Abstract
Aims
Diastolic Ca release (DCR) from sarcoplasmic reticulum (SR) Ca release channel ryanodine receptor (RyR2) has been linked to multiple cardiac pathologies, but its exact role in shaping divergent cardiac pathologies remains unclear. We hypothesize that the SR-mitochondria interplay contributes to disease phenotypes by shaping Ca signalling.
Methods and results
A genetic model of catecholaminergic polymorphic ventricular tachycardia (CPVT2 model of CASQ2 knockout) and a pre-diabetic cardiomyopathy model of fructose-fed mice (FFD), both marked by DCR, are employed in this study. Mitochondria Ca (mCa) is modulated by pharmacologically targeting mitochondria Ca uniporter (MCU) or permeability transition pore (mPTP), mCa uptake, and extrusion mechanisms, respectively. An MCU activator abolished Ca waves in CPVT2 but exacerbated waves in FFD cells. Mechanistically this is ascribed to mitochondria’s function as a Ca buffer or source of reactive oxygen species (mtROS) to exacerbate RyR2 functionality, respectively. Enhancing mCa uptake reduced and elevated mtROS production in CPVT2 and FFD, respectively. In CPVT2, mitochondria took up more Ca in permeabilized cells, and had higher level of mCa content in intact cells vs. FFD. Conditional ablation of MCU in the CPVT2 model caused lethality and cardiac remodelling, but reduced arrhythmias in the FFD model. In parallel, CPVT2 mitochondria also employ up-regulated mPTP-mediated Ca efflux to avoid mCa overload, as seen by elevated incidence of MitoWinks (an indicator of mPTP-mediated Ca efflux) vs. FFD. Both pharmacological and genetic inhibition of mPTP promoted mtROS production and exacerbation of myocyte Ca handling in CPVT2. Further, genetic inhibition of mPTP exacerbated arrhythmias in CPVT2.
Conclusion
In contrast to FFD, which is more susceptible to mtROS-dependent RyR2 leak, in CPVT2 mitochondria buffer SR-derived DCR to mitigate Ca-dependent pathological remodelling and rely on mPTP-mediated Ca efflux to avoid mCa overload. SR-mitochondria interplay contributes to the divergent pathologies by disparately shaping intracellular Ca signalling.
Keywords: CPVT, Calcium signalling, EC-coupling, RyR2
Graphical abstract
Graphical abstract.
1. Introduction
Genetic and acquired defects in the sarcoplasmic reticulum (SR) Ca release channels, ryanodine receptors (RyR2s), have been associated with a spectrum of cardiac disorders, including life-threatening cardiac arrhythmias and heart failure.1 These defects are in general marked by impaired ability of RyR2 to become and stay closed (refractory) during the relaxation phase of the cardiac cycle, thus resulting in enhanced diastolic Ca release (DCR, manifested as Ca sparks and waves). Substantial progress has been made in determining the molecular and cellular mechanisms linking DCR to these different disease states, including the role of spontaneous Ca sparks and waves in arrhythmogenic membrane potential oscillations (i.e. early and delayed afterdepolarizations), cell death, and pathological remodelling.2–5 However, while establishing the key role of DCR in various disease states, these studies also raised the question as to why and how the same underlying defect, i.e. dysregulated RyR2s, is linked to different pathological phenotypes in different disease settings. For example, in settings of catecholaminergic polymorphic ventricular tachycardia (CPVT), a genetic arrhythmia syndrome due to mutations in RyR2 or its accessory proteins, DCR results in arrhythmias without signs of pathological remodelling.2 However, DCR is associated with both arrhythmias and cell death in heart failure1,3 and other types of cardiac diseases, including a metabolic syndrome of pre-diabetic cardiomyopathy (pre-DC).5 This divergence of outcomes suggests additional factors besides dysregulated RyR2s exist that are critical for translating aberrant RyR2 Ca release to a particular disease state.
Mitochondria sense intracellular Ca signals to mediate energy production and cell death.6 They also play an important role in myocyte Ca homeostasis.7 Ca enters mitochondria primarily through the mitochondria Ca uniporter (MCU) complex.6 While mitochondrial Na/Ca exchanger (mNCX) serves as the main mitochondria Ca (mCa) extrusion mechanism, more evidence suggests that mitochondrial permeability transition pore (mPTP) is also employed to extrude Ca to prevent mCa overload.8–10 Mitochondria occupy up to 30% of the total volume of adult cardiomyocytes.11 By taking up Ca, they could act as a significant Ca buffer to shape global Ca dynamics, especially under pathological conditions.12 On the other hand, when overloaded with Ca, excessive generation of mitochondria-derived reactive oxygen species (mtROS) could lead to oxidation/CaMKII phosphorylation of RyR2, exacerbating DCR (mtROS-dependent RyR2 leak) and contributing to cardiac pathologies.13–15 Indeed, the interplay between SR and mitochondria has emerged as an important factor in myocyte Ca handling and in the development of different pathologies, including cardiac arrhythmias and metabolic disease.16–18 However, how this interplay specifically contributes to the development of a particular cardiac disease phenotype is presently unknown.
In this study, we employed two different cardiac disease models marked by enhanced DCR (measured as propensity for Ca waves) to study how SR-mitochondria interplay contributes to the divergent cardiac pathologies. The two models were: (i) a model of congenital arrhythmia, CASQ2 knockout (KO) mice, that mimics subtype 2 of CPVT (CPVT2) caused by mutations of CASQ2;19 and (ii) fructose-fed (FFD) mice5 recapitulating features of pre-DC.5,20,21 While myocytes isolated from the two disease models both exhibit enhanced DCR characteristics under control conditions, they responded differently to pharmacological manipulations of mCa. Mechanistically, this was attributable to different abilities of mitochondria to absorb SR-derived Ca and withstand Ca overload in these two models, thus translating DCR into the divergent pathological phenotypes.
2. Methods
WT, CASQ2 KO (a CPVT2 model),19 MCUfl/fl-MCM, and Cyclophilin D (CypD) KO (CypD−/−) mice were used in this study. Details on the crossbreeding between lines and the generation of FFD models can be found in the Supplementary material online, Methods. Myocytes isolated from mouse models were subjected to fluorescent imaging experiments using an inverted epifluorescent microscope (Olympus IX83). A swelling assay was performed with isolated mitochondria preparation as previously described.22 Western blot was performed to evaluate expression of proteins. Electrocardiographic recordings (ECGs) were recorded on lightly anaesthetized mice to assess vulnerability to ventricular arrhythmias. Mice were anaesthetized by inhaling isoflurane (1.5–2%) supplemented with oxygen during the ECG recording. To fully anaesthetize the mouse, a higher dose of isoflurane (4%) was applied. Experiments mice were adequately anaesthetized and then euthanized by exsanguination secondary to organ/tissue harvesting. The depth of anaesthesia was considered adequate when the animal no longer exhibits reflex responses to a toe pinch. For details regarding methods, please refer to the Methods section in the Supplementary material online.
2.1 Ethical approval
All animal procedures were approved by the Mississippi State University Institutional Animal Care and Use Committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 2011).
2.2 Statistical analysis
Data were compared using unpaired Student’s t-test or one-way ANOVA with Bonferroni post hoc test when appropriate; all values were reported as means ± standard error of the mean (SEM), unless otherwise noted. P-value <0.05 was considered statistically significant. Survival analysis was evaluated using the log-rank test.
3. Results
3.1 Pharmacological modulation of mitochondrial Ca uptake exerted opposite effects on Ca waves in CPVT2 vs. FFD myocytes
SR-mitochondria crosstalk is a known modulator of myocyte Ca cycling that could result in the development of distinct cardiac pathological phenotypes.14,23,24 We pharmacologically manipulated myocyte mCa handling to examine the relationships between mCa and arrhythmogenic Ca waves in two different disease models marked by DCR: CPVT2 and FFD. Intracellular Ca dynamics were examined in WT, CPVT2, and FFD cardiomyocytes using fluorescent microscopy and Fluo-3. Myocytes were perfused with the β-adrenergic agonist isoproterenol (ISO, 100 nM), and paced at 1 or 2 Hz for 5 s followed by a 15 s resting period to assess the frequency of arrhythmogenic Ca waves.
Mitochondria can affect myocyte Ca dynamics through at least two mechanisms: (i) absorbing/buffering of cytosolic Ca; and (ii) modulating RyR2 function via production of ROS with subsequent oxidation or CaMKII-mediated phosphorylation of RyR2.13,15,25,26 Predisposition to DCR in the form of propagating cytosolic Ca waves is an important indicator of abnormal myocyte Ca handling. It is caused by enhanced RyR2-mediated SR Ca leak, and is implicated in arrhythmogenesis and pathological remodelling.1 To assess the role of mitochondria in shaping myocyte Ca handling, we examined the effects of activation and inhibition of MCU on Ca waves. Notably, the MCU activator kaempferol eliminated Ca waves in CPVT2 myocytes, while increasing wave frequency in FFD myocytes (Figure 1A and B). Consistent with the effect of MCU activation, the MCU blocker Ru360 increased the frequency of Ca waves in CPVT2 and reduced them in FFD myocytes (Figure 1C and D). Thus, modulation of mCa uptake led to divergent effects on Ca waves in the two different disease settings. While the effects of MCU modulators on Ca waves in CPVT2 are consistent with mitochondria playing a Ca buffering role (Mechanism 1), the opposite effects in FFD myocytes instead point to mitochondria influencing SR Ca release via ROS (Mechanism 2).
Figure 1.
Pharmacological modulation of MCU Ca uptake exerted divergent effects on Ca wave development in CPVT2 and FFD. (A) Representative traces and fluorescent images of Fluo-3 fluorescence (F/F0) for WT, CPVT2, and FFD both with and without kaempferol. Five still images are shown for each trace: the pacing-induced Ca transient and baseline fluorescence (first two images), and frequency of Ca waves (last three images). The time point for each image is labelled in the representative trace using a red triangle. (B) Mean ± SEM of average of frequency of Ca waves after pacing at 2 Hz for 5 s, with or without MCU activator kaempferol, n = 62–145 cells, N = 4–6 mice, *P < 0.05, unpaired Student’s t-test between the different groups of cells. (C) Representative traces and fluorescent images of F/F0 for CPVT2 and FFD both with and without Ru360. (D) Mean ± SEM of average of frequency of Ca waves after pacing at 2 Hz for 5 s, with or without MCU inhibitor Ru360, n = 71–98 cells, N = 4 mice, *P < 0.05, unpaired Student’s t-test between the different groups of cells.
To directly examine the impact of enhancing mCa uptake on the rate of mitochondria ROS production in the two disease settings, we measured mitochondrial ROS emission with the superoxide-sensitive dye MitoSOX Red in CPVT2 and FFD myocytes. Once again, kaempferol exerted divergent effects, decreasing the mtROS production rate in CPVT2 but increasing it in FFD myocytes (Figure 2B and C). The observed changes in mtROS production supports the role of kaempferol in increasing Ca wave frequency due to mtROS-dependent RyR2 leak in FFD cells. Indeed, when WT and FFD myocytes were pretreated with ROS scavenger MPG, the kaempferol-dependent exacerbation of Ca waves was reversed (Supplementary material online, Figure S1).
Figure 2.
MCU activator kaempferol modulated mitochondria membrane potential and ROS generation differently in CPVT2 and FFD. Mean ± SEM of average of mitochondria ROS production rate and representative images of MitoSOX Red loaded cells treated, both with and without kaempferol, are shown in (A), (B), and (C) for WT, CPVT2, and FFD, respectively, n = 33–59 cells, N = 4–5 mice *P < 0.05, unpaired Student’s t-test between the different groups of cells. (D) Normalized representative trace showing decrease in ΔΨm upon perfusion of kaempferol into cells for 11 min. (E) Mean ± SEM of average level of TMRE fluorescence (F/F0) after kaempferol perfusion, as normalized to the starting level, and (F) Mean ± SEM of average time to reach 50% decrease in TMRE fluorescence, n = 55–101 cells, N = 4–5 mice, *P < 0.05, one-way ANOVA between the different groups of cells.
To evaluate mitochondria’s capacity to take up Ca, we examined the effects of kaempferol on the mitochondria membrane potential (ΔΨm), since ΔΨm provides the driving force for mCa uptake.12,27 ΔΨm was recorded in cardiomyocytes with the voltage-sensitive dye TMRE. In FFD, kaempferol caused a marked and rapid collapse of ΔΨm in myocytes (Figure 2D–F). In CPVT2 myocytes, ΔΨm was relatively resistant to the kaempferol-induced decrease (Figure 2D–F). The fairly well-preserved ΔΨm in CPVT2 cells is consistent with the mitochondria’s ability to function as an efficient Ca buffer in these myocytes. Collectively, these results suggest that in CPVT2 and FFD myocytes, mitochondria interact differentially with SR Ca release, acting as a Ca buffer or as source of ROS, respectively, thereby resulting in different—i.e. pro- or anti-arrhythmic—Ca handling signatures/phenotypes.
3.2 mCa uptake process displayed intrinsic differences in CPVT2 vs. FFD
To assess potential differences in intrinsic mitochondrial Ca uptake properties between the two disease models, we directly examined mCa uptake in saponin-permeabilized myocytes using the Ca-sensitive dye Rhod-2. When challenged with 2 µM free Ca, fluorescence increases as Ca accumulates in the mitochondria. In CPVT2 myocytes, mCa uptake was slower, but exhibited a significantly higher maximum amplitude than in WT and FFD myocytes (Figure 3A–C). Among the three groups of myocytes, mCa uptake occurred most rapidly in FFD (Figure 3C). Thus, when exposed to the same concentration of Ca, CPVT2 cells were able to reach a higher level of mCa, albeit at a slower rate, while FFD cells took up Ca into mitochondria faster, but with a lower total Ca accumulation capacity than CPVT2 myocytes.
Figure 3.
CPVT2 and FFD displayed intrinsic differences in mitochondrial Ca handling. (A) Representative trace showing Rhod-2 fluorescence (F/F0) in permeabilized myocytes upon addition of 2 µM free Ca to the bath. (B) Mean ± SEM of average of maximal Rhod-2 signal at 12 min after addition of Ca to permeabilized myocytes and (C) mean ± SEM of average time to 50% max signal, n = 41–66 cells, N = 4 mice, *P < 0.05, one-way ANOVA between the different groups of cells. Mean ± SEM of average and representative traces of mitochondrial Ca content, indicated by change in Fluo-3 F/F0, in WT, CPVT2, and FFD, were shown in (D) and (E) for baseline conditions and in the presence of 100 nM ISO, respectively. Cells were paced at 0.5 Hz for 10 s before solution was switched to a Li Tyrode solution containing 10 µM thapsigargan for 60 s. Solution was switched to solution with 1 µM FCCP and oligomycin for 45 s to induce mCa release into the cytosol, then 10 mM caffeine was applied. n = 23–66 cells, N = 3–4 mice, *P < 0.05, one-way ANOVA between the different groups of cells.
Next, we examined the ability of mitochondria to accumulate Ca by measuring total mCa content in intact WT, CPVT2, and FFD myocytes (Figure 3D and E). Myocytes were loaded with Fluo-3 and paced at 0.5 Hz to reach a steady state. They were then exposed to 0 Na/0 Ca/0 K Tyrode solution to block the sarcolemmal Na and Ca fluxes and treated with the SERCA inhibitor thapsigargin to inhibit Ca uptake into SR. Application of FCCP (an uncoupler that does not directly modulate RyR2 activity28) abolishes mitochondria membrane potential and causes a rise in the cytosolic Ca signal, thus providing a measure of mCa content.3 As shown in Figure 3D and E, under both baseline settings and in the presence of ISO, CPVT2 myocytes exhibited increased mitochondrial Ca accumulation relative to FFD and WT myocytes. Taken together, these results suggest that CPVT2 and FFD myocytes differ in their intrinsic mCa uptake/accumulation properties, with mitochondria conferring a substantial Ca buffering system in CPVT2.
3.3 Conditional KO of MCU in the CPVT2 mouse caused lethality, severe cardiac remodelling, and exacerbation of ventricular arrhythmias
Our pharmacological studies support the hypothesis that mitochondria act as an important Ca buffer to alleviate aberrant SR Ca release in CPVT2, but not FFD myocytes. To further test this hypothesis, we generated the CPVT2-MCUCKO model by crossbreeding CPVT2 mice with MCU conditional KO (MCUCKO) mice.22 MCU was knocked out in the CPVT2 model in a tamoxifen (TAM)-dependent manner. The mitochondria swelling assay was performed to assess the expression level of MCU. Without TAM induction, CPVT2-MCU control (regular diet) mitochondria displayed Ca-dependent decrease in absorbance due to mitochondria permeability transition following MCU-mediated Ca uptake. In contrast, Ca-dependent mitochondria swelling was largely prevented in mitochondria isolated from CPVT2-MCUCKO (TAM), confirming that MCU was substantially ablated following 3 weeks on TAM diet (Figure 4A and B). Additionally, the expression level of MCU was substantially lower in CPVT2-MCUCKO as compared with CPVT2 mice (Figure 4C).
Figure 4.
Ablation of MCU was lethal in CPVT2. (A and B) mitochondria swelling assay, N = 4 mice. (A) Normalized trace showing decrease in relative absorbance at 540 nm due to 200 µM Ca challenge in isolated mitochondria. (B) Mean ± SEM of average decrease in relative absorbance (540 nm) after addition of 200 µM Ca, *P < 0.05, unpaired Student’s t-test between the different groups of mice. (C) Western blot of MCU expression in cardiac mitochondria. The COX4 subunit of mitochondrial complex IV was used as a loading control. (D) Survival curve of MCUCKO vs. CPVT2-MCUCKO 25 days post-induction, *P < 0.05, log-rank test (N = 14, N = 28 mice, respectively). (E) Mean ± SEM of heart weight normalized to body weight and representative picture of hearts in CPVT2 and CPVT2-MCUCKO (N = 4–5 mice), *P < 0.05, one-way ANOVA. (F) Representative surface ECG traces of CPVT2 (N = 8) and CPVT2-MCUCKO (N = 5) mice after catecholamine challenge (2 mg/kg EPI and 120 mg/kg caffeine, IP). (G) Mean ± SEM of arrhythmia score, *P < 0.05, unpaired Student’s t-test between the different groups of mice.
TAM-treated CPVT2-MCUCKO mice also displayed a profound increase in premature deaths relative to TAM-treated MCUCKO mice (85% and 15%, respectively) (Figure 4D). The premature death of some animals in the TAM-treated MCUCKO group is consistent with previous reports and attributable to cardiac Cre toxicity.29,30 Of note, after 3 weeks back on the normal diet, severe cardiac remodelling, including cardiac hypertrophy, was still observed in CPVT2-MCUCKO hearts, while MCUCKO hearts displayed normal morphology (Figure 4E). Thus, while ablation of MCU alone did not lead to obvious cardiac pathology, it was fatal or highly pathological in the setting of CPVT2.
ECG recordings performed on the CPVT2-MCUCKO mice revealed that ventricular arrhythmias were drastically exacerbated compared with CPVT2 mice. Upon EPI and caffeine injection, a higher percentage of mice from the CPVT2-MCUCKO group (100%, 6 out of 6) developed ventricular tachycardia (VT) as compared with the CPVT2 group (75%, 6 out of 8). The arrhythmia score, which measures the time duration of VT, was substantially higher in the CPVT2-MCUCKO mice than the CPVT2 mice (Figure 4G). Our data suggest that cardiac remodelling combined with an increased vulnerability to arrhythmias contributes to the poor survival of CPVT2-MCUCKO mice.
3.4 Conditional KO of MCU in the FFD mouse alleviated ventricular arrhythmias
We generated FFD-MCUCKO mice by feeding the MCUCKO mice with fructose (10% in drinking water for 3 weeks), after which their susceptibility to ventricular arrhythmias was assessed by performing surface ECG. As the control group, the FFD mice presented only mild ventricular arrhythmias. Half of them displayed ventricular arrhythmias, but mostly in the form of ventricular ectopic beats (50%, 5 out of 10), and only 20% (2 out of 10) of the mice developed VTs (Supplementary material online, Figure S2). The incidence of ventricular arrhythmias was significantly lower in the FFD-MCUCKO mice, with only 12.5% of mice (1 out of 8) from this group exhibiting ectopic beats, and none developing VT (Supplementary material online, Figure S2). Thus, consistent with our pharmacological cellular experiments (Figure 1C and D), the in vivo ECG experiments demonstrate that ablation of MCU protects the FFD mice from ventricular arrhythmias.
3.5 Pharmacological inhibition of mPTP exacerbated Ca waves and mtROS generation in CPVT2 but not FFD myocytes
The aforementioned results suggest that CPVT2 mitochondria are able to accommodate more SR-derived Ca, partially due to increased buffering capacity. Next, we pharmacologically manipulated the mCa extrusion processes to identify additional mechanisms that differentially contribute to mitochondria’s Ca handling capacity in the two disease settings. Since mNCX is considered to be the primary mCa efflux mechanism in mitochondria,6,31 we first examined the effect of mNCX inhibitor CGP-35157 on cytosolic Ca waves. CGP-357157 exacerbated Ca waves in both CPVT2 and FFD cells (Supplementary material online, Figure S3A and B). In addition, CGP-37157 increased the rate of mtROS production in both disease models (Supplementary material online, Figure S3C and D). This result is consistent with the pivotal role of mNCX in mCa extrusion and avoiding mCa overload.31
Recent studies have also proposed the opening of mPTP as an mCa extrusion mechanism in addition to its established role in mitochondria-mediated cell death.9,32 Therefore, we examined the effect of the mPTP inhibitor CsA on Ca waves in the two disease models. Inhibition of mPTP by CsA significantly exacerbated waves in CPVT2 myocytes, but did not have discernable effects on FFD myocytes (Figure 5A and B). Moreover, CsA increased the rate of mtROS production only in CPVT2, but not in FFD myocytes (Figure 5D and E). This result suggests that inhibition of mPTP in CPVT2 exacerbates mtROS-dependent RyR2 leak, which is likely due to potential mCa overload resulting from inhibition of mPTP-mediated Ca extrusion.
Figure 5.
CsA inhibiton of mPTP affected frequency of Ca wave generation and rate of ROS generation differently in CPVT2 and FFD. (A) Representative traces and fluorescent images of Fluo-3 fluorescence (F/F0) for WT, CPVT2, and FFD both with and without CsA. Five still images are shown for each trace: the pacing-induced Ca transient and baseline fluorescence (first two images), and frequency of Ca waves (last three images). The time point for each image is labelled in the representative trace using a red triangle. (B) Mean ± SEM of average of frequency of Ca waves after pacing at 2 Hz for 5 s, with or without CsA, n = 84–154 cells, N = 4–5 mice, *P < 0.05, unpaired Student’s t-test between the different groups of cells. Mean ± SEM of average of mitochondria ROS production rate and representative images of MitoSOX Red loaded cells treated, both with and without CsA, are shown in (C), (D), and (E) for WT, CPVT2, and FFD, respectively, n = 29–65 cells, N = 4–5 mice, *P < 0.05, unpaired Student’s t-test between the different groups of cells.
3.6 Genetic inhibition of mPTP promoted mtROS generation, exacerbated myocyte Ca handling, and ventricular arrhythmias in CPVT2
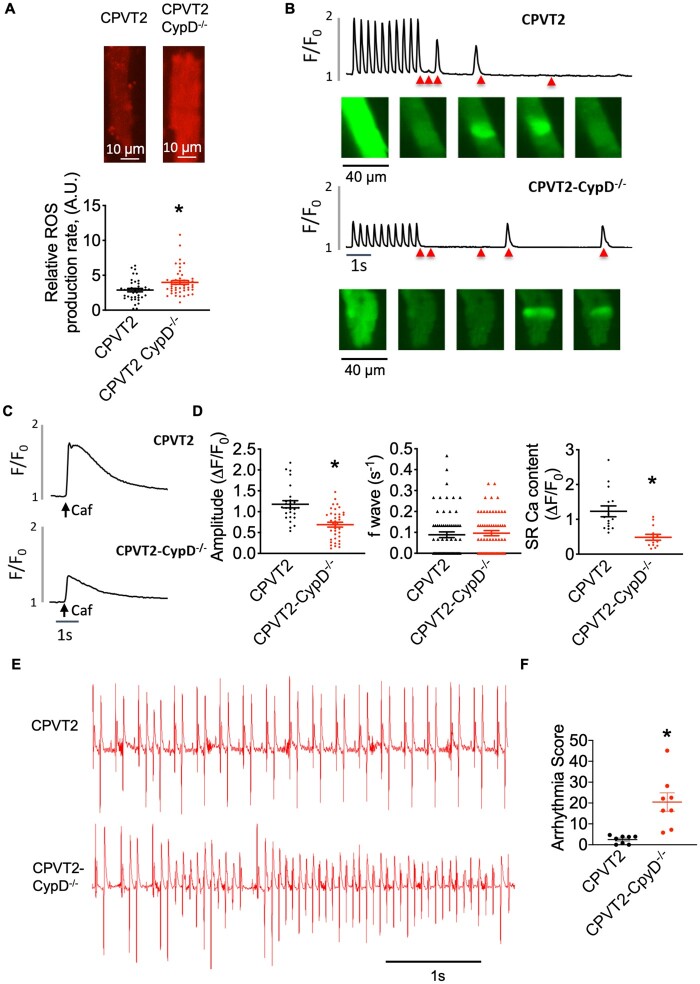
CsA is known to have several intracellular targets,33 but it inhibits mPTP by preventing the activator protein CypD from binding to mPTP.34 CypD is a known regulator of the pore and its ablation prevents mPTP opening.35,36 So, although the molecular identify of mPTP is still unknown, we were able to examine the effect of genetically inhibiting mPTP in CPVT2 settings by crossbreeding the CPVT2 model with CypD−/− mice. Consistent with pharmacological intervention with CsA, cells isolated from CPVT2-CpyD−/− mouse had elevated levels of mtROS generation as compared with CPVT2 cells (Figure 6A). When paced at 2 Hz, ISO treated CPVT2-CypD−/− myocytes exhibited drastically decreased Ca transient amplitude as compared with CPVT2 cells (Figure 6B and D). Despite the substantial decrease in SR Ca content (Figure 6C and D), the CPVT2-CpyD−/− myocytes still displayed frequent Ca waves at a level comparable to CPVT2 myocytes (Figure 6D). Thus, genetic inhibition of mPTP in CPVT2 promoted mtROS generation and exacerbated abnormal myocyte Ca handling. To examine the consequences of impaired myocyte Ca handling in terms of susceptibility to arrhythmias, we performed ECG recordings on the CPVT2-CypD−/− mice to assess their vulnerability to in vivo arrhythmias. Compared with the CPVT2 mice, the CPVT2-CypD−/− mice displayed much more severe VTs, as indicated by the significantly higher arrhythmia score (Figure 6E and F). Taken together, we have obtained evidence from experiments with both pharmacological and genetic inhibitors of mPTP to show that inhibition of mPTP in the setting of CPVT2 is harmful, likely due to mCa overload and subsequent excessive production of mtROS. Thus, increased mPTP-mediated mCa efflux could provide an adaptative/protective mechanism against mCa overload in the setting of enhanced aberrant SR Ca release.
Figure 6.
CPVT2-CypD−/− mice show altered Ca handling, increased mtROS generation, and exacerbated ventricular arrhythmias. (A) Mean ± SEM of average of mitochondria ROS production rate and representative images of MitoSOX Red loaded cells after ISO perfusion in CPVT2 and CPVT2-CypD−/−, n = 39–48 cells, N = 4–5 mice, *P < 0.05, unpaired Student’s t-test between the different groups of cells. (B) Representative traces and fluorescent images of Fluo-3 fluorescence (F/F0) for CPVT2 and CPVT2-CypD−/− with 100 nM ISO perfusion. Five still images are shown for each trace: the pacing-induced Ca transient and baseline fluorescence (first two images), and frequency of Ca waves (last three images). The time point for each image is labelled in the representative trace using a red triangle. (C) Representative trace of SR Ca content (F/F0) after application of 20 mM caffeine. (D) Mean ± SEM of average Fluo-3 amplitude (ΔF/F0), frequency of Ca waves, and SR Ca content (ΔF/F0) in CPVT2 and CPVT2-CypD−/− cells after ISO perfusion (n = 26–38, n = 61–64, n = 12–14 cells, respectively), N = 4–5 mice, *P < 0.05, unpaired Student’s t-test between the different groups of cells. (E) Representative surface ECG traces of CPVT2 (N = 8) and CPVT2-CypD−/− (N = 8) mice after catecholamine challenge (2 mg/kg EPI and 120 mg/kg caffeine, IP). (F) Mean ± SEM of arrhythmia score, *P < 0.05, unpaired Student’s t-test between the different groups of mice.
3.7 mPTP-mediated mCa efflux as an important mechanism to avoid mCa overload in disease models marked by DCR
To further examine the role of mPTP in modulating mitochondrial Ca efflux and its activity in the different disease settings, we directly measured transient mPTP openings using the membrane potential indicator TMRE.9 As mPTP briefly opens to release Ca, there is a transient decrease in membrane potential (ΔΨm) in single mitochondrion, which is referred to as MitoWinks (Figure 7A).9 Normally, MitoWinks are relatively rare events,9 but both CPVT2 and FFD myocytes exhibited elevated frequency of MitoWinks compared to WT, with CPVT2 mitochondria exhibiting the highest rate of MitoWinks (Figure 7B). The duration of MitoWinks was significantly shorter in CPVT2 compared to WT (Figure 7C). These results suggest that mPTP-mediated mCa efflux is more pronounced in disease settings marked by DCR, presumably to alleviate mCa overload. MitoWinks were typically observed in isolation, with at most only a few neighbouring mitochondria displaying MitoWinks simultaneously. Interestingly, CPVT2 exhibited brief, transient low-amplitude ΔΨm alterations we dubbed ‘MiniWinks’ (Figure 7D and E). In contrast with MitoWinks occurring in isolated mitochondria, MiniWinks typically arose in groups of several adjacent mitochondria. Additionally, MiniWinks occurred repeatedly in the same mitochondria over the time span of several minutes. Figure 7E shows an example of MiniWinks occurring repeatedly in single mitochondrion over ∼6 min, where subsequent recovery of ΔΨm was followed by a full MitoWink. These results suggest that in CPVT2 myocytes, it is easier to reach the threshold or setpoint to trigger mPTP-mediated Ca efflux as compared to WT and FFD myocytes. This is in line with our finding that inhibition of mPTP exacerbates Ca waves and mtROS generation in CPVT2 cells.
Figure 7.
CPVT2 cells showed higher level of transient mPTP openings as compared with WT and FFD. (A) Representative image of a MitoWink, whereby mitochondria undergo transient depolarization in membrane potential. (B) Mean ± SEM of average number of MitoWinks per cell per 20 min (n = 41–58 cells, N = 3 mice), (C) Mean ± SEM of average duration of MitoWinks in seconds in WT, CPVT2, and FFD, n = 13–18 cells, N = 3 mice, *P < 0.05, one-way ANOVA between the different groups of cells. (D) The percentage of cells exhibiting MiniWinks in WT, CPVT2, and FFD. Numbers in the grey bars represent cells without MiniWinks, and numbers in black bars represent those that exhibited MiniWinks. *P < 0.05, one-way ANOVA between the different groups of cells. (E) Representative trace of TMRE fluorescence (F/F0) showing transient depolarization for both MiniWinks (blue arrows) and MitoWinks (black arrow).
4. Discussion
Although the harmful role of DCR has been well established,1 it is unknown if SR-derived Ca is handled differently under various disease conditions, and if so, how SR-mitochondria crosstalk contributes to cardiac pathologies. Using two different disease models with enhanced DCR (CPVT2 and FFD), we found that intrinsic disease-dependent differences in mitochondrial Ca handling mechanisms contribute to translating DCR into specific pathological phenotypes. Specifically, we found that mitochondria in CPVT2 are modified to accumulate excessive diastolic cytosolic Ca without substantial mCa-dependent mtROS emission, thus mitigating pathological remodelling. At the same time, FFD mitochondria, while less capable of diastolic Ca accumulation, are more susceptible to mCa-dependent mtROS production. These findings are supported by (i) pharmacological evidence that the same manipulation (inhibition or stimulation) of mitochondrial Ca uptake (by targeting MCU) induces opposite effects on both cytosolic Ca wave formation and mtROS production in the two models, and (ii) genetic evidence that ablation of MCU resulted in severe hypertrophy/decreased survival and exacerbated arrhythmias in CPVT2, but alleviated ventricular arrhythmias in FFD. Through pharmacological or genetic inhibition of mPTP, we also demonstrate that mitochondria in CPVT2 may rely on up-regulated mPTP-mediated Ca efflux to avoid pathologic mCa overload. Taken together, these results demonstrate that mitochondria critically impact arrhythmogenesis and influence cardiac pathophenotypes through shaping cytosolic Ca dynamics. The findings also suggest that therapies based on targeting mitochondria should be tailored to specific pathologies. At the same time, cardioprotective mitochondrial adaptations in response to a constitutive aberrant DCR may provide clues for the development of novel therapies.
4.1 Mitochondria as a Ca buffer
Mitochondria take up Ca to regulate oxidative phosphorylation, but their role in buffering Ca and determining intracellular Ca dynamics is still debated. Although they are not considered as major players under physiological conditions, mitochondria may serve a more important role in shaping intracellular Ca dynamics under pathological conditions associated with sustained elevations of cytosolic Ca.12 Our results demonstrated enhanced mCa uptake plays opposite, i.e. apparently protective or harmful roles in CPVT2 vs. FFD mice. In particular, enhancing mCa uptake alleviated arrhythmogenic Ca waves in CPVT2 myocytes but exacerbated Ca waves in FFD myocytes (Figure 1A and B). Notably, while in FFD myocytes MCU-mediated mCa uptake was accompanied by a drastic decrease of the mitochondria membrane potential, thus preventing further mCa uptake, in CPVT myocytes the mitochondria membrane potential was relatively stable—thus enabling mitochondria to continue taking up Ca (Figure 2D–F). These results suggest that in CPVT2 (but not FFD), mitochondria are adapted to accumulate more Ca to cope with elevated levels of DCR, thus alleviating pathologic consequences, such as activation of Ca-dependent remodelling pathways or cell death. Consistent with this notion, we have observed that permeabilized CPVT2 myocytes take up more Ca to the mitochondria (Figure 3A and B), and that intact CPVT2 cells have a higher level of mCa content compared with FFD cells (Figure 3D and E). Moreover, conditional KO of MCU in the CPVT2 mouse resulted in severe hypertrophy, exacerbated VT, and decreased survival (Figure 4D–G), thus further supporting the buffering role of mitochondria in preventing pathological remodelling in CPVT2.
It remains unclear why mitochondria act as an effective Ca buffer in CPVT2 but not in FFD myocytes. Direct mCa measurements revealed that while the mCa uptake rate actually decreased, maximum mCa uptake capacity/mCa content markedly increased in CPVT2 myocytes compared to both WT and FFD myocytes (Figure 3A, B, D, and E). These results suggest that rather than enhancing the rate of mCa uptake, CPVT2 mitochondria had an increased mCa storage capacity. It has been suggested that a phosphate-based dynamic buffering mechanism contributes to chelating Ca in the mitochondrial matrix.37 When exposed to relatively low [Ca], mCa increases linearly with [Ca]. When challenged with much higher [Ca], mitochondria enter a Ca sink mode that utilizes phosphate as a dynamic, non-linear, high-capacity Ca buffering system.37 Mitochondrial phosphate uptake depends on the activity of the mitochondrial phosphate carrier (mPiC).38 Therefore, we used the mPiC inhibitor, NEM,39 to test the role of the phosphate buffering system in shaping cytosolic Ca dynamics. NEM treatment significantly exacerbated aberrant Ca handling in CPVT2 cells, resulting in more than 60% of cells exhibiting Ca oscillations (Supplementary material online, Figure S4). This result suggests mitochondrial phosphate may indeed affect the ability of mitochondria to sequester excessive cytosolic Ca. However, further studies are needed to fully elucidate the role and working mechanism of the phosphate transport system in buffering mCa and modulating Ca signalling.
4.2 mCa-dependent ROS generation
Increased mCa uptake results in stimulation of dehydrogenases of the tricarboxylic acid (TCA) cycle and increased ATP production, thereby helping myocytes to meet their increased metabolic demands, such as during the fight or flight response. However, this comes at the price of increased release of ROS as potentially toxic byproducts.40 Mitochondria-derived ROS has been shown to alter RyR2 function via oxidation and/or CaMKII-mediated phosphorylation,13,15,25,26 thus giving rise to mtROS-dependent RyR2 leak. Consistent with this mechanism, pharmacologically increasing MCU-mediated mCa uptake increased mtROS generation (Figure 2A and C) and the frequency of Ca waves in WT and FFD cells (Figure 1A and B). Notably, rather than increasing mtROS, stimulating MCU uptake by kaempferol actually decreased mtROS and Ca waves in CPVT2 myocytes. The loss of coupling between mCa uptake and ROS production in CPVT2 myocytes could be attributed to increased mCa buffering capacity, discussed above, along with up-regulated Ca extrusion through mPTP as discussed further below. It is likely that mitochondria in CPVT2 already operate in the Ca sink mode,37 so when kaempferol further enhances mCa uptake, matrix-free Ca decreases—rather than increasing—which explains the decreased mtROS production. Indeed, it has been reported that when bulk Ca is titrated into mitochondria operating in the sink mode, steady state mCa level (free Ca) actually decreased.37
We also observed that inhibition of mNCX-mediated mCa extrusion exacerbated mtROS production and RyR2 leak in both disease models. This is likely to be due to mCa overload subsequent to inhibition of mNCX, the primary mCa extrusion mechanism. Our results are consistent with other pharmacological and genetic studies reporting deleterious effects of mNCX inhibition in rodents.13,31 However, they seem to be at odds with studies with a guinea pig model of heart failure, which showed beneficial effects upon mNCX inhibition.41 This discrepancy could be ascribed to species/disease aetiology-dependent differences in mCa levels and complex relationships between mitochondrial Ca and both ROS production and scavenging, two contributors to total ROS emission.40,42,43 By stimulating the activity of dehydrogenases in the TCA cycle, mCa accelerates electron transport and ROS generation.40 However, TCA cycle acceleration also facilitates the production of NADH/NADPH that determines mitochondrial reducing power, thus facilitating the activity of ROS scavengers and quenching mitochondrial ROS.40 In guinea pig heart failure models, lowered mCa result in decreased NADH/NADPH,41 thus impairing mitochondrial antioxidative capacity and increasing ROS emission. Under these conditions, pharmacological or genetic enhancement of mCa might be expected to decrease net ROS release through increasing NADH/NADPH and promoting ROS scavenging, as observed experimentally in failing guinea pig hearts.41 In contrast, in our mouse cardiac disease models marked by excessive SR Ca leak, excessive ROS generation, secondary to mCa overload, may overwhelm mitochondrial antioxidative capacity resulting in excessive emission of ROS. In this setting, further pharmacological augmentation of mCa might be expected only to further increase ROS emission and exacerbate pathology, as reported in this and previous studies conducted on rodent models.13,31
4.3 mPTP as a mCa ‘safety’ release valve
Recent evidence suggests that besides its well-established role in mitochondria-mediated cell death, mPTP participates in the maintenance of mCa homeostasis through extrusion of mCa.9,32 While the prolonged opening of mPTP is associated with membrane potential collapse, cessation of ATP production and eventually cell death,8 the transient opening mode of mPTP (also dubbed MitoWinks) has been proposed to serve as a physiological mCa efflux mechanism to alleviate mCa overload.9 We found mPTP-mediated mCa efflux plays a special, protective role in CPVT2 myocytes. Specifically, mPTP inhibition with CsA increased mtROS generation and exacerbated Ca waves in CPVT2 myocytes but not FFD and WT myocytes (Figure 5). Further, direct measurement of mPTP openings as MitoWinks revealed an increased frequency of mPTP openings in CPVT2 compared to FFD and WT myocytes (Figure 7B). Moreover, in addition to measuring MitoWinks, in CPVT2 and FFD myocytes, we observed a novel type of mPTP Ca efflux signal we dubbed MiniWinks, characterized by a smaller amplitude and shorter duration than MitoWinks. The frequency of these events was substantially higher in CPVT2 than FFD myocytes and they were absent in WT myocytes (Figure 7D). In further support of the protective role of mPTP-mediated Ca efflux in CPVT2 myocytes, ablation of CypD in the setting of CPVT2 (CPVT2-CypD−/− mouse) resulted in marked aggravation of mtROS production, abnormal Ca handling, and exacerbated in vivo arrhythmias (Figure 6A–F). Thus, similar to MCU-dependent mCa uptake, increased mPTP-mediated Ca efflux appears to be cardioprotective in setting of CPVT2.
Intriguingly, our results suggest that Ca buffering plays a major role in CPVT2 mitochondria; this begs the question of how mPTP-mediated Ca efflux is then activated or up-regulated. First, [Ca] immediately near mPTP is more relevant to reaching the setpoint/threshold to trigger Ca extrusion than the bulk Ca in mitochondria matrix.34 Despite buffering of the bulk Ca, [Ca] may reach sufficient levels in microdomains near mPTP to reach the setpoint to trigger Ca release. Additionally, it has been suggested that instead of responding directly to matrix-free Ca, mPTP responds to total mCa loading,37 which we have shown to be higher in CPVT2 than FFD. Further, it is well documented that phosphate activates mPTP44 under various conditions. Therefore, phosphate accumulation as suggested by increased dynamic mCa buffering in CPVT2 myocytes may also contribute to the seemingly contradictory up-regulation of mPTP-mediated Ca efflux.
4.4 Potential limitations
A genetic CPVT2 model and a dietary-induced pre-DC model of FFD mice, both marked by DCR, are employed in this study to explore how SR-mitochondria crosstalk shapes intracellular Ca signalling to affect disease phenotype. Despite the critical role of RyR2 dysfunction in the pathogenesis of the FFD model,5 other factors, in particular abnormalities in metabolism, are likely to contribute to the divergent phenotype in FFD, which awaits further study.
The CPVT2 model of CASQ2 KO is known to have compensatory changes, including an increase in SR volume. CASQ2 is considered as the major SR Ca buffer protein, estimated to bind ∼50% of total Ca stored in SR.45 Other Ca buffers in SR include histidine-rich Ca binding protein and junctate.46 However, these proteins did not appear to be up-regulated to an extent that compensates for the loss of CASQ2,19 so it is likely that the nearly 2-fold expansion of SR volume helps to maintain the SR Ca storage capacity in the CASQ2 KO mice. Different from SR, the mitochondrial Ca buffering system appears to consist of both fixed and dynamic buffering mechanisms.37,47 The fixed buffer saturates easily, but the phosphate-based dynamic buffering allows mitochondria to operate as a Ca sink and accommodate enormous amounts of Ca by forming Ca-phosphate precipitates.37,47 We speculate that with prolonged DCR in the CASQ2 KO mouse, the mitochondria have already entered the Ca sink mode, due to potential compensatory changes in the phosphate transport process. However, further studies are required to better understand this phosphate buffering mechanism and to pinpoint the adaptive molecular changes in the CASQ2 KO mouse.
While we observed significant differences in MCU-mediated mCa uptake and mPTP-mediated mCa extrusion in the two disease models, we did not detect a difference in expression levels of MCU or the potential candidate of mPTP, FoF1ATP synthase34,48 (Supplementary material online, Figure S5). The molecular identity of MCU was discovered only within the past decade,49,50 while that for mPTP remains unknown. Thus, our understanding about these proteins/mCa handling processes remain limited. It is possible that changes in post-translational modifications to these proteins or altered interactions with their modulator proteins contribute to the observed differences, which awaits future study.
5. Conclusion
Taken together, our results suggest that mitochondria in CPVT2 play a major role in buffering SR-derived DCR to alleviate Ca-dependent cardiac remodelling and rely on up-regulated mPTP-mediated Ca efflux to prevent mCa overload. In contrast, mitochondria in FFD appear to precipitate pathological remodelling due to their vulnerability to mCa overload and mtROS-dependent RyR2 leak. Thus, SR-mitochondria interplay contributes to the distinct pathologies in the two disease models by disparately shaping intracellular Ca signalling.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We thank Dr Jonathan Davis for helpful comments on the manuscript. We also thank Maggie Shepherd for technical assistance.
Contributor Information
Brian D Tow, Department of Biological Sciences, Mississippi State University, 295 Lee Blvd, Starkville, Mississippi, 39762, USA.
Arpita Deb, Department of Biological Sciences, Mississippi State University, 295 Lee Blvd, Starkville, Mississippi, 39762, USA.
Shraddha Neupane, Department of Biological Sciences, Mississippi State University, 295 Lee Blvd, Starkville, Mississippi, 39762, USA.
Shuchi M Patel, Department of Biological Sciences, Mississippi State University, 295 Lee Blvd, Starkville, Mississippi, 39762, USA.
Meagan Reed, Department of Biological Sciences, Mississippi State University, 295 Lee Blvd, Starkville, Mississippi, 39762, USA.
Anna-Beth Loper, Department of Biological Sciences, Mississippi State University, 295 Lee Blvd, Starkville, Mississippi, 39762, USA.
Roman A Eliseev, epartment of Orthopedics, Center for Musculoskeletal Research, University of Rochester, 601 Elmwood Ave, Rochester, New York 14624, USA.
Björn C Knollmann, Department of Medicine, Vanderbilt University School of Medicine, 2215B Garland Ave, Nashville, Tennessee, 37232, USA.
Sándor Györke, Davis Heart and Lung Research Institute and Department of Physiology and Cell Biology, The Ohio State University, 473 W. 12th Avenue, Columbus, Ohio 43210, USA.
Bin Liu, Department of Biological Sciences, Mississippi State University, 295 Lee Blvd, Starkville, Mississippi, 39762, USA.
Funding
This work was supported by the American Heart Association [17SDG33410716 to B.L.] and the National Institute of Health [R15HL154073 to B.L.] and [R35HL144980 to B.C.K.].
Data availability
The data underlying this article are available in the article and in its Supplementary material online.
Translational perspective
It is well-established that RyR2 dysfunction is involved in a spectrum of pathological conditions including cardiac arrhythmias. In this study, two disease models marked by RyR2 dysfunction were employed to explore how the interplay between SR and mitochondria contributes to divergent cardiac pathologies. We found mitochondria act as essential Ca buffer to absorb SR-derived Ca to mitigate pathological remodelling in the genetic arrhythmic syndrome CPVT, but they are more susceptible to Ca overload or ROS-related exacerbation of RyR2 dysfunction in pre-diabetic cardiomyopathy. Thus, tailored therapies should be developed to target SR-mitochondria interplay in the aims of treating these diseases.
References
- 1. Belevych AE, Radwański PB, Carnes CA, Györke S.. ‘Ryanopathy’: causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc Res 2013;98:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gyorke S. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2009;6:123–129. [DOI] [PubMed] [Google Scholar]
- 3. Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH.. Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ Res 2010;106:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalyanasundaram A, Lacombe VA, Belevych AE, Brunello L, Carnes CA, Janssen PM, Knollmann BC, Periasamy M, Gyorke S.. Up-regulation of sarcoplasmic reticulum Ca(2+) uptake leads to cardiac hypertrophy, contractile dysfunction and early mortality in mice deficient in CASQ2. Cardiovasc Res 2013;98:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Federico M, Portiansky EL, Sommese L, Alvarado FJ, Blanco PG, Zanuzzi CN, Dedman J, Kaetzel M, Wehrens XHT, Mattiazzi A, Palomeque J.. Calcium-calmodulin-dependent protein kinase mediates the intracellular signalling pathways of cardiac apoptosis in mice with impaired glucose tolerance. J Physiol 2017;595:4089–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De la Fuente S, Sheu SS.. SR-mitochondria communication in adult cardiomyocytes: a close relationship where the Ca(2+) has a lot to say. Arch Biochem Biophys 2019;663:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamilton S, Terentyeva R, Clements RT, Belevych AE, Terentyev D.. Sarcoplasmic reticulum-mitochondria communication; implications for cardiac arrhythmia. J Mol Cell Cardiol 2021;156:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwong JQ, Molkentin JD.. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab 2015;21:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu X, Kwong JQ, Molkentin JD, Bers DM.. Individual cardiac mitochondria undergo rare transient permeability transition pore openings. Circ Res 2016;118:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernardi P, von Stockum S.. The permeability transition pore as a Ca(2+) release channel: new answers to an old question. Cell Calcium 2012;52:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun N, Finkel T.. Cardiac mitochondria: a surprise about size. J Mol Cell Cardiol 2015;82:213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ.. Mitochondrial calcium uptake. Proc Natl Acad Sci USA 2013;110:10479–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilton S, Terentyeva R, Kim TY, Bronk P, Clements RT, O-Uchi J, Csordás G, Choi B-R, Terentyev D.. Pharmacological modulation of mitochondrial Ca(2+) content regulates sarcoplasmic reticulum Ca(2+) release via oxidation of the ryanodine receptor by mitochondria-derived reactive oxygen species. Front Physiol 2018;9:1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santulli G, Xie W, Reiken SR, Marks AR.. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA 2015;112:11389–11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamilton S, Terentyeva R, Martin B, Perger F, Li J, Stepanov A, Bonilla IM, Knollmann BC, Radwański PB, Györke S, Belevych AE, Terentyev D.. Increased RyR2 activity is exacerbated by calcium leak-induced mitochondrial ROS. Basic Res Cardiol 2020;115:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang KC, Bonini MG, Dudley SC Jr. Mitochondria and arrhythmias. Free Radic Biol Med 2014;71:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D.. The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res 2010;88:30–39. [DOI] [PubMed] [Google Scholar]
- 18. Arruda AP, Hotamisligil GS.. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab 2015;22:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K.. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 2006;116:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rebolledo OR, Marra CA, Raschia A, Rodriguez S, Gagliardino JJ.. Abdominal adipose tissue: early metabolic dysfunction associated to insulin resistance and oxidative stress induced by an unbalanced diet. Horm Metab Res 2008;40:794–800. [DOI] [PubMed] [Google Scholar]
- 21. Francini F, Castro MC, Schinella G, Garcia ME, Maiztegui B, Raschia MA, Gagliardino JJ, Massa ML.. Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Life Sci 2010;86:965–971. [DOI] [PubMed] [Google Scholar]
- 22. Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD.. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep 2015;12:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P, Mao X, Huang K, Xie Z, Zou MH.. Hyperglycemia-driven inhibition of AMP-activated protein kinase alpha2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation 2019;139:1913–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dia M, Gomez L, Thibault H, Tessier N, Leon C, Chouabe C, Ducreux S, Gallo-Bona N, Tubbs E, Bendridi N, Chanon S, Leray A, Belmudes L, Coute Y, Kurdi M, Ovize M, Rieusset J, Paillard M.. Reduced reticulum-mitochondria Ca(2+) transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res Cardiol 2020;115:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho H-T, Liu B, Snyder JS, Lou Q, Brundage EA, Velez-Cortes F, Wang H, Ziolo MT, Anderson ME, Sen CK, Wehrens XHT, Fedorov VV, Biesiadecki BJ, Hund TJ, Györke S.. Ryanodine receptor phosphorylation by oxidized CaMKII contributes to the cardiotoxic effects of cardiac glycosides. Cardiovasc Res 2014;101:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bovo E, Lipsius SL, Zima AV.. Reactive oxygen species contribute to the development of arrhythmogenic Ca(2)(+) waves during beta-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol 2012;590:3291–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mammucari C, Raffaello A, Vecellio Reane D, Gherardi G, De Mario A, Rizzuto R.. Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models. Pflugers Arch 2018;470:1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zima AV, Pabbidi MR, Lipsius SL, Blatter LA.. Effects of mitochondrial uncoupling on Ca(2+) signaling during excitation-contraction coupling in atrial myocytes. Am J Physiol Heart Circ Physiol 2013;304:H983–H993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bersell K, Choudhury S, Mollova M, Polizzotti BD, Ganapathy B, Walsh S, Wadugu B, Arab S, Kuhn B.. Moderate and high amounts of tamoxifen in alphaMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis Model Mech 2013;6:1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA.. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 2009;105:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen X, Madesh M, Houser SR, Elrod JW.. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature 2017;545:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Korge P, Yang L, Yang JH, Wang Y, Qu Z, Weiss JN.. Protective role of transient pore openings in calcium handling by cardiac mitochondria. J Biol Chem 2011;286:34851–34857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halloran PF, Helms LM, Kung L, Noujaim J.. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation 1999;68:1356–1361. [DOI] [PubMed] [Google Scholar]
- 34. Hurst S, Hoek J, Sheu SS.. Mitochondrial Ca(2+) and regulation of the permeability transition pore. J Bioenerg Biomembr 2017;49:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD.. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005;434:658–662. [DOI] [PubMed] [Google Scholar]
- 36. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y.. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005;434:652–658. [DOI] [PubMed] [Google Scholar]
- 37. Wei AC, Liu T, Winslow RL, O'Rourke B.. Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol 2012;139:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seifert EL, Ligeti E, Mayr JA, Sondheimer N, Hajnoczky G.. The mitochondrial phosphate carrier: role in oxidative metabolism, calcium handling and mitochondrial disease. Biochem Biophys Res Commun 2015;464:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leung AW, Varanyuwatana P, Halestrap AP.. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem 2008;283:26312–26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bertero E, Maack C.. Calcium signaling and reactive oxygen species in mitochondria. Circ Res 2018;122:1460–1478. [DOI] [PubMed] [Google Scholar]
- 41. Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP, O’Rourke B.. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ Res 2014;115:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gauthier LD, Greenstein JL, O'Rourke B, Winslow RL.. An integrated mitochondrial ROS production and scavenging model: implications for heart failure. Biophys J 2013;105:2832–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hamilton S, Terentyeva R, Perger F, Hernandez Orengo B, Martin BY, Gorr MW, Belevych AE, Clements RT, Gyorke S, Terentyev D.. MCU overexpression evokes disparate dose-dependent effects on mito-ROS and spontaneous Ca(2+) release in hypertrophic rat cardiomyocytes. Am J Physiol Heart Circ Physiol 2021;321:H615–H632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Varanyuwatana P, Halestrap AP.. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion 2012;12:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. The Netherlands: Springer; 2001. [Google Scholar]
- 46. Prins D, Michalak M.. Organellar calcium buffers. Cold Spring Harb Perspect Biol 2011;3:a004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finkel T, Menazza S, Holmstrom KM, Parks RJ, Liu J, Sun J, Liu J, Pan X, Murphy E.. The ins and outs of mitochondrial calcium. Circ Res 2015;116:1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bernardi P, Rasola A, Forte M, Lippe G.. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 2015;95:1111–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK.. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011;476:341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R.. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011;476:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary material online.
Translational perspective
It is well-established that RyR2 dysfunction is involved in a spectrum of pathological conditions including cardiac arrhythmias. In this study, two disease models marked by RyR2 dysfunction were employed to explore how the interplay between SR and mitochondria contributes to divergent cardiac pathologies. We found mitochondria act as essential Ca buffer to absorb SR-derived Ca to mitigate pathological remodelling in the genetic arrhythmic syndrome CPVT, but they are more susceptible to Ca overload or ROS-related exacerbation of RyR2 dysfunction in pre-diabetic cardiomyopathy. Thus, tailored therapies should be developed to target SR-mitochondria interplay in the aims of treating these diseases.