Abstract
Using spleen cells from mice vaccinated with live Mycobacterium bovis BCG, we previously generated three monoclonal antibodies reactive against a 22-kDa protein present in mycobacterial culture filtrate (CF) (K. Huygen et al., Infect. Immun. 61:2687–2693, 1993). These monoclonal antibodies were used to screen an M. bovis BCG genomic library made in phage λgt11. The gene encoding a 233-amino-acid (aa) protein, including a putative 26-aa signal sequence, was isolated, and sequence analysis indicated that the protein was 98% identical with the M. tuberculosis Lppx protein and that it contained a sequence 94% identical with the M. leprae 38-mer polypeptide 13B3 recognized by T cells from killed M. leprae-immunized subjects. Flow cytometry and cell fractionation demonstrated that the 22-kDa CF protein is also highly expressed in the bacterial cell wall and membrane compartment but not in the cytosol. C57BL/6, C3H, and BALB/c mice were vaccinated with plasmid DNA encoding the 22-kDa protein and analyzed for immune response and protection against intravenous M. tuberculosis challenge. Whereas DNA vaccination induced elevated antibody responses in C57BL/6 and particularly in C3H mice, Th1-type cytokine response, as measured by interleukin-2 and gamma interferon secretion, was only modest, and no protection against intravenous M. tuberculosis challenge was observed in any of the three mouse strains tested. Therefore, the 22-kDa antigen seems to have little potential for a DNA vaccine against tuberculosis, but it may be a good candidate for a mycobacterial antigen detection test.
Tuberculosis remains a widespread and lethal infectious disease affecting millions of people worldwide. The World Health Organization estimates that there are about 8 million new cases each year and that the disease is responsible for at least 3 million deaths annually (5, 12, 23, 34). The eradication of tuberculosis by early diagnosis, efficient therapy, and effective prophylactic vaccination remains an important goal. For the latter purpose, characterization of purified antigens implicated in protective immunity against mycobacterial infections is essential. The protective antigens of Mycobacterium tuberculosis are still not precisely defined, but several observations in experimental animal models have indicated that they predominantly reside within a pool of secreted or surface-exposed proteins that can be found in mycobacterial culture filtrate (CF) (2, 15, 16). Using the powerful technique of DNA vaccination, we and others have shown so far that at least four antigens present in M. tuberculosis CF are able to induce a protective immunity in mice against M. tuberculosis H37Rv infection: the 38-kDa PstS-1 and 40-kDa PstS-3 phosphate binding proteins (36, 41) and the 30- and 32-kDa components of the antigen 85 complex (17, 22). Protective immune responses can also be induced with plasmid DNA encoding the 65-kDa heat shock protein, which is generally considered to be a cytosolic protein but is very abundant in CF from stressed mycobacterial cultures (37). As two-dimensional polyacrylamide gel electrophoresis (PAGE) of M. tuberculosis CF revealed more than 200 different protein spots (35), it is very probable that additional protein components also contribute to the protective efficacy of the mycobacterial CF.
We have previously reported on the production of a number of CF-specific monoclonal antibodies (MAbs) in a study using spleen cells from H-2b haplotype mice (C57BL/6 and BALB.B10) infected with live M. bovis BCG (18). Among these MAbs, VD12-1, VIIIH-1, and 9E5 were all found to react with a CF protein with an estimated molecular mass of 22 kDa. The aims of this work were to clone and characterize the gene encoding this 22-kDa protein from BCG CF and analyze its potency as a DNA vaccine against tuberculosis.
MATERIALS AND METHODS
Screening of a λgt11 M. bovis BCG genomic library with MAbs directed to a 22-kDa CF protein.
A λgt11 BCG genomic DNA library (prepared from Sau3AI partially digested genomic DNA from M. bovis BCG strain 1173P2 [10]) was screened with a mix of the three MAbs VD12-1, 9E5, and VIIIH-1, directed against a 22-kDa protein (18), as previously described (7, 21). Further screening was performed by plaque hybridization (32) with a 25-bp oligonucleotide localized at the 5′ extremity of the clone isolated with the MAbs. Crude lysates from selected λgt11 recombinant lysogens (induced with isopropyl-β-d-thiogalactopyranoside) were prepared and analyzed by sodium dodecyl sulfate (SDS)-PAGE on 8 to 16% gradient gels (Novex) and immunoblotting with MAbs VD12-1, 9E5, and VIIIH-1 as previously described (7).
Large-scale preparation of phage DNA, subcloning, and DNA sequencing.
Large-scale preparation of phage DNA was carried out according to standard procedures (32). C3 recombinant phage was digested with XmnI, and one of the two resulting 2-kb insert fragments was subcloned into the SmaI site of pBluescript II SK+ (this construction is called C3-1). DNA sequencing was carried out on both strands using a Vistra Sequencer 725 (Amersham International plc). Double-stranded plasmid DNA was sequenced using a Thermo Sequenase premixed cycle sequencing kit (Amersham).
Computer analysis.
Computer-aided analyses of the nucleic acid and deduced amino acid sequences were performed with the DNA Strider program (27) and the Genetics Computer Group program (9) of the BEN (Belgian EMB net Node) network facility. Homology searches in the protein sequence data banks were greatly facilitated by use of the National Center for Biotechnology Information BLAST programs (1, 13). Potential sequencing errors and open reading frames (ORFs) were detected with the GenMark 2.1 program (6).
Production and purification of the 22-kDa recombinant protein.
The 22-kDa ORF without the 26 first amino acids (encoding the mycobacterial signal sequence) was amplified by PCR from the C3 phage DNA. Primers used were 5′-GGA AGA TCT CCT CGC CGA CTG ATG CC-3′ (sense) and 5′-GGA AGA TCT ACG CTT CGG CCT AGT C-3′ (antisense). PCR was performed with 25 cycles of 1.5 min at 94°C, 2 min at 55°C, and 3 min at 72°C. The DNA fragment was amplified with primers containing BglII sites. Amplified DNA was then digested with BglII, isolated on a 1% agarose gel, extracted with Prep-A-Gene (Bio-Rad), and inserted in frame with the glutathione S-transferase (GST) coding region into the BamHI site of pGex-5X-3 (Pharmacia Biotech). The resulting plasmid construction was transferred into Escherichia coli DH5α by electroporation. The recombinant fusion protein was purified by chromatography on a GST purification module (Pharmacia Biotech) from 400-ml cultures as described by the manufacturer. The purified protein was subjected to SDS-PAGE (15% gel), and its purity was examined by Coomassie blue staining of the gel. The molecular mass of the purified protein was estimated by comparison with molecular mass markers (midrange protein markers; Promega, Madison, Wis.). After transfer onto nitrocellulose, the filters were incubated with VD12-1, VIIIH-1, and 9E5 and revealed with the Protoblot Western Blot AP system (Promega) according to the manufacturer's instructions.
Flow cytometry analysis.
E. coli JM109 (Promega) culture was grown to an optical density at 600 nm (OD600) of 0.7 in LB (Luria-Bertani) medium. M. bovis BCG was grown as a surface pellicle in synthetic Sauton medium for 10 days, bacteria were decanted and mixed with metallic beads, and the pellicle was homogenized by gentle swirling of the mix for 10 min. One milliliter of each culture was harvested by centrifugation at 3,000 × g at 4°C for 5 min, and the pellet was resuspended in 50 μl of a solution containing 10% fetal calf serum and 0.9% NaCl (solution L). Bacteria were incubated for 1 h at 4°C, with permanent agitation, in presence of one of the following MAbs: VD12-1, 9E5, and VIIIH-1, directed against the 22-kDa protein; and 2A1-2 (18), directed against PstS-2. Cells were washed three times with solution L and incubated for 1 h at 4°C in a final volume of 100 μl with the secondary fluorescein isothiocyanate-conjugated anti-mouse kappa light-chain antibody LO-MK-I (Experimental Immunology Unit, UCL, Brussels, Belgium). After washing, the cell pellets were resuspended in normal saline and used for cytometric analysis, which was performed on a FACSCalibur (Becton Dickinson) driven by CellQuest software. Green fluorescence was studied through a 530-nm band pass filter. Photomultiplier tube pulses were amplified logarithmically. Ten thousand events were measured and stored in list mode data files. Before each sample run, flow cytometer performance was monitored using fluorescent microspheres (Calibrite fluorescent kit; Becton Dickinson). Fluorescence was calibrated using beads of known fluorescence activity ranging from 6.3 × 104 to 1.41 × 106 equivalent soluble molecules of fluorochrome (flow cytometry standards). Bacteria were gated using their morphological properties (forward scatter-side scatter) set on logarithmic mode. The mean fluorescence intensity of the related populations of bacteria was calculated from histograms and expressed in arbitrary units corresponding to an intensity channel number ranging from 0 to 1,023.
Preparation of bacterial cell compartments.
As previously described by Mikusova et al. (28), M. bovis BCG (4 g [wet weight]) was washed twice in buffer A (5 mM 2-mercaptoethanol, 10 mM MgCl2, 50 mM morpholmepropanesulfonic acid [pH 8.0]) at 4°C, resuspended in 20 ml of the same buffer, and disrupted in a French press. The disrupted cells were centrifuged at 27,000 × g for 12 min at 4°C. The cell wall-containing pellet was washed three times with buffer A and resuspended in 2.5 ml of conservation buffer (0.1 mM EDTA, 50% glycerol, 25 mM imidazole-HCl [pH 7.0]) and stored at −20°C (final protein concentration of 9 mg/ml). Membranes of M. bovis BCG were obtained by centrifugation of the 27,000 × g supernatant at 100,000 × g for 1 h at 4°C. The supernatant containing the cytosolic fraction had a final protein concentration of 26 mg/ml; the pellet (membrane components), washed three times in buffer A, was resuspended in conservation buffer to a concentration of 17 mg/ml. Bacterial cell compartments from M. tuberculosis H37Rv were a kind gift from J. Belisle (Colorado State University).
Mice.
C57BL/6 (B6) (H-2b), C3H (H-2k), and BALB/c (H-2d) mice were obtained from the mouse breeding unit of the Pasteur Institute of Brussels. Only female mice, 8 to 10 weeks old at the start of vaccination, were used.
Plasmid construction for DNA vaccination.
The amplicon described above (“Production and purification of the 22-kDa recombinant protein”) was ligated to dephosphorylated BglII-digested V1Jns.tPA vector (17). Recombinant plasmids were transferred into E. coli DH5 (Bethesda Research Laboratories) by electroporation. These cells were then plated on LB agar medium containing kanamycin (50 μg/ml). Recombinant plasmid DNA was amplified in E. coli DH5α and purified on two consecutive cesium chloride-ethidium bromide gradients, followed by 1-butanol and phenol-chloroform extractions and ethanol precipitation. Plasmid DNA was adjusted to a final concentration of 1 mg/ml in saline and stored at −20°C.
DNA vaccination.
Mice were anesthesized by intraperitoneal injection of ketamine-xylazine (100 and 10 mg/kg of body weight, respectively) and injected three times intramuscularly (at 3-week intervals) in both quadriceps with plasmid DNA encoding the 22-kDa protein or with control DNA (empty V1Jns.tPA vector) in saline, using a 0.3-ml insulin syringe (Becton Dickinson). Mice received 100 μg of plasmid DNA at each injection (50 μl of a 1-mg/ml solution in phosphate-buffered saline [PBS] in each hind leg).
Antibody analysis.
Vaccinated mice were sacrificed 3 weeks after the last DNA injection. Specific antibodies directed against the recombinant 22-kDa protein were analyzed using an indirect enzyme-linked immunosorbent assay (ELISA). For each mouse strain, individual sera from five mice vaccinated with plasmid DNA encoding the 22-kDa protein and three mice vaccinated with the empty vector were analyzed. Briefly, microtiter plates were coated overnight at 4°C with 100 μl (5 μg/ml) of the purified 22-kDa antigen (in the form of recombinant GST fusion protein) in borate buffer (pH 9.0). The plates were then washed with a solution of 0.1% Tween 20 in PBS and saturated with skimmed milk proteins (5% in PBS) for 2 h at 37°C. After washing, 100 μl of serial twofold dilutions of serum (starting at 1:50) in 0.1% Tween 20 PBS were added for 2 h at 37°C. Plates were washed, and peroxidase-labeled LO-MK-1 (for total immunoglobulin [Ig] analysis) or rat anti-mouse IgG1, IgG2a, or IgG2b (for isotype analysis) (Experimental Immunology Unit, UCL) was added for 2 h at 37°C. Finally, the plates were washed and developed by the addition of 100 μl of o-phenylenediamine (0.4 mg/ml) diluted in citrate-phosphate buffer (pH 5.6) containing H2O2 (Sigmafast; Sigma, St. Louis, Mo.). The reaction was stopped by addition of 50 μl of 2 M H2SO4, and ODs were read at 492 nm with an automatic Multiskan MCC/340 reader (Titertek). A pool of serum from 22-kDa DNA-immunized mice was arbitrarily assigned a titer of 1,000 and was used as a standard. Total Ig titers in samples were converted to arbitrary units by comparison with the titer of this standard. Data for isotype analysis are expressed as OD values obtained for serum dilutions 1:1,600. This 1:1,600 dilution gives an OD which is at the beginning of the linear part of the ELISA sigmoid for sera with the highest reactivity.
Cytokine production.
Vaccinated mice were sacrificed 3 weeks after the last DNA injection, and spleens were removed aseptically. Spleens from five mice in each group were analyzed individually. Spleen cells were adjusted at a concentration of 4 × 106 cells/ml and cultured in round-bottomed microwell plates (Nunc) in RPMI 1640 medium (Gibco-BRL) supplemented with l-glutamine, 50 μM 2-mercaptoethanol, penicillin, streptomycin, and 10% heat-inactivated fetal calf serum (Gibco-BRL). A volume of 180 μl of cell suspension was added to 20 μl of the recombinant purified 22-kDa antigen (final 22-kDa protein concentration of 5 μg/ml). Cells were incubated at 37°C in a humidified CO2 incubator, and supernatants were harvested after 24 h (for interleukin-2 [IL-2] assays) and 72 h (for IL-6 and gamma interferon [IFN-γ] assays). Supernatants from three separate wells were pooled and stored frozen at −20°C until assayed.
IL-2 assay.
IL-2 activity was measured using a CTLL-2 proliferation assay. Briefly, a volume of 100 μl of 24-h culture supernatant was added to 100 μl of CTLL-2 cells (105/ml) and incubated for 48 h. [3H]thymidine (8.3 Ci/mmol; Amersham) was added (0.4 μCi/well) during the last 6 h of culture. Cells were harvested on a Titertek cell harvester, and the radioactivity recovered on the fiber mats was counted in a Betaplate scintillation counter. IL-2 levels are expressed as mean counts per minute ± standard deviation (SD) (of five mice tested individually). In this assay, 50,000 cpm correspond to about 3.12 IU/ml or about 600 pg/ml, and the detection limit is around 10 pg/ml (100 to 200 cpm).
IFN-γ assay.
IFN-γ activity was quantified on 72-h culture supernatants, using a mouse IFN-γ ELISA (Intertest-γ; Genzyme catalog no. 80-3842-03). Concentrations are expressed as mean picograms per milliliter ± SD (of five mice tested individually). Detection limit in this assay is 10 pg/ml.
IL-6 assay.
IL-6 activity was assessed in triplicate in a colorimetric assay by measuring hexose-aminidase levels of 7TD-1 mouse-mouse hybridoma cell cultures grown in the absence or presence of serial fivefold dilutions of the samples. IL-6 titers are expressed in mean picograms per milliliter ± SD (of five mice tested individually) (20).
M. tuberculosis challenge.
Mice were rested for 2 months after the third DNA vaccination before being infected intravenously in a lateral tail vein with 106 CFU of M. tuberculosis H37Rv grown as a surface pellicle on synthetic Sauton medium for 14 days and stored as a concentrated stock solution at −70° in 20% glycerol. Mice were sacrificed 4 weeks later. Spleens and lungs from individual animals (four to six in each group) were homogenized in PBS supplemented with penicillin (1 μl/ml) and amphotericin B (Fungizone; 2 μl/ml), and serial threefold dilutions were plated on Middlebrook 7H11 agar supplemented with OADC enrichment broth. Plates were incubated at 37° in sealed plastic bags, and the number of CFU was determined after 4 to 5 weeks. Results are presented as mean log10 CFU per spleen or lungs ± SD. For statistical analysis, Student's t test was used. Differences were considered as statistically significant at a P value below 0.05. The experiment was performed twice, and results of one experiment are presented. Protective efficacy of M. bovis BCG was analyzed in independent experiments as described before (36).
Nucleotide sequence accession number.
The sequenced part of the 22-kDa protein gene of M. bovis BCG has been deposited in the EMBL database under accession no. AJ238176.
RESULTS
Identification of the gene encoding the 22-kDa protein.
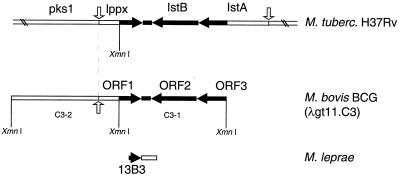
By screening a λgt11 M. bovis BCG expression library with MAbs VD12-1, VIIIH-1, and 9E5 directed against an M. bovis BCG 22-kDa protein present in culture fluid (18), we isolated the recombinant phage C3. Western blot analysis of crude lysates of E. coli C3 lysogen, with the above-mentioned antibodies, surprisingly revealed a protein of about 22 kDa only instead of the expected β-galactosidase fusion protein of 136 kDa (Fig. 1, lane 3). Restriction analysis of the C3 recombinant DNA (with SstI, KpnI, or XmnI) indicated that it contains a 4,000-bp M. bovis BCG DNA fragment insert. The insert contains an internal XmnI site localized in the middle of the mycobacterial DNA. One of the two XmnI restriction fragments (C3-1) of 2,000 bp was subcloned in the XmnI site of pBluescript SK2+. The sequencing of the C3-1 insert DNA showed the presence of three putative ORF's. An incomplete 338-bp ORF (ORF1) was localized at one of the extremities of C3-1. ORF1 overlaps 118 bp of the M. leprae 13B3 DNA fragment previously cloned by Mustafa et al. (29) and 338 bp of the lppx gene of M. tuberculosis (30). The complete 833 bp ORF2, in the middle of the cloned fragment, encodes a protein which is 42% similar to the IstB subunit of E. coli IS21 (25). ORF3 at the other extremity of C3-1 had a size of 444 bp and was also incomplete. Its amino acid sequence presented 20% similarity with the IstA subunit of E. coli IS21 (Fig. 2). The complete C3-1 DNA is 100% identical to an M. tuberculosis DNA region cloned into the SCY24G1 cosmid (31), where an ORF encoding a putative 24.1-kDa lipoprotein (Lppx) is directly followed by two ORFs homologous to E. coli IstB and IstA (Fig. 2). Since the C3 lysogenic bacteria expressed the complete 22-kDa protein (Fig. 1), the 5′ extremity in the mycobacterial DNA fragment C3-2 must contain the NH2-terminal half of the M. bovis BCG 22-kDa protein gene (Fig. 2).
FIG. 1.

Immunoblot analysis of E. coli lysogen containing the gene encoding the 22-kDa protein. Western blotting was performed with MAb 9E5. Lanes 1 to 3 represent CF of M. bovis BCG, a crude lysate of E. coli lysogenized with clone A1 (lysogen of M. tuberculosis PstS-3) as a negative control, and a crude lysate of E. coli lysogenized with clone C3 (lysogen of the M. bovis BCG 22-kDa protein). Positions of molecular weight markers (Rainbow markers; Amersham) are indicated at the left. The arrow indicates the presence of the 22-kDa protein of M. bovis BCG.
FIG. 2.
Alignment of the DNA regions encoding the 22-kDa protein of M. bovis BCG with regions of M. tuberculosis H37Rv (Z83858) and of M. leprae (L29076). DNA sequences homologous to the sequenced M. bovis BCG DNA fragment C3-1 are in black. Arrows indicate orientations of the ORFs. Start codons are indicated by white vertical arrows.
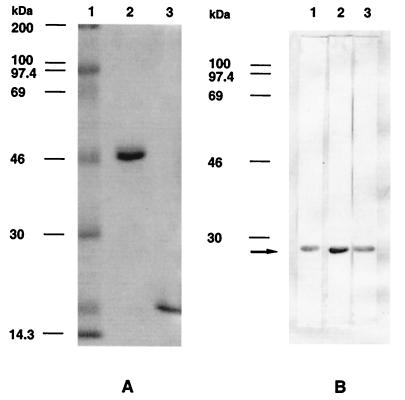
To demonstrate that ORF1 encoded part of the 22-kDa protein recognized by our MAbs, we expressed the putative complete Lppx homolog from BCG in E. coli. Based on the sequence of the 24.1-kDa Lppx protein of M. tuberculosis (EMBL accession no. 283858), primers were chosen to amplify the M. bovis BCG Lppx homolog (without its putative signal sequence) from the mycobacterial DNA inserted in phage C3. The 621-bp amplified DNA fragment was then cloned in frame with the GST coding region into the BamH I site of pGex-5X-3 and used to transform E. coli. Elution of the fusion protein from a gluthatione affinity column followed by SDS-PAGE and staining with Coomassie blue revealed a protein of approximately 47 kDa (Fig. 3A, lane 2), which after cleavage with factor Xa yielded a protein of approximately 22 kDa (Fig. 3A, lane 3). Western blot analysis of this 22-kDa protein encoded by ORF1 showed its recognition by MAbs VD12-1, VIIIH-1, and 9E5 (Fig. 3B), indicating its correspondence with the 22-kDa protein present in M. bovis BCG culture fluid.
FIG. 3.
Electrophoresis and immunoblotting analysis of the 22-kDa protein. (A) Purification by glutathione affinity chromatography of the 22-kDa protein fused to GST. Eluates were analyzed by SDS-PAGE (15% gel) and stained with Coomassie blue. Lane 1, molecular weight markers; lane 2, purified fusion protein; lane 3, fusion protein cleaved by factor Xa. (B) Immunoblotting analysis with MAbs 9E5 (lane 1), VD12-1 (lane 2), and VIIIH-1 (lane 3) of the GST–22-kDa fusion protein following cleavage. The arrow indicates the presence of the 22-kDa protein of M. bovis BCG.
To understand why in the C3 lysogenic bacteria the 22-kDa protein was not expressed as a fusion protein with β-galactosidase (Fig. 1), we analyzed the M. tuberculosis DNA region encoding the Lppx protein (EMBL accession no. 283858, cosmid SCY2461). We observed that the DNA region encoding the signal sequence of the Lppx protein contains regions homologous to typical −35 (complement nucleotides 15041 to 15047) and −10 (complement nucleotides 15014 to 15019) boxes of E. coli promoters. The sequence TTGACAA (6 out of 7 bp homologous to the characteristic TTGACAT −35 consensus sequence of E. coli promoters) was found 18 bp away from an ATGAAT sequence (3 out of 6 bp homologous to the TATAAT −10 consensus sequence of E. coli promoters). A putative Shine-Dalgarno sequence, AGGAGG (AGGTGC localized from complement nucleotides 15030 to 15035), was present 41 bp distant from this probable promotor region; this sequence preceded a possible TTG start codon (4, 33) (in complement position 15018) just neighboring the N-terminal cysteine codon of the mature 22-kDa protein. The presence of this cryptic E. coli-type promotor is a very likely explanation for the observed expression of a mature 22-kDa protein instead of the expected 136-kDa β-galactosidase fusion protein in the λgt11 lysogenic C3 clone.
Localization of the 22-kDa protein.
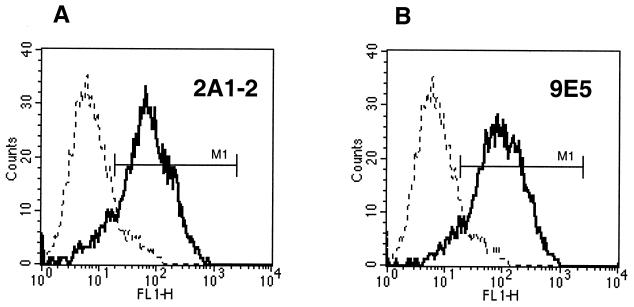
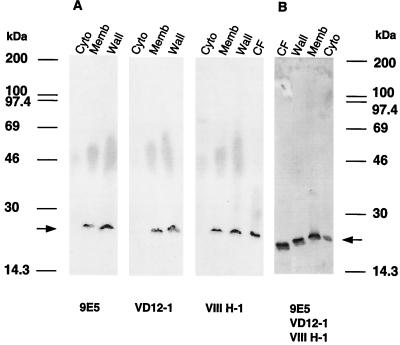
Being homologous to the M. tuberculosis lipoprotein Lppx, the M. bovis BCG 22-kDa protein is predicted to be a lipoprotein which may be anchored onto the bacterial surface. However, the presence of the M. bovis BCG 22-kDa protein in 2-week-old CF suggests that the protein is also released from the bacteria. The association of the 22-kDa protein with the cell envelope was examined by flow cytometry. For that purpose, 10-day surface pellicle cultures of M. bovis BCG homogenized with metallic beads were incubated with the three MAbs VD12-1, VIIIH-1, and 9E5 separately or as a mix. MAb 2A1-2 directed against the surface protein PstS-2 was used as a positive control (18, 26) (Fig. 4A). M. bovis BCG bacilli labeled with MAb 9E5 presented an increase of more than 1 log10 in fluorescence intensity compared to bacilli treated only with the secondary antibody (Fig. 4B). Similar results were obtained with MAbs VD12-1 and VIIIH-1 (results not shown). The best emission of fluorescence was observed with 9E5, and the fluorescence signal could be slightly amplified when the three MAbs were used together. Labeling of the mycobacteria with irrelevant MAbs (anti-human CD3 or CD4 MAbs [data not shown]) was negative. The above results demonstrate that the 22-kDa protein is localized on the mycobacterial surface. These results were also confirmed by Western blot analysis of M. bovis BCG and M. tuberculosis H37Rv compartments (cytosol, membrane, and cell wall), using the three MAbs (Fig. 5). The presence of the 22-kDa protein could be detected in the cell wall, in the membrane compartment, and in the CF but not in the cytosol fraction.
FIG. 4.
Flow cytometric analysis of M. bovis BCG. MAb 2A1-2, recognizing PstS-2 (A), and MAb 9E5, directed to the 22-kDa protein (B), were used. Dotted lines represent the fluorescence of the bacterial population in the absence of the MAbs (negative control), and the heavy lines represent the fluorescence obtained after incubation of M. bovis BCG with the indicated MAbs. M1, range of window used for analysis of positive signals.
FIG. 5.
Immunoblot analysis with MAbs 9E5, VD12-1, and VIIIH-1, directed against the 22-kDa protein of three mycobacterial compartments from M. bovis BCG (A) and from M. tuberculosis H37Rv (B). Lanes Cyto, Memb, Wall, and CF represent cytosolic, membrane, cell wall, and culture filtrate fractions, respectively. The arrow indicates the position of the 22-kDa protein.
Immunogenicity of a plasmid DNA encoding the 22-kDa antigen.
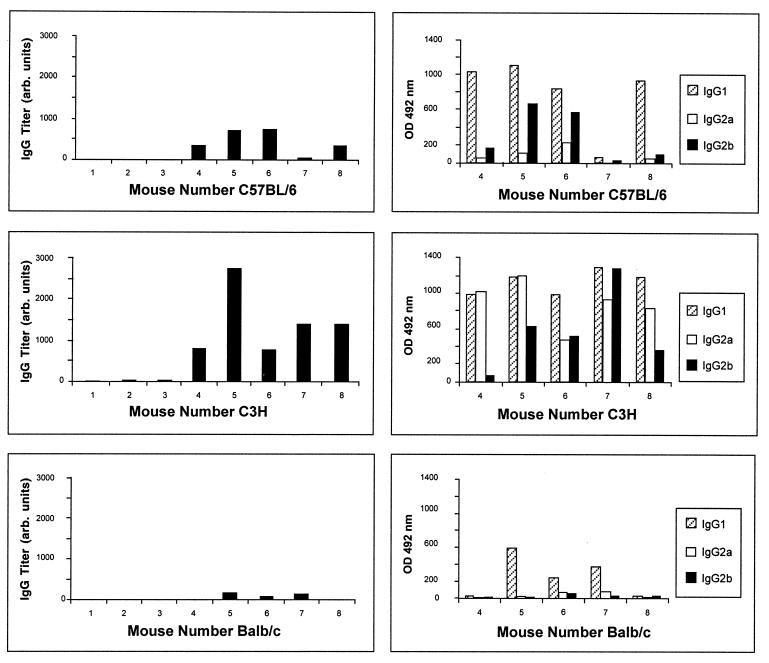
The predicted M. bovis BCG 22-kDa protein contains a region 94% identical with the M. leprae 38-mer polypeptide 13B3 recognized by T cells from killed M. leprae-immunized subjects, and it is even completely identical in its predicted carboxy-terminal part (residues 208 to 219) with peptide P2 of the 13B3 sequence (residues 16 to 27; EMBL accession no. L29076) described by Mustafa et al. (29) as a major human T-cell epitope. For this reason and because of the presence of the 22-kDa protein on the surface and in the CF of M. bovis BCG and M. tuberculosis, we decided to characterize its immunogenicity for B and T lymphocytes. For this purpose, C57BL/6, C3H, and BALB/c mice were immunized with plasmid DNA encoding the M. bovis BCG 22-kDa antigen. Three weeks after the third DNA injection, antibody production and spleen cell cytokine secretion were analyzed. As shown in Fig. 6, mice vaccinated with the empty vector (used as control) had low antibody levels, with values below 100. In contrast, elevated antibody levels were observed in C57BL/6 and C3H mice vaccinated with plasmid DNA encoding the 22-kDa protein. Antibody levels in vaccinated BALB/c mice were also detectable but lower. Analysis of antibody isotype in C3H sera showed high IgG1, IgG2a, and IgG2b isotype serum levels. In C57BL/6 mice, antibodies were mostly of IgG1 and IgG2b isotypes, whereas in BALB/c mice the poor antibody response was exclusively of the IgG1 isotype (Fig. 6).
FIG. 6.
Antibody production directed against the GST–22-kDa fusion protein in DNA-vaccinated mice. Serum samples from three mice vaccinated with the empty V1Jns.tPA vector (columns 1 to 3) and five mice immunized with plasmid DNA encoding the 22-kDa protein (columns 4 to 8) of three different mouse strains (C57BL/6, C3H, and BALB/c) were tested in ELISA. Total anti-GST–22-kDa fusion protein Ig is expressed in arbitrary units (left), and levels of specific Ig isotypes are expressed as OD492 values for a serum dilution of 1:1,600 (right).
Production of the Th1-type spleen cell cytokines IL-2 and IFN-γ was very weak (Table 1) even in C3H mice which demonstrated strong antibody responses with IgG2a isotype. Maximal IL-2 levels were between 100 and 200 pg/ml (830 to 1,660 cpm), and maximal IFN-γ titers were around 850 pg/ml. These values were about 5- to 10-fold lower than those previously observed in mice vaccinated with plasmid DNA encoding Ag85A (17). In contrast, spleen cells from B6 and C3H mice vaccinated with plasmid DNA encoding the 22-kDa antigen produced detectable levels of the Th2-type cytokine IL-6 upon in vitro restimulation with the recombinant protein (Table 1). These antigen-specific IL-6 levels were about 10-fold higher than those previously observed in mice vaccinated with plasmid DNA encoding Ag85A (17).
TABLE 1.
Spleen cell cytokine secretion in B6, C3H, and BALB/c mice vaccinated with plasmid DNA encoding the 22-kDa antigen
| Mouse strain | Cytokine level (mean ± SD)
|
|||||
|---|---|---|---|---|---|---|
| IL-2 (cpm)a
|
IFN-γ (pg/ml)b
|
IL-6 (pg/ml)b
|
||||
| Control | 22 kDa | Control | 22 kDa | Control | 22 kDa | |
| C57BL/6 | 189 ± 27 | 2,236 ± 1,658 | 92 ± 119 | 846 ± 372 | 12 ± 10 | 217 ± 25 |
| C3H | 169 ± 26 | 1,699 ± 674 | 245 ± 219 | 774 ± 188 | 8 ± 4 | 213 ± 30 |
| BALB/c | 539 ± 258 | 641 ± 335 | 64 ± 81 | 160 ± 222 | 14 ± 7 | 133 ± 63 |
In 24-h spleen cell culture supernatants of five mice tested individually.
In 72-h spleen cell culture supernatants of five mice tested individually.
Protective efficacy of a DNA vaccine encoding the 22-kDa antigen.
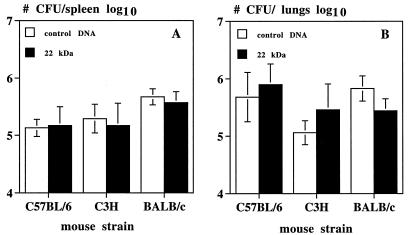
As shown in Fig. 7, vaccination with plasmid DNA encoding the 22-kDa protein could not protect either of the three mouse strains against subsequent intravenous challenge with M. tuberculosis H37Rv. The mean number of CFU in 22-kDa DNA-vaccinated mice was not statistically different either in spleen or in lungs from the mean CFU number in mice vaccinated with empty vector. Vaccination with M. bovis BCG of these three mouse strains resulted in consistent protection, as measured by 1 to 1.5 log10 reductions in CFU counts in spleen and lungs (data not shown).
FIG. 7.
Bacterial replication in spleens and lungs from C57BL/6, C3H, and BALB/c mice vaccinated with empty V1Jns.tPA vector (control) or plasmid DNA encoding the 22-kDa antigen and challenged intravenously with 106 M. tuberculosis H37Rv 8 weeks after the third DNA injection (data expressed as mean ± SD of log10 values of number of CFU per spleen or lungs; four to six mice per group).
DISCUSSION
In this study, we have described the cloning of a gene encoding a 22-kDa protein of M. bovis BCG CF. This protein was found to be 98% identical with the Lppx protein from M. tuberculosis, described as a novel mycobacterial antigen that belongs to a family of secreted lipoproteins (30), and it contained a sequence 94% identical with the 13B3 38-mer peptide of an M. leprae protein recognized by T cells from healthy subjects immunized with killed M. leprae (29).
As predicted from its amino acid sequence, this lipoprotein was also well represented in the M. bovis BCG and M. tuberculosis bacterial membrane and cell wall compartment, and its anchoring onto the cell surface could explain the strong detection by flow cytometry using specific MAbs. Surface-exposed lipoproteins are often very immunogenic for B lymphocytes. As a striking example of this, three proteins from BCG culture filtrate identified as immunodominant B-cell proteins in Western blot analysis with sera from BCG-infected H-2b haplotype mice, i.e., the 22-kDa protein, the 37- to 38-kDa PstS-2, and the 40-kDa PstS-3, have all been characterized as proteins containing a lipoprotein consensus sequence (14, 24, 40). To what extent these lipoproteins are also dominant T-cell antigens with protective potential is less documented. Vordermeier et al. have reported in detail on the T-cell immunogenicity of a fourth lipoprotein, the 38-kDa (PstS-1) protein, and have described strong proliferative responses against this antigen particularly in tuberculosis patients (38). Mice can be protected against intravenous M. tuberculosis challenge by vaccination with plasmid DNA encoding PstS-1 (39), but the protection seems to be short lived, and we have not been able to confirm it when DNA-vaccinated mice were rested for 2 months before challenge (36). In contrast, mice vaccinated with DNA encoding the PstS-3 protein were very well protected in the same experiment (36).
To analyze the possible protective potential of the 22-kDa antigen, we used a similar experimental approach. Mice from three different strains were immunized with plasmid DNA encoding the mature 22-kDa protein and analyzed for humoral and cellular immune response and protection against intravenous M. tuberculosis H37Rv challenge. Although strong IgG2a and IgG2b responses indicative of a Th1-type response were detected in C3H and C57BL/6 mice, the synthesis of IL-2 and IFN-γ was disappointingly low compared to what can be achieved in mice vaccinated with plasmid DNA encoding the Ag85A or the PstS-3 protein (36). Also, vaccination with plasmid DNA encoding the 22-kDa protein was ineffective in protecting mice against an intravenous M. tuberculosis H37Rv challenge, as assessed by enumeration of number of CFU in spleen and lungs. Results obtained in these three mouse strains indicate that the 22-kDa protein is not a good DNA vaccine candidate. Although it could be argued that low expression levels of the 22-kDa protein might be responsible for the observed lack of protection, this is not a very likely explanation in view of the strong antibody responses that could be generated following DNA vaccination. However, we cannot totally exclude that other immunization procedures (such as purified protein in adjuvant or mycobacterial infection) might induce a protective immunity toward this antigen. Also, vaccine efficacy was assessed by determining reductions in CFU counts in spleen and lungs, a standard procedure in mice, but histopathology was not performed, and it is possible that vaccination with the 22-kDa DNA vaccine may have improved lung pathology without reductions in bacterial counts. Finally, it is possible that human lymphocytes react differently against the 22-kDa protein than mouse lymphocytes; stimulation of peripheral blood lymphocytes from BCG vaccinees, healthy purified protein derivative-positive volunteers, and tuberculosis patients with highly purified recombinant 22-kDa protein could give us more information on this issue.
We previously reported that antibody production to the 22-kDa antigen in BCG-vaccinated mice is influenced by genes from the major histocompatibility complex and restricted to H-2b, H-2f and H-2bq1 haplotypes (18, 19). Following DNA vaccination, mice with other H-2 haplotypes, particularly mice expressing the I-Ak allele such as C3H (this report) and B10.BR and B10.A (unpublished data), also produced elevated antibody levels, suggesting that DNA vaccination can broaden the B-cell epitope repertoire, similarly to what we found for the Th1 cell repertoire, which is broadened by DNA vaccination compared to infection with live M. bovis BCG or M. tuberculosis (8).
Flow cytometric analysis with 22-kDa specific MAbs demonstrated a strong surface expression of the antigen on the BCG cell surface. The fluorescence intensity was increased more than 10-fold compared to control staining with the secondary antibody only. It is not entirely clear whether the gene encoding the 22-kDa protein is present in other mycobacterial species, but indirect evidence based on PCR and insertion element analysis indicates that the gene can be found only in mycobacteria belonging to the M. tuberculosis complex and in M. leprae (25). Direct 22-kDa antigen detection based on flow cytometry, particle counting immunoassay (11), or dot blotting (3) using monoclonal and/or polyclonal antibodies against this strongly B-cell immunogenic protein could then be used for a rapid and cheap diagnosis of bacteria belonging to the M. tuberculosis complex. Such tests could be of value for laboratories with limited financial means and also for the analysis of clinical samples such as blood and feces, for which classical PCR tests are hampered by inhibitory substances.
ACKNOWLEDGMENTS
We are very grateful to Kamiel Palfliet, Fabienne Jurion, Vinciane Motte, and Josette Ooms for excellent technical assistance. We also thank Philippe Gilot (Pasteur Institute of Brussels) for revising the manuscript and M. A. Liu (Merck Research Laboratories, West Point, Pa., now at Chiron, Emeryville, Calif.) for providing plasmid V1Jns.tPA.
The protein fractions from M. tuberculosis H37Rv were obtained from Colorado State University's contribution of research material (NIH, NIAID contract NO1 AI-75320). This work was supported by grant G.0355.97 from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen, grant 3.4543.95 from the Fonds de la Recherche Scientifique Médicale, by “de Vrienden van het Instituut Pasteur van Brussel” vereniging zonder winstgevend doel, and by grants PL 96 2167 and 96 2134 from the European Economic Community (BIOMED 2). A.T. holds a grant from the Damiaanaktie Belgium.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley-Hibbert S I, Quan X, Newman T, Huygen K, Godfrey H P. Pathophysiology of antigen 85 in patients with active tuberculosis: antigen 85 circulates as immune complexes with fibronectin and immunoglobulin G. Infect Immun. 1999;67:581–588. doi: 10.1128/iai.67.2.581-588.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black P N, DiRusso C C, Metzger A K, Heimert T L. Cloning, sequencing and expression of the fadD gene of Escherichia coli encoding acyl coenzyme A synthetase. J Biol Chem. 1992;267:25513–25520. [PubMed] [Google Scholar]
- 5.Bloom B R C, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 6.Borodovsky M, McIninch J. GENMARK: parallel gene recognition for both DNA strands. Comput Chem. 1993;17:123–133. [Google Scholar]
- 7.Borremans M, De Wit L, Volckaert G, Ooms J, De Bruyn J, Huygen K, Van Vooren J P, Stelandre M, Verhofstadt R, Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989;57:3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg T P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devereux J, Haeberli J, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Wit L, de la Cuvellerie A, Ooms J, Content J. Nucleotide sequence of the 32-kDa protein gene (antigen 85 A) of Mycobacterium bovis BCG. Nucleic Acids Res. 1990;18:3995. doi: 10.1093/nar/18.13.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drowart A, Cambiaso C L, Huygen K, Serruys E, Yernault J C, Van Vooren J-P. Detection of mycobacterial antigens present in short-term culture media using particle counting immunoassay. Am Rev Respir Dis. 1993;147:1401–1406. doi: 10.1164/ajrccm/147.6_Pt_1.1401. [DOI] [PubMed] [Google Scholar]
- 12.Engers H D Workshop Participants. Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986;51:718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz M A, Lee B W E, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 18.Huygen K, Drowart A, Harboe M, ten Berg R, Cogniaux J, Van Vooren J P. Influence of genes from the major histocompatibility complex on the antibody repertoire against culture filtrate antigens in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1993;61:2687–2693. doi: 10.1128/iai.61.6.2687-2693.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huygen K, Ljungqvist L, ten Berg R, Van Vooren J-P. Repertoires of antibodies to culture filtrate antigens in different mouse strains infected with Mycobacterium bovis BCG. Infect Immun. 1990;58:2192–2197. doi: 10.1128/iai.58.7.2192-2197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huygen K, Vandenbussche P, Heremans H. Interleukin-6 production in Mycobacterium bovis BCG-infected mice. Cell Immunol. 1991;137:224–231. doi: 10.1016/0008-8749(91)90071-i. [DOI] [PubMed] [Google Scholar]
- 21.Huynh T V, Young R A, Davis R W. Constructing and Screening cDNA libraries in λgt10 and λgt11. In: Glover D M, editor. DNA cloning, a practical approach. Vol. 1. Oxford, England: IRL Press; 1985. pp. 47–78. [Google Scholar]
- 22.Kamath A T, Feng C G, MacDonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann S H E, van Embden J D A. Tuberculosis: a neglected disease strikes back. Trends Microbiol. 1993;1:2–5. doi: 10.1016/0966-842x(93)90015-j. [DOI] [PubMed] [Google Scholar]
- 24.Klein P, Somorjai R L, Lau P C. Distinctive properties of signal sequences from bacterial lipoproteins. Protein Eng. 1988;2:15–20. doi: 10.1093/protein/2.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Lefèvre P, Braibant M, Content J, Gilot P. Characterization of a Mycobacterium bovis BCG insertion sequence related to the IS21 family. FEMS Microbiol Lett. 1999;178:211–217. doi: 10.1111/j.1574-6968.1999.tb08679.x. [DOI] [PubMed] [Google Scholar]
- 26.Lefèvre P, Braibant M, De Wit L, Kalai M, Röeper D, Grötzinger J, Delville J-P, Peirs P, Ooms J, Huygen K, Content J. Three different putative phosphate transport receptors are encoded by the Mycobacterium tuberculosis genome and are present at the surface of Mycobacterium bovis BCG. J Bacteriol. 1997;179:2900–2906. doi: 10.1128/jb.179.9.2900-2906.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marck C. ‘DNA Strider’: a ‘C’ program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikusova K, Mikus M, Besra G S, Hancock I, Brennan P J. Biosynthesis of the linkage region of the mycobacterial cell wall. J Biol Chem. 1996;271:7820–7828. doi: 10.1074/jbc.271.13.7820. [DOI] [PubMed] [Google Scholar]
- 29.Mustafa A S, Oftung F, Deggerdal A, Gill H K, Young R A, Godal T. Gene isolation with human T lymphocyte probes. Isolation of a gene that expresses an epitope recognized by T cells specific for Mycobacterium bovis BCG and pathogenic mycobacteria. J Immunol. 1988;141:2729–2733. [PubMed] [Google Scholar]
- 30.Oftung F, Wiker H G, Deggerdal A, Mustafa A S. A novel mycobacterial antigen relevant to cellular immunity belongs to a family of secreted lipoproteins. Scand J Immunol. 1997;46:445–451. doi: 10.1046/j.1365-3083.1997.d01-150.x. [DOI] [PubMed] [Google Scholar]
- 31.Philipp W J, Poulet S, Eiglmeier K, Pascopella L, Balasubramanian V, Heym B, Bergh S, Bloom B R, Jacobs W R, Cole S T. An integrated map of the genome of the tubercle bacillus, Mycobacterium tuberculosis H37Rv, and comparison with Mycobacterium leprae. Proc Natl Acad Sci USA. 1996;93:3132–3137. doi: 10.1073/pnas.93.7.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schneider T D, Storms G D, Gold L. Information content of binding sites on nucleotide sequences. J Mol Biol. 1986;188:415–431. doi: 10.1016/0022-2836(86)90165-8. [DOI] [PubMed] [Google Scholar]
- 34.Snider D, Bart K B, Bloom B, Tokunaga T, David J R, Pio A. Summary, conclusion and recommendations from the International Workshop on “Research towards global control and prevention of tuberculosis: with emphasis on vaccine development.”. J Infect Dis. 1988;158:248–253. [Google Scholar]
- 35.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanghe A, Lefèvre P, Denis O, D'Souza S, Braibant M, Lozes E, Singh M, Montgomery D, Content J, Huygen K. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J Immunol. 1999;162:1113–1119. [PubMed] [Google Scholar]
- 37.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 38.Vordermeier H M, Harris D P, Moreno C, Singh M, Ivanyi J. The nature of the immunogen determines the specificity of antibodies and T cells to selected peptides of the 38 kDa mycobacterial antigen. Int Immunol. 1995;7:559–566. doi: 10.1093/intimm/7.4.559. [DOI] [PubMed] [Google Scholar]
- 39.Vordermeier H M, Zhu X, Harris D P. Induction of CD8+ CTL recognizing mycobacterial peptides. Scand J Immunol. 1997;45:521–526. doi: 10.1046/j.1365-3083.1997.d01-432.x. [DOI] [PubMed] [Google Scholar]
- 40.Young D B, Garbe T R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991;142:55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X J, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]