Abstract
Dexmedetomidine is a selective alpha-2 adrenergic agonist utilized for sedation in critically ill patients.1 We present the case of a morbidly obese critically ill patient who experienced profound hyperthermia, with a maximum temperature of 41.4°C, hours after starting a dexmedetomidine infusion that was otherwise not explained by her clinical diagnoses. The hyperthermia resolved hours following cessation of the infusion. Dexmedetomidine was assessed as probable in terms of causing this adverse effect. Dexmedetomidine may be associated not only with low-grade fever, but as demonstrated in our case, it may be associated with significant temperature elevations requiring cessation of therapy to restore normothermia.
Introduction:
Dexmedetomidine is a selective alpha-2 adrenergic agonist that is commonly used in the intensive care unit (ICU) for sedation in critically ill patients.1 Furthermore, dexmedetomidine is a sympatholytic inhibiting the release of norepinephrine due to binding of alpha-2 adrenoceptors in the brainstem. It is typically dosed as a continuous infusion at a titratable range of 0.2 to 1.5 micrograms/kilograms/hour. Typically, hypotension and bradycardia are the most frequently reported adverse effects with an incidence greater than five percent.2–4 As the clinical use of dexmedetomidine has increased with the drug becoming generic, recent reports have associated dexmedetomidine with low-grade fever.5–6 Here we present the case of severe hyperthermia that developed within hours of initiating dexmedetomidine infusion in a critically ill patient.
Case Description:
A 31-year-old Caucasian female with a past medical history significant for morbid obesity (weight of 300 kg and BMI 94.9 kg/m2) was admitted to the ICU with acute hypoxic respiratory failure requiring mechanical ventilation. A computed tomography scan of her chest revealed a massive pulmonary embolism occluding her right lower lobe pulmonary artery with right heart strain. She received 100 mg of alteplase administered over two hours and was initiated on an unfractionated heparin infusion. The patient was started on hydromorphone and propofol infusions for analgesia and sedation.
Due to concern for concurrent infection with a leukocytosis of 24.9 × 103/microliter, she was initiated on ceftriaxone and azithromycin to cover for possible community-acquired pneumonia. Initial microbiologic cultures included blood and a nasopharyngeal respiratory polymerase chain reaction swab with both resulting in no growth. Her maximum temperature in the first 24 hours of admission was 37.4°C. Additionally, she was in shock requiring low dose norepinephrine infusion that was thought to be cardiogenic in nature secondary to the massive pulmonary embolism.
On hospital day two, she was briefly extubated to high-flow nasal cannula before being re-intubated hours later due to hypercarbia. Her max temperature on this day was 37.3°C and she remained on antibiotics and low-dose norepinephrine infusion. She was re-initiated on propofol and hydromorphone infusions for sedation and analgesia.
On hospital day three, she was transitioned from propofol to a dexmedetomidine infusion to lighten sedation. The propofol drip infusion at 30 mcg/kg/min was stopped two hours prior to the initiation of dexmedetomidine. She was on a propofol infusion for a cumulative time of 34 hours while intubated with a maximum dose of 30 mcg/kg/min. A triglyceride level obtained the next day resulted at 115 milligrams/deciliter. The dexmedetomidine infusion was initiated (no bolus) at 0.4 mcg/kg/hr and titrated up to our institutional maximum dose of 1.4 mcg/kg/hr within nine hours of initiation. Dosing was based on actual body weight. At the time of dexmedetomidine initiation, her temperature was 38.6°C. Antibiotics were empirically broadened 12 hours after starting dexmedetomidine to piperacillin-tazobactam and vancomycin due to new high-grade fevers reaching a temperature of 40.8°C at the time. She was broadly cultured obtaining urine, blood, fungal serology, and endotracheal aspirate to determine if the fever was due to an infectious cause. Of these cultures, one of the four blood cultures grew two biotypes of coagulase negative staphylococcus species. With this organism only growing in one set of the two blood cultures and with it being a common commensal skin bacterium, it was thought that this result was a contaminant and not a true bacteremia. Her temperature progressively rose overnight to a maximum temperature of 41.4°C measured 15 hours following the initiation of dexmedetomidine. Urinary bladder temperatures were recorded throughout this time. These temperatures were confirmed through multiple measurements, including oral and rectal, to validate the readings. All of this was despite a cooling blanket that was placed earlier in the evening and acetaminophen administered every six hours for a total number of four doses. At this time, her other medications prescribed included: acetaminophen, dexmedetomidine infusion, docusate-senna, labetalol, norepinephrine infusion, piperacillin-tazobactam, polyethylene glycol, unfractionated heparin infusion and vancomycin. In the morning of hospital day four, 18 hours following the initiation of dexmedetomidine, the infusion was discontinued due to clinical concern that the infusion was contributing to the hyperthermia. Her temperature responded within hours and down trended throughout the remainder of the day plateauing at a temperature of 37.5°C. She remained on a cooling blanket during this time. The fever curve in relation to dexmedetomidine initiation and cessation is shown in Figure 1. No further doses of acetaminophen were administered following the cessation of the dexmedetomidine infusion. After the dexmedetomidine infusion was stopped, she remained off sedation. Additionally, she was hemodynamically stable in normal sinus rhythm off vasopressors. The piperacillin-tazobactam and vancomycin completed an empiric five-day course, though repeat blood cultures on day 5 were negative. The patient did not exhibit any sequalae of the hyperthermia.
Figure 1:
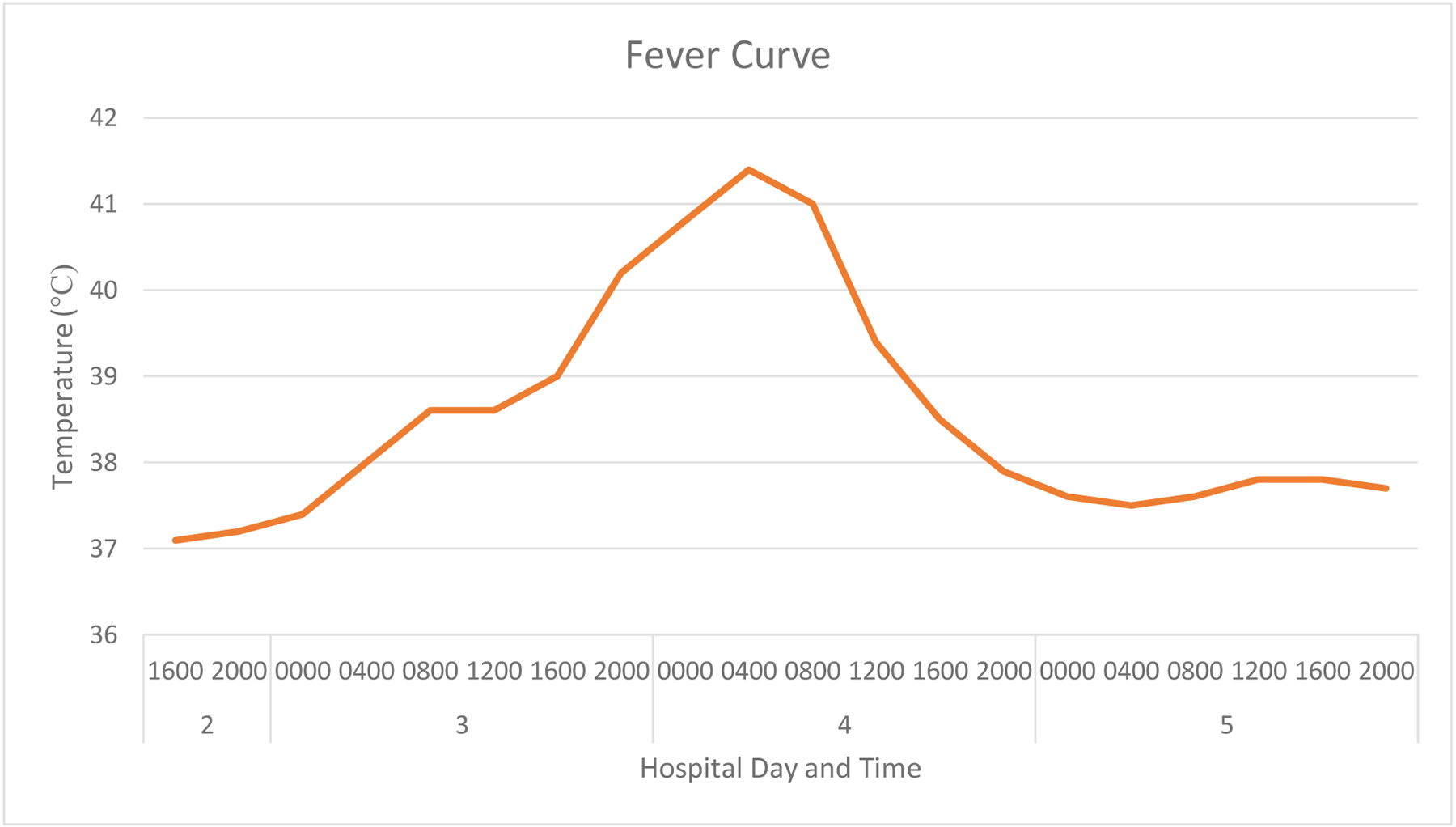
Fever Curve
Discussion:
We present a case of severe hyperthermia that began within hours of dexmedetomidine initiation, was unexplained by infectious causes or the patient’s medical diagnoses, and that rapidly resolved following cessation of dexmedetomidine. Her temperature rose throughout the day and the rise correlated with titrating up the dose on the infusion. The patient’s temperature peaked at 41.4°C while on the dexmedetomidine infusion. Based on her Naranjo Adverse Drug Reaction Probability Scale score of seven, dexmedetomidine was deemed a probable cause for the patient’s hyperthermia (Figure 2).7
Figure 2:

Naranjo Adverse Drug Reaction Probability Score
Additional work-up for the elevated temperatures failed to indicate an alternative source of hyperthermia. Though it is not uncommon for a diagnosis of pulmonary embolism to be associated with fevers, it is however rare for them to be associated with hyperthermia such was seen in our patient.8 The rapid increase in temperature following dexmedetomidine initiation and quick resolution of the hyperthermia corresponding to the discontinuation of dexmedetomidine lowers our suspicion that the pulmonary embolism was the cause. We also felt that the hyperthermia was not due to an infectious source as the only culture that exhibited microbiologic growth was a single blood culture (1 out of 4 bottles). We ruled out propofol-related infusion syndrome as a possible cause as she did not have hallmark symptoms of ventricular tachyarrhythmias, anion-gap metabolic acidosis or hypertriglyceridemia. Additionally, the propofol infusion was stopped before noon on hospital day three. With its half-life of roughly 40 minutes, we would not anticipate that the temperature would continue to rise throughout the day after stopping the infusion. Lastly, none of her medications were well-known sources of drug fever.
Dexmedetomidine has been shown to be associated with fevers, however, it is rare to see severe cases of rapid onset and degree of hyperthermia as seen in our patient.9 In a retrospective cohort study of adult ICU patients across two tertiary hospitals, 15.1% of patients that received six or more hours of dexmedetomidine continuous infusions had at least one temperature greater than or equal to 38.5°C.6 The mean maximum temperature of these patients was 38.9°C with a mean time from dexmedetomidine initiation to fever onset of 26.5 hours. In a retrospective cohort study across medical and surgical ICUs at a single institution, patients exposed to dexmedetomidine had a strong association for temperatures ≥ 103.1°F (39.5°C) compared to patients not exposed to dexmedetomidine (OR 4.5; CI 3.4, 5.9; P < 0.001).10 In a case series which included nine patients with dexmedetomidine drug fever, the maximum temperature of the patients ranged from 101.5°F to 104°F (38.6°C to 40.0°C).11 Our case is distinguished from these prior reports of dexmedetomidine-associated fever by an extreme elevation in temperature that required active intervention and cooling efforts to combat.
The mechanism of dexmedetomidine induced drug fever is not fully explained. One possible cause is that dexmedetomidine reduces central norepinephrine, serotonin, and dopamine release thus altering temperature homeostasis as these neurotransmitters help mediate hypothalamic temperature regulation.12 Obesity may play a role in contributing to dexmedetomidine-induced hyperthermia as the medication is dosed based on actual body weight and has a large volume of distribution. In a single center retrospective cohort study, obesity was associated with a higher incidence of temperatures ≥ 103.1°F (39.5°C) with an OR of 3.44 (CI 1.5, 7.9; P = 0.004).10 A post hoc analysis of the SPICEIII randomized controlled trial found that patients on dexmedetomidine with a body weight greater than 120 kg was associated with an increase in mean body temperature compared to patients with a weight ≤ 120 kg (P < 0.02).5 The group also found that for each additional 1 mcg/kg/hr of dexmedetomidine received there was an associated increase in temperature of 0.30 ± 0.08 °C (p < 0.0002).
The differential diagnosis for severe hyperthermia in a critically ill patient includes a multitude of causes such as sepsis, sympathomimetic intoxication, anticholinergic intoxication, sedative-hypnotic withdrawal, serotonin syndrome, malignant hyperthermia, neuroleptic malignant syndrome, drug fever, venous thromboembolism or severe decompensated hyperthyroidism. It is important for critical care practitioners to be aware that dexmedetomidine may be an offending medication contributing to significant temperature elevations. It is important to stop the medication as part of the fever work-up to evaluate whether it may be the inciting contributor. Drug fever is often benign but temperature elevations this extreme may have clinical consequences.
In summary, we present a 31-year-old critically ill patient with hyperthermia attributed to dexmedetomidine that was otherwise unexplained. While dexmedetomidine is increasingly recognized as a potential source of low-grade fevers, our case suggests that dexmedetomidine can cause dangerous elevations in temperature as well.
References:
- 1.Precedex [Package Insert]. Lake Forest, IL: Hospira Inc; 1999. [Google Scholar]
- 2.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. [DOI] [PubMed] [Google Scholar]
- 3.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–1160. [DOI] [PubMed] [Google Scholar]
- 4.Hughes CG, Mailloux PT, Devlin JW, et al. Dexmedetomidine or Propofol for Sedation in Mechanically Ventilated Adults with Sepsis. N Engl J Med. 2021;384(15):1424–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grayson KE, Bailey M, Balachandran M, et al. The Effect of Early Sedation With Dexmedetomidine on Body Temperature in Critically Ill Patients. Crit Care Med. 2021. Jul 1;49(7):1118–1128. [DOI] [PubMed] [Google Scholar]
- 6.Peterson J, Thomas W, Michaud C, Parker J. Incidence of Fever Associated With Dexmedetomidine in the Adult Intensive Care Unit. J Pharm Pract. 2021. Apr 5 [DOI] [PubMed] [Google Scholar]
- 7.Naranjo CA, Busto U, Sellers EM, et al. A Method for Estimating the Probability of Adverse Drug Reactions. Clin Pharmacol Ther. 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 8.Stein PD, Afzal A, Henry JW, et al. Fever in Acute Pulmonary Embolism. Chest. 2000; 117:39–42. [DOI] [PubMed] [Google Scholar]
- 9.Schurr JW, Ambrosi L, Lastra JL, et al. Fever Associated with Dexmedetomidine in Adult Acute Care Patients: A Systematic Review of the Literature. The Journal of Clinical Pharmacology. 2021, 61 (7) 848–856. [DOI] [PubMed] [Google Scholar]
- 10.Grayson KE, Tobin AE, Lim DT, et al. Dexmedetomidine-associated hyperthermia: a retrospective cohort study of intensive care unit admissions between 2009 and 2016. Anaesth Intensive Care. 2017; 45:727–736. [DOI] [PubMed] [Google Scholar]
- 11.Kruger BD, Kurmann J, Corti N, et al. Dexmedetomidine-Associated Hyperthermia: A Series of 9 Cases and a Review of the Literature. Anesth Analg 2017;125:1898–906 [DOI] [PubMed] [Google Scholar]
- 12.Wu TT, Lupi KE, Dube KM, Devlin JW. You Give Me Fever: Is Dexmedetomidine (or Another Medication) the Cause? Criti Care Med. 2021; 49(7): 1205–1207. [DOI] [PubMed] [Google Scholar]


