Abstract
Introduction:
Transbronchial lung cryobiopsy (TBLC) is increasingly being used as an alternative to video-assisted thoracoscopic surgery (VATS) biopsy to establish the histopathologic pattern in interstitial lung disease (ILD).
Methods:
A systematic literature search of the PubMed and Embase databases, from October 2010 to October 2020, was conducted to identify studies that reported on diagnostic yield or safety of VATS or TBLC in the diagnosis of ILD.
Results:
43 studies were included. 23 evaluated the diagnostic yield of TBLC after multidisciplinary discussion, with a pooled diagnostic yield of 76.8% (95% confidence interval (CI) 70.6–82.1), rising to 80.7% in centres that performed ≥70 TBLC. 10 studies assessed the use of VATS and the pooled diagnostic yield was 93.5% (95% CI 88.3–96.5). In TBLC, pooled incidences of complications were 9.9% (95% CI 6.8–14.3) for significant bleeding (6.9% for centres with ≥70 TBLC), 5.6% (95% CI 3.8–8.2) for pneumothorax treated with a chest tube and 1.4% (95% CI 0.9–2.2) for acute exacerbation of ILD after TBLC. The mortality rates were 0.6% and 1.7% for TBLC and VATS, respectively.
Conclusions:
TBLC has a fairly good diagnostic yield, an acceptable safety profile and a lower mortality rate than VATS. The best results are obtained from more experienced centres.
Short abstract
Transbronchial lung cryobiopsy has a reasonable diagnostic yield of over 80% in experienced centres, with a better safety profile and lower mortality rate than video-assisted thoracoscopic surgery biopsy in interstitial lung diseases. https://bit.ly/3Nqozmn
Background
Interstitial lung diseases (ILDs) are a group of diseases characterised by inflammation and scarring of the lung parenchyma [1, 2] but differ in aetiology, pathological findings, treatment options and prognosis [1, 3]. In patients with suspected ILD, the clinical and imaging findings of chest radiography and conventional computed tomography are generally nonspecific and performing high-resolution computed tomography (HRCT) is essential to improve diagnostic accuracy [4–6]. Still, in many ILDs, clinical setting and HRCT appearances alone are insufficient to provide a definitive diagnosis [6, 7].
Surgical lung biopsy (SLB) has been considered the gold standard method to establish the histopathologic pattern of a specific type of ILD and better guide the treatment [8–10]. When SLB is deemed necessary, the approach of choice is by video-assisted thoracoscopic surgery (VATS), a less invasive technique that has largely replaced open lung biopsy (OLB) [11]. Nevertheless, this surgical technique also carries risks and complications, and some subgroups of patients (older subjects, patients with significant comorbidities and/or advanced respiratory disease) have high operative mortality, which explains the need to consider the risk:benefit ratio before performing such biopsy [11, 12].
Transbronchial lung cryobiopsy (TBLC) is an innovative bronchoscopic method for tissue sampling of patients with suspicious diffuse parenchymal lung diseases. A cryoprobe is inserted distally into the bronchus, cooled at −79°C (using carbon dioxide) or −89°C (using nitrous oxide) within seconds, and then retrieved with the attached frozen lung tissue [13]. It is increasingly being used for assessing ILDs because it provides tissue samples with a higher percentage of alveolar tissue and fewer crush artefacts, when compared to conventional transbronchial biopsies [14]. Furthermore, this technique has attracted considerable interest in the pulmonology community as a promising and potentially safer alternative to SLB [7, 15, 16].
Therefore, we conducted a systematic review and meta-analysis to evaluate the diagnostic yield and safety of TBLC versus VATS lung biopsy in patients with suspected ILD.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement for the present systematic review [17]. The protocol was registered and allocated the identification number CRD42020213326 in the PROSPERO database.
Eligibility criteria
Inclusion criteria were as follows: 1) reported at least one of the procedures (SLB by VATS or TBLC) used for the diagnosis of suspected ILD; 2) reported at least one of the study outcomes; 3) written in English or Portuguese; 4) published within the last 10 years.
To be included in the systematic review, studies had to respect all the inclusion criteria. We excluded abstracts, editorials, reviews, comments and case reports. Given the heterogeneity of parenchymal changes from different ILDs, which potentially impact the biopsy results, studies describing the outcomes of procedures in a sole specific ILD subset were also excluded.
Additionally, when multiple publications from the same centre with overlapping recruitment periods were found, we only included the study with the largest sample size to prevent double counting of patients.
Outcomes evaluated
Two outcomes were defined for this systematic review: diagnostic yield and safety. Diagnostic yield indicates the final clinical diagnosis held by a multidisciplinary team or, when not available, the final histopathological diagnosis. For evaluation of the safety of TBLC, we analysed the incidence of bleeding, pneumothorax and acute exacerbation of ILD. Bleeding was further divided into four categories: “none”; “mild bleeding” (self-limiting bleeding, managed with suction); “moderate bleeding” (controlled with cold saline, vasoconstrictive drugs or by endobronchial blocker); and “severe bleeding” (any bleeding causing hemodynamic or respiratory instability, requiring tamponade or other surgical interventions, transfusions, or admission to the intensive care unit) [7, 18].
As for VATS, we recorded the incidence of recurrent pneumothorax, persistent air leak (defined as an air leak that lasts longer than 5–7 days), pneumonia/empyema, haemothorax, thoracic pain and ILD acute exacerbation. Data from 30-day mortality was collected from both techniques.
Search and selection of the studies
We performed a systematic search of the literature from October 2010 to October 2020 to check all studies that reported relevant information on the diagnostic yield or safety of VATS lung biopsy or TBLC in the diagnosis of ILD. The search was performed in PubMed/MEDLINE and Embase, and the following search terms were used (both as free text and appropriate subject indexing terms): “interstitial lung disease” OR “diffuse parenchymal lung disease” AND “video-assisted thoracic surgery” OR “VATS” OR “cryobiopsy” OR “cryo-transbronchial”. The search did not include the terms “diagnostic yield” or “safety” to maximise the number of research results.
For the selection of the studies to be included in the systematic review, titles and abstracts were independently reviewed for eligibility by two authors (I.R. and R.E.G.). Disagreement on any study selection was resolved by an independent review of a third author (H.N.B.). After the first selection, the nonexcluded studies underwent a full-text review, and data was collected from the studies that were considered eligible.
Data extraction
Before the beginning of the reading of the selected articles, a set of items were established to be extracted: 1) publication details (authors, year of publication and country of origin); 2) study design (cross-sectional, cohort or randomised clinical trial); 3) sample size and population demographics (mean age and sex distribution); 4) details of the procedure (number of biopsies per patient; cryoprobe used and cooling time for TBLC studies); 5) characteristics of biopsy specimens (number, surface area, and largest diameter); 6) diagnostic yield (final clinical-pathologic diagnosis); 7) complications associated to the procedure (severe bleeding, pneumothorax and others); and 8) mortality associated with the procedure.
Quality assessment
The quality and validity of each article were assessed using the CASP (Critical Appraisal Skills Programme) checklists by two reviewers independently. The CASP checklists are a commonly used tool to evaluate the quality appraisal in various types of studies. Based on study design, the CASP cohort study checklist [19], the CASP randomised controlled trial checklist [20] or the CASP diagnostic study checklist [21] were used.
Data analysis
Extracted data were pooled and weighted proportionately to its sample size for all outcomes of interest. The pooled proportions were calculated using the Freeman–Tukey transformation, using a DerSimonian random effects model in the presence of significant heterogeneity. Forest plot graphs were used to illustrate the weighted outcomes as well as the pooled estimation with the 95% confidence interval (CI). Heterogeneity of results was assessed by the I2 statistic, and an I2>40% was considered indicative of significant heterogeneity [22]. The statistical analysis was performed using the Comprehensive Meta-Analysis Package version 2.2.064 (Biostat, Englewood, NJ, USA) statistical software.
Results
Search results
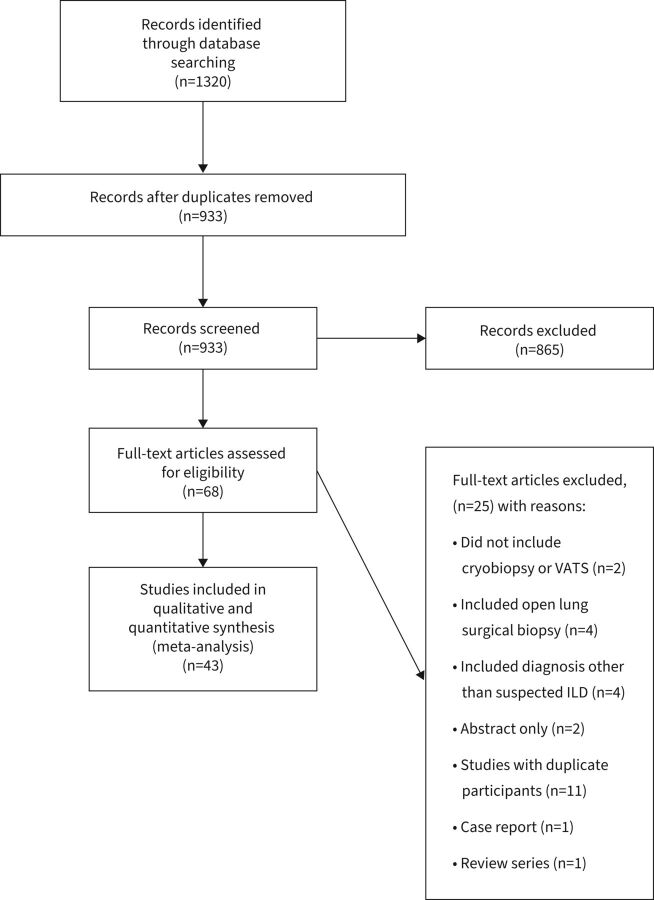
The initial search identified 1320 citations, and 933 titles and abstracts were review after the removal of duplicates (figure 1). Of these, 68 publications were full text reviewed and 43 studies were included in the final systematic review.
FIGURE 1.
Flowchart of study selection. ILD: interstitial lung disease; VATS: video-assisted thoracoscopic surgery.
Study characteristics
The 43 studies included 4550 patients (sample size range 12–359). TBLC was performed in 2824 patients and SLB (by VATS) in 1814. From the included studies (supplementary table S1), 27 [15, 18, 23–47] evaluated the use of TBLC in the diagnosis of suspected ILDs, 13 [2, 48–59] assessed the use of SLB, and three [60–62] compared the use of TBLC and SLB. In two studies [61, 62], both TBLC and SLB were performed sequentially on the same patient. There were 24 retrospective cohorts, 14 prospective cohorts, three cross-sectional studies and two randomised controlled trials comparing TBLC with conventional forceps sampling (supplementary table S2). 41 studies reported mean or median age, ranging from 45.6 to 66.6 years, and 42 studies reported sex distribution, ranging from 33% to 73% male. The number of tissue samples per subject was reported in 33 studies, ranging from one to eight biopsies in the TBLC group and one to three in the SLB group (supplementary table S3). The mean biopsy area was described in eight studies of TBLC, varying from 9 mm2 to 44.4 mm2.
Outcomes of included studies for each technique are summarised in tables 1 and 2. 25 studies reported histopathological diagnostic and 33 reported final diagnostic yield after histopathologic results were combined with clinical and radiological features in a multidisciplinary team discussion. 41 studies reported procedure-related complications.
TABLE 1.
Outcomes of transbronchial lung cryobiopsy (TBLC) of the included studies
| Method of diagnosis | First author (year) [ref.] | Biopsy method (n) | Diagnostic yield, % | Mortality, n (%) | Pneumothorax, n (%) | Chest drain, n (%) | Bleeding, n (%) | ILD acute exacerbation, n (%) | ||
| Mild | Moderate | Severe | ||||||||
| Histology | ||||||||||
| Hernández-González (2015) [43] | TBLC (33) | 79.0 | na | 4 (12) | 1 (3.0) | 3 (9.0) | 7 (21) | 0 (0.0) | na | |
| Ravaglia (2016) [60] | TBLC (297) | 82.8 | 1 (0.3) | 60 (20.2) | 46 (15.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | |
| Linhas (2017) [25] | TBLC (90) | na | 0 (0.0) | 22 (22.4) | 18 (20.0) | 8 (8.9) | 5 (5.6) | 0 (0.0) | 0 (0.0) | |
| Lentz (2018) [47] | TBLC (104) | 44.2 | 0 (0.0) | 3 (3.0) | 3 (3.0) | 100 (96.0) | 0 (0.0) | 4 (4.0) | na | |
| Cooley (2018) [26] | TBLC (159) | 69.0 | 0 (0.0) | 17 (11.0) | 14 (8.8) | 0 (0.0) | 6 (3.8)# | na | ||
| Hagmeyer (2019) [30] | TBLC (61) | 82.0 | 2 (3.2) | 10 (16.4) | na | na | 7 (14.5) | 8 (13.1) | 2 (3.2) | |
| Harari (2019) [29] | TBLC (73) | 88.0 | na | 8 (10.9) | 2 (2.7) | 6 (19.0) | 0 (0.0) | 1 (1.4) | na | |
| Romagnoli (2019) [62] | TBLC (21) | 81.0 | na | 2 (9.5) | 1 (4.8) | na | na | na | 1 (4.8) | |
| Samitas (2019) [15] | TBLC (50) | 80.0 | na | 5 (10.0) | 0 (0.0) | 19 (38.0) | 12 (24.0) | 0 (0.0) | 0 (0.0) | |
| Cho (2019) [24] | TBLC (40) | 85.0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 36 (90.0) | 2 (5.0) | 2 (5.0) | na | |
| Abdelghani (2019) [28] | TBLC (40) | 92.5 | 1 (1.3) | 2 (5.0) | 2 (5.0) | 6 (15.0) | 5 (8.3) | 0 (0.0) | na | |
| Hetzel (2019) [18] | TBLC (359) | na | 1 (0.3) | 27 (7.5) | na | 203 (56.5) | 54 (15.0) | 4 (1.1) | na | |
| Troy (2020) [61] | TBLC (65) | 90.0 | 1 (2.0) | 1 (4.0) | na | 14 (22.0)¶ | 0 (0.0) | 2 (3.1) | ||
| Aburto (2020) [38] | TBLC (257) | 80.2 | 0 (0.0) | 5 (2.0) | na | 18 (7.0)+ | 1 (0.4) | |||
| Bondue (2020) [35] | TBLC (81) | 84.0 | na | 17 (21.0) | 9 (11.0) | 47 (58.0) | 25 (31.0) | 4 (5.0) | 1 (1.2) | |
| Koslow (2020) [34] | TBLC (120) | 52.4 | 2 (1.6) | 6 (5.0) | 1 (0.9) | na | 8 (7.0)# | 1 (0.8) | ||
| Pajares (2020) [36] | TBLC (124) | 54.8 | na | 3 (2.4) | 2 (1.6) | 72 (60.0) | 8 (6.5) | 1 (0.8) | na | |
| Gnass (2020) [39] | TBLC (114) | 79.0 | na | 5 (4.4) | 5 (4.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Wang (2020) [37] | TBLC (70) | 68.6 | 1 (1.4) | 3 (4.3) | 3 (4.3) | 0 (0.0) | 0 (0.0) | 4 (5.7) | 0 (0.0) | |
| MDD | ||||||||||
| Fruchter (2014) [42] | TBLC (75) | 70.0 | na | 2 (2.7) | 0 (0.0) | 3 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Griff (2014) [33] | TBLC (52) | 79.0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | na | na | 0 (0.0) | na | |
| Pajares (2014) [41] | TBLC (39) | 51.4 | na | 3 (7.7) | na | 12 (30.8) | 22 (56.4) | 0 (0.0) | 0 (0.0) | |
| Hernández-González (2015) [43] | TBLC (33) | 79.0 | na | 4 (12) | 1 (3.0) | 3 (9.0) | 7 (21) | 0 (0.0) | na | |
| Cascante (2016) [46] | TBLC (55) | 87.3 | 0 (0.0) | 8 (14.5) | 7 (12.7) | 4 (7.3) | 1 (1.8) | 1 (1.8) | na | |
| Ramaswamy (2016) [45] | TBLC (56) | 66.0 | na | 11 (20) | na | na | na | 1 (2.0) | 0 (0.0) | |
| Kronborg-White (2017) [44] | TBLC (38) | 74.0 | na | 10 (26) | 8 (21.0) | na | 6 (15.0) | 1 (3.0) | 0 (0.0) | |
| Bango-Álvarez (2017) [23] | TBLC (106) | 86.0 | 0 (0.0) | 5 (4.7) | 2 (1.9) | 89 (84.0) | 17 (16) | 0 (0.0) | 0 (0.0) | |
| Lentz (2018) [47] | TBLC (104) | 68.3 | 0 (0.0) | 3 (3.0) | 3 (3.0) | 100 (96.0) | 0 (0.0) | 4 (4.0) | na | |
| Cooley (2018) [26] | TBLC (159) | 79.0 | 0 (0.0) | 17 (11.0) | 14 (8.8) | 0 (0.0) | 6 (3.8)# | na | ||
| Dhooria (2018) [27] | TBLC (128) | 78.1 | 4 (3.1) | 14 (10.9) | 11 (8.6) | 13 (10.2) | 4 (3.1) | 3 (2.3) | 3 (2.3) | |
| Hagmeyer (2019) [30] | TBLC (61) | 75.0 | 2 (3.2) | 10 (16.4) | na | na | 7 (14.5) | 8 (13.1) | 2 (3.2) | |
| Harari (2019) [29] | TBLC (73) | 94.5 | na | 8 (10.9) | 2 (2.7) | 6 (19.0) | 0 (0.0) | 1 (1.4) | na | |
| Romagnoli (2019) [62] | TBLC (21) | 42.9 | na | 2 (9.5) | 1 (4.8) | na | na | na | 1 (4.8) | |
| Samitas (2019) [15] | TBLC (50) | 76.0 | na | 5 (10.0) | 0 (0.0) | 19 (38.0) | 12 (24.0) | 0 (0.0) | 0 (0.0) | |
| Shafiek (2019) [32] | TBLC (12) | 83.3 | na | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | na | |
| Çirak (2020) [31] | TBLC (82) | 91.5 | na | 6 (7.3) | 0 (0.0) | 23 (28.4) | 15 (18.3) | 1 (1.21) | na | |
| Troy (2020) [61] | TBLC (65) | 60.0 | 1 (2.0) | 1 (4.0) | na | 14 (22.0)¶ | 0 (0.0) | 2 (3.1) | ||
| Aburto (2020) [38] | TBLC (257) | 90.3 | 0 (0.0) | 5 (2.0) | na | 18 (7.0)+ | 1 (0.4) | |||
| Bondue (2020) [35] | TBLC (81) | 74.0 | na | 17 (21.0) | 9 (11.0) | 47 (58.0) | 25 (31.0) | 4 (5.0) | 1 (1.2) | |
| Hussein (2020) [40] | TBLC (23) | 95.6 | 0 (0.0) | 2 (8.7) | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (4.4) | 0 (0.0) | |
| Koslow (2020) [34] | TBLC (120) | 55.0 | 2 (1.6) | 6 (5.0) | 1 (0.9) | na | 8 (7.0)# | 1 (0.8) | ||
| Pajares (2020) [36] | TBLC (124) | 47.6 | na | 3 (2.4) | 2 (1.6) | 72 (60.00) | 8 (6.5) | 1 (0.8) | na | |
ILD: interstitial lung disease; MDD: multidisciplinary discussion; na: not available. #: classified as moderate/severe; ¶: classified as mild/moderate; +: bleeding not classified by severity.
TABLE 2.
Outcomes of video-assisted thoracoscopic surgery (VATS) of the included studies
| Method of diagnosis | First author (year) [ref.] | Biopsy method (n) | Diagnostic yield, % | Mortality, n (%) | Pneumothorax, n (%) | Prolonged air leak, n (%) | Pneumonia/empyema, n (%) | Haemothorax, n (%) | Thoracic pain, n (%) | ILD acute exacerbation, n (%) |
| Histology | ||||||||||
| Kayatta (2013) [53] | VATS (196) | 100 | 13 (6.7) | na | na | na | na | na | na | |
| Pompeo (2013) [48] | VATS (30) | 97 | na | na | 0 (0.0) | na | na | na | 0 (0.0) | |
| Morris (2014) [54] | VATS (66) | 74.2 | 1 (1.5) | 7 (10.6) | 1 (1.5) | 4 (6.1) | 0 (0.0) | 3 (4.5) | na | |
| Bagheri (2015) [55] | VATS (38) | 94.7 | na | na | 5 (13.1) | na | na | na | na | |
| Ravaglia (2016) [60] | VATS (150) | 98.7 | 4 (2.7) | na | 5 (3.3) | 3 (2.0) | na | na | 5 (3.3) | |
| Jeon (2018) [59] | VATS (35) | 100 | 0 (0.0) | na | 0 (0.0) | 0 (0.0) | 0 (0.0) | na | 0 (0.0) | |
| Romagnoli (2019) [62] | VATS (21) | 57.1 | na | na | na | na | na | na | na | |
| Cherchi (2020) [51] | VATS (99) | 98 | 1 (1.0) | na | na | na | na | na | na | |
| MDD | ||||||||||
| Fibla (2012) [2] | VATS (224) | 87 | na | 6 (2.7) | 2 (0.9) | 0 (0.0) | 1 (0.4) | 8 (3.7) | 0 (0.0) | |
| Luo (2013) [52] | VATS (32) | 100 | 0 (0.0) | 8 (25.0) | na | 18 (56.3) | 1 (3.1) | na | 0 (0.0) | |
| Kayatta (2013) [53] | VATS (196) | 88.6 | 13 (6.7) | na | na | na | na | na | na | |
| Morris (2014) [54] | VATS (66) | 90.5 | 1 (1.5) | 7 (10.6) | 1 (1.5) | 4 (6.1) | 0 (0.0) | 3 (4.5) | na | |
| Samejima (2015) [56] | VATS (285) | 84.9 | 0 (0.0) | 11 (3.9) | 2 (0.7) | 0 (0.0) | 0 (0.0) | na | 3 (1.0) | |
| Khalil (2016) [57] | VATS (115) | 100 | 0 (0.0) | na | 0 (0.0) | na | na | na | 1 (0.8) | |
| Lieberman (2017) [58] | VATS (45) OLB (2) |
100 | 1 (2.1) | 5 (10.6) | na | na | na | 1 (2.1) | na | |
| Sugino (2019) [49] | VATS (143) | 100 | 0 (0.0) | 5 (3.5) | 4 (2.8) | na | na | na | 2 (1.4) | |
| Troy (2020) [61] | VATS (65) | 74 | 1 (2.0) | na | na | na | na | 1 (2.0) | 2 (3.1) | |
| Pastre (2021) [50] | VATS (268) | 94 | 4 (1.5) | 2 (0.7) | 1 (0.4) | 2 (0.7) | 1 (0.4) | na | 7 (2.6) | |
ILD: interstitial lung disease; MDD: multidisciplinary discussion; na: not available; OLB: open lung biopsy.
Study quality
The quality of full-text studies included in this revision was variable (supplementary tables S4, S5 and S6). Most cohort studies (n=38) were considered to have selected the cohort in an acceptable way, but in relation to the outcomes, only 14 studies (36.8%) had precise results. Two of the three diagnostic studies described the methodology well and had reliable results. Regarding the randomised clinical trials included (n=2), both had nonblinded randomisation and, although the results were not considered totally precise, all important outcomes were evaluated.
Diagnostic accuracy and yield
28 studies evaluated the diagnostic yield of TBLC, and a subanalysis was performed based on the criteria applied by each study to define the diagnostic yield (histopathological pattern alone versus final multidisciplinary diagnosis). 17 studies reported diagnostic yield from histopathologic assessment (figure 2a), with a pooled diagnostic yield of 77.1% (95% CI 70.1–82.8; I2=88.7%, p<0.001); 23 studies reported diagnostic yield after multidisciplinary team discussion, with a pooled diagnostic yield of 76.8% (95% CI 70.6–82.1; I2=86.3%, p<0.001). When looking solely at centres that performed ≥70 TBLCs, which has been suggested as the minimum number to achieve proficiency in the technique [63], the diagnostic yield after multidisciplinary team discussion rises to 80.7% (95% CI 71.6–87.4; I2=92.1%, p<0.001).
FIGURE 2.
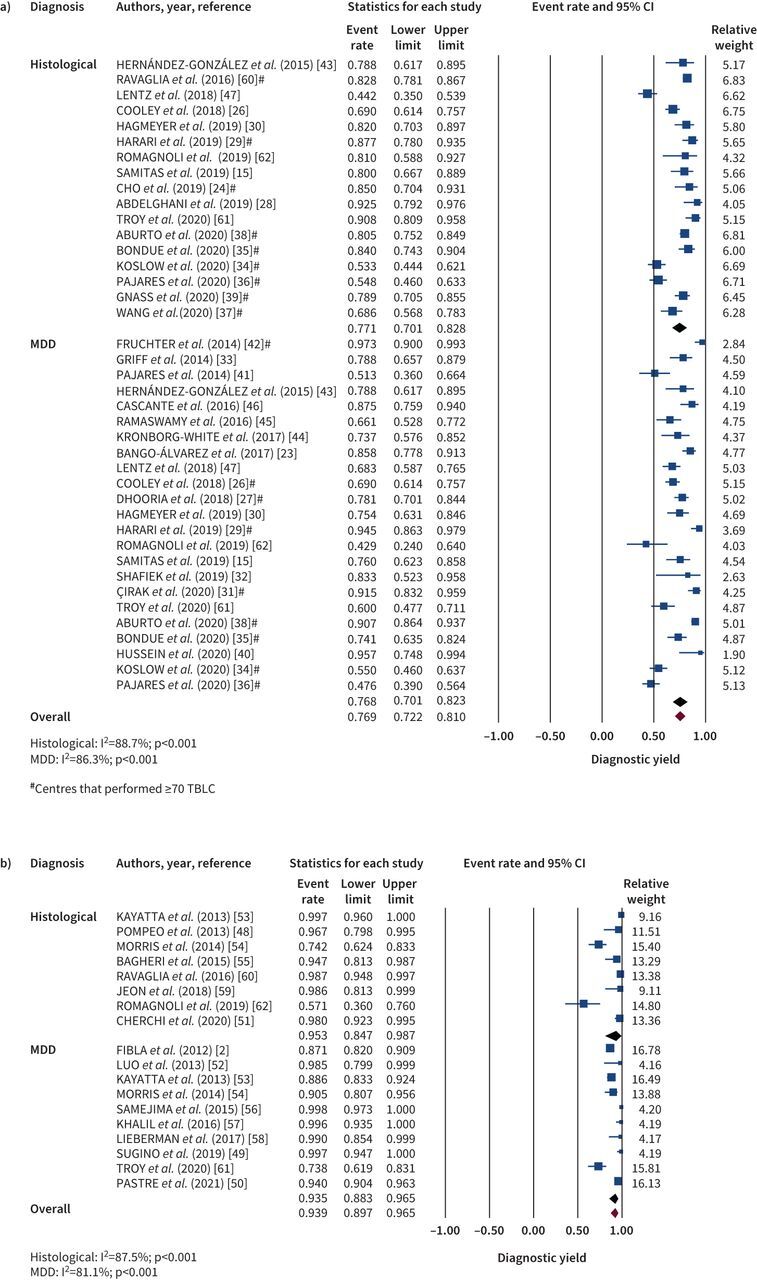
Meta-analysis of the diagnostic yield of a) transbronchial lung cryobiopsy (TBLC) and b) video-assisted thoracoscopic surgery, according to diagnostic criteria. The diamonds indicate the pooled effects. CI: confidence interval; MDD: multidisciplinary discussion.
16 studies analysed the use of SLB in the diagnosis of ILD. Eight studies reported histopathologic diagnosis (figure 2b), with a pooled diagnostic yield of 95.3% (95% CI 84.7–98.7; I2=87.5%, p<0.001); 10 studies reported the diagnostic yield after multidisciplinary team discussion, with a pooled estimate of 93.5% (95% CI 88.3–96.5; I2=81.1%, p<0.001).
Only three studies compared TBLC diagnostic accuracy with the gold standard of SLB, in which two studies [61, 62] compared sequential TBLC followed by SLB performed on the same patient. Romagnoli et al. [62] evaluated sequential TLBC and SLB in 21 patients. A histopathological pattern was obtained in 81% of the TBLCs. However, it was concordant with the SLB histopathological pattern in only 38% of cases (κ=0.22), and with the final diagnosis after multidisciplinary team discussion in 48% (κ=0.31). In the same study, SLB was considered the gold-standard procedure and a histopathological diagnosis was achieved in all cases, which was concordant with the definitive diagnosis after multidisciplinary discussion in 62% of cases (κ=0.51). Troy et al. [61] also performed both techniques sequentially but achieved different results. Agreement between TBLC and SLB on histopathological assessment was present in 70.8% cases (κ=0.7) and, for multidisciplinary discussion final diagnosis, raw agreement between TBLC and SLB was 76.9% (κ=0.62). The final diagnostic yield after multidisciplinary discussion was reached in 60% of the TBLC and 74% of SLB. Ravaglia et al. [60] compared TBLC against SLB performed in different patients. A total of 297 patients were submitted to TLBC and 150 to SLB. Only the histopathological diagnostic rate was described for both techniques, with 82.8% and 98.7% for TBLC and SLB, respectively.
Procedure-related complications
41 studies reported procedure-related complications. In relation to TBLC, 26 studies described the occurrence of bleeding. The mean reported frequency for mild bleeding was 29.9% (range 0–96%), moderate bleeding 9.1% (range 0–56.4%) and severe bleeding 1.6% (range 0–13.1%). Pooled incidence of significant bleeding (data available for moderate to severe degrees) was 9.9% (95% CI 6.8–14.3) (figure 3a). There was significant heterogeneity between studies (I2=85.2%; p<0.001). When analysing studies that performed ≥70 TBLCs, the incidence of significant bleeding falls to 6.9% (95% CI 4.2–11.2; I2=86.5%, p<0.001).
FIGURE 3.
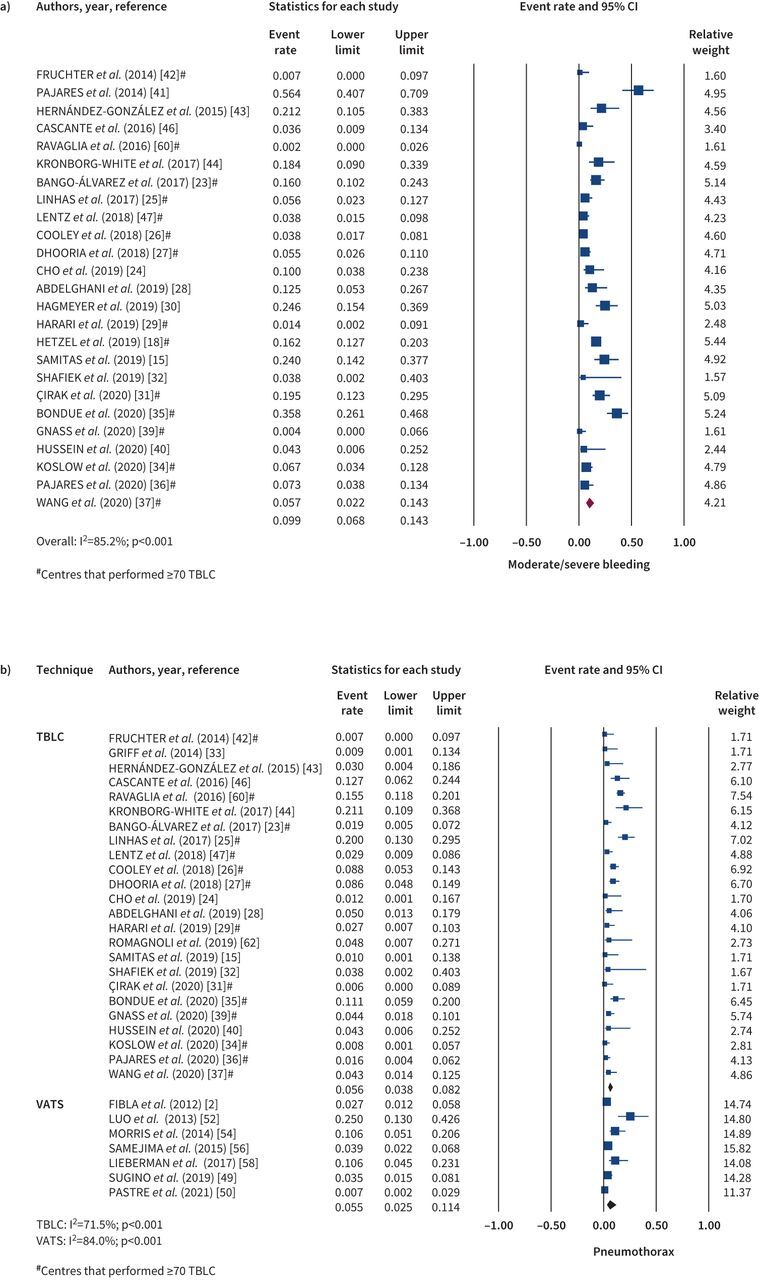
Complications related to the technique: a) moderate/severe bleeding after transbronchial lung cryobiopsy (TBLC); b) pneumothorax after TBLC and video-assisted thoracoscopic surgery (VATS). The diamonds indicate the pooled effects. CI: confidence interval.
Pneumothorax was reported in 30 TBLC studies. It was present in 261 patients, with a mean occurrence rate of 9.2% (range 0–26%). From the studies that reported the need for chest drain, the pooled incidence of patients with pneumothorax that required drainage was 5.6% (95% CI 3.8–8.2) (figure 3b) and significant heterogeneity was observed (I2=71.5%; p<0.001). The incidence of pneumothorax requiring chest drain for studies that performed ≥70 TBLC was 5.3% (95% CI 3.3–8.6; I2=79.2%, p<0.001). Finally, acute exacerbation of ILD after TBLC was reported in 18 studies, with a pooled incidence rate of 1.4% (95% CI 0.9–2.2%), with an I2 of 0% (p=0.851) (supplementary figure S1).
Considering SLB, 13 studies reported procedure-related complications. The pooled incidence rate of recurrent or persistent pneumothorax (figure 3b) was 5.5% (95% CI 2.5–11.4) with significant heterogeneity (I2=84.0%; p<0.001); 2.1% (95% CI 0.3–13.9) for pneumonia or empyema (supplementary figure S2a), with significant heterogeneity (I2=93.3%; p<0.001); 3.4% (95% CI 2.0–5.8%) for thoracic pain (figure S2b), with no significant heterogeneity (I2=0%; p=0.763); 1.8% (95% CI 0.8–4.0) for persistent air leak (figure S2c), with significant heterogeneity (I2=54.5%; p=0.003); and 2.0% (95% CI 1.3–3.1%) for acute ILD exacerbation (figure S2d), with no significant heterogeneity (I2=0%; p=0.635).
30-day mortality was reported in 29 studies. The mortality rate was 0.6% (range 0–3.2%) and 1.7% (range 0–6.7%) in TBLC and SLB, respectively.
Discussion
In this meta-analysis, TBLC was shown to have a pooled diagnostic yield after multidisciplinary discussion of 76.8%, which raises to 80.7% in experienced centres, while SLB had 93.5%. This means that around eight in every 10 cases may achieve confident diagnosis without undergoing surgery. A difference of similar magnitude was observed for histopathological diagnosis with TBLC, compared to SLB. While histopathological diagnostic yield is a simple measure of biopsy efficacy, evaluating specimen adequacy and the presence of a characteristic histological pattern, which could arguably be considered a more direct measure of comparison between biopsy techniques, the contribution to the final ILD diagnosis must be harmonised with other variables in the context of multidisciplinary team discussion. Therefore, we believe that the real impact of the biopsy technique should be discerned within the frame of a multidisciplinary evaluation of the clinical case.
Conversely, the safety profile analysis benefits TBLC, especially when considering the post-procedural 30-day mortality rate, which is 0.6% for TBLC and more than double, 1.7%, in SLB. This figure is in line with a previous meta-analysis that included both VATS and OLB, where the pooled 30-day mortality rate was 2.2%, but could be as high as 7.8% in studies containing mechanically ventilated patients [3]. Possible explanations for the higher mortality in SLB may be the risk of pneumonia, empyema or ILD acute exacerbation in the post-operative period. Additionally, the collapse induced to the lung that will be biopsied during VATS, which is necessary for visualisation of the pleural cavity, may also be deleterious to the patients undergoing SLB. Previous research has linked repetitive alveolar collapse to epithelial cell injury and idiopathic pulmonary fibrosis (IPF) progression [64, 65], and clinical studies have shown that pneumothorax in IPF patients is associated to dismal prognosis, with high in-hospital mortality rate [66, 67]. Although pneumothorax requiring chest drainage after TBLC may also occur, with a pooled incidence rate of 5.6% in our meta-analysis, we found a surprisingly identical rate of recurrent or persistent pneumothorax after SLB, with a pooled frequency of 5.5% of cases, which provides a second hit with lung collapse and potentially drives clinical worsening in susceptible patients.
Clearly, mortality rate and complications associated with SLB can also be influenced by comorbidities, including immunocompromised status and low baseline lung function [3], especially in elderly patients. In a large cohort study of TBLC cases, impaired lung function was also associated with increased rate of post-procedural pneumothorax, but, conversely, was not related with higher mortality [68]. Moreover, we show in our review that TBLC has been performed safely in patients with a wide range of age, further reinforcing its role to diagnose advanced cases of fibrotic ILDs. On the other hand, the most common complication related to TBLC is moderate to severe bleeding (9.9%), although less frequently in the most experienced centres (6.9%) and often manageable with local endoscopic measures. In a previous meta-analysis, the pooled incidence of moderate to severe bleeding differed widely (4.9–39.0%) [60, 69–71]. This difference may be associated with variability in the reporting of this complication and the absence of a prophylactic endobronchial balloon in some studies, which has been associated with lower rate of significant bleeding [27]. Studies suggest that bleeding risk is independent of baseline functional status, probe size, number of samples or multiple site sampling [68].
There are two features that distinguish the present systematic review and meta-analysis from the previous ones. First, we focus on the final diagnostic yield after the multidisciplinary team discussion, as we believe that a simple histopathological yield is not the most clinically informative outcome. Second, our research included two recent trials comparing TBLC with SLB performed sequentially on the same patients [61, 62], which were absent in former meta-analyses. Interestingly, these trials presented contradicting results, with Romagnoli et al. [62] showing poor concordance between TBLC and SLB for the histologic diagnosis and Troy et al. [61] showing that histopathologic agreement between TBLC and SLB was 70.8%, with the concordance increasing to 94.9% in cases of TBLC with high or definite diagnostic confidence at multidisciplinary discussion. Given these conflicting outcomes, an updated meta-analysis was deemed necessary. Sharp et al. [69] reported a pooled diagnostic yield of 84% (based on 10 studies and abstracts) without specifying their diagnostic criteria; Johannson et al. [70] reported 83% (based on five studies) considering histopathological assessment alone and 79% (based on six studies) when TBLC was incorporated into a multidisciplinary discussion; Ravaglia et al. [60] reported 80% (based on eight studies) referring to the identification of a characteristic histological pattern and 81% (based on seven studies) considering multidisciplinary discussion as the final diagnosis; and Iftikhar et al. [71] reported an overall pooled diagnostic yield of 83.7% (based on 16 studies) considering both diagnostic criteria. Here, we calculated a relatively lower diagnostic yield, which may be related to the higher number of studies included in this analysis (23 studies, excluding abstracts), reporting a wide range of diagnostic yield, from 44.2% to 95.6%, possibly explained by technical differences, such as cryoprobe size, number of biopsies and sampling site, duration of freezing time, use of fluoroscopy, method of sedation (moderate/deep), and use of flexible versus rigid bronchoscopy. Both cryoprobe diameters (1.9 mm and 2.4 mm) were shown to perform similarly in terms of diagnostic performance, although associated with significantly lower pneumothorax rate for the 1.9 mm cryoprobe compared with the 2.4 mm (2.7% versus 21.2%) [68]. A prospective study [72] has shown that two samples taken from two different segments significantly increases the diagnostic rate, compared to two biopsies taken from the same segment alone (78% versus 96%). These results were confirmed by a retrospective cohort, in which both histopathological and multidisciplinary team discussion diagnostic yield were significantly better when samples were obtained from two different sites (either different segments in the same lobe or different lobes) [68]. Finally, the incidence for pneumothorax needing drainage in former analyses was similar to ours, with 3% (1–8%) [60], as was the 30-day mortality (0.3–0.7%) [60, 69, 71].
In summary, our results support the clinical use of TBLC, considering its lower invasiveness over SLB, with less morbi-mortality, shorter hospitalisation stay and better cost-effectiveness profile [43]. In fact, the use of TBLC in the diagnostic algorithm of ILD whose differential diagnosis includes fibrotic hypersensitivity pneumonitis has been already suggested [73]. Another strength of the study is that we compared TBLC with SLB performed through VATS, rather than OLB, since the latter has become less recommended due to higher post-operative complications and longer hospital stays [74].
Although we restricted our analysis to studies written in English or Portuguese, a selection bias is unlikely given that only a minority of manuscripts (<2%) were excluded due to language restrictions, and the present review included studies from 21 different countries. Yet, the heterogeneity found between studies for diagnostic accuracy, occurrence of bleeding and pneumothorax, may be pointed out as a potential drawback of our analysis. Heterogeneity may be explained by technical differences in the TBLC procedure between studies and putative selection bias in studies. Overall, better results are found in the most experienced centres. We have previously shown that approximately 70 procedures are needed to achieve technical proficiency in TBLC, which is associated with significant improvement in sample quality and fewer pneumothorax events, and better diagnostic yield, that reflect both proficiency in technical performance and histopathological sample interpretation [63]. Our meta-analysis confirms that the pooled diagnostic yield was higher and complication rates were lower in studies including 70 or more procedures. However, significant heterogeneity was still observed in this subgroup analysis. In order to standardise procedures, Hetzel et al. [16] have published an expert statement on TBLC proposing recommendations in patient selection, contraindications, risks and technical performance. More recently, Maldonado et al. [7] published the first guidelines on the technical aspects of the TBLC procedure. The latter is expected to homogenise the TBLC procedure between centres, which would result in more reliable results in future studies.
Conclusion
In conclusion, our meta-analysis demonstrates that TBLC contributes to the diagnosis in a great number of patients under study for ILD, with a reasonable safety profile, particularly in centres with more experience. Therefore, it should be considered as an alternative to SLB or at least as a first-line procedure for lung tissue sampling. Guidelines on TBLC techniques should improve the diagnostic yield and reduce the risk of complications.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0280-2021.SUPPLEMENT (582.3KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: H. Novais Bastos has received support from Sociedade Portuguesa de Pneumologia, as well as grants or contracts from Fundação para a Ciência e Tecnologia and Fundação Amélia de Mello/CUF; consulting fees from Boehringer-Ingelheim and Roche; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boehringer-Ingelheim and Roche; support for attending meetings and/or travel from Boehringer-Ingelheim; and equipment, materials, drugs, medical writing, gifts or other services from Boehringer-Ingelheim, all outside the submitted work. The remaining authors have nothing to disclose.
Support statement: The authors wish to acknowledge Fundação para a Ciência e Tecnologia for supporting their research under the scope of the project FIBRA-LUNG (PTDC/MEC-RES/0158/2020). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Oliveira DS, Araújo Filho J de A, Paiva AFL, et al. Idiopathic interstitial pneumonias: review of the latest American Thoracic Society/European Respiratory Society classification. Radiol Bras 2018; 51: 321–327. doi: 10.1590/0100-3984.2016.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fibla JJ, Molins L, Blanco A, et al. Video-assisted thoracoscopic lung biopsy in the diagnosis of interstitial lung disease: a prospective, multi-center study in 224 patients. Arch Bronconeumol 2012; 48: 81–85. doi: 10.1016/j.arbres.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Han Q, Luo Q, Xie JX, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015; 149: 1394–1401.e1. doi: 10.1016/j.jtcvs.2014.12.057 [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. doi: 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadoch MA, Cham MD, Beasley MB, et al. Idiopathic interstitial pneumonias: a radiology-pathology correlation based on the revised 2013 American Thoracic Society-European Respiratory Society classification system. Curr Probl Diagn Radiol 2015; 44: 15–25. doi: 10.1067/j.cpradiol.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 6.Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: The British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008; 63: Suppl. 5, 1–58. doi: 10.1136/thx.2008.101691 [DOI] [PubMed] [Google Scholar]
- 7.Maldonado F, Danoff SK, Wells AU, et al. Transbronchial cryobiopsy for the diagnosis of interstitial lung diseases: CHEST guideline and expert panel report. Chest 2020; 157: 1030–1042. doi: 10.1016/j.chest.2019.10.048 [DOI] [PubMed] [Google Scholar]
- 8.Deconinck B, Verschakelen J, Coolen J, et al. Diagnostic workup for diffuse parenchymal lung disease: schematic flowchart, literature review, and pitfalls. Lung 2013; 191: 19–25. doi: 10.1007/s00408-012-9433-5 [DOI] [PubMed] [Google Scholar]
- 9.Thomson CC, Duggal A, Bice T, et al. 2018. Clinical practice guideline summary for clinicians: diagnosis of idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2019; 16: 285–290. doi: 10.1513/AnnalsATS.201809-604CME [DOI] [PubMed] [Google Scholar]
- 10.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 11.Carnochan FM, Walker WS, Cameron EW. Efficacy of video assisted thoracoscopic lung biopsy: an historical comparison with open lung biopsy. Thorax 1994; 49: 361–363. doi: 10.1136/thx.49.4.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson JP, Fogarty AW, McKeever TM, et al. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016; 193: 1161–1167. doi: 10.1164/rccm.201508-1632OC [DOI] [PubMed] [Google Scholar]
- 13.Lentz RJ, Christine Argento A, Colby TV, et al. Transbronchial cryobiopsy for diffuse parenchymal lung disease: a state-of-the-art review of procedural techniques, current evidence, and future challenges. J Thorac Dis 2017; 9: 2186–2203. doi: 10.21037/jtd.2017.06.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganganah O, Guo SL, Chiniah M, et al. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology 2016; 21: 834–841. doi: 10.1111/resp.12770 [DOI] [PubMed] [Google Scholar]
- 15.Samitas K, Kolilekas L, Vamvakaris I, et al. Introducing transbronchial cryobiopsies in diagnosing diffuse parenchymal lung diseases in Greece: implementing training into clinical practice. PLoS One 2019; 14: e0217554. doi: 10.1371/journal.pone.0217554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration 2018; 95: 188–200. doi: 10.1159/000484055 [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetzel J, Eberhardt R, Petermann C, et al. Bleeding risk of transbronchial cryobiopsy compared to transbronchial forceps biopsy in interstitial lung disease – a prospective, randomized, multicentre cross-over trial. Respir Res 2019; 20: 140. doi: 10.1186/s12931-019-1091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Critical Appraisal Skills Programme . CASP cohort study checklist. https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf Date last accessed: 3 April 2021.
- 20.Critical Appraisal Skills Programme . CASP randomised controlled trials checklist. https://casp-uk.b-cdn.net/wp-content/uploads/2020/10/CASP_RCT_Checklist_PDF_Fillable_Form.pdf Date last accessed: 3 April 2021.
- 21.Critical Appraisal Skills Programme . CASP Diagnostic Study Checklist. https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Diagnostic-Checklist-2018_fillable_form.pdf Date last accessed: 3 April 2021.
- 22.Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). London, Cochrane, 2021. www.training.cochrane.org/handbook [Google Scholar]
- 23.Bango-Álvarez A, Ariza-Prota M, Torres-Rivas H, et al. Transbronchial cryobiopsy in interstitial lung disease: experience in 106 cases – how to do it. ERJ Open Res 2017; 3: 00148-2016. doi: 10.1183/23120541.00148-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho R, Zamora F, Gibson H, et al. Transbronchial lung cryobiopsy in the diagnosis of interstitial lung disease: a retrospective single-center experience. J Bronchol Interv Pulmonol 2019; 26: 15–21. doi: 10.1097/LBR.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 25.Linhas R, Marçôa R, Oliveira A, et al. Transbronchial lung cryobiopsy: associated complications. Rev Port Pneumol 2017; 23: 331–337. doi: 10.1016/j.rppnen.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 26.Cooley J, Balestra R, Aragaki-Nakahodo AA, et al. Safety of performing transbronchial lung cryobiopsy on hospitalized patients with interstitial lung disease. Respir Med 2018; 140: 71–76. doi: 10.1016/j.rmed.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 27.Dhooria S, Mehta R, Srinivasan A, et al. The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J 2018; 12: 1711–1720. doi: 10.1111/crj.12734 [DOI] [PubMed] [Google Scholar]
- 28.Abdelghani R, Thakore S, Kaphle U, et al. Radial endobronchial ultrasound-guided transbronchial cryobiopsy. J Bronchol Interv Pulmonol 2019; 26: 245–249. doi: 10.1097/LBR.0000000000000566 [DOI] [PubMed] [Google Scholar]
- 29.Harari S, Cereda F, Pane F, et al. Lung cryobiopsy for the diagnosis of interstitial lung diseases- a series contribution to a debated procedure. Medicina 2019; 55: 606. doi: 10.3390/medicina55090606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagmeyer L, Theegarten D, Wohlschläger J, et al. Transbronchial cryobiopsy in fibrosing interstitial lung disease: modifications of the procedure lead to risk reduction. Thorax 2019; 74: 711–714. doi: 10.1136/thoraxjnl-2018-212095 [DOI] [PubMed] [Google Scholar]
- 31.Çirak AK, Katgi N, Erer OF, et al. Diagnostic approach in parenchymal lung diseases: transbronchial lung biopsy or cryobiopsy? Turk J Med Sci 2020; 50: 1535–1539. doi: 10.3906/sag-1910-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shafiek H, Elbialy S, El Achy SN, et al. Transbronchial cryobiopsy validity in diagnosing diffuse parenchymal lung diseases in Egyptian population. J Multidiscip Healthc 2019; 12: 719–726. doi: 10.2147/JMDH.S208824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griff S, Schönfeld N, Ammenwerth W, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: a retrospective case series. BMC Pulm Med 2014; 14: 171. doi: 10.1186/1471-2466-14-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koslow M, Edell ES, Midthun DE, et al. Bronchoscopic cryobiopsy and forceps biopsy for the diagnostic evaluation of diffuse parenchymal lung disease in clinical practice. Mayo Clin Proc Innov Qual Outcomes 2020; 4: 565–574. doi: 10.1016/j.mayocpiqo.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bondue B, Leduc D, Froidure A, et al. Usefulness of surgical lung biopsies after cryobiopsies when pathological results are inconclusive or show a pattern suggestive of a nonspecific interstitial pneumonia. Respir Res 2020; 21: 231. doi: 10.1186/s12931-020-01487-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pajares V, Núñez-Delgado M, Bonet G, et al. Transbronchial biopsy results according to diffuse interstitial lung disease classification. Cryobiopsy versus forceps: MULTICRIO study. PLoS One 2020; 15: e0239114. doi: 10.1371/journal.pone.0239114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Xu J, Liu C, et al. The significance of multidisciplinary classifications based on transbronchial pathology in possible idiopathic interstitial pneumonias. Medicine 2020; 99: e20930. doi: 10.1097/MD.0000000000020930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aburto M, Pérez-Izquierdo J, Agirre U, et al. Complications and hospital admission in the following 90 days after lung cryobiopsy performed in interstitial lung disease. Respir Med 2020; 165: 105934. doi: 10.1016/j.rmed.2020.105934 [DOI] [PubMed] [Google Scholar]
- 39.Gnass M, Filarecka A, Bartczak A, et al. Transbronchial lung cryobiopsy guided by radial mini-probe endobronchial ultrasound in interstitial lung diseases — a multicenter prospective study. Adv Respir Med 2020; 88: 123–128. doi: 10.5603/ARM.2020.0086 [DOI] [PubMed] [Google Scholar]
- 40.Hussein S, Elhadidy A, Amin H, et al. Transbronchial cryobiopsy as a new tool for lung biopsies in diagnosis of diffuse parenchymal lung diseases. Egypt J Chest Dis Tuberc 2020; 69: 649–658. doi: 10.4103/ejcdt.ejcdt [DOI] [Google Scholar]
- 41.Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology 2014; 19: 900–906. doi: 10.1111/resp.12322 [DOI] [PubMed] [Google Scholar]
- 42.Fruchter O, Fridel L, El Raouf BA, et al. Histological diagnosis of interstitial lung diseases by cryo-transbronchial biopsy. Respirology 2014; 19: 683–688. doi: 10.1111/resp.12296 [DOI] [PubMed] [Google Scholar]
- 43.Hernández-González F, Lucena CM, Ramírez J, et al. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: yield and cost-effectiveness analysis. Arch Bronconeumol 2015; 51: 261–267. doi: 10.1016/j.arbr.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 44.Kronborg-White S, Folkersen B, Rasmussen TR, et al. Introduction of cryobiopsies in the diagnostics of interstitial lung diseases – experiences in a referral center. Eur Clin Respir J 2017; 4: 1274099. doi: 10.1080/20018525.2016.1274099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramaswamy A, Homer R, Killam J, et al. Comparison of transbronchial and cryobiopsies in evaluation of diffuse parenchymal lung disease. J Bronchol Interv Pulmonol 2016; 23: 14–21. doi: 10.1097/LBR.0000000000000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cascante JA, Cebollero P, Herrero S, et al. Transbronchial cryobiopsy in interstitial lung disease are we on the right path? J Bronchol Interv Pulmonol 2016; 23: 204–209. doi: 10.1097/LBR.0000000000000292 [DOI] [PubMed] [Google Scholar]
- 47.Lentz RJ, Taylor TM, Kropski JA, et al. Utility of flexible bronchoscopic cryobiopsy for diagnosis of diffuse parenchymal lung diseases. J Bronchol Interv Pulmonol 2018; 25: 88–96. doi: 10.1097/LBR.0000000000000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013; 95: 445–452. doi: 10.1016/j.athoracsur.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 49.Sugino K, Otsuka H, Matsumoto Y, et al. The role of video-assisted thoracoscopic surgery in the diagnosis of interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis 2019; 36: 148–156. doi: 10.36141/svdld.v36i2.7797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pastre J, Khandhar S, Barnett S, et al. Surgical lung biopsy for interstitial lung disease – safety and feasibility at a tertiary referral center. Ann Am Thorac Soc 2021; 18: 460–467. doi: 10.1513/AnnalsATS.202006-759OC [DOI] [PubMed] [Google Scholar]
- 51.Cherchi R, Grimaldi G, Pinna-Susnik M, et al. Retrospective outcomes analysis of 99 consecutive uniportal awake lung biopsies: a real standard of care? J Thorac Dis 2020; 12: 4717–4730. doi: 10.21037/jtd-20-1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo Q, Han Q, Chen X, et al. The diagnosis efficacy and safety of video-assisted thoracoscopy surgery (VATS) in undefined interstitial lung diseases: a retrospective study. J Thorac Dis 2013; 5: 283–288. doi: 10.3978/j.issn.2072-1439.2013.04.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kayatta MO, Ahmed S, Hammel JA, et al. Surgical biopsy of suspected interstitial lung disease is superior to radiographic diagnosis. Ann Thorac Surg 2013; 96: 399–401. doi: 10.1016/j.athoracsur.2013.04.065 [DOI] [PubMed] [Google Scholar]
- 54.Morris D, Zamvar V. The efficacy of video-assisted thoracoscopic surgery lung biopsies in patients with interstitial lung disease: a retrospective study of 66 patients. J Cardiothorac Surg 2014; 9: 45. doi: 10.1186/1749-8090-9-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bagheri R, Haghi SZ, Attaran D, et al. Efficacy of minimally invasive surgery in diagnosis of interstitial lung disease. Asian Cardiovasc Thorac Ann 2015; 23: 851–854. doi: 10.1177/0218492315593694 [DOI] [PubMed] [Google Scholar]
- 56.Samejima J, Tajiri M, Ogura T, et al. Thoracoscopic lung biopsy in 285 patients with diffuse pulmonary disease. Asian Cardiovasc Thorac Ann 2015; 23: 191–197. doi: 10.1177/0218492314550724 [DOI] [PubMed] [Google Scholar]
- 57.Khalil M, Cowen M, Chaudhry M, et al. Single versus multiple lung biopsies for suspected interstitial lung disease. Asian Cardiovasc Thorac Ann 2016; 24: 788–791. doi: 10.1177/0218492316665551 [DOI] [PubMed] [Google Scholar]
- 58.Lieberman S, Gleason JB, Ilyas MIM, et al. Assessing the safety and clinical impact of thoracoscopic lung biopsy in patients with interstitial lung disease. J Clin Diagn Res 2017; 11: OC57–OC59. doi: 10.7860/JCDR/2017/20281.9626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeon CS, Yoon DW, Moon SM, et al. Non-intubated video-assisted thoracoscopic lung biopsy for interstitial lung disease: a single-center experience. J Thorac Dis 2018; 10: 3262–3268. doi: 10.21037/jtd.2018.05.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ravaglia C, Bonifazi M, Wells AU, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration 2016; 91: 215–227. doi: 10.1159/000444089 [DOI] [PubMed] [Google Scholar]
- 61.Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020; 8: 171–181. doi: 10.1016/S2213-2600(19)30342-X [DOI] [PubMed] [Google Scholar]
- 62.Romagnoli M, Colby TV, Berthet J-P, et al. Poor concordance between sequential transbronchial lung cryobiopsy and surgical lung biopsy in the diagnosis of diffuse interstitial lung diseases. Am J Respir Crit Care Med 2019; 199: 1249–1256. doi: 10.1164/rccm.201810-1947OC [DOI] [PubMed] [Google Scholar]
- 63.Almeida LM, Lima B, Mota PC, et al. Learning curve for transbronchial lung cryobiopsy in diffuse lung disease. Pulmonology 2018; 24: 23–31. doi: 10.1016/j.rppnen.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 64.Lutz D, Gazdhar A, Lopez-Rodriguez E, et al. Alveolar derecruitment and collapse induration as crucial mechanisms in lung injury and fibrosis. Am J Respir Cell Mol Biol 2015; 52: 232–243. doi: 10.1165/rcmb.2014-0078OC [DOI] [PubMed] [Google Scholar]
- 65.Wasnick RM, Korfei M, Piskulak K, et al. Restored alveolar epithelial differentiation and reversed human lung fibrosis upon Notch inhibition. bioRxiv 2019; preprint [ 10.1101/580498]. [DOI] [Google Scholar]
- 66.Nishimoto K, Fujisawa T, Yoshimura K, et al. The prognostic significance of pneumothorax in patients with idiopathic pulmonary fibrosis. Respirology 2018; 23: 519–525. doi: 10.1111/resp.13219 [DOI] [PubMed] [Google Scholar]
- 67.Yamazaki R, Nishiyama O, Gose K, et al. Pneumothorax in patients with idiopathic pulmonary fibrosis: a real-world experience. BMC Pulm Med 2021; 21: 5. doi: 10.1186/s12890-020-01370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravaglia C, Wells AU, Tomassetti S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med 2019; 19: 16. doi: 10.1186/s12890-019-0780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharp C, McCabe M, Adamali H, et al. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease – a systematic review and cost analysis. QJM 2017; 110: 207–214. doi: 10.1093/qjmed/hcw142 [DOI] [PubMed] [Google Scholar]
- 70.Johannson KA, Marcoux VS, Ronksley PE, et al. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. A systematic review and metaanalysis. Ann Am Thorac Soc 2016; 13: 1828–1838. doi: 10.1513/AnnalsATS.201606-461SR [DOI] [PubMed] [Google Scholar]
- 71.Iftikhar IH, Alghothani L, Sardi A, et al. Transbronchial lung cryobiopsy and video-assisted thoracoscopic lung biopsy in the diagnosis of diffuse parenchymal lung disease. A meta-analysis of diagnostic test accuracy. Ann Am Thorac Soc 2017; 14: 1197–1211. doi: 10.1513/AnnalsATS.201701-086SR [DOI] [PubMed] [Google Scholar]
- 72.Ravaglia C, Wells AU, Tomassetti S, et al. Transbronchial lung cryobiopsy in diffuse parenchymal lung disease: comparison between biopsy from 1 segment and biopsy from 2 segments – diagnostic yield and complications. Respiration 2017; 93: 285–292. doi: 10.1159/000456671 [DOI] [PubMed] [Google Scholar]
- 73.Raghu G, Wilson KC, Bargagli E, et al. Diagnosis of hypersensitivity pneumonitis in adults: an official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2020; 202: E36–E69. doi: 10.1164/rccm.202005-2032ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fishbein MC. Diagnosis: to biopsy or not to biopsy: assessing the role of surgical lung biopsy in the diagnosis of idiopathic pulmonary fibrosis. Chest 2005; 128: Suppl. 1, 520S–525S. doi: 10.1378/chest.128.5_suppl_1.520S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0280-2021.SUPPLEMENT (582.3KB, pdf)