Abstract
Allostatic load (AL) exposure may cause detrimental effects on the neuroendocrine system, leading to metabolic syndrome (MetS). The primary mediators of AL involve serum dehydroepiandrosterone sulfate (DHEAS; a functional HPA axis antagonist); further, cortisol, urinary norepinephrine (NE), and epinephrine (EPI) excretion levels (assessed within 12-h urine as a golden standard for the evaluation of the HPA axis activity and sympathetic nervous system activity). However, the evidence of an association between the primary mediators of AL and MetS is limited. This systematic review aimed to critically examine the association between the primary mediators of AL and MetS. PubMed and Web of Science were searched for articles from January 2010 to December 2021, published in English. The search strategy focused on cross-sectional and case–control studies comprising adult participants with MetS, obesity, overweight, and without chronic diseases. The STROBE checklist was used to assess study quality control. Of 770 studies, twenty-one studies with a total sample size (n = 10,666) met the eligibility criteria. Eighteen studies were cross-sectional, and three were case–control studies. The included studies had a completeness of reporting score of COR % = 87.0 ± 6.4%. It is to be noted, that cortisol as a primary mediator of AL showed an association with MetS in 50% (urinary cortisol), 40% (serum cortisol), 60% (salivary cortisol), and 100% (hair cortisol) of the studies. For DHEAS, it is to conclude that 60% of the studies showed an association with MetS. In contrast, urinary EPI and urinary NE had 100% no association with MetS. In summary, there is a tendency for the association between higher serum cortisol, salivary cortisol, urinary cortisol, hair cortisol, and lower levels of DHEAS with MetS. Future studies focusing on longitudinal data are warranted for clarification and understanding of the association between the primary mediators of AL and MetS.
Keywords: allostatic load, cortisol, dehydroepiandrosterone sulfate, epinephrine, norepinephrine, metabolic syndrome, primary marker
1 Introduction
Metabolic syndrome (MetS) is defined as the cluster of co-existence of high blood pressure, abdominal obesity, low high-density lipoprotein (HDL) cholesterol, elevated triglycerides, and hyperglycemia (1, 2). These metabolic abnormalities have been linked to the development of type 2 diabetes (T2DM) and cardiovascular diseases (CVDs) (3). Globally, the prevalence of MetS is estimated to affect over 20% of the adult population in the USA (4), China (5), Europe (6), as well as developing countries (7, 8). The potential causal and influencing factors of MetS may be genetic, environmental (e.g., socioeconomic status, urbanicity), psychosocial (e.g., perceived stress, depression), behavioral (e.g., physical activity), and biographical (e.g., education, childhood adversity) factors that are often conditioned by sex and age (9, 10). A current meta-analysis study that involved total patients (n = 162,450) reported that MetS increased adverse cardiovascular events and mortality rates (11). Similarly, a previous systematic review reported that cumulative stress termed “allostatic load (AL)” is associated with CVDs, diabetes, and MetS (12). A very well-evaluated index for the assessment of chronic stress is the AL index, which reflects the impact of chronic stress on different allosteric systems and pathways (13, 14). Allostasis is an adaptive response mechanism to chronic stress to restore physiological stability through the autonomic nervous system (ANS), the hypothalamic–pituitary–adrenal axis (HPA), the hypothalamic–pituitary–thyroid axis (HPT), somatotropic axes (i.e., growth hormones [GH], insulin-like growth factors [IGF-I and III] and their associated carrier proteins and receptors), gonadal axis (HPG), and the metabolic and immune system (15–18). Moreover, AL is the strain on the body resulting from repeated up and downregulation of physiologic stress response, as well as by the elevated activity of physiologic systems under chronic challenge, the changes in metabolism, and the impact of wear and tear on several organs and tissues that predispose the organism to disease (19, 20).
The concept of the measurement of allostasis and AL is integrated with the AL index, which was first discussed by Seeman et al. (21). Seeman et al. (21) assessed AL using 10 biomarkers. The gold standard for the evaluation of AL is the measurement of 24 biomarkers, which are summarized into an index (22) and theoretically differentiated into primary and secondary mediators of the AL index (23, 24). The primary mediators of AL consist of four biomarkers involving serum dehydroepiandrosterone sulfate (DHEAS; a functional HPA axis antagonist); 12-h urinary cortisol excretion (an integrated measure of 12-h HPA axis activity); and 12-h epinephrine (EPI) and norepinephrine (NE) excretion levels (integrated indices of 12-h sympathetic nervous system activity) (25). The remaining six biomarkers, which are considered secondary mediators of AL, overlap with the biomarkers used in the diagnosis of MetS (14). It has been shown that there is a co-activation of the HPA axis and sympathetic adrenal medullary system (SAM) under stress (26). While the HPA axis secretes glucocorticoids (e.g., cortisol), the SAM secretes catecholamines (e.g., EPI and NE). Stress can alter glucocorticoid function to enhance gluconeogenesis and free fatty acids (FFA) by differentiation of pre-adipocytes leading to central fat accumulation and MetS development (27). On the one hand, cortisol helps to regulate SAM to create optimum homeostasis when an individual encounters acute stress. On the other hand, chronic stress leads to prolonged activation of SAM and alterations in HPA axis function in the cardiovascular, metabolic, immunologic, and central nervous systems (28). Higher cortisol levels lead to obesity and MetS (29, 30). Additionally, both dehydroepiandrosterone (DHEA) and its sulfate ester DHEAS are steroid hormones connected to stress (31). Physiologically, both DHEA and DHEAS exert anti-glucocorticoid activity (32, 33), and catecholamine synthesis and secretion (34). Low DHEAS levels and an age-related decline in DHEAS may cause higher circulating cortisol in peripheral target tissues, contributing to insulin resistance, obesity, and MetS (35, 36).
Furthermore, catecholamines such as EPI and NE modulate corticotrophin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) during both acute and chronic stress challenges (37, 38). Ebert et al. (39) revealed that psychological stress mediated the development of MetS through the release of EPI and NE. Increasing doses of catecholamines show greater lipolytic effects on visceral fats via the β1- and β2-adrenoceptors (40). Furthermore, Ziegler et al. (41) reported that β-adrenergic blocking drugs may lead to impaired metabolism, hyperglycemia, and insulin resistance due to the inhibition of EPI stimulation. There is an emerging interest in understanding how the biomarkers of AL and MetS are connected and influence each other. Current systematic reviews have concentrated on AL and health (42), health risk behaviors and AL (12), basal cortisol levels, and MetS (43). Also, chronic stress effects on glucocorticoids and catecholamines have been reported to be an influencing factor for MetS and CVDs (44). Thus, understanding the linkage between AL and MetS is of clinical relevance. Yet, the evidence for the association between the primary mediators of AL and MetS is limited. Thus, the main aim of the current systematic review is to critically examine the associations of the primary mediators of AL and MetS in the literature. In addition, the study aims to analyze these associations in a wide range of populations.
2 Methods
2.1 Study protocol
The current systematic review was conducted and reported based on the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (45). The completed PRISMA statement checklist is provided as a supplementary material ( Supplementary Tables 1, 2 ).
2.2 Data source and search strategy
Two electronic databases, PubMed and Web of Science, were searched for articles published from January 2010 to December 2021 in English. The search strategy was based on the medical subject heading (MeSH) and non-MeSH search terms of keywords and the Boolean operators AND/OR ([Allostatic load; Allostatic overload; AL; Metabolic syndrome; MetS; Cortisol; Epinephrine; Norepinephrine; Dehydroepiandrosterone sulfate and DHEAS]). For additional information, the Cochrane library and the reference lists of systematic reviews found from the search were screened for related articles.
2.3 Eligibility criteria for study selection
The studies included in this systematic review met the following eligibility criteria: (I) observational studies (i.e., cross-sectional or case–control study) with an adult population (i.e., 18 years and above) that involved (II) study populations affected by MetS, obesity or overweight and control group; (III) studies examining the association between primary AL mediators: cortisol; epinephrine; norepinephrine; dehydroepiandrosterone sulfate and MetS, and (IV) original full-text studies in English. Exclusion criteria used in this systematic review were: (I) reviews, meta-analyses, case reports, expert opinions, trials, studies using animals or children, conference proceedings, and editorials, (II) duplication of the same data and population; and (III) studies using populations with other comorbidities except for individuals with MetS, overweight, or obesity. The Authors (FO and AB) established the search criteria for the study. The searches using the criteria established above for the selection of full-text articles were performed by one author (FO). Disagreements were resolved by a discussion with the second author (AB).
2.4 Data extraction
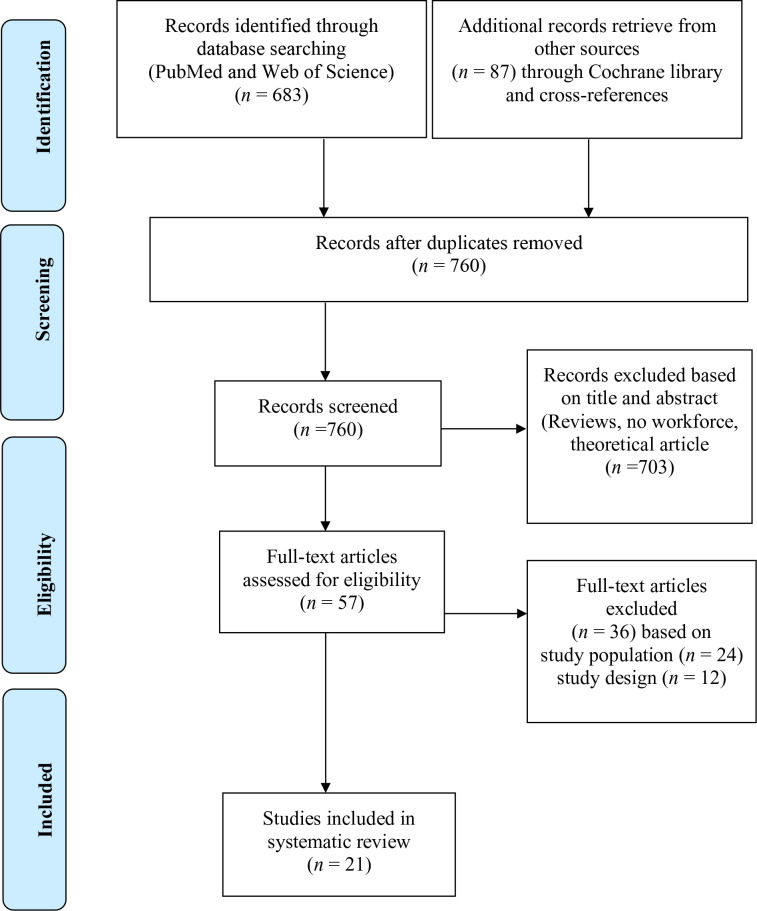
The titles and abstracts of articles identified via the search were screened for relevance and cross-checked for eligibility. Full-text reports of relevant articles were also screened for their eligibility. Information on the search results is provided in Figure 1 . Information from the included studies was extracted (see Table 1 for more details). Data extraction was performed by one author (FO).
Figure 1.
PRISMA flow diagram of search results.
Table 1.
Study characteristics of the association between the primary mediators of AL and MetS (n = 21).
| Author/Date(Country) | MetS diagnosis | Sample | Primary ALmediators | Measurement techniques of primary AL mediators | Association between primary mediators of AL and MetS(Adjustment) | JBI Score | |
|---|---|---|---|---|---|---|---|
| N(sex %) | MetS (%) | ||||||
| 1. Cross-sectional studies | |||||||
| Mazgelytė et al. (46) (Lithuania) |
IDF | 163 adults Men = 100% |
MetS = 23.3 without MetS = 76.7 |
HCC | High-performance liquid chromatography |
Significant association (p <0.005) was observed for higher HCC between participants with MetS (85.73 [150.88] ng/g) in comparison without MetS (36.50 [98.26] ng/g). (Non-adjusted). | 8 |
| Serum cortisol | Enzyme-linked immunoassay | No significant association (p = 0.168) was observed for serum cortisol concentration between participants with MetS (221.78 [94.29] ng/ml) and participants without MetS (200.62 [128.15] ng/ml). (Non-adjusted). | |||||
| Salivary cortisol | Enzyme-linked immunoassay | No significant association (p = 0.193) observed for salivary cortisol concentration between participants with MetS (9.16 [6.78] ng/ml) and participants without MetS (11.09 [9.85] ng/ml). (Non-adjusted). | |||||
| Lehrer et al. (47) (USA) |
NCEP-ATP III (2004) |
228 adults Men = 32% Women = 68% |
Not applicable | HCC | Enzyme-linked immunoassay | Higher HCC was positively associated with MetS severity (b = 0.344, SE = 0.126, 95% CI [0.106, 0.605]).(Adjusted for age, sex, race/ethnicity, income, medication use, physical activity, nervous and calm personality, hair washing, and bleach use). | 7 |
| Martins et al. (48) (Brazil) |
NCEP-ATP III (2001) |
80 adults Men = 43.7% Women = 56.3% |
MetS = 50.0 without MetS = 50.0 |
Salivary cortisol | Radioimmunoassay | No significant association (p = 0.47) was observed for basal salivary cortisol between participants with MetS (44.4 ± 3.1 nmol/L) and participants without MetS (46.5 ± 2.9 nmol/L). (Non-adjusted). | 5 |
| Udenze et al. (49) (Nigeria) |
NCEP-ATP III (2001) |
100 adults Women = 100% |
MetS = 50.0 without MetS = 50.0 |
Serum cortisol | Enzyme-linked immunoassay | No significant association (p = 0.437) was observed for serum cortisol between participants with MetS (12.80 ± 4.79 μg/dl) and participants without MetS (10.83 ± 6.59 μg/dl). (Non-adjusted). | 5 |
| Damgaard–Olesen et al. (50) (Denmark) |
IDF | 303 adults Men = 100% |
Mets = 29.7 without MetS = 70.3 |
DHEAS | TurboFlow-Liquid Chromatography-Mass Spectrometry LC-MS/MS |
No significant association (p = 0.23) was observed for DHEAS between participants with MetS (Geometric Mean = 4,527 nmol/L) and participants without MetS (Geometric Mean = 4,185 nmol/L). (Non-adjusted). | 8 |
| Constantinopoulos et al. (51) (Greece) |
IDF | 37 adults. Men = 47% Women = 53% |
MetS = 51.4 without MetS = 48.6 |
UFC | Chemiluminescence immunoassay | Significant association (p >0.01) was observed for higher 24-h UFC for participants with MetS (116.8 ± 106.6 μg/24-h) in comparison to participants without MetS (71.3 ± 62.7 μg/24-h). (Non-adjusted). | 6 |
| Serum cortisol | Chemiluminescence immunoassay | Significant association (p >0.01) for higher serum cortisol was observed between participants with MetS (16.6 ± 7.2 μg/ml) in comparison to participants without MetS (10.7 ± 4.1 μg/ml). (Non-adjusted). | |||||
| Salivary cortisol |
Chemiluminescence immunoassay | Significant association (p >0.01) was observed for higher salivary cortisol between participants with MetS (0.87 ± 0.4 μg/ml) in comparison with participants without MetS (0.46 ± 0.21 μg/ml). (Non-adjusted). | |||||
| Corbalán-Tutau et al. (52) (Spain) |
IDF | 70 adults Women = 100% |
MetS = 57.0 without MetS = 43.0 |
Salivary Cortisol | Radioimmunoassay | Significant associations (p <0.05), in daily circadian markers for lower salivary cortisol levels (nmol/l) in participants with MetS in comparison participants without MetS. 8 am: MetS (17.1 ± 1.0 nmol/l) vs without MetS (25.3 ± 1.6 nmol/l). 14 pm: MetS (10.6 ± 0.3 nmol/l) vs without MetS (11.9 ± 0.4 nmol/l). 23 pm: MetS (5.0 ± 0.2 nmol/l) vs without MetS (6.3 ± 0.3 nmol/l) (Non-adjusted). |
8 |
| Almadi et al. (53) (Australia) |
IDF | 204 adults Men = 100% |
MetS = 31.9 without MetS = 68.1 |
Salivary Cortisol | Electrochemiluminescence | Significant association (p <0.05) was observed for higher salivary cortisol between stress group with MetS (326.9 ± 153.3 nmol/L) in comparison with non-stress group without MetS (267.3 ± 99.2 nmol/L). (Adjusted for age, type of work, physical activity, awakening time, and work overcommitment). | 8 |
| Fabre et al. (54) (Belgium) |
IDF & NCEP-ATP III (2001) |
149 adults Men = 100% |
MetS = 44.3 without MetS = 55.7 |
Serum Cortisol | Chemiluminescence immunoassay | No significant association (p >0.05) was observed for serum cortisol between participants with MetS (13.7 [5.7–23.6] μg/dl) and participants without MetS (13.3 [5.9–29.4] μg/dl). (Adjusted for age and BMI). | 8 |
| **Mattei et al. (55) (USA) |
AHA/NHLBI | 1318 adults Men = 27.8%Women = 72.2% | MetS = 67.6 without MetS = 32.4 |
UFC | Direct immunoenzymatic colorimetric method |
No significant association (p >0.05) between UFC (mg/g creatinine) (OR = 1, 95% CI [0.995,1.004]) and participants with MetS. (Adjusted for age and sex). | 8 |
| DHEAS | Electrochemiluminescence | No significant association (p >0.05) was observed for DHEAS (OR = 1, 95% CI [1,1] ng/ml) and MetS. (Adjusted for age and sex). |
|||||
| Urinary EPI | Direct immunoenzymatic colorimetric method. |
No significant association (p >0.05) was observed between 12-h urinary EPI (µg/g creatinine) (OR = 0.97, 95% CI [0.938, 1.00]) and MetS. (Adjusted for age and sex). | |||||
| Urinary NE | Direct immunoenzymatic colorimetric method. |
No significant association (p >0.05) was observed between 12-h urinary NE (µg/g creatinine) (OR = 1, 95% CI [0.998, 1]) and MetS. (Adjusted for age and sex). | |||||
| Jang et al. (56) (Korea) |
IDF | 46 adults Men = 59%Women = 41% | MetS = 26.0 without MetS = 74.0 | Salivary Cortisol | Competitive enzyme immunoassay |
Significant association (p = 0.0001) was observed for higher midnight salivary cortisol levels between participants with MetS (70 ± 42.4 ng/dl) in comparison with participants without MetS (48.1 ± 36.8 ng/dl). (Non-adjusted). | 8 |
| Baudrand et al. (57) (Chile) |
NCEP-ATP III (2004) |
221 adults Men = 26.2%Women =73.8% | MetS = 58.8 without MetS = 41.2 | UFC | High-performance liquid Chromatography (HPLC) |
No significant association (p = 0.196) was observed for UFC between participants with MetS (21.13 [11.3–28.1 µg/24 h]) and participants without MetS (24.81 [13.8–31.2 µg/24 h]). (Non-adjusted). |
8 |
| Esteghamati et al. (58) (Iran) |
NCEP-ATP III (2001) |
285 adults Men = 43.5%Women = 56.5% | MetS = 42.1 without MetS = 57.9 | Serum cortisol | Radioimmunoassay | No significance association (p >0.05) was observed for serum cortisol between males and females with MetS (15.16 ± 5.04 µg/dl) and with males and females without MetS (14.56 ± 4.66 µg/dl). (Non-adjusted). Significant association (p <0.05) for higher serum cortisol in males with MetS (17.74 ± 5.1 µg/dl). (Adjusted for age, WC, and BMI). |
6 |
| Park et al. (59) (Korea) |
NCEP-ATP III (2004) |
1881 adults Men = 43.9%Women = 56.1% | Mets = 27.3 without MetS = 72.7 | Serum Cortisol | Radioimmunoassay | Significant association was observed for both males (b = 1.084, SE = 0.021, p = 0.000) and females (b = 1.031, SE = 0.015, p = 0.040) with higher serum cortisol (μg/dl) and MetS. (Adjusted for age and BMI). | 6 |
| Austin-Ketch et al. (60) (USA) |
NCEP-ATP III (2001) | 102 adults Men = 59.8%Women = 40.2% | MetS = 17.7 without MetS = 82.3 | Salivary Cortisol | Chemiluminescence immunoassay | No significant association (p = 0.930) was observed for salivary cortisol and the presence of MetS (F [2, 63] = 0.072; partial η (= 0.002). (Non-adjusted) Significance difference (p = 0.05) was observed in mean diurnal AUC values between males with MetS and males without MetS. (Non-adjusted). |
8 |
| Bengtsson et al. (61) (Sweden) |
NCEP-ATP III (2001) |
175 adults Men = 48%Women = 52% | MetS = 16.6 without MetS = 83.4 | Salivary Cortisol | Radioimmunoassay | Significant association (p = 0.02) was observed for higher salivary cortisol awakening response percentage (CAR%) for women with MetS (CAR% = 91.4 [17.0 nmol/L] in comparison to men without MetS (CAR% = 38.5[13.1nmol/L]. (Non-adjusted). | 8 |
|
62
(Taiwan) |
AHA/NHLBI | 585 adults Men = 100% | MetS = 33.3 without MetS = 66.7 | DHEAS | Electrochemiluminescence | Significant (p >0.001) association was observed for higher DHEAS between participants with MetS (3.1 ± 2.0 µmol/L) in comparison with participants without MetS (2.4 ± 1.6 µmol/L). (Non-adjusted). | 8 |
| Phillips et al. (63) (United Kingdom) |
IDF | 4255 adults Men = 100% |
MetS = 13.7 without MetS = 86.3 |
Serum cortisol | Radioimmunoassay | No significant association between serum cortisol and MetS was observed (OR = 1.31; 95%CI: 0.98, 1.76; p = 0.07). (Adjusted for age, place of service, ethnicity, marital status, alcohol consumption, smoking, household income and education grade). | 8 |
| DHEAS | Radioimmunoassay | Higher DHEAS concentrations significantly reduced MetS (OR = 0.56, 95% CI 0.46–0.69, p <0.001). (Adjusted for age, place of service, ethnicity, marital status, alcohol consumption, smoking, household income and education grade). |
|||||
| 2. Case–control studies | |||||||
| Garcez et al. (64) (Brazil) |
JIS | 250 adults Women = 100% |
MetS = 20.0 Controls = 80.0 |
Salivary Cortisol | Chemiluminescence immunoassay | No significant associations were observed for daily circadian cortisol changes between participants with MetS and participants without MetS. awakening cortisol levels: MetS (5.37 ± 4.10 nmol/l) vs without MetS (6.03 ± 5.39 nmol/l, p = 0.57), salivary cortisol levels after work: MetS (2.78 ± 2.87 nmol/l) vs without MetS (2.78 ± 2.85 nmol/l, p = 0.93). (Adjusted for age). |
9 |
| Kazakou et al. (65) (Greece) |
AHA/NHLBI | I59 adults Men = 42.1% Women = 57.9% |
MetS = 54.1 Controls = 45.9 |
Serum cortisol | Chemiluminescence immunoassay | No significant association (p >0.05) was observed for serum cortisol between participants with MetS (466.27 ± 146.23 nmol/L) and participants without MetS (455.24 ± 168.30 nmol/L). (Non-adjusted). | 9 |
| Özçelik et al. (66) (Turkey) |
NCEP-ATP III (2001) |
55 adults. Women = 100% |
MetS = 63.6 Controls = 36.4 |
UFC | Immunoenzymatic colorimetric method | Significant association (p <0.05) was observed for lower serum DHEAS between participants with MetS (116 [68.00–152.00] µg/dl) in comparison without MetS (166.50[138.00–213.75 µg/dl]). (Non-adjusted). | 7 |
| Serum cortisol | Immunoenzymatic colorimetric method | Significant association (p <0.001) was observed for higher serum cortisol between participants with MetS (18.77 [9.60–25.41] µg/dl) in comparison with participants without MetS (12.71 [11.29–15.70] µg/dl). (Non-adjusted). | |||||
| DHEAS | Electrochemiluminescence | Significant association (p <0.05) was observed for lower DHEAS between participants with MetS (116 [68.00–152.00] µg/dl) in comparison without MetS (166.50 [138.00–213.75] µg/dl). (Non-adjusted). | |||||
*Key: IDF, International Diabetes Federation; AHA/NHLBI, American Heart Association/National Heart, Lung, and Blood Institute; MetS, Metabolic syndrome; DHEAS, Dehydroepiandrosterone sulfate; NCEP-ATP III, National Cholesterol Education Program’s Adult Treatment Panel III; UFC, Urinary free cortisol; CAR%, cortisol awakening response percentage; JIS, Joint Interim Statement; HCC, hair cortisol concentrations; SC, Serum cortisol; BMI, Body mass index; WC, Waist circumference; JBI, The Joanna Briggs checklist for analytical cross-section studies and case–control studies.
**Additional data was obtained from the Authors.
2.5 Assessment of study methodological quality
The Joanna Briggs Institute (JBI) critical appraisal tool was used to assess the methodological quality of the included studies (67). The questions in the JBI included: (a) a clear description of study objectives; (b) clear description of inclusion and exclusion criteria for study participants; (c) a clear description of the population; (d) clearly describing the method of measurement of exposure; (e) characteristics of the mediator/moderator and outcome variables reported; (f) identifying and measuring potential confounders; (g) control of confounders; and (h) appropriate statistics used in answering study objectives. The JBI score assigns a maximum of 8 points (for cross-sectional studies) and 10 points (for case–control studies), indicating the highest study quality. For this systematic review, overall points of ≥5 for all cross-sectional and overall points of ≥6 for case–control studies were considered sufficient for inclusion. The studies were independently reviewed by one author (FO). This JBI tool has been used in other studies, making it a relevant tool to be used in this systematic review (68, 69).
2.6 Assessment of study quality control
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Checklist was used for study quality control assessment (70). The checklist contains a total of 22 items, which evaluated the reporting of each study’s title, abstract, introduction, methodology, results, and discussion. One author (FO) evaluated the studies for each item on the STROBE checklist as “yes,” “no,” or “not applicable” and calculated the number and percentage (%) of the included studies matching each item on the STROBE checklist. The completeness of reporting (COR) was calculated from the formula: COR (%) = (yes ÷ (yes + no) × 100) for each included study. A COR score of (if 0%–49% of items were met) was considered low, (if 50%–74% of items were met) was considered “moderate,” and (if ≥75% of items were met) was considered “high.” A similar protocol has been used in a study published elsewhere (12).
2.7 Statistical methods
All studies derived from the two databases that provided data on primary mediators of AL and MetS were considered eligible for analysis using Microsoft Excel version 16.63.1 (Microsoft Corporation, Redmond-Washington, USA). The included studies reported the associations between primary mediators of AL and MetS, usually using descriptive statistics (i.e., means and standard deviations) and inferential statistical models. Descriptive statistics, mainly frequency distributions, were used to report all the pooled measurements of the primary mediators of AL (i.e., salivary cortisol, serum cortisol, urinary cortisol (UFC), hair cortisol concentration (HCC), DHEAS, urinary EPI, and urinary NE) and their association with MetS.
3 Results
3.1 Main characteristics of studies included
The search of the databases (PubMed, n = 173 and Web of Science, n = 510) yielded 683 studies. Additional records retrieved from other sources through the Cochrane library and cross-references yielded 87 studies, resulting in an overall 770 studies. Out of these studies, 57 studies were assessed for eligibility after excluding 703 studies. Only 21 studies were considered for this systematic review after excluding 24 studies based on the study population and 21 studies based on study design. The included studies had a total number of participants (n = 10,666) with ages between 18 and 75 years. The sample size ranged from 37 to 4,225 participants within different populations (i.e., MetS, without MetS, workers, veterans, overweight, and obese). The included studies were published on different continents, consisting of: Europe (n = 8), Asia (n = 5), North America (n = 3), South America (n = 3), Africa (n = 1), and Australia (n = 1). Eighteen studies were cross-sectional, and three were case–control studies. Studies that reported cortisol as the primary mediator of AL were grouped into long-term cortisol measures (i.e., urinary cortisol [UFC] and hair cortisol concentration [HCC]) and short-term cortisol measures (i.e., salivary and serum cortisol). From the included studies, four studies measured UFC, two studies measured HCC, nine studies measured salivary cortisol, and nine studies measured serum cortisol. DHEAS was measured in six studies as a primary mediator of AL. Urinary EPI and urinary NE were measured in one study as primary mediators of AL (see Table 1 for details).
3.2 Main results
The results are reported based on the different primary mediators of AL and its association with MetS. Afterwards, the results are summarized with the findings. Two studies (55, 57) found no significant associations, whereas two studies (51, 66) found significant associations between UFC and MetS. Two studies (46, 47) found significant associations between HCC and MetS. Four studies (46, 48, 60, 64) found no significant association, whilst six studies (51–53, 56, 60, 61) found significant associations between salivary cortisol and MetS. Six studies (46, 49, 54, 58, 63, 65) found no significant associations, but four studies (51, 58, 59, 66) found significant associations between serum cortisol and MetS. Two studies (50, 55) found no significant associations, while three studies (62, 63, 66) found significant associations between DHEAS and MetS. One study (55) found no significant associations between urinary EPI, urinary NE, and MetS.
3.3 Summary of results
Regarding cortisol, it can be summarized that UFC (12-h or 24-h) showed a significant association with MetS in 50% of the studies, and HCC showed a significant association with MetS in 100% of the studies. Short-term measures including serum cortisol showed a significant association with MetS in 40% of the studies, and salivary cortisol showed a significant association with MetS in 60% of the studies, respectively. In 60% of the studies, DHEAS showed a significant association with MetS. Both urinary EPI and NE (12-h) showed no significant association with MetS in 100% of the studies.
4 Assessment heterogeneity
4.1 Metabolic syndrome diagnoses criteria
There were variations in the diagnosis of MetS in the included studies. Six studies used the “Third National Cholesterol Education Program and Adult Treatment Panel” (NCEP-ATP III) 2001 criteria, and three studies used the 2004 criteria. Seven studies used the “International Diabetes Federation” (IDF) criteria. Three studies used the 2005 criteria of the “American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI). One study used the “Joint Interim Statement” (JIS) criteria. One study used both IDF and NCEP-ATP III (2001) criteria. The different institutional criteria used in the diagnosis of MetS are explained in detail in a study by Alberti et al. (2).
4.2 Assessment criteria for primary allostatic load markers: Measurement of cortisol
UFC (12-h or 24-h) was measured in four studies with chemiluminescence immunoassay (51), direct immunoenzymatic colorimetric method (55), high-performance chromatography (57), and electrochemiluminescence immunoassay (66). HCC was measured in two studies using enzyme-linked immunoassay (47) and high-performance chromatography (46). Salivary cortisol was measured in nine studies with enzyme-linked immunoassay (46), chemiluminescence immunoassay (51, 60, 64), radioimmunoassay (RIA) (48, 52, 61), electrochemiluminescence immunoassay (53), and competitive enzyme immunoassay (56). Serum cortisol was measured in eight studies with enzyme-linked immunoassay (46, 49, 51, 54, 65), RIA (58, 59), and electrochemiluminescent immunoassay (66).
4.3 Assessment criteria for primary allostatic load markers: Measurement of DHEAS
DHEAS was measured in five studies with the turboFlow-LC-MS/MS method (50), chemiluminescent immunoassay (55, 66), electrochemiluminescent immunoassay (62), and RIA (63).
4.4 Assessment criteria for primary allostatic load markers: Measurement of epinephrine and norepinephrine
In one study, urinary EPI and urinary NE (12-h) were measured using a 2-CAT enzyme immunoassay read on a Dynex MRX 96-well plate reader (55).
4.5 Study methodological quality
Applying the JBI tool, twenty studies representing 95.2% were judged very well to excellent (≥6 to ≥10) while one study representing 4.8% was judged fairly good (≥5). The summary of scores of the included studies is presented in Tables 2 , 3 .
Table 2.
Joanna Briggs Institute (JBI) scores for cross-sectional studies.
| Study | Participants and setting described in detail,including similarity of controls | Criteria for inclusion clearly defined and exposures similarly measured | Exposure measured invalid and reliable way | Objective, standard criteria used for measurement of condition | Confounding factors identified | Strategies to deal with confounding factors stated | Outcomes measured invalid and reliable way | Appropriatestatistical analysis used? |
|---|---|---|---|---|---|---|---|---|
| Mazgelytė et al. (46) (Lithuania) | + | + | + | + | + | + | + | + |
| Lehrer et al. (47) (USA) | + | + | + | − | + | + | + | + |
| Martins et al. (48) (Brazil) | + | − | + | + | − | − | + | + |
| Udenze et al. (49) (Nigeria) | + | + | + | + | − | − | + | + |
| Damgaard-Olesen et al. (50) (Denmark) | + | + | + | + | + | + | + | + |
| Constantinopoulos et al. (51) (Greece) | + | + | + | + | − | − | + | + |
| Corbalán-Tutau et al. (52) (Spain) | + | + | + | + | + | + | + | + |
| Almadi et al. (53) (Australia) | + | + | + | + | + | + | + | + |
| Fabre et al. (54) (Argentina) | + | + | + | + | + | + | + | + |
| Mattei et al. (55) (USA) | + | + | + | + | + | + | + | + |
| Jang et al. (56) (Korea) | + | + | + | + | + | + | + | + |
| Baudrand et al. (57) (Chile) | + | + | + | + | + | + | + | + |
| Esteghamati et al. (58) (Iran) | + | + | + | + | − | − | + | + |
| Park etal. (59) (Korea) | + | + | + | + | − | − | + | + |
| Austin-Ketch et al. (60) (USA) | + | + | + | + | + | + | + | + |
| Bengtsson et al. (61) (Sweden) | + | + | + | + | + | + | + | + |
| Chen et al. (62) (Taiwan) | + | + | + | + | + | + | + | + |
| Phillips et al. (63) (United Kingdom) | + | + | + | + | + | + | + | + |
Table 3.
Joanna Briggs Institute (JBI) scores for Case–control studies.
| Study | Group comparable in the presence of disease in cases and absence of diseases in controls | Cases and controls matched appropriately | Same criteria used for identifying cases and controls | Exposure measured invalid and reliable way | Exposure measured in same way as cases and controls | Confounding factors identified | Strategies to deal with confounding factors stated | Outcomes measured in standard, valid and reliable way for cases and controls | Exposure period of interest long enough to be meaningful | Appropriate statistical analysis used? |
|---|---|---|---|---|---|---|---|---|---|---|
| Garcez etal. (64) (Brazil) |
+ | + | + | + | + | + | + | + | – | + |
| Kazakou etal. (65) (Greece) | + | + | + | + | + | + | + | + | – | + |
| Özçelik etal. (66) (Turkey) | + | + | + | + | + | − | − | + | – | + |
4.6 Study quality control
The STROBE Checklist for study quality control assessment was performed on the 21 included studies. From the included studies, one study had moderate score (COR = 50%–74%), and twenty studies had high score (COR = ≥75%). The mean COR score for the included studies was 87.0 ± 6.4% suggesting a higher study quality control (see Table 4 ).
Table 4.
STROBE Statement—A checklist of items and the completeness of reporting score (COR %) for the included studies (n = 21).
| Item No. | Recommendation | Criteria Met (N, %) Yes No N/A | |||
|---|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 21(100) | 0 (0) | 0 (0) |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 21 (100) | 0 (0) | 0 (0) | ||
| Introduction | |||||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 21 (100) | 0 (0) | 0 (0) |
| Objectives | 3 | State-specific objectives, including any prespecified hypotheses | 21 (100) | 0 (0) | 0 (0) |
| Methods | |||||
| Study design | 4 | Present key elements of study design early in the paper | 21 (100) | 0 (0) | 0 (0) |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 21 (100) | 0 (0) | 0 (0) |
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case–control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
N/A 3 (100) 16 (76.1) |
N/A 0 (0) 2 (9.6) |
N/A 0 (0) 3 (14.3) |
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case–control study—For matched studies, give matching criteria and the number of controls per case |
3 (100) | 0 (0) | 0 (0) | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 19 (90.4) | 2 (9.6) | 0(0) |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 21 (100) | 0 (0) | 0 (0) |
| Bias | 9 | Describe any efforts to address potential sources of bias | 19(90.4) | 2 (9.6) | 0 (0) |
| Study size | 10 | Explain how the study size was arrived at | 20 (95.2) | 1 (4.8) | 0 (0) |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 21 (100) | 0 (0) | 0 (0) |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 20 (95.2) | 1 (4.8) | 0 (0) |
| (b) Describe any methods used to examine subgroups and interactions | 21 (100) | 0 (0) | 0 (0) | ||
| (c) Explain how missing data were addressed | 3 (14.3) | 2 (9.6) | 16 (76.1) | ||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case–control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy |
20 (95.2) | 1 (4.8) | 0 (0) | ||
| ( e ) Describe any sensitivity analyses | 20 (95.2) | 0 (0) | 1 (4.8) | ||
| Results | |||||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed | 21 (100) | 0 (0) | 0 (0) |
| (b) Give reasons for non-participation at each stage | 5 (23.8) | 3 (14.3) | 13 (61.9) | ||
| (c) Consider use of a flow diagram | 0 (0) | 21 (100) | 0 (0) | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 19 (90.4) | 1 (4.8) | 1 (4.8) |
| (b) Indicate number of participants with missing data for each variable of interest | 2 (9.6) | 1 (4.8) | 18 (85.6) | ||
| (c) Cohort study—Summarize follow-up time (e.g., average and total amount) | N/A | N/A | N/A | ||
| Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | N/A | N/A | N/A |
| Case–control study—Report numbers in each exposure category, or summary measures of exposure | 3(100) | 0 (0) | 18 (0) | ||
| Cross-sectional study—Report numbers of outcome events or summary measures | 19 (90.4) | 2 (9.6) | 0 (0) | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 19 (90.4) | 2 (9.6) | 0 (0) |
| (b) Report category boundaries when continuous variables were categorized | 13 (61.9) | 1 (4.8) | 7 (33.3) | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | 13 (61.9) | 5 (23.8) | 3 (14.3) | ||
| Other analyses | 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses | 21 (100) | 0 (0) | 0 (0) |
| Discussion | |||||
| Key results | 18 | Summarize key results with reference to study objectives | 21 (100) | 0 (0) | 0 (0) |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 11 (52.4) | 10 (47.6) | 0 (0) |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 15 (71.4) | 6 (28.6) | 0 (0) |
| Generalisability | 21 | Discuss the generalizability (external validity) of the study results | 9 (42.9) | 12 (57.1) | 0 (0) |
| Other information | |||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 13 (61.9) | 7 (33.3) | 1 (4.8) |
| Completeness of Reporting mean of the 21 studies (%) | 87.0 ± 6.4% | ||||
"*" refer to Vandenbroucke et al., (71).
5 Discussion
This systematic review examines the association between primary mediators of AL and the presence of MetS. The systematic review further highlights psychosocial, environmental, anthropometric, and socio-demographic factors influencing the association between the primary mediators of AL and MetS. Regarding the primary AL mediator cortisol, it is to be noted that MetS is associated with higher HCC and in some studies further with UFC, serum cortisol and salivary cortisol. In addition, the other HPA axis-related marker, DHEAS, showed a significant association with MetS. On the other hand, regarding primary mediators of the autonomic nervous system, there is no significant association between urinary EPI, urinary NE, and MetS. The findings of the current systematic review demonstrate that chronic stress leading to higher cortisol levels and low DHEAS levels may be associated with a hyperresponsive HPA axis. In the pathogenesis of MetS, this occurs.
Also, the two studies (51, 66) that reported an association between UFC and MetS involved participants with a body mass index (BMI = 39.3–52.4 kg/m2). In contrast, the other two studies (55, 57) that reported no association between UFC and MetS had participants with a BMI of 29.2–32.9 kg/m2. The results indicate that adults with higher BMI or obesity are most likely to have MetS and a hyperresponsive HPA axis due to increased cortisol levels. The results confirm a previous systematic review that reported that obesity appears to be related to a hyperresponsive HPA axis (72). An increase in body weight may lead to chronic low-grade inflammation, which may provoke an increased production of pro-inflammatory cytokines. The increased production of pro-inflammatory cytokines may cause chronic HPA axis activation, leading to visceral obesity and MetS (73). The discrepancies in the findings on the association between UFC and MetS in this systematic review may be attributed to the ethnicity variation in the diagnosis of MetS. This may be due to the varying measurement techniques employed in the various studies. Alberti et al. (2) reported different ethnicity variations in the diagnosis of MetS. Also, none of the included studies used the gold standard in measuring UFC, i.e., 24-h UFC measured by liquid chromatography with tandem mass spectrometry (LC-MS/MS) (74). Hence, longitudinal research focusing on the gold standard for measuring UFC and its association with MetS across different ethnicities is vital for understanding chronic stress’s effects on metabolic abnormalities.
The literature review showed inconsistent findings based on sex for the association between salivary cortisol and serum cortisol with MetS. Similar findings based on cortisol and sex have been reported in another systematic review (72). Significant associations between higher serum cortisol (59) and higher cortisol awakening response (CAR) (61) were found for both men and women with MetS. Bengtsson et al. (61) further reported an association between CAR and depressive symptoms in women. CAR is the measure of the dynamics of the HPA axis response upon awakening (75). A dampened CAR shows impaired HPA axis reactivity and has been suggested to be associated with metabolic abnormalities (75, 76). On the contrary, Esteghmati et al. (58) found only an association between serum cortisol and MetS in men.This shows that cortisol is a key marker in the stress response in both men and women. This calls for future research to study stress effects on HPA axis dysregulation and metabolic abnormalities in both sexes. Additionally, the literature review found mixed findings for the association between salivary and serum cortisol and MetS in workers. The studies in poultry workers (64) and police officers (60) found no association between salivary cortisol and MetS. In contrast, Almadi et al. (53) found associations between salivary cortisol and MetS in different workers (i.e., veterinary, agricultural, textile, and poultry industries). Also, the only study (63) that measured serum cortisol in veterans of the Vietnam-era USA army found no association with MetS. Notably, a previous systematic review reported that the effects of job strain and MetS appear to be significant (77). This shows that different job strain may affect the neuroendocrine systems differently in the pathogenesis of MetS. Hence, workplace health promotion programs geared toward stress management are needed to prevent the adverse effects of job strain on the neuroendocrine system of workers (78).
In this systematic review, some studies (50, 55) reported no associations between DHEAS and MetS, while others did (62, 63, 66). Furthermore, Chen et al. (62) found that participants with MetS had a higher DHEAS (3.1 ± 2.0 µmol/L) as compared to participants without MetS (2.4 ± 1.6 µmol/L). This could be due to steroid biosynthetic defects of the adrenal glands or functional adrenal hyperplasia, and age-related changes in the adrenal secretory pattern of the participants (Age = 67.8 ± 8.4) employed in their study (79). DHEAS declines with age and may lead to age-specific diseases such as obesity and MetS (44). This age-related decline in DHEAS is attributed to a mechanism termed “adrenopause” (80). There are limited studies investigating the association between DHEAS and MetS. Hence, the interplay between DHEAS and MetS warrants further study.
The only study (55) that reported on urinary NE and urinary EPI found no significant association with MetS. Foremost, Zouhal et al. (40) demonstrated that increased levels of catecholamines lead to lipolytic effects on visceral fats by β1- and β2-adrenoceptors. Conversely, β-adrenergic blocking drugs inhibit EPI stimulation, leading to impaired glucose metabolism, hyperglycemia, and insulin resistance (41). While most of the NE is secreted by the sympathetic nerve endings, the adrenal glands secrete EPI (81). Thus, these catecholamines, which play roles under stress conditions to foster thermogenesis and secretion of insulin, may operate in a divergent fashion in the pathogeneses of MetS (41). From a research perspective, measuring 12-h urine collections for EPI and NE may be labor-intensive and impractical due to poor adherence (14). These findings should be interpreted with caution due to insufficient data.
Most studies used immunoassays for the measurement of cortisol. It should be noted that urine contains conjugated cortisol and other metabolites (82). Assessing UFC and salivary cortisol may lead to cross-reactivity of the antibodies in the immunoassays with other metabolites in urine and steroids in saliva (82, 83). Serum cortisol may not reflect the unbound (free) cortisol levels due to changes in albumin or cortisol binding globulin levels (74). Hence, using the LC-MS/MS to measure 24-h urinary cortisol is the gold standard (74). Mass spectrometry provides reliable cortisol measurement outcomes and prevents cross-reactivity of metabolites (74, 82, 84). Although Alberti et al. (2) released the joint interim statement concerning the diagnosis of MetS, only one study (64) used the joint interim statement criteria for the diagnosis of MetS in the included studies. Thus, caution should be taken when interpreting these results.
5.1 Strengths and limitations
This is the first systematic review to be conducted on the association between primary mediators of AL and MetS using cross-sectional and case–control studies. The large sample size and different populations in the included studies broaden the perspective on how the primary mediators of AL are associated with MetS. Despite these strengths, there are limitations to be reported. The cross-sectional data may prevent the cause–effect relationship between the primary mediators of AL and MetS at the time of measurement due to modifications of these mediators in the long term. The included studies had a wide difference in their methodologies. Most studies used different measurement techniques in measuring the primary mediators of AL, especially cortisol. This makes it difficult to make vivid comparisons and generalizations. Also, the included studies employed different institutional criteria for the diagnosis of MetS. This creates heterogeneity in the diagnosis of MetS. These factors could not be controlled in this systematic review. Additionally, only studies in English were included, which could have omitted potential studies published in other languages for inclusion.
6 Conclusion
The present systematic review revealed that there is a tendency for an association between higher UFC, HCC, serum cortisol, salivary cortisol, and lower DHEAS with MetS. There is no association between urinary NE and urinary EPI with MetS. Different assays for measuring the primary mediators of AL and the association of MetS may yield different outcomes. Research focusing on the standardization of measurement protocols for the primary mediators of AL would be vital for uniformity, comparability, and generalization. It is helpful to identify a cluster of biomarkers from the MetS diagnosis that best reflects the primary mediators of AL in order to foster preventive measures for individuals with altered levels of primary mediators. Future studies focusing on longitudinal data are warranted for clarification and understanding of the association between the primary mediators of AL and MetS.
Data availability statement
The datasets generated for this study are available upon reasonable request to the corresponding author.
Author contributions
FO and AB conceived the research question. FO wrote the first draft of the manuscript with the support of AB. FO, AB and P-MW discussed the results. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The funder does not influence data collection, analysis, and interpretation or writing of the manuscript. The authors declare that they have no competing interests. The paper was funded by the Open Access Publishing Fund by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)-Projektnummer 491466077. The present study was supported by a scholarship from DAAD (grant-number: 57552340) to Francis Osei.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.946740/full#supplementary-material
References
- 1. Thaman RG, Arora GP. Metabolic syndrome: Definition and pathophysiology–the discussion goes on. J Phys Pharm Adv (2013) 3(3):48–56. doi: 10.5455/jppa.20130317071355 [DOI] [Google Scholar]
- 2. Alberti K, Eckel R, Grundy S, Zimmet P, Cleeman J, Donato K, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 3. Kim SH, Abbasi F. Myths about insulin resistance: tribute to Gerald reaven. Endocrinol Metab (2019) 34(1):47–52. doi: 10.3803/EnM.2019.34.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult US population 1999–2010. J Am Coll Cardiol (2013) 62(8):pp.697–703. doi: 10.1016/j.jacc.2013.05.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China health and nutrition survey in 2009. Prev Med (2013) 57(6):867–71. doi: 10.1016/j.ypmed.2013.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vishram JK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jørgensen T, et al. Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in europeans. the MORGAM prospective cohort project. PloS One (2014) 9(9):e107294. doi: 10.1371/journal.pone.0128848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for metabolic syndrome in Asian indians: A community study from urban Eastern India. J Cardiovasc Dis Res (2012) 3(3):204–11. doi: 10.4103/0975-3583.98895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aekplakorn W, Satheannoppakao W, Putwatana P, Taneepanichskul S, Kessomboon P, Chongsuvivatwong V, et al. Dietary pattern and metabolic syndrome in Thai adults. J Nutr Metab (2015):468759. doi: 10.1155/2015/468759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rochlani Y, Pothineni NV, Mehta JL. Metabolic syndrome: does it differ between women and men? Cardiovasc Drugs Ther (2015) 29(4):329–38. doi: 10.1007/s10557-015-6593-6 [DOI] [PubMed] [Google Scholar]
- 10. Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care (2010) 33(11):2457–61. doi: 10.2337/dc10-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Zhai Y, Zhao J, He H, Li Y, Liu Y, et al. Impact of metabolic syndrome and it's components on prognosis in patients with cardiovascular diseases: a meta-analysis. Front Cardiovasc Med (2021) 8:704145. doi: 10.3389/fcvm.2021.704145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suvarna B, Suvarna A, Phillips R, Juster RP, McDermott B, Sarnyai Z. Health risk behaviours and allostatic load: a systematic review. Neurosci Biobehav Rev (2020) 108:694–711. doi: 10.1016/j.neubiorev.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 13. McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology (2016) 41(1):3–23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs (2012) 14(4):311–46. doi: 10.1177/1099800412455688 [DOI] [PubMed] [Google Scholar]
- 15. McEwen BS. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry (2017) 74(6):551–2. doi: 10.1001/jamapsychiatry.2017.0270 [DOI] [PubMed] [Google Scholar]
- 16. Ellis BJ, Del Giudice M. Beyond allostatic load: Rethinking the role of stress in regulating human development. Dev Psychopathol (2014) 26(1):1–20. doi: 10.1017/S0954579413000849 [DOI] [PubMed] [Google Scholar]
- 17. Renaville R, Hammadi M, Portetelle D. Role of the somatotropic axis in the mammalian metabolism. Domest Anim Endocrinol (2002) 23(1-2):351–60. doi: 10.1016/S0739-7240(02)00170-4 [DOI] [PubMed] [Google Scholar]
- 18. Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology.Fisher S, Reason J. (Eds.), Handbook Life stress, cognition Health (1988) (New York: John Wiley & Sons; ):629–49. [Google Scholar]
- 19. Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, et al. Beyond the stress concept: Allostatic load–a developmental biological and cognitive perspective. Developmental Psychopathology (eds and Cicchetti D, Cohen DJ. (2015), 578–628 (New Jersey: John Wiley & Sons Inc; ). doi: 10.1002/9780470939390.ch14 [DOI] [Google Scholar]
- 20. McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Arch Internal Med (1993) 153(18):2093–101. doi: 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- 21. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci (2001) 98(8):4770–5. doi: 10.1073/pnas.081072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med (2012) 74(1):75–83. doi: 10.1016/j.socscimed.2011.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun J, Wang S, Zhang JQ, Li W. Assessing the cumulative effects of stress: The association between job stress and allostatic load in a large sample of Chinese employees. Work Stress (2007) 21(4):333–47. doi: 10.1080/02678370701742748 [DOI] [Google Scholar]
- 24. Goldman N, Turra CM, Glei DA, Lin YH, Weinstein M. Physiological dysregulation and changes in health in an older population. Exp gerontol (2006) 41(9):862–70. doi: 10.1016/j.exger.2006.06.050 [DOI] [PubMed] [Google Scholar]
- 25. Walker FR, Pfingst K, Carnevali L, Sgoifo A, Nalivaiko E. In the search for integrative biomarker of resilience to psychological stress. Neurosci Biobehav Rev (2017) 74:310–20. doi: 10.1016/j.neubiorev.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 26. Nackley BB, Friedman BH. Only time will tell: Acute stress response patterns with time series analysis. Int J Psychophysiol (2021) 166:160–5. doi: 10.1016/j.ijpsycho.2021.05.006 [DOI] [PubMed] [Google Scholar]
- 27. Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol (2017) 68:3–33. doi: 10.1016/j.reprotox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci (2018), 127:1–7. doi: 10.3389/fnbeh.2018.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wester VL, van Rossum EF. Obesity and metabolic syndrome: a phenotype of mild long-term hypercortisolism? In: The hypothalamic-Pituitary-Adrenal axis in health and disease. Cham: Springer; (2017). p. 303–13. [Google Scholar]
- 30. Wester VL, Staufenbiel SM, Veldhorst MA, Visser JA, Manenschijn L, Koper JW, et al. Long-term cortisol levels measured in scalp hair of obese patients. Obesity (2014) 22(9):1956–8. doi: 10.1002/oby.20795 [DOI] [PubMed] [Google Scholar]
- 31. Pluchino N, Drakopoulos P, Bianchi-Demicheli F, Wenger JM, Petignat P, Genazzani AR. Neurobiology of DHEA and effects on sexuality, mood and cognition. J Steroid Biochem Mol Biol (2015) 145:273–80. doi: 10.1016/j.jsbmb.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 32. Karbowska J, Kochan Z. Effects of DHEA on metabolic and endocrine functions of adipose tissue. Hormone Mol Biol Clin Invest (2013) 14(2):65–74. doi: 10.1515/hmbci-2013-0009 [DOI] [PubMed] [Google Scholar]
- 33. McNelis JC, Manolopoulos KN, Gathercole LL, Bujalska IJ, Stewart PM, Tomlinson JW, et al. Dehydroepiandrosterone exerts anti-glucocorticoid action on human preadipocyte proliferation, differentiation, and glucose uptake. Am J Physiol-Endocrinol Metab (2013) 305(9):E1134–44. doi: 10.1152/ajpendo.00314.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maggio M, De Vita F, Fisichella A, Colizzi E, Provenzano S, Lauretani F, et al. DHEA and cognitive function in the elderly. J Steroid Biochem Mol Biol (2015) 145:281–92. doi: 10.1016/j.jsbmb.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 35. da Silva GP, de Melo JT, Monteiro FOB, Ferreira AKP, Carneiro LA, Takeshita RS. Validation of a dehydroepiandrosterone-sulfate assay in three platyrrhine primates (Alouatta caraya, aotus azarae infulatus, and sapajus apella). Int J Primatol (2021) 42(5):722–36. doi: 10.1007/s10764-021-00239-x [DOI] [Google Scholar]
- 36. Stárka L, Dušková M, Hill M. Dehydroepiandrosterone: a neuroactive steroid. J Steroid Biochem Mol Biol (2015) 145:254–60. doi: 10.1016/j.jsbmb.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 37. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci (2006)8(4):383–95. doi: 10.31887/DCNS.2006.8.4/ssmith [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol (2016) 6(2):603. doi: 10.1002/cphy.c150015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, Pfeifer K. Targeted insertion of the cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: A new model for studying the developmental distribution of adrenergic cells. Dev Dynamics (2004) 231(4):849–58. doi: 10.1002/dvdy.20188 [DOI] [PubMed] [Google Scholar]
- 40. Zouhal H, Lemoine-Morel S, Mathieu ME, Casazza GA, Jabbour G. Catecholamines and obesity: effects of exercise and training. Sports Med (2013) 43(7):591–600. doi: 10.1007/s40279-013-0039-8 [DOI] [PubMed] [Google Scholar]
- 41. Ziegler MG, Elayan H, Milic M, Sun P, Gharaibeh M. Epinephrine and the metabolic syndrome. Curr Hypertension Rep (2012) 14(1):1–7. doi: 10.1007/s11906-011-0243-6 [DOI] [PubMed] [Google Scholar]
- 42. Guidi J, Lucente M, Sonino N, Fava GA. Allostatic load and its impact on health: a systematic review. Psychother Psychosomatics (2021) 90(1):11–27. doi: 10.1159/000510696 [DOI] [PubMed] [Google Scholar]
- 43. Garcez A, Leite HM, Weiderpass E, Paniz VMV, Watte G, Canuto R, et al. Basal cortisol levels and metabolic syndrome: A systematic review and meta-analysis of observational studies. Psychoneuroendocrinology (2018) 95:50–62. doi: 10.1016/j.psyneuen.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 44. Teixeira CJ, Veras K, de Oliveira Carvalho CR. Dehydroepiandrosterone on metabolism and the cardiovascular system in the postmenopausal period. J Mol Med (2020) 98(1):39–57. doi: 10.1007/s00109-019-01842-5 [DOI] [PubMed] [Google Scholar]
- 45. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):1–11. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mazgelytė E, Mažeikienė A, Burokienė N, Matuzevičienė R, Linkevičiūtė A, Kučinskienė ZA, et al. Association between hair cortisol concentration and metabolic syndrome. Open Med (2021) 16(1):873–81. doi: 10.1515/med-2021-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lehrer HM, Steinhardt MA, Dubois SK, Laudenslager ML. Perceived stress, psychological resilience, hair cortisol concentration, and metabolic syndrome severity: A moderated mediation model. Psychoneuroendocrinology (2020) 113:104510. doi: 10.1016/j.psyneuen.2019.104510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martins CS, Elias D, Colli LM, Couri CE, Souza MCL, Moreira AC, et al. HPA axis dysregulation, NR3C1 polymorphisms and glucocorticoid receptor isoforms imbalance in metabolic syndrome. Diabetes/metabolism Res Rev (2017) 33(3):e2842. doi: 10.1002/dmrr.2842 [DOI] [PubMed] [Google Scholar]
- 49. Udenze IC, Olowoselu OF, Egbuagha EU, Oshodi TA. Thyroid, cortisol and growth hormone levels in adult nigerians with metabolic syndrome. Pan Afr Med J (2017) 26(52):1–7. doi: 10.11604/pamj.2017.26.52.9909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Damgaard–Olesen A, Johannsen TH, Holmboe SA, Søeborg T, Petersen JH, Andersson AM, et al. Reference ranges of 17-hydroxyprogesterone, DHEA, DHEAS, androstenedione, total and free testosterone determined by TurboFlow-LC–MS/MS and associations to health markers in 304 men. Clin Chimica Acta (2016) 454:82–8. doi: 10.1016/j.cca.2015.12.042 [DOI] [PubMed] [Google Scholar]
- 51. Constantinopoulos P, Michalaki M, Kottorou A, Habeos I, Psyrogiannis A, Kalfarentzos F, et al. Cortisol in tissue and systemic level as a contributing factor to the development of metabolic syndrome in severely obese patients. Eur J Endocrinol (2015) 172(1):69–78. doi: 10.1530/EJE-14-0626 [DOI] [PubMed] [Google Scholar]
- 52. Corbalán-Tutau D, Madrid JA, Nicolás F, Garaulet M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol Behav (2014) 123:231–5. doi: 10.1016/j.physbeh.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 53. Almadi T, Cathers I, Chow CM. Associations among work-related stress, cortisol, inflammation, and metabolic syndrome. Psychophysiology (2013) 50(9):821–30. doi: 10.1111/psyp.12069 [DOI] [PubMed] [Google Scholar]
- 54. Fabre B, Grosman H, Mazza O, Nolazco C, Machulsky NF, Mesch V, et al. Relationship between cortisol, life events and metabolic syndrome in men. Stress (2013) 16(1):16–23. doi: 10.3109/10253890.2012.676112 [DOI] [PubMed] [Google Scholar]
- 55. Mattei J, Bhupathiraju S, Tucker KL. Higher adherence to a diet score based on American heart association recommendations is associated with lower odds of allostatic load and metabolic syndrome in Puerto Rican adults. J Nutr (2013) 143(11):1753–9. doi: 10.3945/jn.113.180141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jang YM, Lee EJ, Kim DL, Kim SK, Song KH. The association between midnight salivary cortisol and metabolic syndrome in Korean adults. Diabetes Metab J (2012) 36(3):245–50. doi: 10.4093/dmj.2012.36.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, et al. Increased urinary glucocorticoid metabolites are associated with metabolic syndrome, hypoadiponectinemia, insulin resistance and β cell dysfunction. Steroids (2011) 76(14):1575–81. doi: 10.1016/j.steroids.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 58. Esteghamati A, Morteza A, Khalilzadeh O, Noshad S, Novin L, Nakhjavani M. Association of serum cortisol levels with parameters of metabolic syndrome in men and women. Clin Invest Med (2011) 34(3):E131–7. doi: 10.25011/cim.v34i3.15185 [DOI] [PubMed] [Google Scholar]
- 59. Park SB, Blumenthal JA, Lee SY, Georgiades A. Association of cortisol and the metabolic syndrome in Korean men and women. J Korean Med Sci (2011) 26(7):914–8. doi: 10.3346/jkms.2011.26.7.914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Austin-Ketch TL, Violanti J, Fekedulegn D, Andrew ME, Burchfiel C, Hartley T, et al. Metabolic syndrome and salivary cortisol: Is there dysregulation among a group of active duty urban police officers? Diabetes Metab Syndrome: Clin Res Rev (2010) 4(2):82–8. doi: 10.1016/j.dsx.2010.01.008 [DOI] [Google Scholar]
- 61. Bengtsson I, Lissner L, Ljung T, Rosengren A, Thelle D, Währborg P. The cortisol awakening response and the metabolic syndrome in a population-based sample of middle-aged men and women. Metabolism (2010) 59(7):1012–9. doi: 10.1016/j.metabol.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 62. Chen YC, Chang HH, Wen CJ, Lin WY, Chen CY, Hong BS, et al. Elevated serum dehydroepiandrosterone sulphate level correlates with increased risk for metabolic syndrome in the elderly men. Eur J Clin Invest (2010) 40(3):220–5. doi: 10.1111/j.1365-2362.2009.02248.x [DOI] [PubMed] [Google Scholar]
- 63. Phillips AC, Carroll D, Gale CR, Lord JM, Arlt W, Batty GD. Cortisol, DHEA sulphate, their ratio, and all-cause and cause-specific mortality in the Vietnam experience study. Eur J Endocrinol (2010) 163(2):285–92. doi: 10.1530/EJE-10-0299 [DOI] [PubMed] [Google Scholar]
- 64. Garcez A, Weiderpass E, Canuto R, Lecke SB, Spritzer PM, Pattussi MP, et al. Salivary cortisol, perceived stress, and metabolic syndrome: a matched case-control study in female shift workers. Hormone Metab Res (2017) 49(07):510–9. doi: 10.1055/s-0043-101822 [DOI] [PubMed] [Google Scholar]
- 65. Kazakou P, Kyriazopoulou V, Michalaki M, Ierodiakonou V, Psyrogiannis A, Habeos I. Activated hypothalamic pituitary adrenal axis in patients with metabolic syndrome. Hormone Metab Res (2012) 44(11):839–44. doi: 10.1055/s-0032-1311632 [DOI] [PubMed] [Google Scholar]
- 66. Özçelik E, Uslu S, Kebapçı N, Kara M, Dokumacıoğlu A, Musmul A. Interrelations of serum leptin levels with adrenocorticotropic hormone, basal cortisol and dehydroepiandrosterone sulphate levels in patients with metabolic syndrome. Diabetes Metab Syndrome: Clin Res Rev (2010) 4(1):13–17. doi: 10.1016/j.dsx.2010.01.005 [DOI] [Google Scholar]
- 67. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In:Aromataris E, Munn Z. (Editors) Joanna Briggs institute reviewer's manual(Australia:The Joanna Briggs Institute; ) (2017). https://jbi.global/critical-appraisal-tools. [Google Scholar]
- 68. Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ (2020) 369:m1642. doi: 10.1136/bmj.m1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Med Res (2020) 7(1):1–11. doi: 10.1186/s40779-020-00238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Von Elm E, Althman DG, Egger M, Pocock SJ, Gotzche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines fro reporting observational studies. Br J Med (2007) 335(7624):806–8. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann Intern Med (2007) 147(8):W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 72. Rodriguez ACI, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology (2015) 62:301–18. doi: 10.1016/j.psyneuen.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 73. Marazziti D, Rutigliano G, Baroni S, Landi P, Dell'Osso L. Metabolic syndrome and major depression. CNS spectrums (2014) 19(4):293–304. doi: 10.1017/S1092852913000667 [DOI] [PubMed] [Google Scholar]
- 74. El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva–are our assays good enough? Ann Clin Biochem (2017) 54(3):308–22. doi: 10.1177/0004563216687335 [DOI] [PubMed] [Google Scholar]
- 75. Kuehl LK, Hinkelmann K, Muhtz C, Dettenborn L, Wingenfeld K, Spitzer C, et al. Hair cortisol and cortisol awakening response are associated with criteria of the metabolic syndrome in opposite directions. Psychoneuroendocrinology (2015) 51:365–70. doi: 10.1016/j.psyneuen.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 76. Lamers F, Vogelzangs N, Merikangas KR, De Jonge P, Beekman ATF, Penninx BWJH. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry (2013) 18(6):692–9. doi: 10.1038/mp.2012.144 [DOI] [PubMed] [Google Scholar]
- 77. Watanabe K, Sakuraya A, Kawakami N, Imamura K, Ando E, Asai Y, et al. Work-related psychosocial factors and metabolic syndrome onset among workers: a systematic review and meta-analysis. Obes Rev: an official J the Intern Assoc for the Study of Obesity (2018) 19(11):1557–568. doi: 10.1111/obr.12725 [DOI] [PubMed] [Google Scholar]
- 78. Glazer S, Liu C. Work, stress, coping, and stress management. In: Oxford Research encyclopedia of psychology (2017) (Oxford, England; Oxford University Press; ). [Google Scholar]
- 79. Shojaie M, Rajpout MY, Abtahian A, Pour AE, Ghobadifar MA, Akbarzadeh A. Dehydroepiandrosterone sulfate as a risk factor for premature myocardial infarction: a comparative study. Korean J Family Med (2015) 36(1):1–9. doi: 10.4082/kjfm.2015.36.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hornsby PJ. Adrenopause. In: Conn's handbook of models for human aging. (Elsevier B.V. Amsterdam, The Netherlands: Academic Press; ) (2018). p. 131–7. [Google Scholar]
- 81. Chokroverty S, Bhat S. Functional neuroanatomy of the peripheral autonomic nervous system. In: Autonomic nervous system and sleep. Cham: Springer; (2021). p. 19–28. [Google Scholar]
- 82. Casals G, Hanzu FA. Cortisol measurements in cushing's syndrome: immunoassay or mass spectrometry? Ann Lab Med (2020) 40(4):285–96. doi: 10.3343/alm.2020.40.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aranda G, Careaga M, Hanzu FA, Patrascioiu I, Ríos P, Mora M, et al. Accuracy of immunoassay and mass spectrometry urinary free cortisol in the diagnosis of Cushing’s syndrome. Pituitary (2016) 19(5):496–502. doi: 10.1007s11102-016-0730-5 [DOI] [PubMed] [Google Scholar]
- 84. Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol (2015) 173(2):D1–12. doi: 10.1530/EJE-15-0338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available upon reasonable request to the corresponding author.