Abstract
Background
Half of older people are prescribed unnecessary/inappropriate medications that are not routinely deprescribed in hospital hence there is a need for deprescribing trials. We aimed to develop a Core Outcome Set (COS) for deprescribing trials for older people under the care of a geriatrician during hospital admission.
Methods
We developed a list of potentially relevant outcomes from the literature. Using a two-round Delphi survey of stakeholder groups representing older people and carers, hospital clinicians, hospital managers, and ageing/deprescribing researchers, each outcome was scored according to Grading of Recommendations Assessment, Development and Evaluation, followed by two consensus workshops to finalise the COS.
Results
Two hundred people completed Round 1 and 114 completed Round 2. Representing all stakeholder groups, 10 people participated in workshop 1 and 10 in workshop 2. Six outcomes were identified as most important, feasible and acceptable to collect in a trial: number of prescribed medicines stopped; number of prescribed medicines with dosage reduced; quality of life; mortality; adverse drug events and number of hospital stays. Three other outcomes were identified as important, but currently too burdensome to collect: number of potentially inappropriate medicines prescribed; burden from medication routine; and medication-related admissions to hospital.
Conclusions
A COS represents the minimum outcomes that should be collected and reported. Whilst uncommon practice for COS development, the value of considering outcome collection feasibility is demonstrated by the removal of three potential outcomes that, if included, may have compromised COS uptake due to challenges with collecting the data.
Keywords: deprescribing, core outcome set, older people, hospital
Key Points
A Core Outcome Set (COS) is the minimum outcomes that all trials within a particular clinical area should measure.
Nine outcomes were identified as important to measure in trials about deprescribing in hospital for older people.
Six outcomes were selected as the most important, acceptable and feasible to measure and included in the COS.
A further three outcomes were identified as important but are currently not feasible to collect in the context of trials.
Background
Approximately 50% of older people are prescribed one or more medicines where the risk of harm outweighs chances of benefit, predisposing them to adverse outcomes including morbidity, hospitalisation, and mortality [1]. There is an expectation from older people and carers that during a hospital admission, any medicines deemed to have an unfavourable balance of benefit and harm are stopped before harm occurs [2]. This is termed ‘proactive deprescribing’ in contrast to ‘reactive deprescribing’ which is stopping a medicine in response to present harm. Working towards the World Health Organisation target of reducing medication-related harm by 50% [3], interventions to increase proactive deprescribing in hospital are being developed. Such interventions should ideally be rigorously evaluated before implemented in clinical practice. An agreed set of outcomes to measure enables results from trials to be compared, and avoids bias in the selection of trial outcomes that are reported [4].
A Core Outcome Set (COS) is an agreed minimum set of standardised outcomes that should be reported in all trials for a specific clinical area or intervention [4]. A COS is yet to be developed for proactive deprescribing in hospital. Important outcomes may differ between the patient population for whom medicines are proactively deprescribed, thus it is important that the population is specified [4, 5]. Older people are a high-risk population for medication-related harm, and therefore a frequently included group in proactive deprescribing interventions. There is therefore a need to develop a COS for this population.
Existing COS for medication reviews in older people with polypharmacy [6] and for addressing polypharmacy in older people in primary care [7] are relevant for informing a new hospital proactive deprescribing COS; however, neither focused on the deprescribing process itself nor involved the views of stakeholders relevant to this activity in a hospital setting. The CompreHensive geriAtRician-led MEdication Review (CHARMER) study is a National Institute for Health Research (NIHR) funded research programme to develop and test an intervention to support geriatricians and pharmacists to work with older people and their carers to deprescribe in hospital. As part of the CHARMER programme of research, this study aimed to develop a COS for use in all trials evaluating the effectiveness of deprescribing interventions for older people under the care of a geriatrician in hospital.
Methods
We followed accepted conduct and reporting guidelines for the COS [4, 8] (detail provided in Supplementary file 1) including registration on the COMET (Core Outcome Measures in Effectiveness Trials) database (https://www.comet-initiative.org/Studies/Details/1825). Ethical approval was obtained from South Central -Berkshire Research Ethics Committee (Project ID: 20/SC/0375).
The research team
Academics with relevant topic and methodological expertise, Patient and Public Involvement (PPI) members, and clinicians working with older people in hospital and primary care settings, were involved in all stages of the study.
Phase 1: Identifying and reviewing potentially relevant outcomes
We extracted and reviewed for relevance all reported outcomes in a primary care polypharmacy COS [7], a medication review COS [6] two systematic reviews of deprescribing trials [9, 10]. We retained potentially relevant outcomes and proposed additional outcomes specific to proactive deprescribing.
To develop plain English definitions for the outcomes, we reviewed the literature, existing COS and contacted their authors; for any remaining potential outcomes, research team members (DB, DT, SS, JT) developed definitions. We reviewed and refined definitions to improve clarity, organising similar outcomes into domains: use of medicines; patient-reported outcomes; carer-reported outcomes; use of healthcare resources; death; adverse events; costs; processes of care (detailed in Supplementary file 2).
Phase 2: modified Delphi
Participants
We sought representation from four stakeholder groups: older people (and their informal carers) under the care of a geriatrician and taking ≥5 medicines; clinicians involved in the care of older people in hospital (geriatricians, pharmacists, occupational therapists, physiotherapists, nurses); hospital managers; and academics with an interest in older people’s medicine and/or deprescribing. The first three stakeholder groups were in England and academics were worldwide. Estimating ≤30% attrition at Round 1 and 20% in Round 2 [6, 7, 11], we aimed to recruit a minimum of 40 clinicians (to ensure sufficient numbers across professions), and a minimum of 30 participants for each of the other stakeholder groups. Participants provided demographic details including age, gender, location, ethnicity, profession, and years practising (clinicians, hospital managers, academics only).
Recruitment
All hospitals in England were invited to express interest in study participation by providing details of a staff member to act as their gatekeeper. The gatekeeper identified hospital staff (clinicians, hospital managers) and/or older people taking ≥5 medicines and carers (detail within Supplementary file 3).
Researchers were identified via the research team’s networks and by contacting authors of hospital deprescribing articles. JMK contacted academic researchers by email, providing them with the Delphi survey link.
Survey participants expressing an interest in attending a workshop to discuss the Delphi results and finalise the COS were recruited. We also invited patient research ambassadors from one site to attend workshops to increase the older people and carer voice.
Delphi survey
We hosted the Delphi survey using DelphiManager (COMET Initiative) and planned up to three rounds dependent upon the extent to which each round approached consensus [4].
In Round 1, participants assessed the importance of each outcome from Phase 1 on a Likert scale of 1–9 using three scoring categories: 1–3 (not important), 4–6 (important but not critical) and 7–9 (very important or critical) [12]. There was an option of ‘unable to rate’ and opportunity to add comments for each outcome. Outcomes reaching consensus to retain were removed after Round 1 and progressed for discussion at Workshop 1. We invited participants to suggest additional outcomes with a rating of importance. We also extracted outcomes from a review of interventions to reduce inappropriate medicines [13] identified during the Delphi process. Additional outcomes were reviewed by the research team to determine overlap with existing outcomes.
Outcomes failing to reach consensus in Round 1, plus new proposed outcomes, progressed to Round 2 for re-rating. In Round 2, we directed participants to choose either a score of 1-3 if they felt the outcome was not important, or 7–9 if they felt the outcome was very important. Participants received their Round 1 rating and the rating for their own and other stakeholder groups using histograms. To facilitate rating by participants completing by phone or post, we provided their rating plus a mean of overall outcome score achieved in Round 1.
After Round 2, outcomes that were compound, or would be covered in a cost-effectiveness evaluation, or were distal from the intervention, were removed.
Data analysis
Statistical analysis was undertaken using SPSS and Microsoft Excel. We a priori defined consensus to retain an outcome as >70% of participants in all stakeholder groups rating it as ‘very important or critical (7-9)’. We planned to exclude outcomes if the opposite were true [4, 5, 11, 14–16]. To account for the different sizes of each stakeholder group we calculated an adjusted lower boundary for each group for each outcome for the proportion that rated it as 7-9. The lower boundary was based on a one-sided test of the null hypothesis that the percentage of participants rating as 7-9 was 70%. Thus, the boundary was conservative as it was always less than the 70% and for small samples was lower than for larger samples when it was closer to 70%.
Phase 3: consensus workshops
We held two 90-minute online workshops each with 10–12 people representing the four stakeholder groups to discuss the outcomes of the Delphi. Both workshops were recorded audio-visually and facilitators took notes of decisions during the workshops and re-visited the recordings to ensure all views and decisions were accurately captured. We organised each outcome for discussion into its relevant core area of ‘death’, ‘life impact’, ‘pathophysiological manifestations’, and ‘resource use’ [17]. Death was automatically included in the COS as it was the only outcome in the core area of ‘death’.
At Workshop 1, we asked participants to select outcomes prioritised from the Delphi, to include in the COS. We presented the outcomes in two categories; those rated as very important (7–9) by all stakeholders and those by at least one stakeholder group. At Workshop 2, we presented outcomes selected from Workshop 1 (Table 2) and asked participants to assess both their importance and feasibility and acceptability of measuring them. We presented tools that could be used for measuring each outcome to help guide their assessment.
Table 2.
Summary of decisions in the consensus workshops
| Core area | Outcomes | Measurement tool considered during workshop 2 | Delphi (All groups rated > 70% as 7-9)a | Important (workshop 1) | Important (workshop 2) | Feasibility | Acceptability |
|---|---|---|---|---|---|---|---|
| Life impact | Activities of daily living | Barthel Index of Daily Living | ✓ | ✓ | ✗ | ✓ | ✗ |
| Burden from the medicine routine | Medication-related burden Quality of Life (MRB-QoL) [21] | ✓ | ✓ | ✓ | ✗ | ✗ | |
| Cognitive function | Mini-COG MMSE | ✓ | ✓ | ✗ | ✓ | ✗ | |
| Quality of life | Preference-based health-related quality of life tools: EQ5D and ICECAP-O | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Resource use | Number of prescribed medicines that are stopped (discontinued) | Routine data collection from electronic records (primary and secondary care) | ✗ | ✓ | ✓ | ✓ | ✓ |
| Number of prescribed medicines with dosage reduced | Routine data collection from electronic records (primary and secondary care) | ✗ | ✓ | ✓ | ✓ | ✓ | |
| Number of prescribed medicines | Routine data collection from electronic records (primary and secondary care) | ✓ | ✓ | ✗ | ✓ | ✓ | |
| Number of potentially inappropriate medicines prescribed | Screening tool providing a list of medicines and situations under which they should not be prescribed is used. | ✓ | ✓ | ✓ | ✗ | ✓ | |
| Cost of medicines | Collected from patient medical records by research team | ✗ | ✓ | ✗ | ✓ | ✓ | |
| Number of hospital stays | In the UK, Hospital Episode Statistics (HES) | ✓ | ✓ | ✗ | ✓ | ✓ | |
| Pathophysiological manifestations/Adverse events | Medication-related admissions to hospital | Tool developed in Sweden [22] | ✓ | ✓ | ✓ | ✗ | ✓ |
| Adverse drug events | Bespoke reporting process for the trial | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Death | Mortality/death | Not discussed; data available from ONS | ✓ | n/a | n/a | n/a | n/a |
For both workshops, we used informal consensus techniques and facilitators ensured that all participants could put forward their view on the outcomes. We worked in smaller groups at times to encourage more discussion; PPI team members supported participants representing the patient and carer voice. At the end of each workshop, we presented a summary of the decisions and gave participants the opportunity to discuss these and provide feedback to ensure we had captured the discussion and decisions accurately.
Synthesis meeting
Following the workshops, the research team, including representation from clinical trial unit members, discussed the COS proposed by workshop participants, and their comments regarding feasibility and acceptability. We used this to identify approaches for measuring included outcomes and any barriers to the COS implementation. We also used the meeting to discuss practicalities of using the COS within our deprescribing trial.
Results
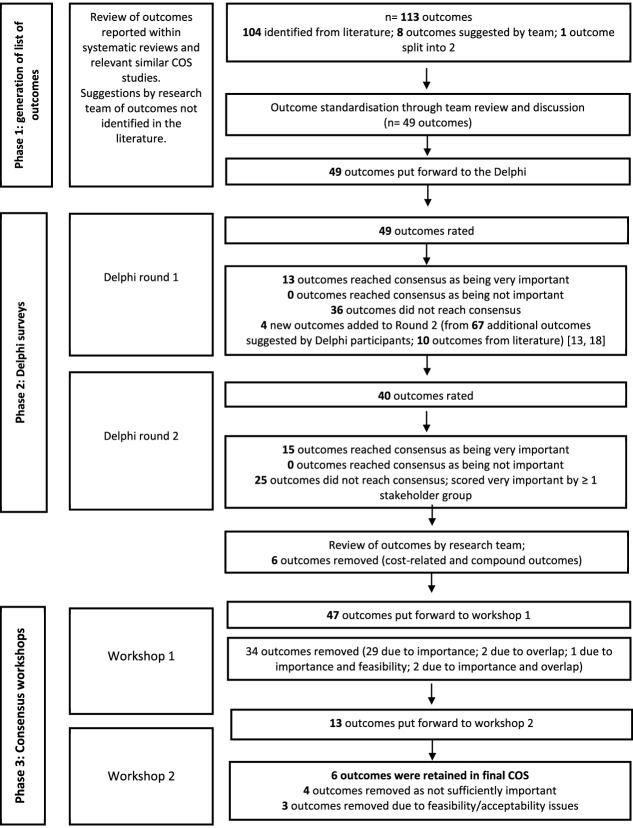
Figure 1 summarises the process to develop the COS.
Figure 1.
Overview of COS process.
Phase 1—identification of outcomes
The 113 outcomes identified in phase 1 were reduced to 49 by outcome standardisation discussions by the multidisciplinary research team leading to outcome removal due to duplication, being related to deprescribing specific medicines or being related to process evaluation. Supplementary file 4 lists the outcomes and definitions, included in Round 1 of the Delphi.
Phase 2: Delphi survey
Participants
Fifteen hospitals participated; five hospitals recruited staff and older people/carers and 10 hospitals recruited only staff. From the 238 who consented, 212 people took part; 200 completed Round 1 and 114 completed Round 2. Table 1 summarises participants (more detail in Supplementary file 5). Each hospital recruited between 2 and 21 participants. Eighteen older people and carers participated in the study. In the workshops, two research ambassadors also participated (one representing the carer voice and one representing the patient voice).
Table 1.
Characteristics of participants in the study
| Round 1 (completed) | Round 1 (not completed) | Round 2 (completed) | Workshops | |
|---|---|---|---|---|
| Total participants | n = 200 | n = 12 | n = 114 | n = 20 |
| Older people and carers | n = 15 | n = 3 | n = 12 | n = 5a |
| Clinicians | n = 140 | n = 8 | n = 81 | n = 12 |
| Hospital managers | n = 17 | n = 1 | n = 8 | n = 1 |
| Academic researchers | n = 28 | n = 0 | n = 13 | n = 2 |
aThree Delphi participants and two research ambassadors representing older people’s views.
Round 1 survey
Figure 1 provides the flow of outcomes rated in Round 1 (February–June 2021). Thirteen of the 49 outcomes were rated as very important by >70% of participants in all stakeholder groups and thus removed for Round 2; no outcomes met the criteria for exclusion. The 67 additional proposed outcomes and 10 additional identified outcomes from the literature [13, 18] are provided, including four outcomes agreed as being distinct from existing outcomes and relevant to hospital deprescribing trials for older people (Supplementary file 6).
Round 2 survey
In Round 2 (July 2021) a further 15 outcomes reached consensus for consideration in the COS (providing 28 in total across two rounds) The remaining 25 outcomes did not reach consensus and were rated as very important (scored 7–9) by at least one stakeholder group (Supplementary file 7 and 8 summarise the ratings in Rounds 1 and 2).
The outcome review and categorisation by the research team prior to Workshop 1 led to the removal of compound outcomes: Falls Risk Assessment Tool (FRAT score) and cost-effectiveness (costs of hospital, social care, primary care) and ‘whether hospital discharge medication guidance is implemented in community’ which is distal to the intervention hospital setting.
Phase 3: consensus workshops
Twenty participants (18 from the Delphi surveys and two patient research ambassadors) took part in workshops (10 in each workshop) during August 2021. The list of outcomes reviewed in workshop 1 is Supplementary file 9. Of the 13 outcomes selected for the COS, 10 had been rated as very important by all four stakeholder groups in the Delphi (Table 2). One outcome, ‘number of new prescribed medicines’ was refined by the workshop participants to ‘number of prescribed medicines’.
In workshop 2, participants agreed that seven outcomes should be removed from the COS proposed by workshop 1 participants (Table 2). Two outcomes were identified as not important enough to include in the COS: ‘number of prescribed medicines’ as participants felt that this was not specific to deprescribing and would relate to other factors e.g. multimorbidity and medical complexity; ‘cost of medicines’ was removed as participants felt it did not reflect the therapeutic benefit of deprescribing.
Two outcomes were considered important but not feasible or acceptable to measure: ‘number of potentially inappropriate medicines prescribed’ and ‘medication-related admissions to hospital’. These outcomes either do not have a suitable tool or would not be able to be collected in hospital trials in England due to workload burden associated with data collection. ‘Medication-related admissions’ was identified as difficult to collect, and because of this, ‘number of hospital stays’ which was feasible to collect (and will include medication-related hospital admissions) was included in the final COS. Two outcomes were not felt by older people to be important or acceptable to collect: ‘cognitive function’ and ‘activities of daily living’; both involving answering questions that older people did not like. Older people and carers particularly commented that activities that are possible do not necessarily reflect the person’s perception of their wellbeing or goals. Participants also felt that aspects of ‘activities of daily life’ were captured within ‘quality of life’. ‘Burden from medicine routine’ was identified by participants as not acceptable or feasible due to the completion of lengthy questionnaires (>40 questions).
Final core outcome set
Table 3 includes the six outcomes retained in the COS and provides definitions and suggestions for measurement of these. The definition for Adverse Drug Events (ADEs) was clarified to emphasise that Adverse Drug Withdrawal Events (ADWEs) are included within ADEs, acknowledging the importance of capturing events believed to be directly associated with withdrawal of a medicine in deprescribing trials [21]. This is supported by the literature acknowledging that ADWEs are a subset of ADEs [21,22]. Supplementary file 10 provides an overview of every outcome identified within the study and the decisions made throughout the study.
Table 3.
Final COS with definitions, core area and suggested measurement tools
| Outcome | Definition (plain English) | Core area | Suggested measurement tool |
|---|---|---|---|
| Quality of life | The standard of health, comfort and happiness experienced by an individual, including quality of life relating to medication use. | Life impact | EQ5D or SF-36 |
| Number of prescribed medicines that are stopped (discontinued) | The number of prescribed medicines that are stopped (i.e. no longer prescribed). | Resource use | Routine data collection from electronic records (secondary care) |
| Number of prescribed medicines with dosage reduced | The number of prescribed medicines that have been reduced; e.g., the number of tablets or strength of medication has been reduced. | Resource use | Routine data collection from electronic records (secondary care) |
| Number of hospital stays | The number of admissions or re-admissions to hospital for treatment or monitoring health. Can be planned or unplanned. | Resource use | Routine data collection from electronic records (primary and/or secondary care) |
| ADEs | An unwanted or harmful reaction caused by a patient’s medicine or the withdrawal of a patient’s medicine.a | Pathophysiological manifestations/adverse events | Data from primary care (GP) and bespoke collection of data in Care Record Forms in hospital. |
| Mortality/death | The death of a patient for any reason. | Death | Data from Office of National Statistics (ONS) |
aDefinition for ADEs was refined by the research team to remove ambiguity and include emphasis on withdrawal events, as well as other ADEs, to ensure consistent outcome data collection in deprescribing trials
Discussion
We report a COS comprising six outcomes that were the most important to all stakeholders and feasible and acceptable to be implemented in trials. These outcomes are the minimum that should be collected and reported by all trials of deprescribing in hospital for older people under the care of a geriatrician. The study also provides recommendations for tools to measure the outcomes within the COS. Our COS builds on existing COS for medication reviews in older people with polypharmacy [6], for addressing polypharmacy in older people in primary care [7] and for optimising prescribing in older people in care homes [23]. These COS whilst relevant to our own COS, would not have been suitable for use in hospital deprescribing trials for older people under the care of a geriatrician. This is because a COS needs to be developed in conjunction with the stakeholders that will be affected by the COS [4]—in our case this includes older people under the care of a geriatrician, and relevant hospital staff—who can decide which outcomes are most important to include in the COS. The outcomes included within the COS are relevant to all medicines, which is important as deprescribing is undertaken across all medicines. Three outcomes were excluded based on feasibility/acceptability but have some overlap or similarities with outcomes in the COS (for example, ‘medication-related hospital admissions’ will be captured by ‘number of hospital stays’). Having sought input from key stakeholders involved in hospital deprescribing for older people under the care of a geriatrician, outcomes within the COS could be measured by organisations that are not undertaking a deprescribing trial but wish to evaluate their deprescribing practices.
Challenges to COS implementation include cost/resource requirements of outcome data collection and burden on patients [24–27]. To support the development of easily implementable COS, identifying how to measure outcomes through assessing the validity, feasibility and acceptability of using a measurement instrument is a recognised stage in COS development [5, 28–30]. However, a review of COS studies reported that only 38% included recommendations about how to measure the outcomes in the COS [30, 31]. It was recently noted that a COS should only be considered complete when the measurement instrument has been identified [32].
We used an efficient modified Delphi design to identify the most important outcomes for the different stakeholder groups (that others can use to select additional outcomes) and to select the most important outcomes for the COS that are also acceptable and feasible to measure in a trial context. The COS does not preclude other outcomes being collected in addition to the core outcomes and is not meant to ‘stifle the development and use of other outcomes’ [33]. Our reporting allows researchers designing hospital deprescribing trials to review outcomes that were important to stakeholder groups but were not identified as very important to include in the COS. Implementation of a COS is important in trials, otherwise this constitutes research waste [34]. A recent review highlighted several barriers to a COS being implemented including burden to patients and researchers of lengthy outcome data collection, costs of collecting the full COS, lack of consensus of measurement tools and lack of clarity [24]. Another recent review demonstrated that many clinical trials do not adopt a COS despite a relevant COS being available [23]. Reported reasons for low adoption included difficulty identifying validated instruments to collect data, excessive cost related to the required data collection, and perceived burden for trial participants in terms of data collection. We believe that by adopting a flexible and pragmatic approach that assessed feasibility and acceptability throughout the study and included identifying measurement tools that can be utilised in a trial that this COS is more likely to be adopted in future research. This will help to ensure that the results of hospital deprescribing trials for older people under the care of a geriatrician can be compared and aggregated in the future, thus generating more robust evidence about the effectiveness of deprescribing interventions. Adoption of the COS also facilitates better reporting of deprescribing trials by supporting researchers to completely define pre-specified outcomes as recommended by Blom et al 2020 to report and describe deprescribing trials [35].
Strengths and limitations
Adherence to COS development and reporting guidelines affords rigour and transparency to the findings as does the input from the four stakeholder groups. Whilst requiring adjustments to facilitate engagement by adapting the Delphi survey completion to be by post or telephone for older people and carers, the value of including the service user voice was most evident in guiding decision-making regarding acceptability of outcomes and measurement tools. We incorporated a second workshop, which is not usual practice but was beneficial for our process to develop a COS that can be implemented in hospital deprescribing trials in older people under the care of a geriatrician.
The study was limited to English speakers, healthcare staff, older people and carers from England. Despite our recruitment efforts, it was not possible to involve older people who lived in care homes and we also experienced difficulty recruiting carers. These are potential limitations of the study. Workshops were held online, due to COVID-19, which facilitated representation of stakeholders from a wide geography but restricted participation from potential participants without access to the internet or those uncomfortable attending an online event.
Conclusion
We have worked with key stakeholders to identify six outcomes which form a COS and should be collected and reported in all trials of hospital deprescribing in older people under the care of a geriatrician. This COS could have a major impact if consistently implemented in all trials of hospital deprescribing interventions for older people. Most notably, the implementation of this COS would enable synthesis of results across similar trials so that results can be compared and a robust evidence base established.
Supplementary Material
Acknowledgments
The authors would like to thank all participants of the Delphi surveys and consensus meetings. We thank all participating sites for identifying and approaching eligible people for the study, in particularly difficult times. We thank VOICE for promoting this study to assist with recruitment of older people and carers to the study. We thank Professor Anne Spinewine and Dr Jean-Baptiste Beuscart for providing us with definitions for outcomes used with their study developing a COS for medication reviews in older patients with polypharmacy. MDW acknowledges support from the NIHR Newcastle Biomedical Research Centre.
Contributor Information
Jacqueline Martin-Kerry, School of Healthcare, University of Leicester, Leicester LE1 7RH, UK; Department of Health Sciences, University of York, York YO10 5DD, UK.
Jo Taylor, Department of Health Sciences, University of York, York YO10 5DD, UK.
Sion Scott, School of Healthcare, University of Leicester, Leicester LE1 7RH, UK.
Martyn Patel, Norfolk and Norwich University Hospital, Norwich NR4 7UY, UK; Norwich Medical School, University of East Anglia, Norwich NR4 7TJ, UK.
David Wright, School of Healthcare, University of Leicester, Leicester LE1 7RH, UK; School of Pharmacy, University of Bergen, Bergen 5008, Norway.
Allan Clark, Norwich Medical School, University of East Anglia, Norwich NR4 7TJ, UK.
David Turner, Norwich Medical School, University of East Anglia, Norwich NR4 7TJ, UK.
David Phillip Alldred, School of Healthcare, University of Leeds, Leeds LS2 9JT, UK.
Katherine Murphy, Patient and Public Involvement Lead, School of Healthcare, University of Leicester, Leicester, LE1 7RH, UK.
Victoria Keevil, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB2 0QQ, UK.
Miles D Witham, Newcastle Biomedical Research Centre, Newcastle University, Newcastle upon Tyne NE4 5PL, UK; Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne NE7 7DN, UK.
Ian Kellar, School of Psychology, University of Leeds, Leeds LS2 9JU, UK.
Debi Bhattacharya, School of Healthcare, University of Leicester, Leicester LE1 7RH, UK.
Conflict of Interest
None.
Ethical approval
This study was approved by the South Central -Berkshire Research Ethics Committee (REC Reference number 20/SC/0375) and the UK Health Research Authority (IRAS ID 288286).
Funding
This research is funded by the National Institute for Health and Care Research (NIHR) Programme Grants for Applied Research stream (award ID PGfAR 200874). This study is supported by the National Institute for Health and Care Research Applied Research Collaboration East of England (NIHR ARC EoE) at Cambridge and Peterborough NHS Foundation Trust. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
- 1.Gallagher P, Lang PO, Cherubini Aet al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol 2011; 67: 1175–88. 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- 2.Scott S, Clark A, Farrow Cet al. Attitudinal predictors of older peoples' and caregivers' desire to deprescribe in hospital. BMC Geriatr 2019; 19: 108. 10.1186/s12877-019-1127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation . Global Patient Safety Challenge: Medication Without Harm 2017 2017. [Available from: http://apps.who.int/iris/bitstream/10665/255263/1/WHO-HIS-SDS-2017.6-eng.pdf?ua=1&ua=1
- 4.Williamson PR, Altman DG, Bagley Het al. The COMET Handbook: version 1.0. Trials 2017; 18: 280. 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson PR, Altman DG, Blazeby JMet al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012; 13: 132. 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuscart JB, Knol W, Cullinan Set al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med 2018; 16: 21. 10.1186/s12916-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rankin A, Cadogan CA, In Ryan Cet al. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc 2018; 66: 1206–12. 10.1111/jgs.15245. [DOI] [PubMed] [Google Scholar]
- 8.Gargon E, Williamson PR, Altman DG, Blazeby JM, Tunis S, Clarke M. The COMET Initiative database: progress and activities update (2015). Trials 2017; 18: 54. 10.1186/s13063-017-1788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloomfield HE, Greer N, Linsky AMet al. Deprescribing for community-dwelling older adults: a systematic review and meta-analysis. J Gen Intern Med 2020; 35: 3323–32. 10.1007/s11606-020-06089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thillainadesan J, Gnjidic D, Green S, Hilmer SN. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: A systematic review of randomised trials. Drugs Aging 2018; 35: 303–19. 10.1007/s40266-018-0536-4. [DOI] [PubMed] [Google Scholar]
- 11.Hall DA, Smith H, Heffernan Eet al. Recruiting and retaining participants in e-Delphi surveys for core outcome set development: Evaluating the COMiT'ID study. PLoS One 2018; 13: e0201378. 10.1371/journal.pone.0201378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Kunz Ret al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64: 395–400. 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Aubert CE, Kerr EA, Maratt JK, Klamerus ML, Hofer TP. Outcome measures for interventions to reduce inappropriate chronic drugs: a narrative review. J Am Geriatr Soc 2020; 68: 2390–8. 10.1111/jgs.16697. [DOI] [PubMed] [Google Scholar]
- 14.Garfinkel D. Poly-de-prescribing to treat polypharmacy: efficacy and safety. Ther Adv Drug Saf 2018; 9: 25–43. 10.1177/2042098617736192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargon E, Gurung B, Medley Net al. Choosing important health outcomes for comparative effectiveness research: a systematic review. PLoS One 2014; 9: e99111. 10.1371/journal.pone.0099111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manage 2004; 42: 15–29. 10.1016/j.im.2003.11.002. [DOI] [Google Scholar]
- 17.Boers M, Kirwan JR, Gossec Let al. How to choose core outcome measurement sets for clinical trials: OMERACT 11 approves filter 2.0. J Rheumatol 2014; 41: 1025–30. 10.3899/jrheum.131314. [DOI] [PubMed] [Google Scholar]
- 18.NSW Therapeutic Advisory Group Inc . Resource kit for measuring strategies to reduce harm from polypharmacy in Australian Hospitals: QUM indicators, patient reported experience measures and risk stratification tools 2020. [Available from: https://www.nswtag.org.au/polypharmacy-qum-indicators-and-resources/.
- 19.Mohammed MA, Moles RJ, Chen TF. Factors associated with medication-related burden quality of life (MRB-QoL) in community-dwelling adults with long-term conditions: an exploratory study. Patient Relat Outcome Meas 2021; 12: 55–63. 10.2147/PROM.S245534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempen TGH, Hedström M, Olsson Het al. Assessment tool for hospital admissions related to medications: development and validation in older patients. Int J Clin Pharmacol 2019; 41: 198–206. 10.1007/s11096-018-0768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanlon JT, Gray SL. Deprescribing trials: A focus on adverse drug withdrawal events. J Am Geriatr Soc 2022; 70: 2738–41. 10.1111/jgs.17883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald EG, Wu PE, Rashidi Bet al. The MedSafer study-electronic decision support for deprescribing in hospitalized older adults: a cluster randomized clinical trial. JAMA Intern Med 2022; 182: 265–73. 10.1001/jamainternmed.2021.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millar AN, Daffu-O'Reilly A, Hughes CMet al. Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes. Trials 2017; 18: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes KL, Clarke M, Williamson PR. A systematic review finds Core Outcome Set uptake varies widely across different areas of health. J Clin Epidemiol 2021; 129: 114–23. 10.1016/j.jclinepi.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathioudakis AG, Janssens W, Sivapalan Pet al. Acute exacerbations of chronic obstructive pulmonary disease: in search of diagnostic biomarkers and treatable traits. Thorax 2020; 75: 520–7. 10.1136/thoraxjnl-2019-214484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matvienko-Sikar K, Avery K, Blazeby JMet al. Use of core outcome sets was low in clinical trials published in major medical journals. J Clin Epidemiol 2022; 142: 19–28. 10.1016/j.jclinepi.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Veysey EC, Ingram JR, Apfelbacher CJ, Drucker AM. Core outcome set implementation supported by the BJD. Br J Dermatol 2021; 184: 987–9. 10.1111/bjd.20050. [DOI] [PubMed] [Google Scholar]
- 28.Boers M, Kirwan JR, Wells Get al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol 2014; 67: 745–53. 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Prinsen CA, Vohra S, Rose MRet al. How to select outcome measurement instruments for outcomes included in a ``Core Outcome Set'' - a practical guideline. Trials 2016; 17: 449. 10.1186/s13063-016-1555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson PR, Ávila OR, Clarke Met al. Assessing the relevance and uptake of core outcome sets (an agreed minimum collection of outcomes to measure in research studies) in Cochrane systematic reviews: a review. BMJ Open 2020; 10: e036562. 10.1136/bmjopen-2019-036562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorst SL, Gargon E, Clarke M, Blazeby JM, Altman DG, Williamson PR. Choosing important health outcomes for comparative effectiveness research: an updated review and user survey. PLoS One 2016; 11: e0146444. 10.1371/journal.pone.0146444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt J, Kottner J, Lange T. Controversy and Debate Series on Core Outcome Sets. Paper 6: Improving the generalizability, credibility and implementation of core outcome sets - the example of the Cochrane Skin-Core Outcome Set Initiative (CS-COUSIN). J Clin Epidemiol 2020; 125: 229–31. 10.1016/j.jclinepi.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007; 8: 39. 10.1186/1745-6215-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevance A, Tran VT, Ravaud P. Controversy and Debate Series on Core Outcome Sets. Paper 1: Improving the generalizability and credibility of core outcome sets (COS) by a large and international participation of diverse stakeholders. J Clin Epidemiol 2020; 125: 206–12.e1. 10.1016/j.jclinepi.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Blom JW, Muth C, Glasziou Pet al. Describing deprescribing trials better: an elaboration of the CONSORT statement. J Clin Epidemiol 2020; 127: 87–95. 10.1016/j.jclinepi.2020.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.