Abstract
An enzyme-linked immunosorbent assay and a Western blot analysis were developed to study the antibody response to Pneumocystis carinii in serum and bronchoalveolar lavage fluid from 27 human immunodeficiency virus 27 (HIV)-infected patients with P. carinii pneumonia (Pcp), 32 patients without Pcp, and 51 HIV-negative controls. Urea was used for the correct dilution of epithelial lining fluid, and albumin was used to evaluate transudation from plasma for the assessment of local production of antibodies to P. carinii. By contrast with those of immunoglobulin G (IgG), IgA responses to P. carinii were increased in serum from HIV-positive patients compared to negative controls. Local production of antibodies to P. carinii, especially IgA, was decreased in patients with Pcp. In a study of 10 patients of each group, IgG and IgA responses to gp116 from P. carinii were lower in patients with Pcp than in other groups. These results suggest that, in addition to alveolar macrophages, local antibodies may play a role in host defense against P. carinii.
Pneumocystis carinii is a ubiquitous microorganism which can infect many mammalian species, including humans. It is a frequent cause of pneumonia and mortality in patients with AIDS (25). It is usually localized in the alveolar lung mucosa. In addition to alveolar macrophages, antibodies seem to play an important role in host defense against this microorganism since their administration can protect against pneumonia in SCID mice and reduce the number of cysts in the lung (7). P. carinii is exposed to locally produced antibodies in the bronchoalveolar mucosa, which could have roles in host defense to P. carinii more important than those of serum antibodies. The serum immunoglobulin (Ig) response to P. carinii has been extensively explored; however, the local antibody response to this microorganism in the bronchopulmonary tract is not well known.
We studied antibody responses to P. carinii by enzyme-linked immunosorbent assay (ELISA) and Western blotting of serum and bronchoalveolar liquid (BAL) of human immunodeficiency virus (HIV)-infected patients with and without P. carinii pneumonia (Pcp) and compared the results to results from HIV-negative control subjects. We have estimated local production of IgG and IgA against P. carinii using the urea concentration in BAL and in serum as a dilution factor of epithelial lining fluid (ELF) and the albumin concentration as a transudation factor of antibodies from plasma.
MATERIALS AND METHODS
Patients.
BAL and serum specimens were obtained from 59 HIV-seropositive patients and 51 HIV-seronegative controls. All HIV patients received zidovudine or didanosine therapy, except for five patients who were not treated at that time. Twenty-seven AIDS patients had respiratory symptoms due to Pcp as confirmed by bronchoscopy and direct detection of P. carinii in BAL by classical Giemsa and Gomori-Grocott techniques. This group contained 11 patients with active pneumonia and 16 patients with previous pneumonia (BAL was assayed 6 to 12 months after Pcp). The population included 25 males and 2 females of mean age 36.5 years (range, 27 to 52 years). Twenty-four had CD4-cell counts <150 cells/mm3, those for 2 were 2 between 150 and 300 cells/mm2, and that for 1 was >300 cells/mm3.
Thirty-two HIV-positive patients had respiratory symptoms which justified bronchoalveolar lavage. P. carinii infection was not demonstrated in these patients, and four of them were dropped from this study because they had high levels of albumin in BAL, suggesting transudation of serum to the BAL. This group included 29 males and 3 females of mean age 39.7 years (range, 28 to 51 years). Twenty-eight had CD4-cell counts <150 cells/mm3, those for 3 were between 150 and 300 cells/mm3, and that for 1 was >300 cells/mm3.
BAL samples were examined for the presence of P. carinii by Giemsa staining and Gomori-Grocott silver staining and for other bacteria, mycobacteria, viruses, and fungi by microscopy and in vitro culture methods. Fifty-one HIV-seronegative patients matched for sex and age that were subjected to bronchoalveolar lavage because of initial suspicion of lung cancer were used as controls, but the diagnosis was not confirmed.
Bronchoalveolar lavage protocols.
The lavage was performed using an Olympus BF IT 10 bronchoscope. Briefly, following local anesthesia of the naso-oropharynx, the bronchoscope was inserted and wedged into a subsegmental bronchus of the right middle lobe. Five 20-ml fractions of 0.9% sterile saline serum were injected and allowed to remain for no more than a 4-min dwell time to minimize urea diffusion from the bloodstream; they were recovered by a gentle aspiration (22). Lavage fluid samples were filtered through a single layer of sterile gauze to remove mucus and centrifuged for 5 min at 800 × g.
All sera and BAL were heated at 56°C for 30 min to inactivate HIV. These conditions did not affect IgG and IgA activities (9). The samples were aliquoted immediately and frozen at −80°C until use.
Urea and albumin measurements.
Urea was used to determine the dilution of ELF recovered by the bronchoalveolar lavage. Urea is freely diffusible through most body compartments including the lung, such that plasma and ELF urea concentrations are essentially identical. An immediate freezing of sampled BAL was performed to avoid urea splitting by organisms potentially present in BAL. Using this approach, we were able to determine the initial concentration of Igs in the ELF recovered by bronchoalveolar lavage (24, 30). To determine the concentration of urea recovered from BAL and serum samples, a commercially available kit (Biomerieux, Marcy l'Etoile, France) was used. This kit allows the determination of urea concentration by a glutamate dehydrogenase method.
In order to estimate the level of locally produced antibodies in lung mucosa, albumin was used to estimate transudation from plasma. Albumin is a protein with a molecular mass of 67 kDa, which is produced by liver but not by lung cells. It can diffuse from plasma into lung secretions in the same manner as antibodies do. Thus, transudation of antibodies can be estimated by a method previously described to quantify locally produced antibodies in the central nervous system in patients with herpes simplex encephalitis (12) and in the lung mucosa (17, 24). Albumin concentrations in BAL and serum were determined by immunoephelometry using a protein analyzer (BN 10; Behringwerke AG, Marburg, Germany). The antibody used to detect albumin in serum and in BAL reacted only with the 67-kDa band as demonstrated by native polyacrylamide gel electrophoresis (PAGE) and immunoblotting (data not shown), suggesting that albumin quantified in serum and in BAL had the same molecular weight (MW).
Antigen preparation.
Three week-old New Zealand White rabbits (250 g) were immunosuppressed by oral administration of 20 mg of azathioprine/kg of body weight three times weekly. Amoxicillin was added to drinking water daily (20 mg/liter) to inhibit bacterial infection (32, 34). After 2 weeks, rabbits were euthanized and aseptic lung biopsies were performed. Imprints from each lung were observed after Giemsa and Gomori-Grocott staining to detect P. carinii cysts. A severe Pcp had developed in most of the animals.
Human P. carinii antigens were prepared from human lung obtained from autopsy specimens from one AIDS patient. This human lung was provided by C. Contini (Institute of Infectious and Respiratory Diseases, University of Ferrara, Ferrara, Italy).
Lungs infected with P. carinii were used for extraction of cysts. The P. carinii antigens were purified by enzymatic digestion of lung tissue and a discontinuous Percoll gradient as described by Walzer et al. (36) with some modifications. Briefly, lung tissue was homogenized and digested twice in 0.2% collagenase–0.2% hyaluronidase dissolved in Hanks balanced salt solution (HBSS) supplemented with Ca2+ and Mg2+ (HBSS+) at 37°C for 30 min. The final suspension was washed twice in HBSS, and the pellet was resuspended in 1 ml of phosphate-buffered saline (PBS) buffer, loaded in a discontinuous gradient of three 3-ml layers of Percoll in saline solution (30, 20, and 10%), and centrifuged at 800 × g for 15 min. Fractions with purified P. carinii cysts were collected and washed in PBS. Then the pellets were resuspended in 1 ml of PBS, and 10-μl samples were used to estimate the relative number of cysts by Gomori-Grocott staining. Antigen preparations were stored at −80°C until use.
Antibodies to P. carinii.
Hyperimmune serum to P. carinii was obtained by immunization of rabbits with rabbit P. carinii. New Zealand White rabbits were inoculated intradermally with a suspension of 5 × 106 cysts emulsified in Freund's complete adjuvant. The animals were boosted twice, at monthly intervals, with similar doses of antigen in incomplete adjuvant. Blood was collected 10 days after the last injection by heart puncture, and the sera were stored at −80°C. A monoclonal antibody (85-1-5E12) that reacts with gpA of P. carinii (6, 8) was kindly provided by F. Gigliotti (University of Rochester School of Medicine, Rochester, N.Y.)
Analysis of rabbit P. carinii by SDS-PAGE and immunoblotting.
Considering the lack of availability of human P. carinii, we have substituted rabbit P. carinii. Rabbit P. carinii antigens were analyzed by sodium dodecyl sulfate (SDS)-PAGE and immunoblotting using rabbit hyperimmune serum, human serum, and a monoclonal antibody to major glycoprotein gpA. Rabbit P. carinii antigens, human P. carinii antigens, and human and rabbit uninfected lung lysates were compared.
ELISA.
IgG and IgA responses to P. carinii in serum and BAL were measured by a micro-ELISA technique as previously described (26). Cysts (2 × 106) were diluted in distilled water and sonicated (20,000 times/s) on ice four times for 5 min and then ultracentrifuged at 108,000 × g for 60 min. The sediment was resuspended in 1 ml of PBS, and the protein concentration was determined by the method of Lowry et al. (19). Ninety-six-well microtiter plates (Immunoplate I; Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl of sonicated P. carinii antigens (2 μg/ml) in 0.1 M carbonate buffer (pH 9.6) and then washed five times and blocked for 2 h at 37°C with 5% bovine serum albumin (BSA; Sigma, St. Louis, Mo.) in carbonate buffer. The plates were washed as before, and 100 μl of human serum or BAL diluted in 0.05 M phosphate buffer (pH 7.3) with 5% BSA and 0.05% Tween 20 was added to each well. Plates were incubated for 1 (IgG) or 2 h (IgA) at 37°C. All samples were run in duplicate, and positive (serum antibodies to P. carinii tested by immunofluorescence assay) and negative (sera of 6-month-old children without P. carinii antibodies) controls were included in each run. After the washing, peroxidase-conjugated goat anti-human IgG or IgA (Cappel, Cochranville, Pa.) was added to each well and the wells were incubated at 37°C for 60 min and then rinsed as before. One hundred microliters of freshly prepared substrate (4 mM orthophenylene diamine in 0.02 M citrate-phosphate buffer [pH 5] and 0.04% hydrogen peroxide) was added to the plates, and the plates were kept for 15 min at 20°C in darkness. The reaction was stopped with 50 μl of HCl, 1 N and the absorbance at 492 nm was determined on a Titertek Multiskan reader (Flow Labs, Irvine, United Kingdom). Results were expressed as an optical density (OD) ratio (OD of the sample/OD of the positive control) (20).
For measurement of the IgA antibody response, IgG was removed from serum and BAL samples by absorption with sheep anti-human IgG (RF absorbent; Behringwerke) before incubation in plates (29). IgM was not detectable in BAL (less than 2 mg/liter).
Electrophoresis and immunoblotting.
To study the specificities of antibodies to P. carinii, SDS-PAGE and immunoblotting were performed by the method of Laemmli (15) and Towbin et al. (35). Briefly, P. carinii antigens were diluted 1/2 in lysis buffer containing 2% SDS, 0.06 M Tris-HCl (pH 6.8), 5% 2-mercaptoethanol, 10% glycerol, and 0.002% bromophenol blue. The solution was heated at 100°C for 5 min, and the equivalent of 5 × 105 cysts were applied to each lane of a discontinuous slab gel (15 by 14 cm) composed of a 4% stacking gel and a 7.5% separating gel. MW standards were applied as markers in one lane on the gels. Electrophoresis was run at 4°C under 24 mA. After completion of the run, proteins were transferred from gel to a nitrocellulose filter (Protran nitrocellulose; pore size, 0.45 μm; Scheicher and Schuell, Dassel, Germany). Gels were soaked for 30 min in transfer buffer (39 mM glycine, 48 mM Tris, 0.037% SDS, 20% methanol; pH 8.3) and transferred in the same buffer in an electrophoretic blotting apparatus (Multiphore II; Pharmacia LKB, Uppsala, Sweden) for 120 min at 0.8 mA/cm2 of gel area. After transfer, lanes containing the MW standards were stained with amido black and the remainder of the filter was used in a Western blot assay, which involved incubation for 2 h in blocking solution (5% nonfat milk, 0.01% antifoam A, 0.02% Tween 20 in PBS buffer) to block potential nonspecific binding. The filter then was immediately incubated with test serum diluted 1/10 or BAL diluted 1/2 in the same buffer for 2 h at room temperature (IgG) or overnight at 4°C (IgA). Strips were then washed five times in blocking solution without milk and incubated for 1 to 2 h with horseradish peroxidase-conjugated goat anti-human IgG or IgA (Cappel) diluted 1/100 in blocking solution. After being washed five times in PBS, the strips were revealed by chromogen substrate (0.05% diaminobenzidine tetrahydrochloride–0.03% H2O2 in 0.1 M Tris buffer [pH 7.6]).
Statistical analysis.
The results for the three groups were expressed as mean values ± standard errors. The various experimental groups were compared using an analysis of variance followed by posthoc Scheffé's pairwise comparisons. When the variances were too large, we used robust statistical procedures such as a Kruskal-Wallis or a Mann-Whitney test.
RESULTS
Dilution of ELF and transudation.
The dilution of ELF was estimated by the ratio of serum to BAL urea. This ratio did not differ significantly among the HIV-seropositive patients with Pcp (mean value, 43.6 ± 8.9) or without Pcp (mean value, 49.6 ± 8.7) and control patients (mean value, 48.0 ± 4.6). Each BAL sample dilution was corrected individually. The transudation of antibodies from plasma to ELF was assessed by determining the ratio of serum to ELF albumin. The ratio did not differ significantly among the three groups, the HIV-seropositive patients with Pcp (mean value, 22.73 ± 3.43) or without Pcp (mean value, 17.90 ± 3.87) and control patients (mean value, 22.85 ± 3.01).
Rabbit P. carinii analysis.
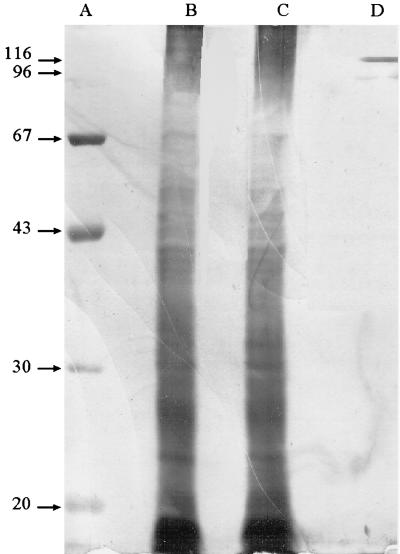
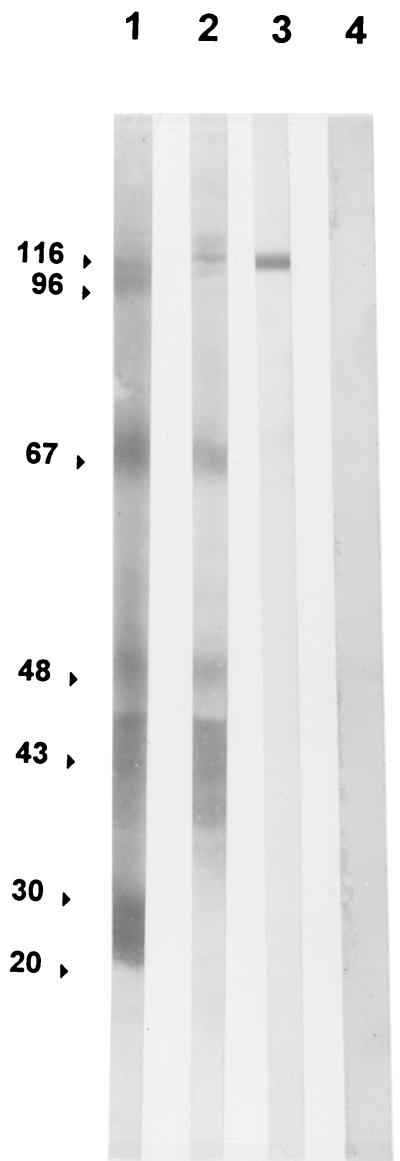
The rabbit P. carinii preparation contained many proteins with molecular masses ranging between 10 and 140 kDa, as shown by numerous bands in SDS-PAGE gel and silver stain (Fig. 1). Rabbit and human sera recognized only a few of them (116, 96, 67, 50 to 40, 30, and 20 kDa) (Fig. 2, lanes 1 and 2). The 116-kDa band was gpA, as determined by use of the 85-1-5E12 monoclonal antibody (Fig. 2, lane 3). Rabbit P. carinii had an antigen profile comparable to that of human P. carinii (data not shown). Enzyme digestion used in preparation of the antigen had no apparent effect on gpA since the 85-1-5E12 monoclonal antibody detected only one band with a 116-kDa molecular mass (Fig. 2, lane 3). Human serum did not react with rabbit lung antigens (Fig. 2, lane 4).
FIG. 1.
SDS-PAGE and silver stain analysis of a P. carinii preparation from lungs of rabbits with Pcp. Lanes A and D, MW markers; lanes B and C, P. carinii preparations from two different rabbits.
FIG. 2.
Western immunoblot analysis of rabbit P. carinii. Antibody reactivities of rabbit hyperimmune serum (lane 1), human serum (lane 2), and the 85-1-5E12 mouse IgM monoclonal antibody to gpA (lane 3) with rabbit P. carinii antigens are shown. Lane 4, antibody reactivity of human serum with uninfected rabbit lung lysate.
ELISA.
Measurements were performed in duplicate for samples from all patients of each group except for four HIV-positive patients without Pcp, who were dropped from the study because they showed high levels of albumin in BAL, suggesting a serum transudation. We retained 11 HIV-infected patients with active Pcp and 16 with prior Pcp in one group of 27 HIV patients because they had similar results; there were 28 HIV-positive patients without Pcp and 51 HIV-negative controls.
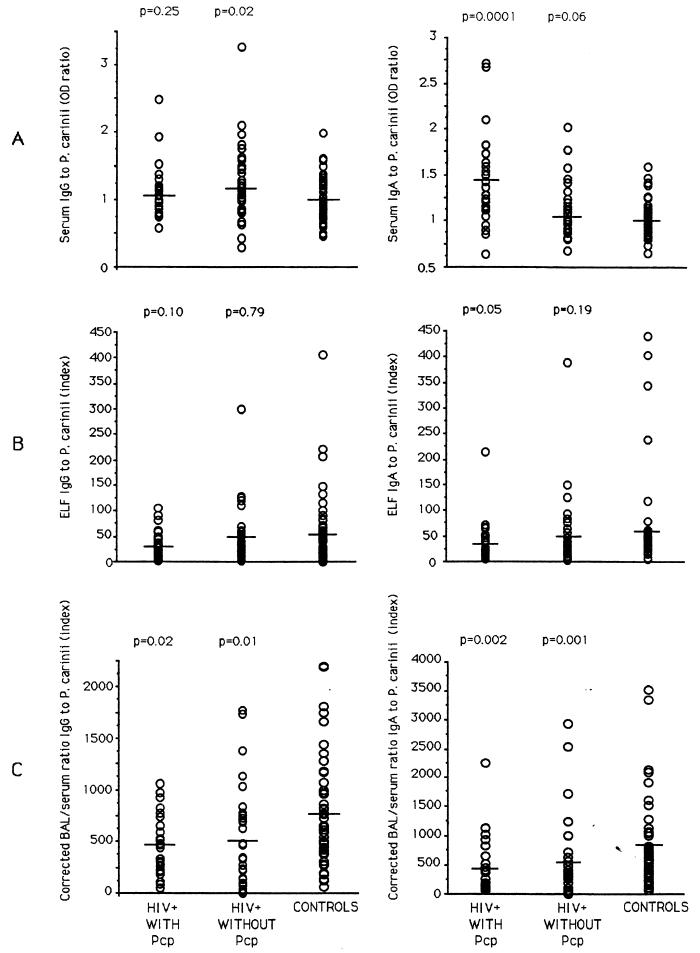
IgG and IgA to P. carinii were detected in all serum samples. The IgG OD ratio for the HIV-seropositive patients with Pcp (mean value, 1.10 ± 0.07) was not significantly different from that for control patients (mean value, 0.99 ± 0.04). HIV-positive patients without Pcp had an IgG OD ratio (mean value, 1.22 ± 0.10) that was significantly higher than that for control patients (P = 0.02). The difference between the values for the two HIV groups was not significant (Fig. 3). By contrast, the IgA OD ratio for serum from HIV-positive patients with Pcp (mean value, 1.41 ± 0.09) was significantly higher than that for HIV-negative controls (mean value, 1 ± 0.02) (P < 0.01). HIV-positive patients without Pcp had a serum IgA response (mean value, 1.11 ± 0.05) that was not significantly different from that of the control group. The difference between the two HIV groups was significant (Fig. 3).
FIG. 3.
IgG and IgA responses to P. carinii in serum (A) and ELF (B) from, and locally produced by (C), HIV-positive patients (27 with and 28 without Pcp) and 51 HIV-seronegative controls. The mean value for each group is indicated with a line, and each parameter measured for HIV-positive patients was compared with the corresponding value for normal controls by the Mann-Whitney U test.
The P. carinii IgG response in ELF from bronchoalveolar lavage corrected by the dilution factors was lower in HIV-positive patients with Pcp (31.54 ± 5.27) than in patients without Pcp (50.83 ± 10.52) and in control patients (56.11 ± 9.68), although this difference was not statistically significant. By contrast, the IgA response in HIV-positive patients with Pcp was significantly lower (34.66 ± 7.79) than in control patients (62.03 ± 12.87) (P = 0.05). In HIV-positive patients without Pcp, the IgA response (39.48 ± 6.49) was higher than in HIV-positive patients with Pcp and lower than in control patients, although not statistically different (Fig. 3).
When antibodies in ELF were corrected by use of albumin as an estimate of transudation, it was possible to assess levels of locally produced antibody. Corrected for albumin, the level of IgG to P. carinii was very low in HIV-positive patients with Pcp (mean value, 474 ± 58) and without Pcp (mean value, 504 ± 87) compared to control patients (mean value, 761 ± 76) (P < 0.05). IgA responses to P. carinii were very low in HIV-positive patients with Pcp (461 ± 90) and without Pcp (528 ± 122) compared to control patients (835 ± 103) (P < 0.05). Levels in HIV-positive patients with Pcp were lower than in HIV-positive patients without Pcp, but the difference was not significant (Fig. 3).
Immunoblotting.
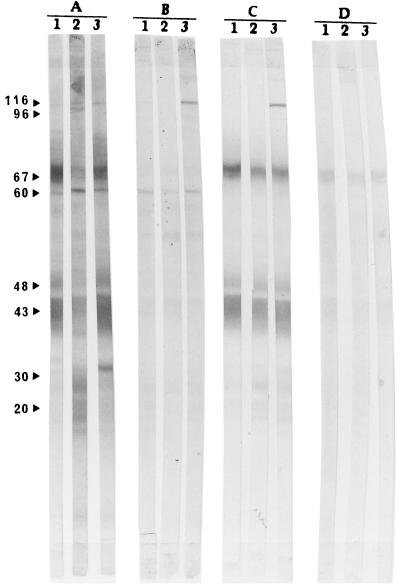
In order to study the antibody response to major P. carinii antigens, we focused on the 116-kDa antigen, which is frequently referred to as surface glycoprotein A (gpA), since it is the first surface molecule of P. carinii to be isolated (5) and on the 43-kDa antigen, which is the most commonly detected protein in the lungs or BAL of subjects with Pcp (37). Serum and BAL of 10 patients from each group were tested by immunoblotting to study the specificities of IgG and IgA to major P. carinii antigens. The numbers and percentages for patients with detectable IgG and IgA antibodies to gp116 and p43, major P. carinii antigens in serum and BAL, are shown in Table 1, and Western immunoblot analysis demonstrating their reactivity to the P. carinii antigen is presented in Fig. 4.
TABLE 1.
Numbers and percentages of patients with detectable antibody to major P. carinii antigens in serum and BAL
| Source of antibody | Group (10 patients tested) | No. (%) of patients with major P. carinii antigen:
|
|
|---|---|---|---|
| gp116 | gp43 | ||
| Serum IgG | HIV+ with Pcp | 4 (40) | 9 (90) |
| HIV+ without Pcp | 6 (60) | 10 (100) | |
| Controls | 8 (80) | 10 (100) | |
| Serum IgA | HIV+ with Pcp | 3 (30) | 5 (50) |
| HIV+ without Pcp | 3 (30) | 5 (50) | |
| Controls | 4 (40) | 5 (50) | |
| BAL IgG | HIV+ with Pcp | 1 (10) | 9 (90) |
| HIV+ without Pcp | 1 (10) | 9 (90) | |
| Controls | 1 (10) | 10 (100) | |
| BAL IgA | HIV+ with Pcp | 1 (10) | 4 (40) |
| HIV+ without Pcp | 0 (0) | 4 (40) | |
| Controls | 1 (10) | 5 (50) | |
FIG. 4.
Western immunoblot analysis demonstrating reactivity to P. carinii antigens. IgG in serum (A), IgA in serum (B), IgG in BAL (C), and IgA in BAL (D) are shown. Lane 1, HIV-positive patient with Pcp; lane 2, HIV-positive patient without Pcp; lane 3, HIV-negative control subject. The same three patients were used in panels A to D.
DISCUSSION
In addition to the role of alveolar macrophages, humoral immune responses also may be important in defenses against P. carinii. So we attempted to investigate the antibody responses to P. carinii in serum and BAL of HIV-infected patients with and without Pcp and compared them to that of HIV-negative control subjects.
Because of the lack of availability of human P. carinii, we used rabbit P. carinii for our studies. We chose rabbits because young animals develop Pcp spontaneously and rapidly. Moreover, 2 weeks of immunosuppression increases the number of P. carinii cysts in lungs (33, 34), in contrast to what is found for rats, which require corticoid treatment for 8 to 12 weeks (1). Furthermore, rat and human P. carinii cysts are antigenically different (13, 37). Human serum recognized rabbit and human P. carinii antigens in nearly the same way. Approximately the same antigens that are identified by rabbit anti-P. carinii antibodies are also identified by human serum, as shown by immunoblotting. Further, a monoclonal antibody which recognizes human P. carinii gpA (6) recognized the rabbit P. carinii 116-kDa protein. These results suggest that rabbit P. carinii can be used to study the human antibody response to P. carinii.
By contrast to what was found for HIV-positive patients with Pcp, an increase of serum IgG to P. carinii was found in HIV-positive patients without Pcp, suggesting that some patients could develop a specific humoral immune response potentially capable of protecting them from P. carinii infection. In several previous studies, the serum IgG responses to P. carinii in patients and controls did not differ significantly (10, 11). Nevertheless, other authors found reduced IgG antibody activities in HIV-infected patients with Pcp (4).
The serum IgA response to P. carinii was higher in HIV-positive patients with Pcp than in control patients. This increase can be partly the result of a polyclonal activation of B cells, which is known to occur frequently in HIV-infected patients (16). However, patients with Pcp had a higher IgA response to P. carinii in serum than patients without Pcp, suggesting that the IgA response may have been preferentially stimulated by the infection. Serum IgG and IgA responses to P. carinii may not necessarily reflect the role of these antibodies in local defense against P. carinii in the lung because these antibodies may not be in direct contact with microorganisms.
A study of a rabbit model of P. carinii infection was previously performed by our research group. Levels of specific antibodies to P. carinii in serum and in BAL were measured during the resolution of naturally acquired Pcp in young rabbits. Specific antibody titers in BAL rose rapidly concomitantly with P. carinii cyst elimination, whereas titers in serum rose later (data not published), suggesting a potential role of locally produced antibodies in immune defenses against this fungus.
After correction for dilution, the IgA response to P. carinii in ELF was lower in HIV-infected patients with Pcp than in control subjects. These data are consistent with an antibody deficit that may predispose some patients to P. carinii infection and an alteration of IgA secretion by the mucosal immune system in HIV-positive patients, as previously shown (24).
Antibodies present in ELF are both locally produced and transudated from plasma. Local production of antibodies was previously assessed in the central nervous system compartments of patients with herpes simplex encephalitis (12) and in lung mucosae of patients with AIDS (24). Corrected IgG and IgA responses to P. carinii were very low in HIV-positive patients with and without Pcp compared to those in control subjects. Decreased production of mucosal antibodies to P. carinii in HIV-infected patients may be due to a defect of plasmocystic function or number and may be related to the depletion of antigen-presenting cells in the lung mucosa, as is known to occur in the gut mucosa (18).
Immunoreactivity was used to find antibodies to P. carinii in BAL from HIV-positive patients with and without Pcp as well as in HIV-negative controls, but no attempt to quantify these antibodies was made (3). Laursen et al. estimated the amount of antibody to P. carinii in BAL by indirect immunofluorescence after concentrating and adjusting all lavage fluids with PBS to the same ratio of albumin concentration in BAL to albumin concentration in serum. They found a decreased response of specific IgG to P. carinii, and an increased response of IgA and IgM, in HIV-positive patients with respect to control subjects (17).
A decrease of serum IgG and IgA reactive with gp116 was found in HIV-positive patients with Pcp. No difference in antibody reactive with gp43 between patients and controls was detected. In bronchoalveolar lavage fluid, antibodies to gp116 were detected in only a few patients. Activity levels may have been too low to be detected by the technique used.
No study of BAL was previously reported. gp116 is a membrane glycoprotein responsible for P. carinii attachment to alveolar epithelial cells (28). Antibodies to these antigens may participate in the clearance of P. carinii organisms by blocking their attachment to alveolar cells. A monoclonal antibody to gp116 can significantly reduce the number of P. carinii cysts in lungs of animals when administered throughout a period of immunosuppression (7). Analysis of the serologic response to P. carinii antigens by the immunoblot technique showed that the 40- and 116-kDa glycoproteins are the major P. carinii antigens recognized by serum antibodies of healthy people and immunosuppressed patients (14, 27).
Considering that HIV-positive patients with Pcp have a decreased specific local antibody response, mucosal humoral immunity may play an important role in the clearance of P. carinii from the lung. Previous studies have shown that antibodies can have a protective role against P. carinii. A pneumonia developed in transgenic B-cell-deficient mice (21). Administration of hyperimmune serum to SCID mice resulted in effective immunity to P. carinii (31). Blumenfeld et al. demonstrated the presence of IgG as well as IgA on the surfaces of P. carinii cysts obtained from AIDS subjects (2). These antibodies may contribute to elimination of the microorganism and may play a role in local control of the infection. Antibodies may act as opsonins. In a previous study, Masur and Jones showed that antibodies to P. carinii were required for ingestion and intracellular killing of fungi by freshly isolated alveolar macrophages (23). On the other hand, immunoglobulins attached to the surfaces of P. carinii cysts, especially with anti-gp116, could also block attachment of the fungi to alveolar epithelial cells (2, 28).
In conclusion, this study demonstrates that local production of IgA and IgG to P. carinii was lower in HIV-positive patients than in controls and raises the possibility that the occurrence of Pcp in these patients was due not only to a decrease of CD4-cell number and to an alveolar macrophage defect but also to a decrease in local humoral immunity.
ACKNOWLEDGMENTS
We thank F. Gigliotti for the monoclonal antibody, P. Rouk for human P. carinii, F. Duplat for excellent technical assistance, and M. Rehailia for statistical analysis.
This work was supported by a grant from Région Rhône-Alpes, France.
REFERENCES
- 1.Bauer N, Paulsrud J, Bartlett M, Smith J, de Wilde C E. Pneumocystis carinii organisms obtained from rats, ferrets, and mice are antigenically different. Infect Immun. 1993;61:1315–1319. doi: 10.1128/iai.61.4.1315-1319.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenfeld W, Mandrell R E, Jarvis G A, Griffis J M. Localization of host immunoglobulin G to the surface of Pneumocystis carinii. Infect Immun. 1990;58:456–463. doi: 10.1128/iai.58.2.456-463.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenfeld W, McCook O, McLeod G. Detection of antibodies to Pneumocystis carinii in bronchoalveolar lavage fluid by immunoreactivity to Pneumocystis carinii within alveoli, granulomas, and disseminated sites. Mod Pathol. 1992;5:107–113. [PubMed] [Google Scholar]
- 4.Burns S M, Read J A, Yap P L, Brettle R P. Reduced concentrations of IgG antibodies to Pneumocystis carinii in HIV infected patients during active Pneumocystis carinii infection and possibility of passive immunization. J Infect. 1990;20:33–39. doi: 10.1016/s0163-4453(90)92280-x. [DOI] [PubMed] [Google Scholar]
- 5.Gigliotti F. Antigenic variation of a major surface glycoprotein of Pneumocystis carinii. J Protozool. 1991;38:4S–5S. [PubMed] [Google Scholar]
- 6.Gigliotti F. Host species-specific antigenic variation of a mannosylated surface glycoprotein of Pneumocystis carinii. J Infect Dis. 1992;165:329–336. doi: 10.1093/infdis/165.2.329. [DOI] [PubMed] [Google Scholar]
- 7.Gigliotti F, Hughes W T. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Investig. 1988;81:166–168. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigliotti F, Stokes D C, Cheatham A B, Davis D S, Hughes W T. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis. 1986;154:315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- 9.Gleeson M, Herd L, Burns C. Effect of heat inactivation of HIV on specific serum proteins and tumour markers. Ann Clin Biochem. 1990;27:592–594. doi: 10.1177/000456329002700611. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann B, Odum N, Platz P, Ryder L P, Svejgaard A, Nielsen P B, Andersen W H, Gerstoft J, Nielsen J O, Mojon M. Humoral responses to Pneumocystis carinii in patients with acquired immunodeficiency syndrome and in immunocompromised homosexual men. J Infect Dis. 1985;152:838–840. doi: 10.1093/infdis/152.4.838. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann B, Nielsen P B, Odum N, Gerstoft J, Platz P, Ryder L P, Poulsen A G, Mathiesen L, Dickmeiss E, Norrild B, Andersen H K, Westergaard B F, Neilsen C M, Andersen W H, Mojon M, Nielsen J O, Svejgaard A. Humoral and cellular responses to Pneumocystis carinii, CMV, and herpes simplex in patients with AIDS and in controls. Scand J Infect Dis. 1988;20:389–394. doi: 10.3109/00365548809032473. [DOI] [PubMed] [Google Scholar]
- 12.Klapper P E, Laing I, Lonson M. Rapid non-invasive diagnosis of herpes encephalitis. Lancet. 1981;ii:607–609. doi: 10.1016/s0140-6736(81)92744-6. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs J, Lundgren B, Masur H. Identification of antigens specific for Pneumocystis carinii. J Protozool. 1989;36:67S–69S. doi: 10.1111/j.1550-7408.1989.tb02706.x. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs J A, Halpern J L, Swan J C, Moss J, Parrillo J E, Masur H. Identification of antigens and antibodies specific for Pneumocystis carinii. J Immunol. 1988;140:2023–2031. [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lane H C, Masur H, Edgar L C, Whalen G, Rook A H, Fauci A S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 17.Laursen A L, Jensen B N, Andersen P L. Local antibodies against Pneumocystis carinii in bronchoalveolar lavage fluid. Eur Respir J. 1994;7:679–685. doi: 10.1183/09031936.94.07040679. [DOI] [PubMed] [Google Scholar]
- 18.Lim S G, Condez A, Poulter L W. Mucosal macrophage subsets of the gut in HIV: decrease in antigen-presenting cell phenotype. Clin Exp Immunol. 1993;92:442–447. doi: 10.1111/j.1365-2249.1993.tb03418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Lundgren B, Lundgren J D, Nielsen T, Mathiesen L, Nielsen J O, Kovacs J A. Antibody responses to a major Pneumocystis carinii antigen in human immunodeficiency virus-infected patients with and without Pneumocystis carinii pneumonia. J Infect Dis. 1992;165:1151–1155. doi: 10.1093/infdis/165.6.1151. [DOI] [PubMed] [Google Scholar]
- 21.Marcotte H, Levesque D, Delanay K, Bourgeault A, La Durantaye R D, Brochu S, Lavoie M C. Pneumocystis carinii infection in transgenic B cell-deficient mice. J Infect Dis. 1996;173:1034–1037. doi: 10.1093/infdis/173.4.1034. [DOI] [PubMed] [Google Scholar]
- 22.Marcy T W, Merrill W W, Rankin J A, Reynolds H Y. Limitations of using urea to quantify epithelial lining fluid recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1987;135:1276–1280. doi: 10.1164/arrd.1987.135.6.1276. [DOI] [PubMed] [Google Scholar]
- 23.Masur H, Jones T C. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1978;147:157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moja P H, Jalil A, Quesnel A, Perol M, Cotte L, Livrozet J M, Boibieux A, Chamson A, Vergnon J M, Lucht F, Tran R, Pozzetto B, Genin C. Humoral immune response within the lung in HIV-1 infection. Clin Exp Immunol. 1997;110:341–348. doi: 10.1046/j.1365-2249.1997.4231441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray J F, Felton C P, Garay S M, Gottlieb M S, Hopewell P C, Stover D E, Teirstein A S. Pulmonary complications of the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:1682–1688. doi: 10.1056/NEJM198406213102529. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen P D, Mojon M. Enzyme-linked immunosorbent assay compared with indirect immunofluorescence test for detection of Pneumocystis carinii specific immunoglobulins G, M, and A. APMIS. 1988;96:649–654. doi: 10.1111/j.1699-0463.1988.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 27.Peglow S L, Smulian G, Linke M J, Pogue C L, Nurre S, Crisler J, Phair J, Gold J W M, Armstrong D, Walzer P D. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis. 1990;161:296–306. doi: 10.1093/infdis/161.2.296. [DOI] [PubMed] [Google Scholar]
- 28.Pottratz S T, Paulsrud J, Smith J S, Martin W J., II Pneumocystis carinii attachment to cultured lung cells by Pneumocystis gp120, a fibronectin binding protein. J Clin Investig. 1991;88:403–407. doi: 10.1172/JCI115318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quesnel A, Pozzetto B, Moja P, Grattard F, Lucht F, Touraine J L, Gaudin O G, Genin C. Prognostic value of serum immunoglobulin A antibodies to pol gene products during human immunodeficiency virus 1 infection. Clin Exp Immunol. 1993;91:237–240. doi: 10.1111/j.1365-2249.1993.tb05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennard S I, Basset G, Lecossier D, O'Donnell K M, Pinkston P, Martin P G, Crystal R G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 31.Roths J B, Sidman C L. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J Clin Investig. 1992;90:673–678. doi: 10.1172/JCI115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheldon W H. Experimental pulmonary Pneumocystis carinii infection in rabbits. J Exp Med. 1959;110:147–160. doi: 10.1084/jem.110.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soulez B, Dei-Cas E, Camus D. The rabbit, experimental host of Pneumocystis carinii. Ann Parasitol Hum Comp. 1988;63:5–15. doi: 10.1051/parasite/19886315. . (In French.) [DOI] [PubMed] [Google Scholar]
- 34.Soulez B, Dei-Cas E, Charet P, Mougeot G, Caillaux M, Camus D. The young rabbit: a non-immunosuppressed model for Pneumocystis carinii pneumonia. J Infect Dis. 1989;160:355–356. doi: 10.1093/infdis/160.2.355. [DOI] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walzer D P, Rutledge M E, Yoneda K, Stahr B J. A new method of separating Pneumocystis carinii from infected lung tissue. Exp Parasitol. 1979;47:356–368. doi: 10.1016/0014-4894(79)90088-2. [DOI] [PubMed] [Google Scholar]
- 37.Walzer P, Linke M. A comparison of the antigenic characteristics of rat and human Pneumocystis carinii by immunoblotting. J Immunol. 1987;138:2257–2265. [PubMed] [Google Scholar]