Abstract
Despite historical controversy, pharmacologic ascorbate is emerging as promising cancer therapy via pro-oxidant chemistry. In this issue of Cancer Cell, Schoenfeld et al. describe how intracellular iron pools and reactive oxygen species drive pharmacologic ascorbate’s selective toxicity to cancer cells in vitro, in mice, and in humans.
Ascorbic acid (ascorbate, vitamin C) in cancer treatment has been an adventure in serendipity (Padayatty and Levine, 2000; reviewed in Levine et al., 2011). In 1954, William McMormick hypothesized that ascorbate could treat cancer by making collagen stronger, thereby preventing local invasiveness. Ewan Cameron widened the hypothesis and began treating patients with high, or pharmacologic, doses of ascorbate: 10 g daily. He and two-time Nobel Laureate Linus Pauling published a total of 100 cases suggesting efficacy of these doses in prolonging the lives of some patients with terminal cancers. Following quickly, investigators at Mayo Clinic demonstrated absolutely no efficacy, in two double-blind placebo-controlled trials. Oncologists pooh-poohed ascorbate, and even today many believe this was the end of the story. However, new data intervened, independent of cancer. Vitamin C pharmacokinetics discoveries in humans revealed that vitamin C concentrations are tightly controlled, mediated by ascorbate-specific transporters. Intravenous ascorbate bypasses tight control until homeostasis is restored by renal excretion. The cancer cases described by Cameron received intravenous and oral ascorbate, but the patients enrolled by Mayo investigators received only oral ascorbate (Padayatty and Levine, 2000; Levine et al., 2011). Pharmacologic ascorbate, given intravenously as a drug in humans and additionally by intraperitoneal injection in animals, produced concentrations up to 1,000 times higher than those from oral ascorbate (Chen et al., 2008). Concurrent findings showed that pharmacologic ascorbate was a pro-drug in cancer treatment by generating hydrogen peroxide (H2O2) in extracellular fluid at concentrations 10- to 100-fold higher than those found physiologically (Chen et al., 2007, 2008). Extracellular H2O2 formation was an essential prerequisite for cancer cell death, without harming normal cells. New excitement about pharmacologic ascorbate was based on many affected cancer cell types, coupled with data showing surprising safety (Padayatty et al., 2010) and clinical reduction in adverse effects from chemotherapy (Ma et al., 2014).
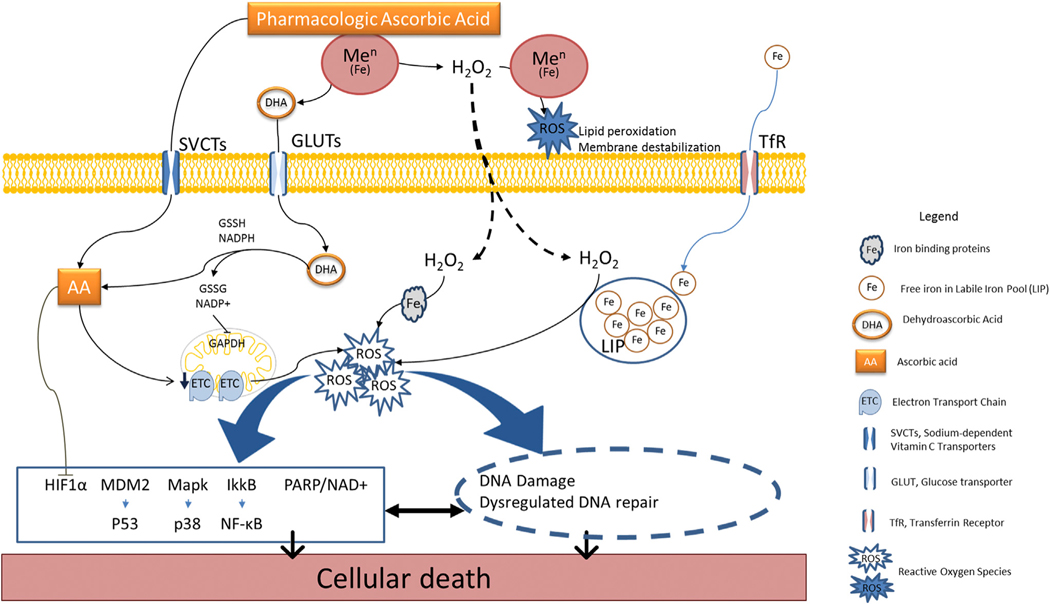
But how did extracellular H2O2 specifically cause the death of many types of cancer cells without harming normal cells? Reactive oxygen species (ROS) were believed to be effectors (Figure 1). They could form via Fenton reactions, either outside or inside cells (Chen et al., 2005; Verrax and Calderon, 2009). In theory, extracellular action is more straightforward. ROS could form from extracellular Fenton reactions driven by pharmacologic ascorbate, H2O2, and metal centers from extracellular fluid proteins or in cell membrane proteins with extracellular domains. Once formed, ROS could damage external-facing domains of cancer cell membranes. Internal damage to cancer cells would be initiated by extracellular formation of H2O2 followed by entry into cancer cells, likely by diffusion. Intracellular ROS formation by Fenton reactions requires metal centers and electrons. The electrons would again come from ascorbate, found in millimolar concentrations in most normal and cancer cells in vivo. Once intracellular ROS are formed, multiple intracellular pathways would be disrupted.
Figure 1. Pharmacologic Ascorbate Mediation of Cancer Cell Death.
Pharmacologic ascorbate is a pro-drug for formation of extracellular H2O2. H2O2 diffuses intracellularly and interacts with iron or other metal centers to form reactive oxygen species (ROS). ROS modulate multiple downstream pathways and targets that induce cancer cell death.
In this issue of Cancer Cell, Schoenfeld and colleagues examine the sources of these intracellular metal centers and their roles in mediating ascorbate-induced cancer cell death in an elegant bench-to-bedside presentation (Schoenfeld et al., 2017). Selected models are non-small-cell lung cancer and glioblastoma, both appropriate because the survival of patients with either has not improved substantially for approximately two decades. The pre-clinical dataset shows that cancer cells, but not normal cells, were sensitive to pharmacologic ascorbate and that pharmacologic ascorbic acid as an external H2O2 generator initiated cancer cell death. The survival of mice implanted with tumors was longest when treated with conventional chemotherapy plus radiationin combination with pharmacologic ascorbate, compared to treatments without combination. Dehydroascorbic acid, or oxidized ascorbic acid, although previously reported to kill colon cancer cell lines, was not toxic in the cell lines studied here, which have more complex genetic backgrounds. Lack of toxicity was shown both directly with dehydroascorbic acid itself and indirectly by using a glucose analog that competes with dehydroascorbic acid entry into cells. In the tumor lines tested by Schoenfeld et al., both H2O2 and intracellular redox active metals were necessary for cell death. Increased superoxide generation from mitochondria led to a basal increase in the labile iron pool (LIP) via increased expression of transferrin receptors. This iron was then able to interact with H2O2 generated by ascorbate, as well as with intracellular ascorbate. Future studies will reveal whether these findings about labile iron pool findings from lung and brain cancer cells are generalizable. Complementing this mechanism, ascorbate induced an increase in intracellular labile iron that appeared to be mediated by intracellular H2O2. The mechanism involved intracellular ascorbate interacting with dysregulated mitochondrial electron transfer complexes, or iron-sulfur protein complexes. Although not directly addressed, the source of H2O2 must be at least in part extracellular, initially generated by pharmacologic ascorbate. Otherwise, physiologic ascorbate concentrations achieved by oral doses only would treat cancer in humans, which unfortunately does not seem to be true.
A great strength of the new findings by Schoenfeld and colleagues is direct translation to patients with glioblastoma and advanced non-small-cell lung cancer in two clinical trials. Patients in both trials received conventional therapy plus pharmacologic ascorbate. In the 11 glioblastoma patients, progression-free survival increased by 40% and overall survival by nearly 30% compared to historical controls. Eight glioblastoma patients had an aberrant 06-methylguanine DNA methyltransferase gene promoter. Historically, these patients have worse overall survival. With the addition of pharmacologic ascorbate to conventional treatment here, overall survival was nearly doubled. The 14 patients with non-small-cell lung cancer received platinum-doublet chemotherapy plus pharmacologic ascorbate. Compared to historical controls, disease control rate was more than doubled, and confirmed objective response rate also approached being doubled. Adverse events were similar or reduced compared to conventional therapy.
Limitations of these clinical data are small numbers and historical controls. Limitations of pharmacologic ascorbate are the need for prolonged repetitive therapy given intravenously. Solutions are readily available for both obstacles. For the latter, home treatment can be utilized, similar to other chronic intravenous therapies for parenteral nutrition or prolonged antibiotic administration. For the former, advancement of clinical trials is the clear way forward.
Despite their inherent shortcomings, the clinical results here are extremely promising and are consistent with prior encouraging results from small phase I trials. As seen here and elsewhere, pharmacologic ascorbate has a terrific safety profile in comparison to most agents (Padayatty et al., 2010; Ma et al., 2014). Although ascorbate has endured a tortuous path as a cancer treatment agent, data here add to the increasing evidence supporting pharmacologic ascorbate’s potential. Indeed, it is our firm belief that the time is now for pharmacologic ascorbate to be advanced to rigorous prospective cancer treatment trials for glioblastoma, non-small-cell lung cancers, and other cancers. Iron-rich tumors such as non-Hodgkin’s lymphomas are ripe for such trials. Other candidates include metastatic pancreatic cancer, metastatic ovarian cancer, non-resectable hepatoma, metastatic renal-cell cancer, and invasive bladder cancer. A thoughtfully designed trial for metastatic prostate cancer has recently opened (NCT02516670). Multiple myeloma can be added to the list if renal function is followed especially carefully. Pharmacologic ascorbate should be added early in treatment, as was done admirably by Schoenfeld and colleagues.
Pharmacologic ascorbate as a pro-oxidant therapy is not initially intuitive, as ascorbate is commonly thought of as an antioxidant. Analogously, pharmacologic ascorbate has promiscuous actions in cancer treatment. Although promiscuous treatment at first read would seem disadvantageous, due to possible harm, again the evidence here and elsewhere indicates that this tenet doesn’t apply to pharmacologic ascorbate. Rather, we should exploit pharmacologic ascorbate’s promiscuity. Patients deserve no less.
ACKNOWLEDGMENTS
The authors were supported by Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. NCT02516670 is funded by the Marcus Foundation. M.L. is a co-principal investigator on the trial. Opinions stated here are those of the authors and are not official positions of the National Institutes of Health.
REFERENCES
- Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, and Levine M (2005). Proc. Natl. Acad. Sci. USA 102, 13604–13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, and Levine M (2007). Proc. Natl. Acad. Sci. USA 104, 8749–8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, and Levine M (2008). Proc. Natl. Acad. Sci. USA 105, 11105–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Padayatty SJ, and Espey MG (2011). Adv. Nutr. 2, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, and Chen Q (2014). Sci. Transl. Med. 6, 222ra18. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, and Levine M (2000). J. Am. Coll. Nutr. 19, 423–425. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, and Levine M (2010). PLoS One 5, e11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, Sandhu S, Carlisle TL, Smith MC, Abu Hejleh T, et al. (2017). Cancer Cell 31, this issue, 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrax J, and Calderon PB (2009). Free Radic. Biol. Med. 47, 32–40. [DOI] [PubMed] [Google Scholar]