Abstract
Persistent breathlessness >28 days after acute COVID-19 infection has been identified as a highly debilitating post-COVID symptom. However, the prevalence, risk factors, mechanisms and treatments for post-COVID breathlessness remain poorly understood. We systematically searched PubMed and Embase for relevant studies published from 1 January 2020 to 1 November 2021 (PROSPERO registration number: CRD42021285733) and included 119 eligible papers. Random-effects meta-analysis of 42 872 patients with COVID-19 reported in 102 papers found an overall prevalence of post-COVID breathlessness of 26% (95% CI 23–29) when measuring the presence/absence of the symptom, and 41% (95% CI 34–48) when using Medical Research Council (MRC)/modified MRC dyspnoea scale. The pooled prevalence decreased significantly from 1–6 months to 7–12 months post-infection. Post-COVID breathlessness was more common in those with severe/critical acute infection, those who were hospitalised and females, and was less likely to be reported by patients in Asia than those in Europe or North America. Multiple pathophysiological mechanisms have been proposed (including deconditioning, restrictive/obstructive airflow limitation, systemic inflammation, impaired mental health), but the body of evidence remains inconclusive. Seven cohort studies and one randomised controlled trial suggested rehabilitation exercises may reduce post-COVID breathlessness. There is an urgent need for mechanistic research and development of interventions for the prevention and treatment of post-COVID breathlessness.
Short abstract
A sizable proportion of patients with COVID-19 experienced post-COVID breathlessness, and the prevalence estimate varied by population characteristics and methodological approaches. Further research on mechanisms and interventions for this sequela is needed. https://bit.ly/3P5ayv6
Introduction
Long-COVID or post-COVID syndrome, defined as ongoing otherwise unexplained symptoms lasting for over 4 weeks after getting COVID-19 [1], has become an urgent international health challenge. Persistent breathlessness is one of the most prevalent and debilitating symptoms experienced by COVID-19 survivors with long-COVID [2]. In this paper, we used the term post-COVID breathlessness to describe the experience of breathlessness >28 days following acute COVID-19 infection.
Several meta-analyses on post-COVID symptoms have found that between 24–37% of hospitalised and nonhospitalised patients with COVID-19 experienced short-term persistent breathlessness (2 weeks to 7 months post-COVID) [2–7]. However, most previous meta-analyses have not distinguished the prevalence estimate of post-COVID breathlessness by different definitions of persistent breathlessness, initial severities of illness, follow-up lengths and demographic characteristics. With a fast-growing body of data on post-COVID breathlessness now becoming available, a detailed synthesis of longer-term follow-up data (especially 7–12 months post-COVID) is required to better understand the natural history of post-COVID breathlessness and provide evidence to guide ongoing healthcare provision.
Furthermore, there is an urgent need to better understand risk factors and characterise underlying mechanisms of post-COVID breathlessness. These important evidence gaps make it challenging to develop targeted interventions or rehabilitation therapies for at-risk or affected patients [8].
To inform these deliberations, we conducted a systematic review and meta-analysis to comprehensively evaluate the prevalence of post-COVID breathlessness across different populations, using differing criteria and methodological approaches, and investigate changes in prevalence over time. We also synthesised data from studies that examined risk factors, mechanisms and potential interventions for post-COVID breathlessness to inform strategies for prevention and clinical management of post-COVID breathlessness.
Methods
Registration and reporting
The study protocol is registered in PROSPERO with the registration number: CRD42021285733. The reporting of this study followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guideline [9].
Search strategy
We systematically searched PubMed and Embase databases to identify studies that reported data on persistent breathlessness in patients with COVID-19 published in English between 1 January 2020 and 1 November 2021. Studies were identified using search terms related to COVID-19, long-term follow-up and breathlessness (or dyspnoea). Detailed search strategies and procedures are presented in the supplementary material.
Inclusion/exclusion criteria
For the estimation of prevalence of post-COVID breathlessness in COVID-19 survivors, studies were included if they used a cohort, cross-sectional or case series design and reported data required for meta-analyses. To comply with this criterion of long-COVID symptoms [1] and our practical definition of post-COVID breathlessness, we only included studies with a mean/median follow-up time of >28 days after acute COVID-19 infection, symptom onset, initial COVID-19 diagnosis/positive test, or hospital discharge, depending on the follow-up time information provided by individual studies. Studies were excluded from meta-analysis if they did not report follow-up time, only recruited participants who had residual symptoms/complications following COVID-19 (e.g. all study participants were sufferers of post-COVID syndrome), or only recruited participants with a specific comorbidity. We also excluded studies with ≤50 COVID-19 survivors because of concerns regarding the precision and potential bias of prevalence estimates. For studies using the Medical Research Council (MRC) or modified MRC (mMRC) dyspnoea scale, we excluded those using cut-off points different from mMRC score ≥1 (or equivalently, MRC score ≥2) to make sure the prevalence estimates were comparable across included studies.
For the synthesis of evidence on risk factors and mechanisms for post-COVID breathlessness, we included relevant cohort, case–control, cross-sectional or case series studies. Cohort/case–control studies and randomised/nonrandomised trials investigating the effectiveness of interventions for post-COVID breathlessness were also included.
Data extraction
We extracted the following information from eligible studies for meta-analysis: 1) sample size and prevalence of post-COVID breathlessness; 2) definition of post-COVID breathlessness and relevant scales and cut-off points; 3) methodological characteristics (i.e. follow-up period, source of study population, follow-up method); 4) population characteristics (i.e. country, age, sex, ethnicity/race, severity of COVID-19 infection); and 5) subgroup prevalence estimates (e.g. by sex). Graphical prevalence data [10–14] were extracted using PlotDigitizer (www.plotdigitizer.sourceforge.net/).
The following data from studies on risk factors, mechanisms, or interventions were also extracted: 1) sample size, population characteristics, and follow-up time and method; 2) assessed risk factors, clinical parameters, or interventions; 3) definition of post-COVID breathlessness; and 4) statistical methods and results.
Study quality assessment
The study quality of included papers for meta-analyses was evaluated using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Studies Reporting Prevalence Data [15]. We assigned an overall quality rating of high, moderate or low to each study after a qualitative evaluation based on the nine items in the checklist.
Data analysis and synthesis
Random-effects meta-analyses were conducted to estimate the pooled prevalence of post-COVID breathlessness and its confidence interval [16] in COVID-19 survivors. In consideration of the heterogeneity in the definition and measurement of breathlessness, we separated the overall analysis by three definition categories: studies that did not use a breathlessness scale (i.e. directly measuring the presence or absence of the symptom); studies that used the MRC or the mMRC dyspnoea scale; and studies using other scales to measure breathlessness. Study-specific 95% CIs were estimated using the Wilson's score CI method [16]. The heterogeneity of prevalence estimates across studies was assessed by the I2 statistic and Q test. A funnel plot for the prevalence estimate after logit transformation was created to identify publication bias, followed by an Egger's test.
For studies directly measuring the presence or absence of the symptom, we also stratified the meta-analysis by follow-up periods, i.e. 1–6 months and ≥7 months, and by hospitalised and nonhospitalised patients. Further subgroup meta-analyses were conducted to investigate potential risk factors for post-COVID breathlessness and potential sources of heterogeneity, such as mean/median age, sex, continent of study population, definition of post-COVID breathlessness (whether defined as new/worse breathlessness than pre-COVID baseline level or not), severity of COVID-19 infection (intubation/intensive care unit (ICU)/World Health Organization (WHO) clinical progression scale [17] ≥6 versus nonsevere) and follow-up method. Between-group heterogeneity was tested based on Q statistics and DerSimonian-Laird subgroup weights [16]. In the subgroup analyses by sex and severity of infection, since several studies contributed to multiple estimates of breathlessness prevalence (e.g. a single study reported the prevalence estimates for men and women separately), multilevel meta-analysis models with study ID as random effects were used to address the intra-study correlation [18]. No subgroup meta-analyses were conducted for studies using MRC/mMRC dyspnoea scale or other scales due to insufficient number of eligible studies.
Several sensitivity analyses were performed by: 1) excluding studies with any children/adolescents (<18 years old); 2) excluding studies in which at least one patient was followed for ≤28 days (e.g. a study reported follow-up data between 14–176 days post-infection [19]); 3) excluding studies rated as low quality; 4) excluding studies based on electronic health record data to reduce methodological heterogeneity; and 5) repeating the meta-analyses using prevalence estimates after logit transformation or Freeman-Tukey double arcsine transformation [16]. We also assessed the influence of each study by recalculating the pooled prevalence after removing that study.
Finally, data from studies on risk factors, mechanisms or interventions of post-COVID breathlessness were qualitatively synthesised due to substantial methodological heterogeneity or limited number of studies.
Statistical analyses were performed using Stata (version 14, StataCorp, College Station, TX) and the metafor package [18] in R (version 4.1.2, R Core Team). All statistical tests were two-sided and the significance level was defined as p<0.05.
Results
Search results and study characteristics
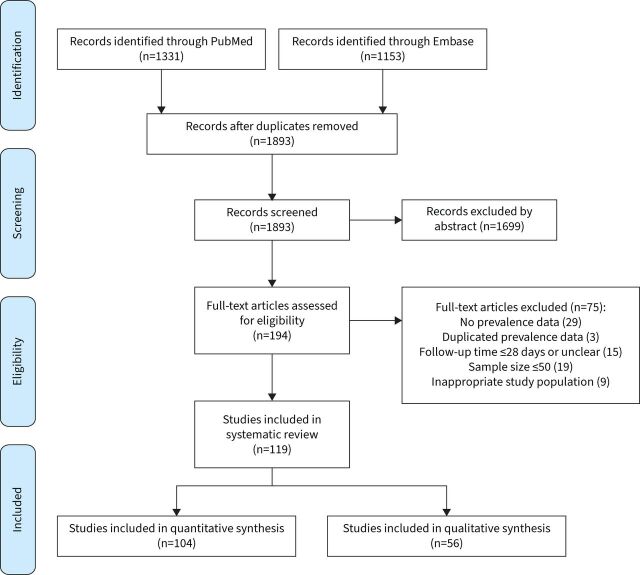
The literature search yielded 1 893 records. After screening, 119 eligible papers remained, of which 104 were included in the meta-analyses (two papers were only included in the subgroup analyses) [10–14, 19–27, 29–44, 47–50, 52–66, 77, 85–138]; 56 were included in the qualitative synthesis (41 contributed to both) (figure 1).
FIGURE 1.
PRISMA flowchart.
Characteristics of the 104 papers included in meta-analyses are presented in table 1. Two pairs of papers [20–23] reported follow-up data from identical/overlapping populations, but at different time points, thus two papers [21, 23] were only included in subgroup meta-analyses but not the overall meta-analysis (i.e. leaving 102 papers). Most studies were conducted in the UK (n=16), Italy (n=12), China (n=11), the USA (n=11), Spain (n=7), France (n=8) and Turkey (n=5). The sample size of the included papers varied from 55 to 9 816 (median 154). The mean/median follow-up length ranged from 1–12 months. Most papers reported follow-up data of hospitalised patients with COVID-19 (n=61) or mixed samples of hospitalised/nonhospitalised patients (n=34). Most studies recruited adult patients only, while only two studies [24, 25] focused on children/adolescents with COVID-19 and seven studies included a small proportion of children/adolescents. Thirty-seven papers used clinical scales to measure post-COVID breathlessness, such as the MRC/mMRC dyspnoea scale (n=22) and the New York Heart Association Functional Classification (n=3) which are functional measures of breathlessness rather than direct measures of presence of the symptom. Twenty-nine papers used self-reported breathlessness level before COVID-19 as a baseline when assessing post-COVID breathlessness. Only seven papers included a control group without COVID-19. The most common follow-up method was in-person research/clinic visit (n=54), followed by phone interview (n=27) and online survey (n=7); three studies were based on electronic health records.
TABLE 1.
Study characteristics of 104 papers included in the meta-analyses
| First author | Sample size | Country | Mean/median age, years | Only adults | Male proportion | Scale for breathlessness | Compared with pre-COVID level | Breathlessness prevalence | Hospitalisation | Follow-up method | Follow-up period, months |
| Abdelrahman [86] | 172 | Egypt | 42 | Yes | 0.34 | Yes | 0.22 | Mixed | Phone | – | |
| Anaya [49] | 100 | Colombia | 49 | Yes | 0.47 | No | 0.24 | Mixed | Visit | 7–12 | |
| Aparisi [26] | 70 | Spain | 55 | Yes | 0.36 | NYHA functional class ≥2 | No | 0.586 | Mixed | Visit | 1–6 |
| Ares-Blanco [87] | 155 | Spain | 59 | Yes | 0.48 | No | 0.31 | Mixed | Phone | 1–6 | |
| Armange [88] | 214 | France | 39 | Yes | 0.40 | No | 0.402 | Nonhosp | Online | 1–6 | |
| Arnold [89] | 110 | UK | 55 | Yes | 0.62 | No | 0.39 | Hosp | Visit | 1–6 | |
| Asadi-Pooya [24] | 58 | Iran | 12 | No | 0.48 | Yes | 0.12 | Hosp | Phone | – | |
| Augustin [90] | 442 (4 months), 353 (7 months) | Germany | 43 | Yes | 0.48 | No | 0.086 (4 months), 0.136 (7 months) | Nonhosp | Visit | 1–6, 7–12 | |
| Aul [91] | 387 (370) | UK | 63 | Yes | 0.57 | No | 0.365 | Hosp | Phone | 1–6 | |
| Aydin [63] | 116 | Turkey | 49 | No | 0.48 | No | 0.19 | Hosp | Phone | 1–6 | |
| Baldini [62] | 55 | Argentina | 55 | – | 0.73 | No | 0.55 | Hosp | Visit | 1–6 | |
| Bell [92] | 303 | USA | 44 | No | 0.30 | No | 0.257 | Nonhosp | Online | 1–6 | |
| Blomberg [93] | 312 | Norway | 46 | No | 0.49 | No | 0.21 | Mixed | Visit | 1–6 | |
| Boari [94] | 94 | Italy | 71 | – | 0.67 | Yes | 0.36 | Hosp | Visit | 1–6 | |
| Çalik Kütükcü [95] | 100 | Turkey | 37 | Yes | 0.41 | No | 0.58 | Mixed | Visit | 1–6 | |
| Carfì [96] | 143 | Italy | 57 | Yes | 0.63 | No | 0.434 | Hosp | Visit | 1–6 | |
| Carvalho-Schneider [97] | 150 | France | 49 | Yes | 0.44 | Yes | 0.367 | Mixed | Phone | 1–6 | |
| Cheng [98] | 113 | UK | 58 | Yes | 0.68 | MRC increase | Yes | 0.363 | Hosp | Visit | 1–6 |
| Cortés-Telles [59] | 186 | Mexico | 47 | – | 0.61 | No | 0.376 | Mixed | Visit | 1–6 | |
| Damanti [99] | 67 | Italy | 63 | – | 0.85 | mMRC ≥1 | No | 0.478 | Hosp | Visit | 1–6 |
| Dankowski [100] | 102 | Poland | 52 | Yes | 0.44 | No | 0.422 | Mixed | Visit | 1–6 | |
| Darcis [101] | 199 | Belgium | 61 | Yes | 0.63 | No | 0.47 | Hosp | Visit | 1–6 | |
| Daynes [10] | 131 | UK | 60 | – | 0.59 | COPD assessment test | No | 0.731 | Hosp | Phone | 1–6 |
| D'Cruz [35] | 119 (115) | UK | 59 | Yes | 0.62 | mMRC increase, numerical rating scale ≥4 | Yes | 0.443, 0.322 | Hosp | Visit | 1–6 |
| de Graaf [61] | 81 | Netherlands | 61 | Yes | 0.63 | NYHA functional class ≥2 | No | 0.617 | Hosp | Visit | 1–6 |
| Diaz-Fuentes [102] | 111 | USA | 60 | Yes | 0.47 | No | 0.559 | Mixed | Visit | 1–6 | |
| Dreyer [103] | 1518 (977) | USA | 42 | Yes | 0.12 | No | 0.119 | Mixed | Online | 1–6 | |
| Erol [25] | 121 | Turkey | 9 | No | 0.54 | No | 0.0826 | Mixed | Visit | 1–6 | |
| Evans [104] | 1077 (767) | UK | 58 | Yes | 0.64 | Numerical rating scale increase | Yes | 0.481 | Hosp | Visit | 1–6 |
| Faverio [50] | 312 (283) | Italy | 62 | Yes | 0.73 | mMRC ≥1 | No | 0.31 | Hosp | Visit | 1–6 |
| Fernández-de-Las-Peñas [105] | 1950 | Spain | 61 | – | 0.53 | Yes | 0.233 | Hosp | Phone | 7–12 | |
| Fortini [36] | 59 | Italy | 68 | – | 0.53 | Yes | 0.373 | Hosp | Visit | 1–6 | |
| Froidure [37] | 126 | Belgium | 60 | – | 0.59 | mMRC ≥1 | No | 0.357 | Hosp | Visit | 1–6 |
| Gaber [106] | 138 | UK | – | Yes | 0.08 | No | 0.399 | Nonhosp | Online | 1–6 | |
| Galván-Tejada [19] | 141 | Mexico | 39 | – | 0.49 | No | 0.099 | – | – | 1–6 | |
| Gamberini [55] | 178 | Italy | 64 | – | 0.73 | mMRC ≥1 | No | 0.584 | Hosp | Visit | 7–12 |
| Garrigues [53] | 120 | France | 63 | – | 0.63 | mMRC ≥1, no | No | 0.533, 0.417 | Hosp | Phone | 1–6 |
| Gautam [42] | 200 (144) | UK | 57 | – | 0.63 | mMRC ≥1 | No | 0.632 | Hosp | Visit | – |
| Ghosn [11] | 948 (3 months), 1065 (6 months) | France | 61 | – | 0.63 | No | 0.304 (3 months), 0.263 (6 months) | Hosp | Visit | 1–6 | |
| González [107] | 60 | Spain | 60 | Yes | 0.74 | mMRC ≥1 | No | 0.467 | Hosp | Visit | 1–6 |
| Halpin [108] | 100 | UK | 67 | Yes | 0.54 | Likert scale increase | Yes | 0.5 | Hosp | Phone | 1–6 |
| Horwitz [21] | 126 | USA | 62 | Yes | 0.60 | PROMIS® dyspnea characteristics instrument ≥1 | No | 0.63 | Hosp | Online/phone | 7–12 |
| Huang [22] | 1615 | China | 57 | Yes | 0.52 | mMRC ≥1 | No | 0.259 | Hosp | Visit | 1–6 |
| Huang [23] | 1276 (1271) | China | 59 | Yes | 0.53 | mMRC ≥1 | No | 0.3 | Hosp | Visit | 7–12 |
| Huang [12] | 382 | USA | 55 | No | 0.41 | Yes | 0.17 | Nonhosp | EHR | – | |
| Italia [43] | 123 | Italy | 62 | – | 0.68 | NYHA functional class ≥2 | No | 0.341 | Hosp | Visit | 1–6 |
| Jacobs [109] | 128 | USA | 57 | Yes | 0.62 | No | 0.453 | Hosp | Online/phone | 1–6 | |
| Karaarslan [110] | 300 | Turkey | 53 | Yes | 0.60 | Likert scale | No | 0.263 | Hosp | Phone | 1–6 |
| Klein [111] | 103 | Israel | 35 | Yes | 0.62 | Yes | 0.078 | – | Phone | 1–6 | |
| Landi [112] | 131 | Italy | 56 | Yes | 0.61 | Yes | 0.44 | Hosp | Visit | 1–6 | |
| Lerum [29] | 103 | Norway | 59 | Yes | 0.52 | mMRC ≥1 | No | 0.54 | Hosp | Visit | 1–6 |
| Liang [60] | 76 | China | 41 | Yes | 0.28 | 0–4 grade ≥1 | No | 0.605 | Hosp | Visit | 1–6 |
| Lindahl [30] | 101 (93) | Finland | 60 | Yes | 0.53 | mMRC ≥1 | No | 0.645 | Hosp | Online/printed | 1–6 |
| Lund [77] | 9816 | Denmark | 50 | No | 0.42 | Yes | 0.014 | Mixed | EHR | – | |
| Maestre-Muñiz [113] | 543 | Spain | 65 | Yes | 0.51 | Yes | 0.193 | Mixed | Phone | 7–12 | |
| Mahmud [114] | 355 | Bangladesh | 40 | Yes | 0.58 | Yes | 0.07 | Hosp | Phone | 1–6 | |
| Mallia [115] | 401 | UK | 59 | Yes | 0.60 | MRC increase, no | Yes | 0.408, 0.464 | Mixed | Visit | 1–6 |
| Mandal [116] | 384 | UK | 60 | – | 0.62 | 0–10 scale ≥1 | No | 0.53 | Hosp | Phone/visit | 1–6 |
| Mechi [44] | 112 | Iraq | 51 | – | 0.66 | No | 0.3036 | Mixed | Visit | 7–12 | |
| Meije [56] | 294 | Spain | 69 | Yes | 0.57 | No | 0.299 | Hosp | Visit | 1–6 | |
| Menges [31] | 431 (395) | Switzerland | 47 | Yes | 0.50 | mMRC ≥1 | No | 0.24 | Mixed | Online | 7–12 |
| Moradian [117] | 200 | Iran | 56 | – | 0.80 | No | 0.185 | Hosp | Phone | 1–6 | |
| COMEBAC Study Group [85] | 478 | France | 61 | Yes | 0.42 | Yes | 0.163 | Hosp | Phone | 1–6 | |
| Motiejunaite [64] | 114 | France | 57 | Yes | 0.67 | No | 0.4 | Mixed | Visit | 1–6 | |
| Mumoli [118] | 88 | Italy | 63 | Yes | 0.74 | No | 0.494 | Hosp | Visit | 1–6 | |
| Munblit [119] | 2649 (2620) | Russia | 56 | Yes | 0.49 | No | 0.174 | Hosp | Phone | 7–12 | |
| Naik [54] | 1234 | India | 41 | Yes | 0.69 | No | 0.061 | Mixed | Visit/phone | 1–6 | |
| Nehme [120] | 479 (30–45 days), 410 (7–9 months) | Switzerland | 43 | Yes | 0.38 | Yes | 0.111 (30–45 days), 0.117 (7–9 months) | Nonhosp | Online/phone | 1–6, 7–12 | |
| O'Keefe [121] | 290 | USA | 44 | Yes | 0.25 | No | 0.141 | Mixed | Online | 1–6 | |
| O'Sullivan [122] | 155 | UK | 39 | – | 0.82 | No | 0.767 | Mixed | Phone | 1–6 | |
| Peluso [13] | 143 (4 months), 68 (8 months) | USA | 48 | Yes | 0.56 | Yes | 0.224 (4 months), 0.206 (8 months) | Mixed | Visit | 1–6, 7–12 | |
| Qin [47] | 647 | China | 58 | – | 0.44 | Yes | 0.087 | Hosp | Visit | 1–6 | |
| Raman [48] | 58 | UK | 55 | – | 0.59 | MRC ≥2 | No | 0.643 | Hosp | Visit | 1–6 |
| Righi [123] | 448 | Italy | 56 | Yes | 0.55 | Yes | 0.11 | Mixed | Phone/visit | 1–6 | |
| Riou [52] | 81 | France | 61 | – | 0.73 | No | 0.2 | Hosp | Visit | 1–6 | |
| Sathyamurthy [124] | 279 | India | 71 | Yes | 0.64 | No | 0.018 | Hosp | Phone | 1–6 | |
| Seeßle [41] | 146 (5 months), 96 (12 months) | Germany | 57 | Yes | 0.49 | Yes | 0.271 (5 months), 0.375 (12 months) | Mixed | Visit | 1–6, 7–12 | |
| Shah [32] | 73 | Canada | 65 | Yes | 0.60 | UCSD-SOBQ >10 | No | 0.425 | Hosp | Visit | 1–6 |
| Shang [27] | 796 | China | 62 | Yes | 0.51 | No | 0.204 | Hosp | Phone | 1–6 | |
| Shendy [125] | 81 | Egypt | 34 | Yes | 0.32 | Numerical rating scale (0–10) ≥1 | No | 0.741 | Mixed | Phone | 1–6 |
| Shoucri [126] | 364 | USA | 61 | Yes | 0.52 | No | 0.159 | Hosp | EHR | 1–6 | |
| Sigfrid [33] | 327 | UK | 60 | Yes | 0.59 | MRC increase | Yes | 0.468 | Hosp | Post/phone/visit | 7–12 |
| Skjorten [38] | 156 (126) | Norway | 56 | Yes | 0.62 | mMRC ≥1 | No | 0.47 | Hosp | Visit | 1–6 |
| Sonnweber [58] | 145 (133) | Austria | 57 | Yes | 0.55 | No | 0.36 | Mixed | Visit | 1–6 | |
| Stavem [127] | 451 | Norway | 50 | Yes | 0.44 | No | 0.16 | Nonhosp | Post/online | 1–6 | |
| Suárez-Robles [128] | 134 | Spain | 59 | – | 0.46 | No | 0.403 | Hosp | Phone | 1–6 | |
| Sultana [129] | 186 | Bangladesh | 35 | Yes | 0.66 | No | 0.102 | Mixed | Phone | 1–6 | |
| Sun [130] | 932 | China | 58 | No | 0.40 | No | 0.072 | Hosp | Phone | 1–6 | |
| Szekely [66] | 71 | Israel | 53 | Yes | 0.66 | No | 0.225 | Mixed | Visit | 1–6 | |
| Tawfik [14] | 120 | Egypt | 34 | Yes | 0.42 | No | 0.647 | Mixed | – | 1–6 | |
| Taylor [131] | 675 | UK | 56 | – | 0.58 | MRC increase, no | Yes | 0.578, 0.344 | Mixed | Online/phone | 1–6 |
| Todt [132] | 251 | Brazil | 53 | Yes | 0.60 | mMRC increase | Yes | 0.279 | Hosp | Phone | 1–6 |
| Tosato [133] | 165 | Italy | 73 | Yes | 0.62 | No | 0.515 | Hosp | Visit | 1–6 | |
| Varghese [134] | 116 | Germany | 41 | Yes | 0.85 | No | 0.06 | Mixed | Visit | 1–6 | |
| Venturelli [135] | 767 | Italy | 63 | Yes | 0.67 | mMRC ≥1, no | No | 0.298, 0.218 | Mixed | Visit | 1–6 |
| Vijayakumar [65] | 80 | UK | 59 | Yes | 0.66 | No | 0.46 | Hosp | Visit | 1–6 | |
| Weerahandi [20] | 152 | USA | 62 | Yes | 0.63 | PROMIS® dyspnea characteristics instrument ≥1 | No | 0.743 | Hosp | Online/phone | 1–6 |
| Wu [136] | 132 | China | 45 | No | 0.55 | mMRC ≥1 | No | 0.068 | Hosp | Visit | 1–6 |
| Wu [57] | 83 | China | 60 | Yes | 0.57 | mMRC ≥1 | No | 0.81 (3 months), 0.05 (12 months) | Hosp | Visit | 1–6, 7–12 |
| Yin [40] | 337 | China | 54 | – | 0.51 | 0–4 grade ≥1 | Yes | 0.27 | Hosp | Visit | 7–12 |
| Yomogida [34] | 366 | USA | 39 | Yes | 0.43 | No | 0.128 (2 months), 0.104 (7 months) | Mixed | Phone | 1–6, 7–12 | |
| Zayet [137] | 354 | France | 50 | Yes | 0.37 | Yes | 0.11 | Mixed | Online | 7–12 | |
| Zhang [39] | 2433 | China | 60 | Yes | 0.50 | Yes | 0.041 | Hosp | Phone | 7–12 | |
| Zhao [138] | 55 | China | 48 | Yes | 0.58 | No | 0.1455 | Hosp | Visit | 1–6 |
Sample sizes in brackets refer to valid cases in the prevalence calculation. Studies that did not use a scale to measure breathlessness defined it as the presence/absence of the symptom. NYHA: New York Heart Association; Nonhosp: nonhospitalised patients; Hosp: hospitalised patients; MRC: Medical Research Council Dyspnoea Scale; mMRC: modified Medical Research Council Dyspnoea Scale; UCSD-SOBQ: University of California San Diego–Shortness of Breath Questionnaire; EHR: electronic health record.
Quality assessment
The study quality of most papers for meta-analysis was rated as moderate (n=49) or low (n=32), while 23 studies had high overall quality [15]. The commonest sources of potential bias were lack of representativeness of the target population, small sample size, low response rate, lack of validated measures for breathlessness, and lack of reliable follow-up methods (supplementary table 1).
Prevalence of post-COVID breathlessness among COVID-19 survivors
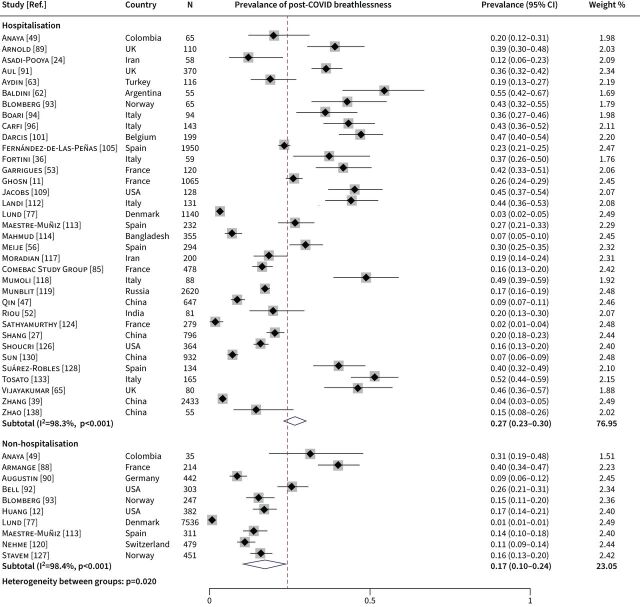
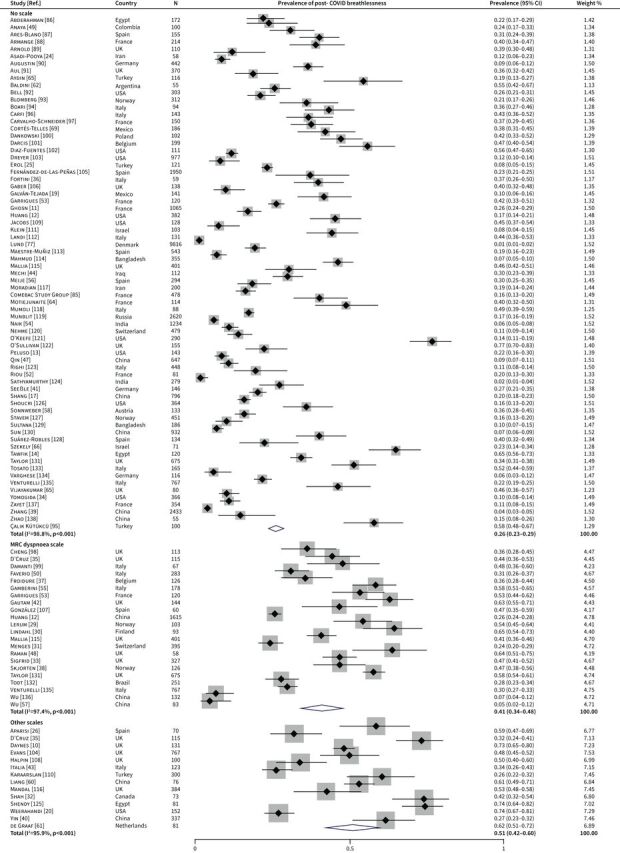
After excluding two papers reporting data from duplicated populations [21, 23], data from the remaining 102 papers with a total of 42 872 COVID-19 survivors were synthesised in the meta-analysis. The meta-analysis was conducted separately by different definitions of breathlessness: no scale (71 papers), MRC or mMRC dyspnoea scale (22 papers) and other scales (14 papers); 5 of the 102 papers measured post-COVID breathlessness using more than one definition. The pooled prevalence of post-COVID breathlessness was 26% (95% CI 23–29) in studies directly measuring presence or absence of the symptom, 41% (95% CI 34–48) in studies using MRC/mMRC dyspnoea scale and 51% (95% CI 42–60) in studies using other scales (figure 2). Substantial heterogeneity across studies was observed in each of the three meta-analyses (I2=98.8%, 97.4% and 95.9%, respectively; p<0.001).
FIGURE 2.
Forest plot for the overall prevalence of post-COVID breathlessness by definition of breathlessness. MRC: Medical Research Council Dyspnoea Scale.
We further differentiated between hospitalised and nonhospitalised COVID-19 survivors for studies directly measuring presence or absence of the symptom. There were 35 papers reporting data from hospitalised patients and 10 from nonhospitalised patients, of which four papers with mixed samples reported subgroup prevalence estimates by hospitalisation or not. The subgroup meta-analysis demonstrated a higher prevalence of post-COVID breathlessness in hospitalised patients (27%, 95% CI 23–30) compared with nonhospitalised patients (17%, 95% CI 10–24) (figure 3). The between-subgroup heterogeneity was statistically significant (p=0.020).
FIGURE 3.
Forest plot for the prevalence of post-COVID breathlessness in hospitalised versus nonhospitalised patients.
We also compared papers reporting follow-up data at 1–6 months (n=60) and 7–12 months post-COVID (n=14), of which six papers reported data from multiple time points. This subgroup meta-analysis showed a decreased prevalence of post-COVID breathlessness over time, with estimates of 28% (95% CI 25–32) and 20% (95% CI 15–26), respectively (figure 4). Significant between-subgroup heterogeneity was detected (p=0.014).
FIGURE 4.
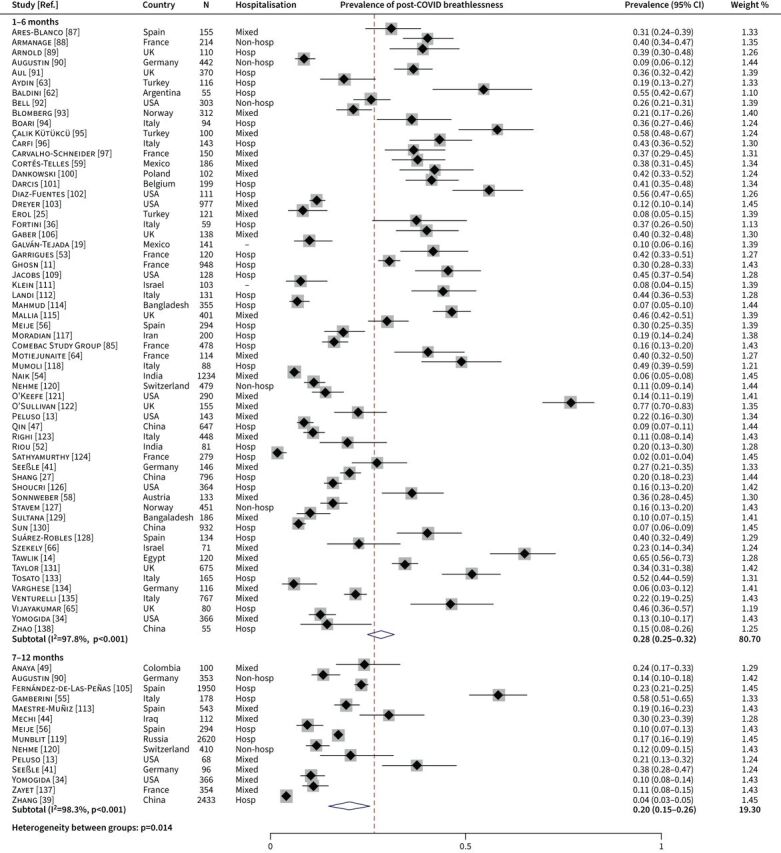
Forest plot for the prevalence of post-COVID breathlessness at different follow-up time points. Hosp: hospitalised patients; Nonhosp: nonhospitalised patients.
Both the funnel plots (supplementary figure 1) and Egger's tests (p>0.10) suggested no evidence of publication bias. The results of the sensitivity analyses showed prevalence estimates consistent with the main analyses (supplementary table 2). The influential analysis indicated no single study had a major impact on the pooled prevalence estimate (supplementary table 3).
Subgroup analyses for risk factors of post-COVID breathlessness
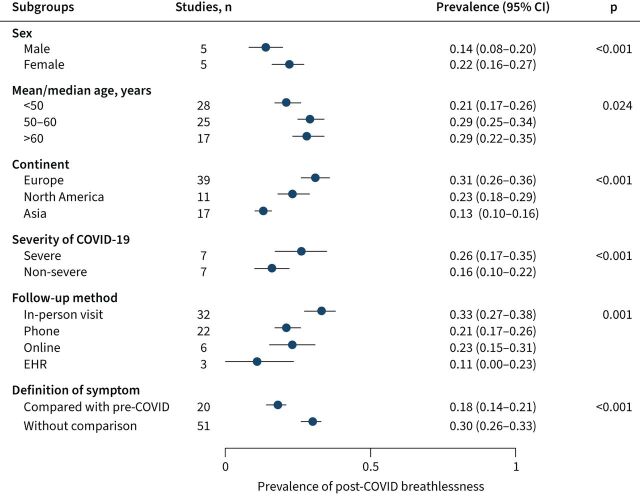
Further subgroup meta-analyses were conducted in studies directly measuring presence or absence of the symptom (i.e. without scales). Based on the sex-specific data reported from five papers, female survivors were more likely to report post-COVID breathlessness than males (22% versus 14%; p<0.001) (figure 5). Studies with mean/median patient age below 50 years reported lower prevalence of post-COVID breathlessness than studies with the mean/median patient age between 50–60 years or above 60 years (21% versus 29% or 29%, respectively; p=0.024). Patients in studies conducted in Asia had lower prevalence of post-COVID breathlessness than patients in Europe or North America (13% versus 31% or 23%; p<0.001). Patients with severe/critical COVID-19 infection (intubation/ICU/WHO scale [17] ≥6) had higher prevalence of post-COVID breathlessness than nonsevere patients (26% versus 16%; p<0.001).
FIGURE 5.
Subgroup meta-analyses for the prevalence of post-COVID breathlessness. Between-group heterogeneity was tested based on Q statistics and DerSimonian-Laird subgroup weights (except for a multilevel meta-analysis for heterogeneity by sex or severity of COVID-19). EHR: electronic health record.
As for methodological heterogeneity, the prevalence of post-COVID breathlessness varied by different follow-up methods (figure 5). The pooled prevalence estimates were 33%, 21% and 23% for in-person visit, phone interview and online survey, whereas the estimate based on electronic health record data was much lower (11%) despite reflecting period prevalence instead of point prevalence. In addition, studies that defined post-COVID breathlessness in comparison to recalled pre-COVID breathlessness level reported a lower prevalence than the other studies that defined post-COVID breathlessness based solely on the post-COVID level (18% versus 30%; p<0.001).
Qualitative synthesis of studies on risk factors, mechanisms and treatments of post-COVID breathlessness
Of the 56 papers included in the qualitative synthesis (supplementary tables 4–5), 46 reported data on risk factors or mechanisms of post-COVID breathlessness and 10 evaluated rehabilitation interventions or potential therapies.
Inconsistent results for multiple risk factors were reported. Nine studies [26–34] showed a higher prevalence of post-COVID breathlessness in female patients than males (four with statistical significance and five with borderline statistical significance), but another five studies [35–39] did not detect an association and one study [40] showed the opposite association. Two studies [39, 40] showed a positive association between age and post-COVID breathlessness; one study [41] reported that patients aged between 50–59 years were more likely to report post-COVID breathlessness than those aged 60 years and over; and 11 studies [26, 27, 29, 31–38] did not detect a significant association with age. Four papers [31, 32, 39, 40] found no association between smoking and post-COVID breathlessness. Several studies identified that obesity (n=3) [31, 38, 42], hypertension (n=2) [39, 42], cardiovascular diseases (n=2) [42, 43] or diabetes (n=2) [42, 44] was significantly associated with higher risk of post-COVID breathlessness; however, two [35, 45], four [26, 28, 32, 35], four [26, 28, 32, 39] and six studies [26, 32, 35, 38, 39, 46], respectively, did not detect the associations. Regarding pre-morbid obstructive lung diseases, three studies [35, 39, 42] identified an association with a higher risk of post-COVID breathlessness but another five studies [26, 28, 31, 32, 36] did not. Three studies [28, 31, 40] found a significant association with overall comorbidity while two studies [33, 36] found no association. Clinical severity of acute COVID-19 infection was positively associated with post-COVID breathlessness in five studies [22, 39, 40, 47, 48], but nine studies [27, 31, 36, 37, 42, 49–52] did not identify this association. Only one study [40] (out of eight studies [28, 29, 31, 35, 39, 40, 42, 53]) showed a higher prevalence of post-COVID breathlessness in patients managed in ICU. In contrast, two studies [31, 54] showed a higher prevalence estimate in hospitalised versus nonhospitalised patients, and three [28, 39, 40] and one [55] studies found positive associations with lengths of hospital stay and days of invasive mechanical ventilation. One paper [56] reported that individuals with a ratio of arterial oxygen partial pressure to fractional inspired oxygen (PAO2/ FIO2) <200 during hospitalisation were at higher risk of post-COVID breathlessness. Mixed results were shown for the trajectory of post-COVID breathlessness. Four papers [21, 39, 57, 58] reported a decreased prevalence over time, while two papers [23, 41] found an increased trend and three papers [31, 32, 40] detected no change.
Regarding underlying mechanisms, several papers reported that post-COVID breathlessness was correlated with reduced spirometry parameters (n=5) [32, 42, 58–60], lower diffusion capacity for carbon monoxide (DLCO) (n=7) [32, 36, 55, 58, 59, 61, 62] and lung imaging abnormalities (computed tomography (CT) (n=3) [40, 58, 63], lung ultrasound (n=1) [36], chest radiograph (n=1) [42]), but four [26, 37, 55, 61], four [26, 37, 60, 64] and three papers [35, 37, 65] found no significant associations with these three measurements, respectively. One paper [26] reported that patients with post-COVID breathlessness had reduced exercise capacity based on the 6-minute walk test, lower predicted peak oxygen consumption and worse performance in cardiopulmonary exercise testing; another paper [59] also found a reduced 6-minute walk distance and lower end-exercise oxygen saturation, and two additional papers [38, 66] supported the findings in cardiopulmonary exercise testing. Four papers [26, 32, 61, 66] assessed echocardiogram results during follow-up but only one [66] detected an association with post-COVID breathlessness. One paper [63] identified a correlation with higher C-reactive protein (CRP) level at the follow-up visit, but another paper [42] did not. Three papers [32, 35, 48] reported significant associations between post-COVID breathlessness and symptoms of depression and anxiety, one of which also reported an association with post-traumatic stress disorder [35].
Two randomised controlled trials (RCT) [67, 68] assessed interventions for the prevention or treatment of post-COVID breathlessness. One RCT in Iran [67] showed that among 55 outpatients with mild COVID-19 infection, those receiving sofosbuvir/daclatasvir plus hydroxychloroquine had a lower risk of persistent dyspnoea at 1-month follow-up compared with a control arm receiving hydroxychloroquine alone (15% versus 42%, p=0.035).
The other RCT in China [68] evaluated a home-based telerehabilitation programme for COVID-19 (breathing control and thoracic expansion, aerobic and lower limb muscle strength exercise) and showed that, among 120 formerly hospitalised COVID-19 survivors with residual dyspnoea, the intervention group had a lower mMRC dyspnoea level than controls immediately after the 6-week intervention period (p=0.001), but not at the 28-week follow-up.
Seven observational studies [69–75] also suggested that rehabilitation exercises were associated with reduced persistent breathlessness in hospitalised or mild cases of COVID-19. Another small-scale observational study [76] showed that the use of Pycnogenol-Centellicum supplementation was associated with improved breathlessness after COVID-19.
Discussion
Our meta-analyses showed that 26% of COVID-19 survivors reported the presence of breathlessness symptom >4 weeks post-infection, and 41% of survivors reported reduced physical capacity due to post-COVID breathlessness based on the MRC/mMRC dyspnoea scale. The pooled prevalence of self-reported breathlessness symptom decreased significantly over time from 28% at 1–6 months post-COVID to 20% at 7–12 months. Significant variations in the prevalence estimate were observed across different clinical and population characteristics and methodological approaches.
The overall prevalence estimate was consistent with previous meta-analyses on post-COVID symptoms [2–7]. Post-COVID breathlessness has been associated with reduced quality of life [26], posing limitations to survivors’ everyday life and challenges in returning to normal [35]. The pooled prevalence of post-COVID breathlessness obtained from previous meta-analyses ranged from 24% to 37% among COVID-19 survivors [2–7]. However, these studies had a relatively limited time frame (<7 months post-COVID) and did not capture the large number of more recent data with longer-term follow-up of COVID-19 survivors. Although the time course of persisting breathlessness has aroused a controversy due to inconsistent findings from previous studies with multiple follow-up visits [41, 57, 58], our meta-analysis by follow-up duration supports the trend of decreasing prevalence over time. Nevertheless, one in five survivors still suffered from breathlessness 7–12 months after their acute illness, implying that this is not a symptom that simply requires more time to recover and highlighting the unmet need in those affected survivors for timely medical intervention or treatment. We should also bear in mind that the medical needs are likely to vary among patients due to the underlying pathophysiological aetiology of this symptom, especially given the protean effects of this coronavirus (e.g. in respiratory and cardiovascular systems), which are complicated by potential consequences of treatment during acute infection (e.g. prolonged immobilisation and ventilator-induced lung injury).
When interpreting the prevalence of post-COVID breathlessness among COVID-19 survivors, it is important to distinguish from their pre-existing breathlessness symptom before infection or the population's baseline level. Our subgroup meta-analysis restricted to 20 studies that used recalled pre-COVID breathlessness level as baseline reference when defining self-reported post-COVID breathlessness showed 18% (95% CI 14–21) of patients with COVID-19 reported new or worse breathlessness at the follow-up visit compared with their pre-COVID level. In addition, five of the included studies compared the prevalence of breathlessness between COVID-19 survivors and non-COVID-19 controls. Four of these [19, 23, 48, 77] demonstrated significantly higher prevalence in COVID-19 survivors than controls; the other study [66] observed higher prevalence of breathlessness in controls due to the specific selection criteria (i.e. historical nonpatients with COVID-19 who performed combined cardiopulmonary exercise testing and stress echocardiography in their institution). Similar to patients with COVID-19, a large proportion of survivors of severe acute respiratory syndrome (SARS) also experienced persistent breathlessness. A study on 1-year outcomes of 117 SARS survivors showed that 44%, 49% and 45% of the survivors reported breathlessness at the 3-, 6- and 12-month follow-up visits, respectively [78]. Another follow-up study of 50 long-term survivors of acute respiratory distress syndrome (ARDS) showed that 32% of them complained of breathlessness on moderate exercise [79].
We observed a significant difference in pooled prevalence of self-reported post-COVID breathlessness between hospitalised and nonhospitalised patients (27% versus 17%). In addition, the pooled prevalence was significantly higher in patients treated for severe or critical acute COVID-19 compared with nonsevere patients (26% versus 16%). Female sex was also shown to be a risk factor for post-COVID breathlessness; it is worth further investigation whether the observed sex difference could be explained by differences in absolute spirometric volumes or ventilatory capacity, as suggested by previous data in the general population [80, 81] and in patients with COPD [82]. Despite the limited data on ethnicity/race reported by included papers, we found that studies in Asia had lower pooled prevalence than studies in Europe or North America, which highlights the ongoing need to investigate the ethnic heterogeneity in post-COVID symptoms and also raises the possibility of cultural differences in post-COVID symptom assessment/reporting. Other potential risk factors reported by previous studies included age, obesity and comorbidities (e.g. obstructive lung diseases), but the existing evidence for these risk factors was inconsistent and inconclusive. Future confirmatory research of these risk factors could pave the way for personalised risk prediction of post-COVID breathlessness and the stratification of high-risk individuals for targeted intervention or preventive therapies. Moreover, studies with multiple regression models mutually adjusting for these variables are needed to ascertain their relative contributions to post-COVID breathlessness and to account for potential confounding bias.
Our meta-analysis also revealed substantial methodological heterogeneity in the estimation of prevalence of post-COVID breathlessness, including different definitions of post-COVID breathlessness and different follow-up methods. This emphasises the need to account for specific methodological approaches used when interpreting results from individual studies on post-COVID symptoms [83].
The available evidence is insufficient to draw firm conclusions about the underlying mechanisms of post-COVID breathlessness. Previous studies reported inconsistent results for the role of impaired lung function or lung pathologies (based on pulmonary function tests and imaging data), oxygen desaturation related to exertion, and systemic inflammation, though correlations between mental health disorders (depression and anxiety) and post-COVID breathlessness appear to be more robust [32, 35, 48]. In addition, two clinical investigations [84, 85] of COVID-19 survivors who reported persistent breathlessness identified a range of potential causes, such as a cardiorespiratory cause (parenchymal abnormality, pulmonary embolism, cardiac complications), fibrotic changes, dysfunctional breathing, underlying chronic lung diseases or physical deconditioning. Together, these results suggest that the experience of post-COVID breathlessness may be shaped by multiple factors. Consistent evidence suggests that pulmonary rehabilitation can prevent or reduce post-COVID breathlessness [68–75], although confirmatory evidence from large-scale multicentre RCTs is needed. Corrective measures for specific underlying physiological sequelae should also be considered on a case-by-case basis.
Several limitations in this systematic review should be noted. Substantial between-study heterogeneity was detected in our meta-analyses despite our efforts to identify sources of heterogeneity, which is commonly observed in meta-analyses of prevalence data. Future studies with similar methodological approaches and population characteristics could allow a set of more precise subgroup meta-analyses. Since different starting points for the follow-up period were used by different studies, we applied an operational definition of post-COVID breathlessness as >28 days after either acute COVID-19 infection, symptom onset, initial COVID-19 diagnosis/positive test or hospital discharge. This is a conservative definition because the symptom onset, initial COVID-19 diagnosis/positive test, or hospital discharge occurred after acute COVID-19 infection. In addition, little information on COVID-19 strains or variants was reported in the included papers and the patient recruitment period was either missing or had a wide range in many papers which could not be used to infer underlying variants reliably. Whether different COVID-19 variants (especially the Omicron variant) differ in their long-term respiratory consequences warrants further research. Finally, the heterogeneous or limited evidence on risk factors, mechanisms and treatments of post-COVID breathlessness precluded our ability to perform quantitative syntheses.
In conclusion, this systematic review and meta-analysis demonstrated that over one-quarter of COVID-19 survivors reported post-COVID breathlessness. The prevalence of post-COVID breathlessness decreased over longer-term follow-up and is likely to be influenced by population characteristics (initial disease severity, sex, and continent) and methodological approaches. Given inconsistencies in the available data, no firm conclusion can yet be drawn regarding the pathophysiological mechanisms of post-COVID breathlessness. The limited body of available evidence supports the implementation of rehabilitation exercises in COVID-19 survivors, while confirmatory trials are awaited. Future mechanistic research into the pathophysiology and targeted preventive interventions or treatments for post-COVID breathlessness are needed to meet the growing need for health services of at-risk or affected COVID-19 survivors.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0071-2022.SUPPLEMENT (483.2KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: A. Sheikh and B. Zheng contributed to the conception and design of the work. B. Zheng wrote the first draft of the manuscript with input from L. Daines and A. Sheikh. B. Zheng and Q. Han contributed to the acquisition and analysis of data. All authors critically reviewed the manuscript and approved the final version before submission.
Conflict of interest: A. Sheikh is a member of the Scottish Government Chief Medical Officer's COVID-19 Advisory Group and its Standing Committee on Pandemics, and a member of the UK Government's Risk Stratification Subgroup and AstraZeneca's Thrombotic Thrombocytopenic Taskforce; all roles are unremunerated. P. Pfeffer reports grants from NIHR, outside the submitted work. M. Shankar-Hari reports grants from National Institute for Health Research, outside the submitted work. C.E. Brightling reports grants from UKRI-MRC/DHSC-NIHR. R.A. Evans reports a grant from NIHR Clinician Scientist Fellowship, outside the submitted work. L.V. Wain reports grants from GSK, grants from Orion, outside the submitted work. L.G. Heaney reports personal fees from Novartis, Hoffman la Roche/Genentech Inc, Sanofi, Evelo Biosciences, GlaxoSmithKline, AstraZeneca, Teva, Theravance and Circassia; grants from Medimmune, Novartis UK, Roche/Genentech Inc, GlaxoSmithKline, Amgen, Genentech/Hoffman la Roche, AstraZeneca, Medimmune, Aerocrine and Vitalograph; and other support from Boehringer Ingelheim, Chiesi and Napp Pharmaceuticals, outside the submitted work. All other authors declare no competing interests.
Support statement: This study was supported by a grant to the University of Leicester from the MRC–UK Research and Innovation (UKRI) and the Department of Health and Social Care (DHSC) through the National Institute for Health Research (NIHR) rapid response panel to tackle COVID-19 (grants: MR/V027859/1 and COV0319). The study was also supported by the UK Health Data Research BREATHE Hub and the Chief Scientist Office of the Scottish Government (COV/LTE/20/15). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.National Institute for Health and Care Excellence . COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. www.nice.org.uk/guidance/ng188. Date last updated: November 11, 2021. Date last accessed: April 16, 2022. [PubMed]
- 2.Iqbal FM, Lam K, Sounderajah V, et al. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine 2021; 36: 100899. doi: 10.1016/j.eclinm.2021.100899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-de-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med 2021; 92: 55–70. doi: 10.1016/j.ejim.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cares-Marambio K, Montenegro-Jiménez Y, Torres-Castro R, et al. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Chron Respir Dis 2021; 18: 14799731211002240. doi: 10.1177/14799731211002240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11: 16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health 2021; 6: e005427. doi: 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Ramirez DC, Normand K, Zhaoyun Y, et al. Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines 2021; 9: 900. doi: 10.3390/biomedicines9080900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adeloye D, Elneima O, Daines L, et al. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med 2021; 9: 1467–1478. doi: 10.1016/S2213-2600(21)00286-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daynes E, Gerlis C, Briggs-Price S, et al. COPD assessment test for the evaluation of COVID-19 symptoms. Thorax 2021; 76: 185–187. doi: 10.1136/thoraxjnl-2020-215916 [DOI] [PubMed] [Google Scholar]
- 11.Ghosn J, Piroth L, Epaulard O, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect 2021; 27: 1041. doi: 10.1016/j.cmi.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Pinto MD, Borelli JL, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: looking for clarity in the haze of the pandemic. medRxiv 2021; preprint [ 10.1101/2021.03.03.21252086] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peluso MJ, Kelly JD, Lu S, et al. Rapid implementation of a cohort for the study of post-acute sequelae of SARS-CoV-2 infection/COVID-19. medRxiv 2021; preprint [ 10.1101/2021.03.11.21252311]. [DOI] [Google Scholar]
- 14.Tawfik HM, Shaaban HM, Tawfik AM. Post-covid-19 syndrome in Egyptian healthcare staff: highlighting the carers’ sufferings. Electron J Gen Med 2021; 18: em291. doi: 10.29333/ejgm/10838 [DOI] [Google Scholar]
- 15.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015; 13: 147–153. doi: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 16.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39. doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20: e192–e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 19.Galván-Tejada CE, Herrera-García CF, Godina-González S, et al. Persistence of COVID-19 symptoms after recovery in Mexican population. Int J Environ Res Public Health 2020; 17: 9367. doi: 10.3390/ijerph17249367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weerahandi H, Hochman KA, Simon E, et al. Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med 2021; 36: 738–745. doi: 10.1007/s11606-020-06338-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwitz LI, Garry K, Prete AM, et al. Six-month outcomes in patients hospitalized with severe COVID-19. J Gen Intern Med 2021; 36: 3772–3777. doi: 10.1007/s11606-021-07032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397: 220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398: 747–758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asadi-Pooya AA, Nemati H, Shahisavandi M, et al. Long COVID in children and adolescents. World J Pediatr 2021; 17: 495–499. doi: 10.1007/s12519-021-00457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erol N, Alpinar A, Erol C, et al. Intriguing new faces of COVID-19: persisting clinical symptoms and cardiac effects in children. Cardiol Young 2021: 32: 1085–1091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aparisi A, Ybarra-Falcon C, Garcia-Gomez M, et al. Exercise ventilatory inefficiency in post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med 2021; 10: 2591. doi: 10.3390/jcm10122591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang YF, Liu T, Yu JN, et al. Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their risk factors. J Intern Med 2021; 290: 444–450. doi: 10.1111/joim.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Fatigue and dyspnoea as main persistent post-COVID-19 symptoms in previously hospitalized patients: related functional limitations and disability. Respiration 2021; 101: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerum TV, Rodriguez JR, Meltzer C, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J 2021; 57: 2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindahl A, Aro M, Reijula J, et al. Women report more symptoms and impaired quality of life: a survey of Finnish COVID-19 survivors. Infect Dis (Lond) 2021: 54: 53–56. [DOI] [PubMed] [Google Scholar]
- 31.Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One 2021; 16: e0254523. doi: 10.1371/journal.pone.0254523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah AS, Ryu MH, Hague CJ, et al. Changes in pulmonary function and patient-reported outcomes during COVID-19 recovery: a longitudinal, prospective cohort study. ERJ Open Res 2021; 7: 00243–02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigfrid L, Drake TM, Pauley E, et al. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur 2021; 8: 100186. doi: 10.1016/j.lanepe.2021.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yomogida K, Zhu S, Rubino F, et al. Post-acute sequelae of SARS-CoV-2 infection among adults aged ≥18 years - Long Beach, California, April 1–December 10, 2020. MMWR Morb Mortal Wkly Rep 2021; 70: 1274–1277. doi: 10.15585/mmwr.mm7037a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Cruz RF, Waller MD, Perrin F, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res 2021; 7: 00655–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortini A, Torrigiani A, Sbaragli S, et al. COVID-19: persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection 2021; 49: 1007–1015. doi: 10.1007/s15010-021-01638-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froidure A, Mahsouli A, Liistro G, et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir Med 2021; 181: 106383. doi: 10.1016/j.rmed.2021.106383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skjorten I, Ankerstjerne OAW, Trebinjac D, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J 2021; 58: 2100996. doi: 10.1183/13993003.00996-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open 2021; 4: e2127403. doi: 10.1001/jamanetworkopen.2021.27403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin X, Xi X, Min X, et al. Long-term chest CT follow-up in COVID-19 survivors: 102–361 days after onset. Ann Transl Med 2021; 9: 1231. doi: 10.21037/atm-21-1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis 2022; 74: 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautam N, Madathil S, Tahani N, et al. Medium-term outcome of severe to critically ill patients with SARS-CoV-2 infection. Clin Infect Dis 2022; 74: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Italia L, Ingallina G, Napolano A, et al. Subclinical myocardial dysfunction in patients recovered from COVID-19. Echocardiography 2021; 38: 1778–1786. doi: 10.1111/echo.15215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mechi A, Al-Khalidi A, Al-Darraji R, et al. Long-term persistent symptoms of COVID-19 infection in patients with diabetes mellitus. Int J Diabetes Dev Ctries 2022; 42: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-de-Las-Peñas C, Torres-Macho J, Elvira-Martínez CM, et al. Obesity is associated with a greater number of long-term post-COVID symptoms and poor sleep quality: a multicentre case-control study. Int J Clin Pract 2021; 75: e14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernández-de-Las-Peñas C, Guijarro C, Torres-Macho J, et al. Diabetes and the risk of long-term post-COVID symptoms. Diabetes 2021; 70: 2917–2921. doi: 10.2337/db21-0329 [DOI] [PubMed] [Google Scholar]
- 47.Qin W, Chen S, Zhang Y, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J 2021; 58: 2003677. doi: 10.1183/13993003.03677-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021; 31: 100683. doi: 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anaya JM, Rojas M, Salinas ML, et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun Rev 2021; 20: 102947. doi: 10.1016/j.autrev.2021.102947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faverio P, Luppi F, Rebora P, et al. Six-month pulmonary impairment after severe COVID-19: a prospective, multicentre follow-up study. Respiration 2021; 100: 1078–1087. doi: 10.1159/000518141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milanese M, Anselmo M, Buscaglia S, et al. COVID-19 6 months after hospital discharge: pulmonary function impairment and its heterogeneity. ERJ Open Res 2021; 7: 00196–2021. doi: 10.1183/23120541.00196-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riou M, Marco TC, Oulehri W, et al. Respiratory follow-up after hospitalization for COVID-19: Who and when? Eur J Clin Invest 2021; 51: e13603. doi: 10.1111/eci.13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81: e4–e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naik S, Haldar SN, Soneja M, et al. Post COVID-19 sequelae: a prospective observational study from Northern India. Drug Discov Ther 2021; 15: 254–260. doi: 10.5582/ddt.2021.01093 [DOI] [PubMed] [Google Scholar]
- 55.Gamberini L, Mazzoli CA, Prediletto I, et al. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir Med 2021; 189: 106665. doi: 10.1016/j.rmed.2021.106665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meije Y, Duarte-Borges A, Sanz X, et al. Long-term outcomes of patients following hospitalization for coronavirus disease 2019: a prospective observational study. Clin Microbiol Infect 2021; 27: 1151–1157. doi: 10.1016/j.cmi.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X, Liu X, Zhou Y, et al. 3-month, 6–month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 2021; 9: 747–754. doi: 10.1016/S2213-2600(21)00174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J 2021; 57: 2003481. doi: 10.1183/13993003.03481-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cortés-Telles A, López-Romero S, Figueroa-Hurtado E, et al. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol 2021; 288: 103644. doi: 10.1016/j.resp.2021.103644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang L, Yang B, Jiang N, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35: e418. doi: 10.3346/jkms.2020.35.e418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Graaf MA, Antoni ML, Ter Kuile MM, et al. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine 2021; 32: 100731. doi: 10.1016/j.eclinm.2021.100731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baldini M, Chiapella MN, Fernández A, et al. Evaluation of the pulmonary function of patients with severe coronavirus 2019 disease three months after diagnosis. Medicina (B Aires 2021; 81: 715–721. [PubMed] [Google Scholar]
- 63.Aydin S, Unver E, Karavas E, et al. Computed tomography at every step: long coronavirus disease. Respir Investig 2021; 59: 622–627. doi: 10.1016/j.resinv.2021.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motiejunaite J, Balagny P, Arnoult F, et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur Respir J 2021; 58: 2101578. doi: 10.1183/13993003.01578-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vijayakumar B, Tonkin J, Devaraj A, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology 2022; 303: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szekely Y, Lichter Y, Sadon S, et al. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr 2021; 34: 1273–1284. doi: 10.1016/j.echo.2021.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roozbeh F, Saeedi M, Alizadeh-Navaei R, et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. J Antimicrob Chemother 2021; 76: 753–757. doi: 10.1093/jac/dkaa501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Xia W, Zhan C, et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax 2021; 77: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zha L, Xu X, Wang D, et al. Modified rehabilitation exercises for mild cases of COVID-19. Ann Palliat Med 2020; 9: 3100–3106. doi: 10.21037/apm-20-753 [DOI] [PubMed] [Google Scholar]
- 70.Ahmed I, Inam AB, Khalil W, et al. Effectiveness of aerobic exercise training program on cardio-respiratory fitness and quality of life in patients recovered from COVID-19. Eur J Physiother 2021; 1–6. doi: 10.1080/21679169.2021.1909649 [DOI] [Google Scholar]
- 71.Bouteleux B, Henrot P, Ernst R, et al. Respiratory rehabilitation for COVID-19 related persistent dyspnoea: a one-year experience. Respir Med 2021; 189: 106648. doi: 10.1016/j.rmed.2021.106648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curci C, Negrini F, Ferrillo M, et al. Functional outcome after inpatient rehabilitation in postintensive care unit COVID-19 patients: findings and clinical implications from a real-practice retrospective study. Eur J Phys Rehabil Med 2021; 57: 443–450. [DOI] [PubMed] [Google Scholar]
- 73.Dalbosco-Salas M, Torres-Castro R, Rojas Leyton A, et al. Effectiveness of a primary care telerehabilitation program for post-COVID-19 patients: a feasibility study. J Clin Med 2021; 10: 4428. doi: 10.3390/jcm10194428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayden MC, Limbach M, Schuler M, et al. Effectiveness of a three-week inpatient pulmonary rehabilitation program for patients after COVID-19: a prospective observational study. Int J Environ Res Public Health 2021; 18: 9001. doi: 10.3390/ijerph18179001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stavrou VT, Tourlakopoulos KN, Vavougios GD, et al. Eight weeks unsupervised pulmonary rehabilitation in previously hospitalized of SARS-CoV-2 infection. J Pers Med 2021; 11: 806. doi: 10.3390/jpm11080806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cesarone MR, Hu S, Belcaro G, et al. Pycnogenol(R)-Centellicum(R) supplementation improves lung fibrosis and Post-Covid-19 lung healing. Minerva Med 2022; 113: 135–140. [DOI] [PubMed] [Google Scholar]
- 77.Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis 2021; 21: 1373–1382. doi: 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med 2007; 167: 1312–1320. doi: 10.1001/archinte.167.12.1312 [DOI] [PubMed] [Google Scholar]
- 79.Schelling G, Stoll C, Vogelmeier C, et al. Pulmonary function and health-related quality of life in a sample of long-term survivors of the acute respiratory distress syndrome. Intensive Care Med 2000; 26: 1304–1311. doi: 10.1007/s001340051342 [DOI] [PubMed] [Google Scholar]
- 80.Ekström M, Schiöler L, Grønseth R, et al. Absolute values of lung function explain the sex difference in breathlessness in the general population. Eur Respir J 2017; 49: 1602047. doi: 10.1183/13993003.02047-2016 [DOI] [PubMed] [Google Scholar]
- 81.Ekström M, Sundh J, Schiöler L, et al. Absolute lung size and the sex difference in breathlessness in the general population. PLoS One 2018; 13: e0190876. doi: 10.1371/journal.pone.0190876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ekström M, Bornefalk-Hermansson A, Wysham N, et al. Spirometric volumes and breathlessness across levels of airflow limitation: the COPDGene study. Am J Respir Crit Care Med 2018; 198: 678–681. doi: 10.1164/rccm.201803-0594LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Office for National Statistics . Technical Article: Updated Estimates of the Prevalence of Post-Acute Symptoms Among People with Coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021. Date last accessed: April 16, 2022. Date last updated: September 16, 2021.
- 84.Hall J, Myall K, Lam JL, et al. Identifying patients at risk of post-discharge complications related to COVID-19 infection. Thorax 2021; 76: 408–411. doi: 10.1136/thoraxjnl-2020-215861 [DOI] [PubMed] [Google Scholar]
- 85.The Writing Committee for the COMEBAC Study Group . Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325: 1525–1534. doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abdelrahman MM, Abd-Elrahman NM, Bakheet TM. Persistence of symptoms after improvement of acute COVID19 infection, a longitudinal study. J Med Virol 2021; 93: 5942–5946. doi: 10.1002/jmv.27156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ares-Blanco S, Pérez Álvarez M, Gefaell Larrondo I, et al. SARS-CoV-2 pneumonia follow-up and long COVID in primary care: a retrospective observational study in Madrid city. PLoS One 2021; 16: e0257604. doi: 10.1371/journal.pone.0257604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armange L, Bénézit F, Picard L, et al. Prevalence and characteristics of persistent symptoms after non-severe COVID-19: a prospective cohort study. Eur J Clin Microbiol Infect Dis 2021; 40: 2421–2425. doi: 10.1007/s10096-021-04261-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2021; 76: 399–401. doi: 10.1136/thoraxjnl-2020-216086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021; 6: 100122. doi: 10.1016/j.lanepe.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aul DR, Gates DJ, Draper DA, et al. Complications after discharge with COVID-19 infection and risk factors associated with development of post-COVID pulmonary fibrosis. Respir Med 2021; 188: 106602. doi: 10.1016/j.rmed.2021.106602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bell ML, Catalfamo CJ, Farland LV, et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the Arizona CoVHORT. PLoS One 2021; 16: e0254347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27: 1607–1613. doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boari GEM, Bonetti S, Braglia-Orlandini F, et al. Short-term consequences of SARS-CoV-2-related pneumonia: a follow up study. High Blood Press Cardiovasc Prev 2021; 28: 373–381. doi: 10.1007/s40292-021-00454-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Çalik Kütükcü E, Çakmak A, Kinaci E, et al. Reliability and validity of the Turkish version of post-COVID-19 functional status scale. Turk J Med Sci 2021; 51: 2304–2310. doi: 10.3906/sag-2105-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021; 27: 258–263. doi: 10.1016/j.cmi.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng D, Calderwood C, Skyllberg E, et al. Clinical characteristics and outcomes of adult patients admitted with COVID-19 in East London: a retrospective cohort analysis. BMJ Open Respir Res 2021; 8: e000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Damanti S, Ramirez GA, Bozzolo EP, et al. 6-month respiratory outcomes and exercise capacity of COVID-19 acute respiratory failure patients treated with CPAP. Intern Med J 2021; 51: 1810–1815. doi: 10.1111/imj.15345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dankowski R, Sacharczuk W, Duszynska D, et al. Depression and anxiety in patients recently recovered from coronavirus disease (COVID-19). Neuropsychiatr Neuropsychol 2021; 16: 11–16. doi: 10.5114/nan.2021.108028 [DOI] [Google Scholar]
- 101.Darcis G, Bouquegneau A, Maes N, et al. Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: a monocentric prospective observational cohort study. Int J Infect Dis 2021; 109: 209–216. doi: 10.1016/j.ijid.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diaz-Fuentes G, Roa-Gomez G, Reyes O, et al. Coronavirus pneumonia: outcomes and characteristics of patients in an inner-city area after 3 months of infection. J Clin Med 2021; 10: 3368. doi: 10.3390/jcm10153368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dreyer N, Petruski-Ivleva N, Albert L, et al. Identification of a vulnerable group for post-acute sequelae of SARS-CoV-2 (PASC): People with autoimmune diseases recover more slowly from COVID-19. Int J Gen Med 2021; 14: 3941–3949. doi: 10.2147/IJGM.S313486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. doi: 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fernández-de-Las-Peñas C, Guijarro C, Plaza-Canteli S, et al. Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: a multicenter study. Lung 2021; 199: 249–253. doi: 10.1007/s00408-021-00450-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gaber TAK, Ashish A, Unsworth A. Persistent post-covid symptoms in healthcare workers. Occup Med (Lond) 2021; 71: 144–146. doi: 10.1093/occmed/kqab043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.González J, Benítez ID, Carmona P, et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest 2021; 160: 187–198. doi: 10.1016/j.chest.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93: 1013–1022. doi: 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 109.Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15: e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karaarslan F, Demircioğlu Güneri F, Kardeş S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: prospective follow-up by phone interviews. Rheumatol Int 2021; 41: 1263–1271. doi: 10.1007/s00296-021-04882-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect 2021; 27: 769–774. doi: 10.1016/j.cmi.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Landi F, Carfì A, Benvenuto F, et al. Predictive factors for a new positive nasopharyngeal swab among patients recovered from COVID-19. Am J Prev Med 2021; 60: 13–19. doi: 10.1016/j.amepre.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. J Clin Med 2021; 10: 2945. doi: 10.3390/jcm10132945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mahmud R, Rahman MM, Rassel MA, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS One 2021; 16: e0249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mallia P, Meghji J, Wong B, et al. Symptomatic, biochemical and radiographic recovery in patients with COVID-19. BMJ Open Respir Res 2021; 8: e000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mandal S, Barnett J, Brill SE, et al. “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021; 76: 396–398. doi: 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moradian ST, Parandeh A, Khalili R, et al. Delayed symptoms in patients recovered from COVID-19. Iran J Public Health 2020; 49: 2120–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mumoli N, Bonaventura A, Colombo A, et al. Lung function and symptoms in post-COVID-19 patients: a single-center experience. Mayo Clin Proc Innov Qual Outcomes 2021; 5: 907–915. doi: 10.1016/j.mayocpiqo.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munblit D, Bobkova P, Spiridonova E, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy 2021; 51: 1107–1120. doi: 10.1111/cea.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nehme M, Braillard O, Chappuis F, et al. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med 2021; 174: 1252–1260. doi: 10.7326/M21-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Keefe JB, Minton HC, Morrow M, et al. Postacute sequelae of SARS-CoV-2 infection and impact on quality of life 1–6 months after illness and association with initial symptom severity. Open Forum Infect Dis 2021; 8: ofab352. doi: 10.1093/ofid/ofab352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Sullivan O, Barker-Davies RM, Thompson K, et al. Rehabilitation post-COVID-19: cross-sectional observations using the Stanford Hall remote assessment tool. BMJ Mil Health 2021; in press [ 10.1136/bmjmilitary-2021-001856]. [DOI] [PubMed] [Google Scholar]
- 123.Righi E, Mirandola M, Mazzaferri F, et al. Long-term patient-centred follow-up in a prospective cohort of patients with COVID-19. Infect Dis Ther 2021; 10: 1579–1590. doi: 10.1007/s40121-021-00461-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sathyamurthy P, Madhavan S, Pandurangan V. Prevalence, pattern and functional outcome of post COVID-19 syndrome in older adults. Cureus 2021; 13: e17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shendy W, Elsherif AA, Ezzat MM, et al. Prevalence of fatigue in patients post COVID-19. Eur J Mol Clin Med 2021; 8: 1330–1340. [Google Scholar]
- 126.Shoucri SM, Purpura L, DeLaurentis C, et al. Characterising the long-term clinical outcomes of 1190 hospitalised patients with COVID-19 in New York City: a retrospective case series. BMJ Open 2021; 11: e049488. doi: 10.1136/bmjopen-2021-049488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stavem K, Ghanima W, Olsen MK, et al. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2021; 76: 405–407. doi: 10.1136/thoraxjnl-2020-216377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suárez-Robles M, Iguaran-Bermúdez MDR, García-Klepizg JL, et al. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call check list. Pan Afr Med J 2020; 37: 289. doi: 10.11604/pamj.2020.37.289.27110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sultana S, Islam MT, Salwa M, et al. Duration and risk factors of post-COVID symptoms following recovery among the medical doctors in Bangladesh. Cureus 2021; 13: e15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun LL, Wang J, Wang YS, et al. Symptomatic features and prognosis of 932 hospitalized patients with coronavirus disease 2019 in Wuhan. J Dig Dis 2021; 22: 271–281. doi: 10.1111/1751-2980.12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taylor RR, Trivedi B, Patel N, et al. Post-COVID symptoms reported at asynchronous virtual review and stratified follow-up after COVID-19 pneumonia. Clin Med (Lond) 2021; 21: e384–e391. doi: 10.7861/clinmed.2021-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Todt BC, Szlejf C, Duim E, et al. Clinical outcomes and quality of life of COVID-19 survivors: a follow-up of 3 months post hospital discharge. Respir Med 2021; 184: 106453. doi: 10.1016/j.rmed.2021.106453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tosato M, Carfì A, Martis I, et al. Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc 2021; 22: 1840–1844. doi: 10.1016/j.jamda.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Varghese J, Sandmann S, Ochs K, et al. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci Rep 2021; 11: 12775. doi: 10.1038/s41598-021-91270-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect 2021; 149: e32. doi: 10.1017/S0950268821000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu Q, Hou X, Li H, et al. A follow-up study of respiratory and physical function after discharge in patients with redetectable positive SARS-CoV-2 nucleic acid results following recovery from COVID-19. Int J Infect Dis 2021; 107: 5–11. doi: 10.1016/j.ijid.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zayet S, Zahra H, Royer PY, et al. Post-COVID-19 syndrome: nine months after SARS-CoV-2 infection in a cohort of 354 patients: data from the first wave of COVID-19 in Nord Franche-Comté Hospital, France. Microorganisms 2021; 9: 1719. doi: 10.3390/microorganisms9081719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25: 100463. doi: 10.1016/j.eclinm.2020.100463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0071-2022.SUPPLEMENT (483.2KB, pdf)