Abstract
Psoriasis is a chronic inflammatory disease which mostly affects skin. Tildrakizumab is a specific anti-interleukin -23p19 monoclonal antibody approved for the treatment of plaque psoriasis in adults. Herein, we report about a patient who came to our attention for a moderate-to-severe plaque psoriasis, involving primarily upper limbs, elbow, abdomen and knees (PASI 18 – DLQI 22). His medical history was relevant for epilepsy controlled pharmacologically. In addition, an eczematous and edematous appearance of the tibial area was detected; the histologic findings did not contradict the diagnostic hypothesis of subacute spongiotic dermatitis. The patient was treated with Tildrakizumab. After 12 weeks the clinical lesions improved significantly, and the eczematous component disappeared in the tibial area bilaterally. The clinical improvement was maintained even after one year of therapy. Tildrakizumab showed excellent results in the control of psoriasis, with an excellent safety profile. The promising results of clinical trials have been confirmed in a real-life setting. There are no reports about its safety in epileptic patients. In our case, neurological adverse events did not verify and tildrakizumab managed to control both the psoriatic and eczematous components.
Key words: Psoriasis, Eczema, Tildrakizumab
Introduction
Psoriasis is a chronic inflammatory disease which mostly affects skin, but may also affect the joints and different organ systems.
Its severity can vary depending on numerous genetic and environmental factors. 1 The IL-23 and the downstream Thelper cell 17 (Th17) pathway are key drivers of psoriasis pathogenesis.1,2
The treatment of psoriasis has undergone a revolution with the advent of biologic therapies that target specific components of the immune system.3
Tildrakizumab is a specific anti-interleukin -23p19 monoclonal antibody approved in US and Europe for the treatment of moderate-to-severe chronic plaque psoriasis,4 which binds specifically to the p19 protein subunit of the cytokine 23 (IL- 23) inhibiting its interaction with the specific IL-23 receptor.5
The promising results of phase one studies I terms of efficacy of Tildrakizumab (Kopp et al.)6 have been confirmed by double- blind randomized phase III trials (reSURFACE1 ad reSURFACE2);7 the rate of severe infections, malignancies, skin cancers, major cardiovascular events, and drug-related hypersensitivity reactions was low.
Case Report
Herein, we describe the case of a 45- year-old man with moderate-to-severe plaque psoriasis.
He had a history of cortical dysplasia, cerebellar vermis atrophy, corpus callosum dysplasia and mono-lateral microgyria determining prominent progressive spastic ataxia, sensory motor axonal neuropathy mild intellectual disability and frequent epileptic events controlled pharmacologically.
The patient came to our attention for a moderate-to-severe plaque psoriasis, involving primarily upper limbs, elbow, abdomen and knees (PASI 18 – DLQI 22). He referred itching, burning and skin tightness. On skin examination skin dryness, cracking, scaling, flaking, redness and bleeding were detectable. In addition, skin lesions not attributable to psoriatic disease were detected on clinical examination. Indeed, eczematous areas and edematous appearance of the tibial areas with hyperpigmented reddish-brown spots were detected. For this reason, an incisional biopsy on tibial area was performed before starting therapy. Histological examination showed a mild and focal orthokeratosis hyperkeratosis, hyper granulosis, spongiotic vesicles with neutrophilic granulocytic infiltrate and sporadic images of lymphocytic exocytosis. Moreover, an inflammatory lymphocytic infiltrate circumscribed the ectasic vessels of the superficial dermis. The morphologic findings described did not contradict the diagnostic hypothesis of subacute spongiotic dermatitis.
The patient had previously been treated with cyclosporine, acitretin and systemic steroids, which proved ineffective.
Considering this clinical information, we started treatment with Tildrakizumab 100 mg every 12 weeks in March 2020. After 12 weeks the clinical lesions improved significantly, and the eczematous component disappeared in the tibial area bilaterally. The clinical improvement was maintained even after one year of therapy with Tildrakizumab as showed in Figure 1.
Figure 1.
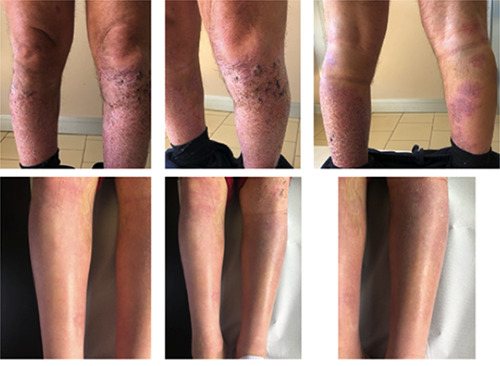
Clinical improvement after 12 weeks of treatment with tildrakizumab.
Discussion
As demonstrated elsewhere, tildrakizumab showed excellent results in the control of psoriasis in the short term, with an excellent safety profile. The promising results of the clinical trials have been confirmed even in a real-life setting.8
Consistently with the literature, our case confirms the long-lasting durability of the therapeutic effects of tildrakizumab. This sustained response over time has been related to inhibition of IL-23, which acts upstream in the IL-23/Th17 pathway.9
Tildrakizumab is a valid therapeutic choice in special population including patients with IBD, cardiovascular disease, metabolic syndrome, advanced age, and history of malignancy; however, there are no reports about its safety in epileptic patients. In our case, neurological adverse events did not verify. Furthermore, there is a growing body of evidence about the involvement of inflammatory mediatorsreleased by brain cells and peripheral immune cells in both the origin of individual seizures and the epileptogenic process, which include the IL23-Th17 axis.10
In addition, our patient had a histologically demonstrated eczematous component. Historically, psoriasis and eczema were considered as opposite diseases, especially regarding their pathophysiology, psoriasis being Th17 dominant and eczema being Th2 dominant. However, it is now recognized that these entities can co-exist.11 In our case, it is interesting to note that tildrakizumab managed to control both the psoriatic and eczematous components. Moreover, there are anecdotical reports of IL12/23 inhibitors in atopic dermatitis with good outcomes, highlighting that the impact of anti-IL-12/IL-23p40 therapy in eczematous diseases is still unclarified.12
Conclusions
Our case highlights that tildrakizumab is a promising drug for the biological treatment of plaque psoriasis in the moderate or severe stage and confirms its safety and efficacy even in special populations and the complex settings offered by the real-life practice.
Funding Statement
Funding: None.
References
- 1.AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis - comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol 2020;59:566-71. [DOI] [PubMed] [Google Scholar]
- 2.Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci 2019;20:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivamani RK, Goodarzi H, Garcia MS, et al. Biologic therapies in the treatment of psoriasis: a comprehensive evidencebased basic science and clinical review and a practical guide to tuberculosis monitoring. Clin Rev Allergy Immunol 2013;44:121-40. [DOI] [PubMed] [Google Scholar]
- 4.Reich K, Warren RB, Iversen L, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol 2020;182:605-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tildrakizumab Summary of Product Characteristics. [Google Scholar]
- 6.Kopp T, Riedl E, Bangert C, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 2015;521:222-6. [DOI] [PubMed] [Google Scholar]
- 7.Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390:276-88. [DOI] [PubMed] [Google Scholar]
- 8.Drerup KA, Seemann C, Gerdes S, Mrowietz U. Effective and Safe Treatment of Psoriatic Disease with the Anti-IL-23p19 Biologic Tildrakizumab: Results of a Real-World Prospective Cohort Study in Nonselected Patients. Dermatology 2021;1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 2009;129:1339-50. [DOI] [PubMed] [Google Scholar]
- 10.Mao LY, Ding J, Peng WF, et al. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia 2013;54:e142-5. [DOI] [PubMed] [Google Scholar]
- 11.Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis - a retrospective review. J Dermatolog Treat 2021;32:716-20. [DOI] [PubMed] [Google Scholar]
- 12.Husein-ElAhmed H, Steinhoff M. Effectiveness of ustekinumab in patients with atopic dermatitis: analysis of real-world evidence. J Dermatolog Treat 2021;1-6. [DOI] [PubMed] [Google Scholar]


