-induced rosacea mice increased but decreased after treatment with thalidomide (Scale Bar=200 μm).
Figure 8.
Western blot of N-cadherin, COL1, and vimentin protein expression in dorsal skin from mice. Data are presented as the mean ± SD, n=3 for each group.
Discussion
The aim of this study was to establish a long-term model of rosacea, induce the formation of a pre-fibrotic phenotype, and investigate the therapeutic effect of thalidomide on rosacea mice. Our results suggested that LL-37 could mimic the pathogenesis of rosacea and induce an inflammatory model of rosacea. In our experiment, prolonged the induction time can stimulate the generation of fibrosis, while thalidomide can maintain skin homeostasis and improve the occurrence and development of rosacea by inhibiting inflammation, angiogenesis, and fibrosis.
At present, many studies believe that inflammation and fibrosis are gradual and complementary processes. In the case of a wound, the repair process involves hemostasis, inflammation generation, cell proliferation, re-epithelialization, and wound reconstruction.19 After skin damage is caused by stimulating factors, the first physiological reaction is platelet coagulation and hemostasis, and then neutrophils accumulate in the skin lesion to induce inflammation. Sustained inflammation at the damaged site develops into chronic stimulation, and dysregulation of tissue repair response leads to pathological wound repair.20 In skin lesions, both keratinocytes and resident epithelial cells transform into mesenchymal cells, which migrate at the wound site. They are involved in epithelial re-formation and extracellular matrix deposition.21 At the same time, inflammatory cells and their secreted inflammatory factors activate the proliferation of fibroblasts, leading to abnormal tissue remodeling and the formation of keloids or hyperplastic scars.22 For pathological skin diseases without wounds caused by cell dysfunction, such as scleroderma, immune dysregulation and microvascular injury in the skin induce T cell activation, activates proinflammatory and profibrotic signals, and lead to the occurrence of disease.23 The initial inflammatory stage is characterized by the thickening of collagen fibers in the dermis and dense inflammatory infiltration in the surrounding blood vessels and sweat glands. In the later stage of fibrosis, collagen fibers become highly dense, the dermis and subcutaneous tissues are replaced by collagen, and blood vessels and the sweat glands atrophy and disappear.24 In the early stage, rosacea only shows capillary dilation, flushing, gradual formation of inflammation, papules, and pustules, finally, it is characterized by sebaceous gland hyperplasia, skin fibrosis, and nasal vegetation growth. Previous studies only constructed an inflammatory model of rosacea but did not show whether LL-37 could induce fibrosis. Therefore, our study mimicked the long-term pathogenesis of rosacea and developed a pre-fibrotic model through the reciprocal transformation of inflammation and fibrosis. This study may lay a foundation for our further exploration and construction of the rosacea fibrosis model.
As an antimicrobial peptide, LL-37 plays an important role in the regulation of inflammation, immune response, and angiogenesis.8 Previous studies have suggested that LL-37 expression is enhanced in skin wounds. It can induce keratinocyte migration through transactivation of epidermal growth factor receptor-mediated by heparin-binding epidermal growth factor. The use of anti-LL-37 antibodies can inhibit the re-epithelialization of the wound.25 LL-37 also plays an inevitable role in the pathogenesis of rosacea which is related to a variety of factors. Toll-like receptor 2 (TLR2) signaling pathway can be activated in response to external stimuli such as UV irradiation and microbial infection.1,26 Previous bioinformatics analyses have also suggested that the expression of this pathway is up-regulated in rosacea patients.26 TLR2 enhances the activity of serine kinase peptide-releasing enzyme 5 (KLK5), which activates and cleaves the LL-37 fragment of antimicrobial peptide.27 At the same time, TLR2 induces the excessive release of LL-37 from keratinocytes, thereby stimulating inflammation and angiogenesis.26,28 Our study confirmed the inflammatory induction of LL-37 by measuring true epidermal thickness and dermal inflammatory cell infiltration. At the same time, LL-37 can also stimulate neutrophils to produce proinflammatory factors such as IL-1β and TNF-α.27 These target genes were positively expressed in the skin of rosacea patients.29 As can be seen from our results, the expression of these inflammatory factors was indeed up-regulated. In addition, LL-37 can activate mast cells (MC), promote their degranulation, and release MC-associated proteases, histamine, Matrix metallopeptidase 9 (MMP9), etc. These mediators can promote skin inflammation and angiogenesis, leading to fibroblast proliferation, participate in tissue remodeling, and promote the formation of fibrosis.1,30 MMP9 is involved in the degradation of ECM and the regulation of inflammatory response.31 Yumiko Muto et al found no inflammation in LL-37-induced MC deficient mice, and the expression of MMP9 was inhibited by the use of MC stabilizer cromolyn.30 Tissue fibrosis is a late stage of various biological processes, during which ECM is excessively deposited, including fibronectin, collagen, and vimentin. Epithelial-mesenchymal transition (EMT) can be identified by the expression of mesenchymal markers, such as vimentin and N-cadherin, in the process of fibrosis. Early and sustained activation of EMT is thought to promote inflammation and fibrosis during the repair of skin tissue damage. EMT can increase the expression of ECM-related proteins such as α-SMA and vimentin.21,32 Our results confirmed that stimulation with LL-37 up-regulated the expression of COL1, α-SMA, vimentin, and N-cadherin and promoted the increase of collagen content in the dermis. Therefore, in this study, LL-37 was used as the starting point to establish a mouse model of rosacea. The findings indicate that LL-37 can promote inflammatory response and the production of fibrosis, but its specific mechanism needs to be further explored.
As an immunosuppressant, thalidomide can be used in the treatment of a variety of skin diseases, such as erythema nodosum leprae,33 Pruritus nodosum,34 AIDS,35 etc., and other systemic diseases, such as multiple myeloma36 and pulmonary fibrosis.14 Previous studies by our group have demonstrated that thalidomide can inhibit ER stress, TLR4-NF-κB signaling pathway, and inflammatory response to treat silica-induced silicosis. TNF-α, as a downstream factor of the TLR4-NF-κB signaling pathway, is significantly down-regulated.37 This is consistent with our experimental results, in our rosacea mice model, thalidomide could reduce the skin lesion area and inhibit inflammation by reducing the infiltration of inflammatory cells and the expression of TNF-α and IL-1β. Intestinal fibrosis is considered to be a common phenomenon in inflammatory bowel diseases. Studies have found that thalidomide can inhibit collagen deposition and down-regulate the expression of COL1 and α-SMA in mice with TNBS-induced colitis,15 which is consistent with the phenomenon that thalidomide inhibits rosacea fibrosis in our model mice. However, in the previous study, the down-regulated vimentin was reversed by thalidomide, and in the current study, the expression of vimentin was decreased in the treatment group compared with the model group, which may be due to the inconsistent animal models constructed or different diseases studied. These findings thus suggested that thalidomide may have a therapeutic effect on all stages of rosacea. However, thalidomide dosage should be strictly controlled because of its teratogenic effects and neurotoxicity. With the development of drug research, in order to reduce its side effects, the derivatives of thalidomide, such as lenalidomide and pomalidomide, are also being gradually applied.38 In future studies, we will continue to look for more appropriate ways of administration in the later stage, for example, thalidomide derivatives with higher water solubility and better skin permeability were used to explore the effect of topical application. At the same time, it may be necessary to explore the specific mechanism of thalidomide and its analogs in rosacea by combining animal, cell, and clinical experiments.
Conclusion
In conclusion, prolonging the modeling period of LL-37 could better simulate the occurrence and development of rosacea, and repeated inflammatory stimulation could induce its development to fibrosis. Moreover, thalidomide had potential therapeutic effects on these phenotypes of rosacea. The present study thus provides a basis for studying the pathogenesis for the early or late intervention of rosacea.
Acknowledgments
We would like to thank EditorBar for English language editing.
Funding Statement
This work was funded by the National Natural Science Foundation of China (82204006), the Natural Science Foundation of Hebei Province (2022209039), Hebei Provincial Department of Education Project (QN2022007).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Farshchian M, Daveluy S. Rosacea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 2.Hampton PJ, Berth-Jones J, Duarte Williamson CE, et al. British association of dermatologists’ clinical standards unit. British Association of Dermatologists guidelines for the management of people with rosacea 2021. Br J Dermatol. 2021;185:725–735. doi: 10.1111/bjd.20485 [DOI] [PubMed] [Google Scholar]
- 3.Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722–1729. doi: 10.1016/j.jaad.2018.08.049 [DOI] [PubMed] [Google Scholar]
- 4.Aimee M T, Wiggin W, Richard L, et al. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72:749–758. doi: 10.1016/j.jaad.2014.08.028 [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Kroumpouzos G, Kassir M, et al. Rosacea management: a comprehensive review. J Cosmet Dermatol. 2022;21:1895–1904. doi: 10.1111/jocd.14816 [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Gong W, Huang J, et al. The potentials of short fragments of human anti-microbial peptide LL-37 as a novel therapeutic modality for diseases. Front Biosci. 2021;26:1362–1372. [DOI] [PubMed] [Google Scholar]
- 7.Simonetti O, Cirioni O, Goteri G, et al. Efficacy of cathelicidin LL-37 in an MRSA wound infection mouse model. Antibiotics. 2021;10(10):1210. doi: 10.3390/antibiotics10101210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G, Narayana JL, Mishra B, et al. Design of antimicrobial peptides: progress made with human cathelicidin LL-37. Adv Exp Med Biol. 2019;1117:215–240. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Zhang M, Wang Y, et al. Murine models of rosacea: a review. J Cosmet Dermatol. 2022;21:905–909. doi: 10.1111/jocd.14164 [DOI] [PubMed] [Google Scholar]
- 10.Sampaio EP, Sarno EN, Galilly R, et al. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173:699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain K, Patel P, Roberts N. The role of thalidomide in dermatology. Clin Exp Dermatol. 2022;47:667–674. doi: 10.1111/ced.15019 [DOI] [PubMed] [Google Scholar]
- 12.Maronese CA, Pimentel MA, Li MM, et al. Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol. 2022;23(5):615–634. doi: 10.1007/s40257-022-00699-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chasset F, Tounsi T, Cesbron E, et al. Efficacy and tolerance profile of thalidomide in cutaneous lupus erythematosus: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;78(2):342–350.e4. doi: 10.1016/j.jaad.2017.09.059 [DOI] [PubMed] [Google Scholar]
- 14.Dong X, Li X, Li M, et al. Antiinflammation and antioxidant effects of thalidomide on pulmonary fibrosis in mice and human lung fibroblasts. Inflammation. 2017;40:1836–1846. doi: 10.1007/s10753-017-0625-2 [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Haixia X, Luo L, et al. Thalidomide prevented and ameliorated pathogenesis of crohn’s disease in mice via regulation of inflammatory response and fibrosis. Front Pharmacol. 2019;10:1486. doi: 10.3389/fphar.2019.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Xie H, Chen Z, et al. Thalidomide ameliorates rosacea-like skin inflammation and suppresses NF-κB activation in keratinocytes. Biomed Pharmacother. 2019;116:109011. doi: 10.1016/j.biopha.2019.109011 [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Zhang Y, Yangfan L, et al. Bioinformatics and network pharmacology identify the therapeutic role and potential mechanism of melatonin in AD and rosacea. Front Immunol. 2021;12:756550. doi: 10.3389/fimmu.2021.756550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai Y, Woods EL, Dally J, et al. Myofibroblasts: function, formation, and scope of molecular therapies for skin fibrosis. Biomolecules. 2021;11(8):1095. doi: 10.3390/biom11081095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues M, Kosaric N, Bonham CA, et al. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z-C, Zhao W-Y, Cao Y, et al. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. 2020;11:603187. doi: 10.3389/fimmu.2020.603187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone RC, Pastar I, Ojeh N, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365:495–506. doi: 10.1007/s00441-016-2464-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18. doi: 10.3390/ijms19010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattoo H, Pillai S. Idiopathic pulmonary fibrosis and systemic sclerosis: pathogenic mechanisms and therapeutic interventions. CMLS. 2021;78:5527–5542. doi: 10.1007/s00018-021-03874-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. JEADV. 2017;31:1401–1424. doi: 10.1111/jdv.14458 [DOI] [PubMed] [Google Scholar]
- 25.Tokumaru KS, Shirakata Y. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662 [DOI] [PubMed] [Google Scholar]
- 26.Margalit A, Kowalczyk MJ, Żaba R, et al. The role of altered cutaneous immune responses in the induction and persistence of rosacea. J Dermatol Sci. 2016;82:3–8. doi: 10.1016/j.jdermsci.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 27.Roh K-B, Ryu D-H, Cho E, et al. Coptis chinensis franch directly inhibits proteolytic activation of kallikrein 5 and cathelicidin associated with rosacea in epidermal keratinocytes. Molecules. 2020;25:5556. doi: 10.3390/molecules25235556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140:645–655. doi: 10.1016/j.jid.2019.08.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casas C, Paul C, Lahfa M, et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol. 2012;21:906–910. doi: 10.1111/exd.12030 [DOI] [PubMed] [Google Scholar]
- 30.Muto Y, Wang Z, Vanderberghe M, et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728–2736. doi: 10.1038/jid.2014.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544–548. doi: 10.1111/j.1468-3083.2010.03825.x [DOI] [PubMed] [Google Scholar]
- 32.Kuo Y-L, Jou I-M, Jeng S-F, et al. Hypoxia-induced epithelial-mesenchymal transition and fibrosis for the development of breast capsular contracture. Sci Rep. 2019;9:10269. doi: 10.1038/s41598-019-46439-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan I, Dorjay K, Anwar P. Thalidomide in dermatology: revisited. Indian J Dermatol. 2015;60:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sardana K, Gupta A, Sinha S. An observational analysis of low-dose thalidomide in recalcitrant prurigo nodularis. Clin Exp Dermatol. 2020;45:92–96. doi: 10.1111/ced.14015 [DOI] [PubMed] [Google Scholar]
- 35.Asatsuma-Okumura T, Ito T, Handa H. Molecular mechanisms of the teratogenic effects of thalidomide. Pharmaceuticals. 2020;13:95. doi: 10.3390/ph13050095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noonan K, Colson K. Immunomodulatory agents and proteasome inhibitors in the treatment of multiple myeloma. Semin Oncol Nurs. 2017;33:279–291. doi: 10.1016/j.soncn.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Cai W, Jin F, et al. Thalidomide alleviates pulmonary fibrosis induced by silica in mice by inhibiting ER stress and the TLR4-NF-κB pathway. Int J Mol Sci. 2022;23(10):5656. doi: 10.3390/ijms23105656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahony C, Erskine L, Niven J, et al. Pomalidomide is nonteratogenic in chicken and zebrafish embryos and nonneurotoxic in vitro. Proc Natl Acad Sci U S A. 2013;110:12703–12708. doi: 10.1073/pnas.1307684110 [DOI] [PMC free article] [PubMed] [Google Scholar]