Abstract
Background: The purpose of the study was to determine the maximum tolerated dose of systemic oxaliplatin (oxal), 5-fluorouracil (5-FU) and leucovorin (LV) that could be administered with hepatic arterial infusion (HAI) of floxuridine (FUDR) and dexamethasone (Dex) in the adjuvant setting after hepatic resection.
Methods: Thirty-five patients with resected liver metastases were entered into a phase I trial using HAI FUDR/Dex with escalating doses of oxal and 5-FU.
Results: The initial dose of HAI FUDR was fixed at 0.12 mg/kg × pump volume divided by pump flow rate plus Dex infused over the first 2 weeks of a 5-week cycle. Systemic chemotherapy was delivered on days 15 and 29 with the doses of oxal escalated from 85 to 100 mg/m2 and the 5-FU 48-h continuous infusion doses from 1000 to 2000 mg/m2. The LV dose was fixed at 400 mg/m2. Dose-limiting toxic effects were diarrhea, 8.5%, and elevated bilirubin, 8.5%. With a median follow-up of 43 months, the 4-year survival and progression-free survival were 88% and 50%, respectively.
Conclusions: Adjuvant therapy after liver resection with HAI FUDR/Dex plus systemic oxal at 85 mg/m2 and 5-FU by continuous infusion at 2000 g/m2 with LV at 400 mg/m2 is feasible and appears effective. Randomized studies comparing this regimen to systemic FOLFOX are suggested.
Key words: adjuvant HAI FUDR, oxaliplatin/FU/LV, liver resection, colorectal cancer
introduction
Nearly 149 000 new cases of colorectal cancer are diagnosed each year in the United States [1]. Approximately 15% will have hepatic metastases at the time of diagnosis and 60% of patients with metastatic disease will develop hepatic metastases [2, 3]. Some of these patients will be suitable for surgical resection, which, alone, affords a 5-year survival of 30%–50% [3, 4]. Unfortunately, 70% of the patients who undergo liver resection will recur, either in the liver or another site [5]. Several studies evaluated the use of adjuvant therapy after liver resection. Two looked at systemic 5-fluorouracil (5-FU) and leucovorin (LV), but neither showed a significant improvement in disease-free survival (DFS) [6, 7]. European Organization for Research and Treatment of Cancer (EORTC) evaluated both pre- and postoperative therapy with systemic FOLFOX after liver resection and demonstrated an increase in progression-free survival (PFS) of 8.1% at 3 years, 36.2% and 28.1% for those with perioperative chemotherapy versus no chemotherapy, respectively [8]. The use of hepatic arterial infusion (HAI) with or without systemic therapy demonstrated in three of four randomized studies a significant increase in DFS [9., 10., 11., 12., 13.]. The use of HAI dexamethasone (Dex) with floxuridine (FUDR) in a randomized study versus Dex alone decreased hepatic toxicity [14]. Therefore, Dex was added to HAI FUDR with systemic oxaliplatin (oxal)/5-FU/LV in a phase I study of previously treated patients with unresectable liver metastases (13). The response rate was 86% [15] and the median survival was 22 months. Since our first HAI adjuvant trial [9] demonstrated increased systemic toxicity when HAI and systemic therapy were combined, the present study was designed to evaluate the appropriate dose of systemic oxal + 5-FU/LV with HAI FUDR/Dex after liver resection.
patients and methods
eligibility criteria and pretreatment evaluation
All patients had histologically confirmed colorectal adenocarcinoma with completely resectable hepatic metastases. Patients were excluded from the study for the following reasons: extrahepatic disease, prior hepatic radiation, prior use of oxal, infection, Karnofsky performance status of less than sixty, concurrent malignancy (within 5 years), white blood cell (WBC) count <3000 cells/μl, absolute neutrophil count (ANC) ≤1.5 K/μl, platelet count <100 000 cells/μl and total bilirubin ≥1.5 mg/dl. Prior chemotherapy (with the exception of oxal) was permitted provided the last dose was given ≥3 weeks before study entry. Computed tomography (CT) scans of the chest, abdomen and pelvis were carried out within 6 weeks before surgery. All patients signed informed consents; the protocol and informed consent were approved by the Memorial Sloan Kettering Cancer Center institutional review board.
Pretreatment evaluation included a complete history, physical examination and laboratory studies, including complete blood count, total bilirubin, alkaline phosphatase, aspartate aminotransferase (AST), carcinoembryonic antigen (CEA) and lactic dehydrogenase, obtained within 48 h before commencing chemotherapy. All patients underwent preoperative hepatic CT angiogram, including visualization of the celiac and superior mesenteric arteries, to evaluate hepatic arterial blood supply. Guidelines for pump placement have been previously reported [16]. The common hepatic, proper hepatic and gastroduodenal artery are completely dissected and all branches are divided. The bile duct is not dissected. A cholecystectomy is carried out to prevent chemotherapy-induced cholecystitis. The catheter is placed into the gastroduodenal artery and tested with methylene blue or fluorescein to ensure perfusion of the liver and exclude extrahepatic perfusion. Additional accessory and/or replaced branches are ligated and divided. An intraoperative injection of dye (either fluorescein or methylene blue) was used to check the flow immediately after placement. Postoperatively, a perfusion study utilizing technetium-99m macroaggregated albumin via the pump's sideport was compared with a standard sulfur colloid liver–spleen scan to confirm that distribution of the pump effluent was confined to the liver and that adequate perfusion of the liver remnant was achieved.
chemotherapy administration and toxicity evaluation
Chemotherapy was scheduled to start no sooner than 4 weeks after surgery. Chemotherapy was administered on a 5-week cycle. Pump therapy with FUDR and Dex was administered on day 1 of each cycle; the pump was emptied of drug and refilled with heparin and normal saline on days 15 and 29. Systemic chemotherapy was administered on days 15 and 29 of each cycle, and treatment was recycled on day 36. The HAI FUDR dose was fixed at 0.12 mg/kg × pump volume (30) divided by pump flow rate (supplied by the pump manufacturer, Codman Inc., Johnson and Johnson, Raynham, MA). In patients who were 25% above ideal weight, the actual dose of FUDR was calculated using an average between the patient's actual weight and the patient's ideal weight. Pump doses were reduced as outlined in Table 1 [17]. During FUDR administration, Dex was administered concurrently, at the rate of 1.0 mg × pump volume (30) divided by pump flow rate, and combined with 30 000 U heparin and normal saline in a quantity sufficient to fill the pump reservoir to 30 ml. On days 15 and 29 of each cycle, the residual was removed from the pump and measured to ensure maintenance of adequate flow rate and the pump was refilled with 30 ml heparinized saline. Oxal dose was initially fixed at 85 mg/m2, administered over a 2-h infusion, given concurrently with LV at 400 mg/m2 via a Y connection (over 2 h) followed by 5-FU 1000 mg/m2 delivered via an external pump for a 48-h continuous infusion (Table 2). The 5-FU dose was escalated to 1200 and then 1400 mg/m2. Because significant toxic effects were seen using the combination of systemic bolus 5-FU/LV and HAI FUDR [8], the starting dose of 5-FU was lower, and bolus 5-FU was excluded. After escalation of the 5-FU dose to 1400 mg/m2, the initial oxal dose of 85 mg/m2 was increased to 100 mg/m2, while 5-FU escalation continued. All toxic effects were graded according to the National Cancer Institute—Common Toxicity Criteria (3.0). Because patients entered the study with varying degrees of hepatic enzyme abnormality (Table 3), hepatic toxicity from treatment was defined as a significant increase over individual baseline values (twofold or greater for alkaline phosphatase, threefold or greater for AST and bilirubin ≥3 mg/dl). During treatment, toxicity was also measured against ‘reference values’, defined as the hepatic enzyme levels on the date the patient last received FUDR treatment. In the case of elevated transaminases, total bilirubin or both, FUDR administration was lowered or held as indicated in Table 1. Epigastric pain prompted an evaluation with an upper gastrointestinal endoscopy. If an ulcer or gastroduodenitis was documented, HAI therapy was withheld for 1 month to allow healing. Severe abdominal pain or diarrhea during HAI treatment resulted in immediate emptying of drug from the pump and instillation of heparinized saline or glycerol (50%) until further evaluation was completed (including a repeat flow scan to rule out extrahepatic perfusion) and/or the symptoms resolved.
Table 1.
FUDR dose modification
| Reference valuea | % FUDR dose | |
|---|---|---|
| AST (at pump emptying or day of planned retreatment, whichever is higher) | 0 to <2 × reference value | 100 |
| 2 to <3 × reference value | 80 | |
| 3 to <4 × reference value | 50 | |
| >4 × reference value | Hold | |
| Alk Phos (at pump emptying or day of planned retreatment, whichever is higher) | 0 to <1.2 × reference value | 100 |
| 1.2 to <1.5 × reference value | 50 | |
| >1.5 × reference value | Hold | |
| Total bilirubin (at pump emptying or day of planned retreatment, whichever is higher) | 0 to <1.2 × reference value | 100 |
| 1.2 to <1.5 × reference value | 50 | |
| >1.5 × reference value | Hold |
FUDR, floxuridine; AST, aspartate aminotransferase; Alk Phos, alkaline phosphatase.
If a patient's alkaline phosphatase or total bilirubin showed a continual rise from day 1 of treatment, then the day 1 value was used as the reference value for that patient when determining whether to hold treatment and time of retreatment after hold.
Table 2.
Final dose schema
 |
Table 3.
Summary of variables
| Variable | No. of patients | % | |
|---|---|---|---|
| Gender | |||
| Male | 25 | 71 | |
| Female | 10 | 29 | |
| Primary tumor | |||
| Colon | 26 | 74 | |
| Rectum | 9 | 26 | |
| Dukes' stage | |||
| B | 13 | 37 | |
| C | 22 | 63 | |
| Synchronous | |||
| Yes | 15 | 43 | |
| No | 20 | 57 | |
| No. of pathological lesions | |||
| 1 | 12 | 34 | |
| 2–4 | 18 | 51 | |
| >4 | 5 | 14 | |
| DFI | |||
| <12 months | 20 | 55 | |
| ≥12 months | 15 | 45 | |
| Margins | |||
| >1 cm | 18 | 51 | |
| <1 cm | 17 | 48 | |
| Positive margins (≤0.1 cm) | 4 | 11 | |
| Previous chemotherapy | |||
| Yes | 25 | 71 | |
| No | 10 | 29 | |
| Previous CPT-11, yes | 12 | 34 | |
| Age, years | |||
| Median | 58 | ||
| Range | 41–75 | ||
| Preoperative CEA | |||
| >30 ng/ml | 4 | 11 | |
| Postoperative CEA | |||
| >5 ng/ml | 4 | 11 | |
| Size of metastatic lesion | |||
| ≥5 cm | 7 | 20 | |
| Metastatic lesions | |||
| Bordering vessels, yes | 16 | 46 | |
| ≥2 vessels, yes | 2 | 6 | |
| Central, yes | 4 | 11 | |
| Bordering vena cava, yes | 2 | 6 | |
| Bilobar | 14 | 40 | |
| Above normal | |||
| Preoperative CEA | 19 | 54 | |
| Postoperative CEA | 4 | 11 | |
| LDH | 0 | 0 | |
| Alkaline phosphatase | 22 | 63 | |
| Bilirubin | 5 | 14 | |
| AST | 3 | 9 |
DFI, disease free interval; CEA, carcinoembryonic antigen; LDH, lactic dehydrogenase; AST, aspartate aminotransferase.
For the purposes of dose escalation, dose-limiting toxicity (DLT) was defined as any of the following occurring within the first two cycles of treatment: grade 4 neutropenia or thrombocytopenia; neutropenic fever, defined as grade 3 or 4 neutropenia plus fever higher than 38.3°C; grade 3 or 4 diarrhea; serum creatinine ≥1.8 mg/dl; ANC <1000 or platelet count <75 000 or total bilirubin ≥3.0 mg/dl not caused by disease progression (i.e. biliary obstruction as a result of tumor had been ruled out). Liver function tests were evaluated on days 1 and 15 of each cycle. Changes in doses of FUDR were calculated from the changes in liver function tests (Table 1). Patients were required to have a WBC count of 2500 U/l and an ANC >1500 U/l for subsequent cycles of systemic chemotherapy to be administered. If counts were below these levels on date of scheduled treatment, therapy was delayed for 1 week. CT of the chest/abdomen/pelvis was carried out every 3 months for the first 2 years. From year 2 to 4 after resection, scans and blood tests (including CEA and liver function tests) were repeated every 4 months; at 4 years every 6 months and at ≥5 years annually.
study design and statistical analysis
The primary objective was to define the maximum tolerated dose (MTD) of i.v. oxal plus systemic 5-FU/LV given in combination with hepatic arterial FUDR and Dex administered via an implanted pump in the adjuvant setting after resection of hepatic metastases from colorectal cancer. Three patients were enrolled at each dose level; barring DLT, as outlined above, enrollment proceeded to the next dose level. If one of three patients experienced DLT, then three additional patients would be enrolled. If two of the three patients experienced DLT, dose escalation would stop and three additional patients would be accrued to the previous dose level for further study. If no patients out of the additional three exhibited DLT, then next dose level was opened for enrollment. If one or more patients out of the additional three experienced DLT, dose escalation would stop and the previous dose level would be accepted as MTD. Once MTD is determined, 8–12 additional patients will be treated at this dose level to further explore the tolerability of this level.
These additional patients in the expanded cohort will provide a higher confidence of the true toxicity rate. Thus, if 10 patients are treated at the MTD, and if, for example, two patients have toxicity, there is a 90% chance that the true toxicity rate is between 4% and 51%. However, if four additional DLTs occur in this expanded cohort, the 95% confidence interval is 11% to 59% for the true toxicity rate. If four or more DLTs occur, we would recommend the next lower dose level for further evaluation of this drug combination.
results
patient characteristics
Thirty-five patients who underwent liver resection, one with concurrent radiofrequency ablation, were entered into the study. Patient characteristics are listed in Table 3. Poor prognostic indicators that were present included disease-free interval from primary resection <12 months (55%), stage III primary (64%), positive margins (12%), >1 metastatic lesion (64%), preoperative CEA >30 ng/ml (12%), postoperative CEA >5 ng/ml (11%) and lesion size >5 cm (21%). Prior adjuvant therapy after the primary colorectal resection was administered in 17 (49%) patients of whom four had FOLFIRI while the rest had FU/LV. Another eight patients had FOLFIRI for the treatment of metastatic disease for an average of 6 months before resection, and six of the eight had a partial response.
dose escalation and toxicity
The initial dose of oxal was 85 mg/m2 given on days 15 and 29 of the cycle. 5-FU dose was initially 1000 mg/m2 given over 48 h. The 5-FU dose was escalated until a dose of 1400 mg/m2 was attained with no toxic effects seen during the first two cycles. Then, the oxal dose was increased to 100 mg/m2 and the 5-FU dose was escalated up to 1800 mg/m2. At this dose, there were two DLTs in three patients. One patient was admitted for grade 4 diarrhea and another patient was admitted for grade 4 nausea and vomiting (Table 4).
Table 4.
Systemic and hepatic toxic effects
| Dose |
Cycles 1 and 2 |
Cycles 1 and 2 |
Cycles 1 and 2 |
Cycles 3–6 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea |
Nausea |
|||||||||||
| Cohort | Oxal (mg/m2) | 5-FU (mg/m2) | Total patients | Patients with DLT | Grade 3 | Grade 4 | Grade 3 | Grade 4 | Alk Phos ≥2 × RV | AST ≥3 × RV | Alk Phos ≥2 × RV | AST ≥3 × RV |
| 1 | 85 | 1000 | 3 | 1 | 1 | |||||||
| 2 | 85 | 1200 | 3 | 1 | 2 | 1 | ||||||
| 3 | 85 | 1400 | 3 | 1 | 2 | 1 | ||||||
| 4 | 100 | 1400 | 3 | 1 | ||||||||
| 5 | 100 | 1600 | 3 | 1 | 1 | |||||||
| 6 | 100 | 1800 | 3 | 2 | 1 | 1 | 1 | |||||
| 7 | 85 | 1600 | 9 | 1 | 6 | 4 | 2 | |||||
| 8 | 85 | 1800 | 6 | 1 | 1 | 1 | 2 | |||||
| 85 | 2000 | 2 | ||||||||||
Oxal, oxaliplatin; 5-FU, 5-fluorouracil; DLT, dose-limiting toxicity; Alk Phos, alkaline phosphatase; RV, reference value; AST, aspartate aminotransferase.
One could conclude that dose level 5 was our MTD; however, since a recent publication demonstrated a correlation between the use of oxal and hepatic toxicity [18], and our patients receiving oxal at 100 mg/m2 (e.g. cohorts 4 and 5 above) were only able to tolerate two full-dose cycles of HAI FUDR and then required dose reduction, the protocol was amended to reduce oxal to 85 mg/m2. Patients were then entered to dose level 7 (i.e. 5-FU dose of 1600 mg/m2) and after entering nine patients with no toxicity, the protocol was amended to allow escalation of 5-FU dose to 1800 and then 2000 mg/m2. No systemic toxic effects have been seen at these dose levels. Accrual to this protocol has slowed since many patients are now receiving preoperative oxal (which was an exclusionary factor). Therefore, we feel it would take too long to complete further 5-FU escalation. In looking at the dose intensity of oxal, 18 of 26 patients who were treated with oxal at 85 mg/m2 received ≥75% of the planned dose, but only four of nine who were treated with oxal at 100 mg/m2 received ≥75% of the planned dose.
Hepatic toxic effects are listed in Table 4. Elevations in alkaline phosphatase of two times the reference value occurred in 10 patients and AST elevations of three times the reference value occurred in four, for which mandatory FUDR dose reductions were made. Of the 35 patients, three (8.5%) had bilirubin elevations and one (2.8%) required a stent. A 60-year-old male in cohort 5 developed a bilirubin of 2.7 mg/dl in cycle 3, which went down to <1.5 one week later. After therapy was completed, an endoscopic retrograde cholangiopancreatography revealed a short stricture of the common hepatic duct and a biliary stent was placed. The patient is now 2.5 years from stent placement without recurrence of disease or jaundice. A 56-year-old male in cohort 1 developed an elevated bilirubin of 12.0 mg/dl 6 months after completing therapy which spontaneously resolved within 1 month, without subsequent elevation (it is now 4 years since that episode without recurrence in the liver). The last patient, a 43-year-old male in cohort 7, developed a bilirubin of 2.1 mg/dl, 11 months after starting therapy, which returned to normal; 1 year later (when initiating chemotherapy for recurrent disease in the liver), his bilirubin rose to 3.0 mg/dl.
Over all cycles and cohorts, seven patients required admissions: three patients for grade 3 diarrhea, two for abdominal pain, one for grade 4 vomiting and one for a bleeding ulcer. All toxic effects resolved and patients were able to be retreated.
progression and survival
Accrual to the study has been slow as the study mandated that patients may not have received prior oxal; the first patient to start treatment was on 19 March 2003 and the last was on 16 January 2008. For this reason, there is a long median follow-up of 43 months. One-year survival was 100%, 2-year survival 97% and 3-, 4- and 5-year survival 88% (Figure 1). Median survival has not been reached. Hepatic PFS was 94% at 1 and 2 years and 86% at 3, 4 and 5 years (Figure 2); four patients (11%) recurred in the liver and three responded to further HAI therapy. Overall PFS was 81% at 1 year, 58% at 2 years and 50% at 3, 4 and 5 years (Figure 3). Of the 13 extrahepatic progressions, 10 were in the lung and six patients were able to undergo resection of all disease. There were three recurrences in nodes in the abdomen which were resected. There were no brain or bone metastases. The median PFS is 37.7 months (95% CI 16.5 to infinity).
Figure 1.
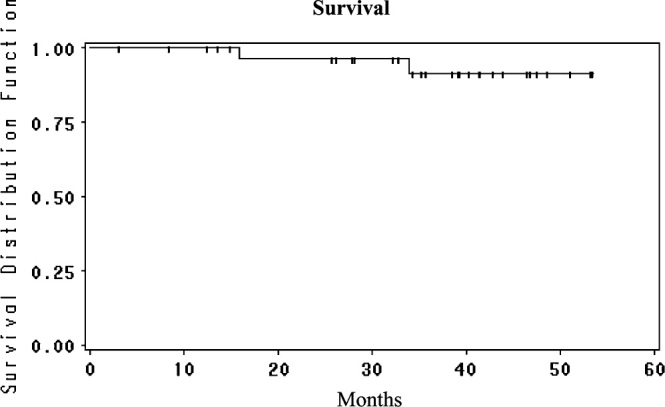
Overall survival from the time of liver resection for the patients treated with adjuvant HAI FUDR/Dex plus systemic oxaliplatin/5-FU/LV. HAI, hepatic arterial infusion; FUDR, floxuridine; Dex, dexamethasone; 5-FU, 5-fluorouracil; LV, leucovorin.
Figure 2.
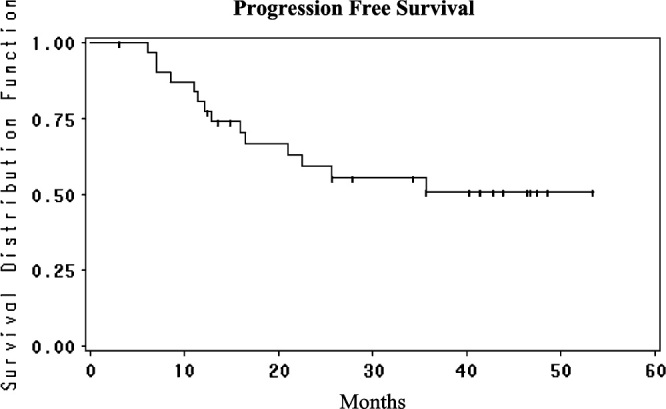
Progression-free survival from the time of liver resection for the patients treated with adjuvant HAI FUDR/Dex plus systemic oxaliplatin/5-FU/LV. HAI, hepatic arterial infusion; FUDR, floxuridine; Dex, dexamethasone; 5-FU, 5-fluorouracil; LV, leucovorin.
Figure 3.
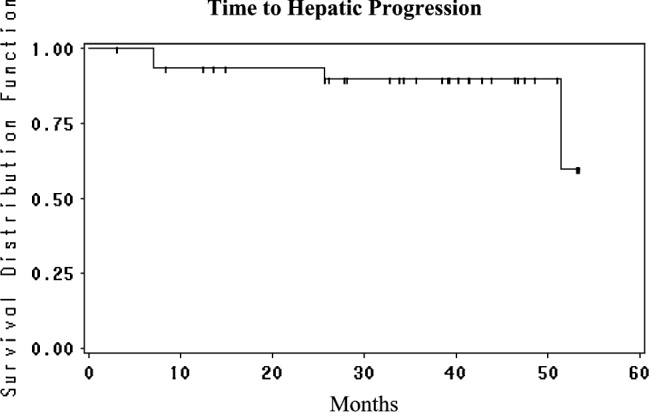
Time to hepatic progression from the time of liver resection for the patients treated with adjuvant HAI FUDR/Dex plus systemic oxaliplatin/5-FU/LV. HAI, hepatic arterial infusion; FUDR, floxuridine; Dex, dexamethasone; 5-FU, 5-fluorouracil; LV, leucovorin.
discussion
The liver is the most common site for metastatic disease from colorectal cancer. For patients with disease confined to the liver, resection of hepatic metastases improves survival. However, the relapse rate within the first 2 years is high, and the liver is the most common site of recurrence. These hepatic relapses may be related to the persistence of micrometastases not detected by CT or intraoperative inspection. After neoadjuvant chemotherapy and hepatic resection, hepatic metastases may be undetected due to clinical complete responses [19] or because of chemotherapy-induced changes in the liver parenchyma [18]. Therefore, whether or not neoadjuvant chemotherapy is administered with hepatic resection, adjuvant therapy is needed. Systemic 5-FU/LV has not produced a significant increase in survival in the adjuvant setting after hepatic resection, and systemic adjuvant FOLFIRI (Irinotecan, 5FU, LV) after liver resection showed no benefit compared with 5-FU/LV [20]. The EORTC [8] administered perioperative therapy with FOLFOX 3 months before and 3 months after surgery and reported a DFS at 2, 3, 4 and 5 years of 50%, 42.4%, 38% and 28%, respectively, in resected patients. In resected patients, there was a 9% increase in DFS compared with no therapy after resection, but the study did not answer two questions: is postoperative FOLFOX or preoperative FOLFOX beneficial and is it more useful than 5-FU/LV alone?
Several studies have demonstrated the usefulness of HAI after liver resection with a significant increase in DFS in three of four studies comparing HAI + systemic to systemic alone or control (Table 5). Studies utilizing irinotecan and oxal have been initiated to improve results. Using systemic irinotecan combined with HAI FUDR/Dex therapy, the 2- and 5-year survivals were 87% and 60%, respectively [17]. The addition of systemic oxal/5-FU/LV with HAI in the present study seems to improve results. Although not randomized comparisons, the groups have similar patient characteristics. The proportion of patients with more than one metastasis was 56% in the present study versus 32% in the Portier study [6] and 49% in the EORTC study [8]. The possible improvement in survival with the use of adjuvant oxal/FU/LV after liver resection is similar to the reports in adjuvant chemotherapy for stage III colon cancer where FOLFOX increased DFS [21]. The improved survival seen in this report compared with historical controls may also be due to the fact that survival for all patients is improving. Andres et al. reported on an improvement in survival comparing the time periods 1984–1992 to 1999–2005. They reported that the 3- and 5-year survivals for the time period of 1984–1992 were 30% and 23%, respectively, while it was 69% and 46%, respectively, for the time period from 1995 to 2005 [22]. DFS did not improve in the later time period. Survival may have improved due to repeat resections, newer chemotherapy and improved general medical care.
Table 5.
Randomized trials of HAI + systemic versus systemic or control after liver resection
| Study | Number of patients | Disease-free survival |
P value | |||
|---|---|---|---|---|---|---|
| % 2-year survival |
% 5-year survival |
|||||
| HAI | Systemic | HAI | Systemic | |||
| MSKCC [9] | 156 | 55 | 45 | 40 | 30 | 0.02 |
| ECOG [12] | 75a | 60 | 40b | 40 | 20b | 0.03 |
| German [10] | 186a | Median | 20/12.6b | NS | ||
| Greek [11] | 122 | 66 | 48 | 60 | 35 | 0.0002 |
HAI, hepatic arterial infusion; ECOG, Eastern Cooperative Oncology Group; NS, not significant.
Patients treated in study.
No treatment in control arm.
The toxicity of systemic oxal 5-FU/LV plus HAI FUDR/Dex was less than reported with HAI plus systemic 5-FU/LV. The original adjuvant study used bolus 5-FU for 5 days (and in addition, the doses administered were almost full doses of 5-FU/LV whereas in this report the doses of 5-FU are lower). Additionally, the FUDR dose has been lowered: the original dose was 0.14 mg/kg × 30 divided by the flow rate, while in this study, the dose is 0.12 mg/kg. Thirty-nine percent of patients were hospitalized in the original study for toxic effects such as diarrhea, leukopenia and small bowel obstruction, while 20% of patients were hospitalized in the present study when all cycles were included.
It is possible that the 5-FU dose could be increased beyond 2000 g/m2, but this dose achieved excellent results without significant toxicity. However, if higher doses could be administered, it is possible that there would be a decrease in extrahepatic metastases. In the study using combined HAI and systemic oxal/5-FU/LV therapy for metastatic disease, the MTD for 5-FU was 1400 mg when used with oxal at 100 mg/m2; therefore, it is unlikely that a much higher dose would be achievable.
This study demonstrates that it is possible to combine an attenuated dose of oxal/5-FU/LV with HAI therapy in the adjuvant setting after liver resection with acceptable toxicity. The maximum dose without toxicity was oxal 85 mg/m2 and 5-FU 2000 mg/m2 by continuous infusion for 2 days with FUDR (0.12 mg/kg × 30 divided by flow rate plus Dex) and LV at 400 mg/m2. With a median follow-up of 43 months, the 2- and 4-year survivals were 97% and 88%, respectively, with 2- and 4-year DFS of 58% and 50%, respectively. Randomized trials are suggested to compare HAI FUDR/Dex and systemic oxal/5-FU/LV with systemic FOLFOX in the adjuvant setting after resection of hepatic metastases from colorectal carcinoma.
funding
Sanofi Aventis (43900).
references
- 1.Jemal A., Siegel R., Ward E., et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Weiss L., Grandmann E., Torhost J., et al. Hematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y.C., Fortner A.M., Enker J.G., et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15(3):938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 4.Choti M.A., Sitzmann J.V., Tiburi M.F., et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235(6):759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes K.S., Simon R., Songhorabodi S., et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100(2):278–284. [PubMed] [Google Scholar]
- 6.Portier G., Elias D., Bouche O., et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24(31):4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 7.Mitry E., Fields A.L., Bleiberg H., et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer. A meta-analysis of two randomized trials. J Clin Oncol. 2006;24(18S):1. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 8.Nordlinger B., Sorbye H., Glimelius B., et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemeny N., Huang Y., Cohen A.M., et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341(27):2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz M.M., Schramm H.H., Gassel H., et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen) Ann Surg. 1998;228(6):756–762. doi: 10.1097/00000658-199812000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lygidakis N.J.S., Vlachos G., Raptis L., et al. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection. Results of a prospective randomized study. Hepatogastroenterology. 2001;48(42):1685–1691. [PubMed] [Google Scholar]
- 12.Kemeny M.M., Adak S., Gray B., et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an intergroup study. J Clin Oncol. 2002;20:1499–1505. doi: 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 13.Kemeny N., Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352(7):734–735. doi: 10.1056/NEJM200502173520723. [DOI] [PubMed] [Google Scholar]
- 14.Kemeny N., Seiter K., Niedzwiecki D., et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992;69(2):327–334. doi: 10.1002/1097-0142(19920115)69:2<327::aid-cncr2820690209>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Kemeny N., Jarnagin W., Paty P., et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23(22):4888–4896. doi: 10.1200/JCO.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 16.Kemeny N., Daly J., Oderman P., et al. Hepatic artery pump infusion toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol. 1984;2:595–600. doi: 10.1200/JCO.1984.2.6.595. [DOI] [PubMed] [Google Scholar]
- 17.Kemeny N., Jarnagin W., Gonen M., et al. A phase I/II study of hepatic arterial therapy with floxuridine and dexamethasone in combination with intravenous irinotecan as adjuvant treatment after resection of hepatic metastases from colorectal cancer. J Clin Oncol. 2003;21:3303–3309. doi: 10.1200/JCO.2003.03.142. [DOI] [PubMed] [Google Scholar]
- 18.Rubbia-Brandt L.A., Sartoretti V., Roth P., et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15(3):460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 19.Benoist S., Brouquet A., Penna C., et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24(24):3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 20.Ychou M., HW, Thezenas S., et al. Randomized phase III trial comparing infused 5-fluorouracil/folinic acid (LV5FU) versus LV5FU+irinotecan (LV5FU+IRI) as adjuvant treatment after complete resection of liver metastases from colorectal cancer (LMCRC). (CPT-GMA-301) J Clin Oncol. 2008;26(Suppl) (Abstr LBA4013) [Google Scholar]
- 21.Andre T., Boni C., Mounedji-Boudiaf L., et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 22.Andres A., Majno P.E., Morel P., et al. Improved long-term outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of a severity score. Ann Surg Oncol. 2008;15(1):134–143. doi: 10.1245/s10434-007-9607-1. [DOI] [PubMed] [Google Scholar]


