Abstract
Non-Aspergillus filamentous fungi causing invasive mould infections have increased over the last years due to the widespread use of anti-Aspergillus prophylaxis and increased complexity and survival of immunosuppressed patients. In the few studies that have reported on invasive mould infection epidemiology, Mucorales are the most frequently isolated group, followed by either Fusarium spp. or Scedosporium spp. The overall incidence is low, but related mortality is exceedingly high. Patients with haematological malignancies and haematopoietic stem cell transplant recipients comprise the classical groups at risk of infection for non-Aspergillus moulds due to profound immunosuppression and the vast use of anti-Aspergillus prophylaxis. Solid organ transplant recipients also face a high risk, especially those receiving lung transplants, due to direct exposure of the graft to mould spores with altered mechanical and immunological elimination, and intense, associated immunosuppression. Diagnosing non-Aspergillus moulds is challenging due to unspecific symptoms and radiological findings, lack of specific biomarkers, and low sensitivity of cultures. However, the advent of molecular techniques may prove helpful. Mucormycosis, fusariosis and scedosporiosis hold some differences regarding clinical paradigmatic presentations and preferred antifungal therapy. Surgery might be an option, especially in mucormycosis. Finally, various promising strategies to restore or enhance the host immune response are under current evaluation.
Short abstract
Invasive fungal disease caused by non-Aspergillus moulds is increasing and is associated with exceedingly high mortality. Diagnosis and treatment challenges are discussed in this review. https://bit.ly/3PVozfp
Introduction
Invasive fungal diseases (IFDs) are a major cause of morbidity and mortality worldwide [1]. Over the last few years, an epidemiological change in fungal infections has taken place, with an increase in invasive mould infections (IMIs) and a decrease in yeast infections [2]. Filamentous fungi or moulds typically cause lung infections due to inhalation of spores from the environment, although disseminated infections are not uncommon.
The most common fungi causing IMIs are Aspergillus spp. However, extensive use of anti-Aspergillus prophylaxis in different settings, together with increased complexity and survival of immunosuppressed patients, has partly driven the emergence of non-Aspergillus mould infections [3, 4]. Also, the advent of microbiological molecular techniques has favoured a better recognition of these fungi, typically described in patients with immunosuppression, although there have been reports in immunocompetent patients after natural disasters or viral infections [5]. Of note, most accepted definitions on IFDs are mainly applicable in patients with haematological malignancies or receiving transplants [6, 7]. That stated, non-Aspergillus IMIs are probably underrecognised and underreported in several at-risk populations.
Non-Aspergillus mould infections are associated with an exceedingly high mortality. This is mainly due to intrinsic resistance to multiple antifungal drugs. However, a lack of proper diagnostic tools has also been associated with delays in specific treatments, which in turn can lead to a poorer prognosis. In this setting, both improved diagnostic tests and novel antifungals and therapeutic strategies are urgently needed.
In this article, we will review the recent literature concerning data on non-Aspergillus mould lung infections, focusing on different at-risk populations, diagnostic challenges, specific causative agents, and current and future therapeutic strategies. We decided to not include other important types of fungi that cause lung infections, e.g. Pneumocystis jirovecii, or dimorphic fungi such as Histoplasma spp., Coccidioides spp. or Sporothrix schenckii. The reasons for this include space constraints and a desire to focus the review with a group of more similar mould infections.
Non-Aspergillus IMI epidemiology
There are a few studies focusing on the epidemiology of non-Aspergillus IMIs, especially in immunosuppressed patients. Slavin et al. [5] reported 162 non-Aspergillus mould infections from across 15 hospitals in Australia and New Zealand. Mucormycosis was the most frequent IMI (46%), followed by scedosporiosis (33%), phaeohyphomycosis (10%) and fusariosis (8%). Mucormycosis was associated with prior voriconazole prophylaxis, while scedosporiosis and fusariosis were more common in solid organ transplant (SOT) and neutropenic patients, respectively. Around 70 patients had pulmonary involvement, with half presenting a disseminated infection. Overall, 90-day mortality was >44%.
Lee et al. [8] reported another large cohort of 689 patients with IMIs from a South Korean tertiary centre. Only ∼7% of IMIs were due to moulds other than Aspergillus, with Mucorales being the most frequent causative agent once again, followed by Fusarium spp. The most common site of IMIs was pulmonary, with 18% being disseminated infections and 24% being breakthrough episodes. Interestingly, almost 20% of patients had a mixed fungal infection (all with Aspergillus) and >50% presented a coexisting infection other than IMIs, a reflection of the severe net state of immunosuppression in patients. Finally, 12-week mortality was 39%. The authors did not find a significant increase in the rate of non-Aspergillus/Aspergillus infections over the 7-year study period.
The Transplant Associated Infections Surveillance Net (TRANSNET), a multicentre network of 23 transplantation hospitals in the USA, reported IFD epidemiology during 2001–2006 [9]. Of the 1208 cases of proven or probable IFDs in SOT recipients and the 983 cases in haematopoietic stem cell transplant (HSCT) recipients, 169 (7.7%) cases were due to non-Aspergillus moulds. Mucorales (105 patients) were the most common, followed by Fusarium spp. (37 patients) and Scedosporium spp. (27 patients). The lower respiratory tract was the most commonly involved site and 17.4% of all cases had disseminated infection. The overall crude mortality rate at 90 days was 56.6% (90 out of 169). Globally, cumulative 12-month incidence was low, with allogenic HSCT (0.2–0.8%, depending on human leukocyte antigen relatedness) and lung and liver transplants (∼0.15%) being the most elevated [9]. Interestingly, high variability was observed between centres in both incidence and agents causing non-Aspergillus IMIs. This outlines the importance of environmental and climate conditions as these relate to growth and sporulation of these moulds. It underpins, at the same time, the expectable variability of certain species predominance between distant regions. That stated, in a prospective cohort of eight Brazilian transplant centres, the leading IFD was fusariosis (35%), followed by aspergillosis (30%), invasive candidiasis (17%) and hyalohyphomycosis (12%) [10].
Some other cohorts also report data on IFDs caused by non-Aspergillus moulds [11–15]. The Prospective Antifungal Therapy (PATH) Alliance Registry documented proven or probable IFDs in HSCT recipients from across 16 North American medical centres between 2004 and 2007 [11]. 14% of the episodes were caused by Mucorales (half of them) or other moulds. In another study conducted in Taiwan [14], the investigators reported 27 diagnosed cases of non-Aspergillus IMIs throughout a 9-year period; 12 (44.4%) were caused by Fusarium spp., seven (25.9%) by Paecilomyces spp., five (18.5%) by Mucorales and three (11.1%) by Scedosporium spp. Overall, 12-week mortality was 40.7%, reaching 76.9% in patients with haematological malignancies.
Table 1 summarises some of the most important studies regarding non-Aspergillus IMIs. In all of these studies, reported clinical features among the different moulds were almost indistinguishable (except for that of bloodstream infection, which was more frequent in fusariosis). In this sense, thorough knowledge of local epidemiology is mandatory. Additionally, the presence of a non-Aspergillus mould could result from antifungal selection pressure. For that reason, expected breakthrough IFD epidemiology per prior antifungal therapy has been suggested [2]. Importantly, mortality rates were variable, although commonly stated as >50%. This variability could be explained by the wide variety of hosts involved in the different studies, as well as the potentially different virulence of the various causative moulds.
TABLE 1.
Important studies regarding non-Aspergillus invasive mould infections (IMIs)
| First author [ref.], year# | Country | Study type, period | Infections (n) | Baseline disease | IMI epidemiology | Mortality |
| Neofytos [11 ], 2009 | USA and Canada | Prospective, 2004–2007 | 250 IFDs, 35 non-Aspergillus mould infections | HSCT recipients | 51% Mucorales, 11% Fusarium spp., 38% other moulds (not specified) | 12-week mortality: 72% |
| Hsiue [14], 2009 | Taiwan | Retrospective, 2000–2008 | 27 non-Aspergillus mould infections | 58% haematological malignancies, 12% solid tumour, 12% SOT, 8% diabetes mellitus, 8% HIV | 44% Fusarium spp., 26% Paecilomyces spp., 19% Mucorales, 11% Scedosporium spp. | 12-week mortality: 41% |
| Kontoyiannis [9], 2010 | USA | Prospective, 2001–2005 | 1208 IFDs, 169 non-Aspergillus mould infections | 73% HSCT, 27% SOT | 62% Mucorales, 25% Fusarium spp., 13% Scedosporium spp. | 90-day mortality: 56.6% |
| Nucci [10], 2013 | Brazil | Prospective, 2007–2009 | 66 IFDs, 35 non-Aspergillus mould infections | 61% HSCT, 39% acute leukaemia | 69% Fusarium spp., 23% hyaline moulds, 8% Mucorales | 6-week mortality: 47% |
| Girmenia [15], 2014 | Italy | Prospective, 2008–2010 | 164 IFDs, 11 non-Aspergillus mould infections | HSCT recipients | 54% Mucorales, 27% Fusarium spp., 9% Scedosporium spp., 9% other hyaline moulds | 100-day mortality: 75% |
| Slavin [5], 2014 | Australia and New Zealand | Retrospective, 2004–2012 | 162 non-Aspergillus mould infections | 46% haematological malignancies, 23% diabetes mellitus, 17% HSCT, 16% COPD, 15% no comorbidities, 14% SOT | 46% Mucorales, 33% Scedosporium spp., 10% phaeohyphomycetes, 8% Fusarium spp., 5% other hyaline moulds | 90-day mortality: 44.4% |
| Fox [12], 2014 | Spain | Retrospective, 2009–2011 | 7 non-Aspergillus mould infections | HSCT recipients | 72% Mucorales, 14% Scedosporium spp., 14% other hyaline moulds | Overall mortality: 85% |
| Peghin [13], 2016 | Spain | Retrospective, 2003–2013 | 10 non-Aspergillus mould infections | Lung transplant recipients | 40% Purpureocillium lilacinum, 40% Scedosporium apiospermum, 20% other hyaline moulds | Overall mortality: 40% |
| Lee [8], 2020 | South Korea | Retrospective, 2011–2018 | 689 IMIs, 46 non-Aspergillus mould infections | All cases in patients with haematological diseases | 57% Mucorales, 19% Fusarium spp., 4% Scedosporium spp., 15% other hyaline moulds, 4% phaeohyphomycetes | 12-week mortality: 39.1% |
IFD: invasive fungal disease; HSCT: haematopoietic stem cell transplant; SOT: solid organ transplant. #: publications listed in chronological order.
Non-Aspergillus mould infections in specific populations and settings
Patients with haematological malignancies and HSCT recipients
IFD is a common cause of morbidity and mortality in patients with haematological malignancies and HSCT recipients. Highly toxic chemotherapies associated with profound and prolonged aplasia, along with an increased survival of patients, make this population highly susceptible to fungal infection [16]. Classically, patients with acute myeloid leukaemia (AML) and allogeneic HSCT recipients have comprised the groups associated with the highest risk of fungal infection. However, in recent years, IFD has been increasingly reported in other populations, such as those with acute lymphoblastic leukaemia, lymphoma or multiple myeloma. This holds especially true in those patients who have received multiple prior lines of treatment [17, 18]. IFD epidemiology in patients with haematological malignancies has been highly influenced by the widespread introduction of antifungal prophylaxis in high-risk populations [2]. At the beginning of the current century, fluconazole prophylaxis became the standard of care in neutropenic patients with haematological malignancies, since some trials had demonstrated a reduction in fungal infection and mortality [19–21]. After fluconazole prophylaxis, IFD epidemiology shifted from the clear predominance of invasive candidiasis (reaching ∼90% of all fungal infections in some older studies) to a scenario in which invasive aspergillosis became the most frequent IFD [9, 11, 15]. As previously presented, non-Aspergillus moulds already caused ∼6–14% of all fungal infections in such studies. However, later trials also showed that anti-Aspergillus prophylaxis with posaconazole or voriconazole lowered the risk of IFD in both high-risk patients with either AML or myelodysplastic syndrome and allogenic HSCT recipients with graft-versus-host disease receiving corticosteroid therapy [22–24]. Large cohorts describing breakthrough IFD epidemiology in patients receiving posaconazole or voriconazole prophylaxis are missing. However, these episodes will be expected to be associated with an increase in non-Aspergillus resistant fungi rates. For example, Lamoth et al. [25] compared 24 microbiologically documented breakthrough IMIs (eight for posaconazole and 16 for voriconazole) with 66 non-breakthrough episodes. They showed a clear increase in rates of IMIs caused by Mucorales and other moulds. Importantly, >50% of breakthrough episodes were due to voriconazole- and posaconazole-resistant species, and 17% of strains were also amphotericin B resistant. Larger prospective studies reporting current breakthrough fungal infection epidemiology in patients with haematological malignancies are needed.
SOT recipients
IFDs pose a significant threat to patients undergoing SOT. Risk and features of fungal infection vary widely depending on the organ transplanted, surgery performed, baseline disease and comorbidities, and type of immunosuppression. Over the last few years, mucormycosis and other non-aspergillosis IMIs have been increasingly reported in SOT, especially in those of heart and lung, and typically late after transplantation [26–28]. The most common presentation forms are fungal pneumonia and rhino-sinus infection. Lung transplant recipients are at a particularly higher risk of IMIs for several reasons: 1) the transplanted lung has decreased mucociliary function and lymphatic drainage dysfunction, 2) the graft is directly exposed to the ubiquitous environmental mould spores, 3) an altered blood supply complicates the delivery of antifungal treatments, and 4) immunosuppression therapies are frequently more intense than in other SOTs. Remarkably, mortality as high as 40–80% has been reported [29–31]. In this setting, antifungal prophylaxis is often used in these patients, often with nebulised amphotericin B-based regimens. However, there is a lack of consensus as it relates to the best prophylactic agent or duration of treatment. A Spanish cohort recently reported the epidemiology of non-Aspergillus IMIs in patients undergoing lung transplantation [13]. Of the 412 lung transplantations, 10 (2.42%) patients developed non-Aspergillus mould infections: three (30%) cases of simple tracheobronchitis, three (30%) ulcerative tracheobronchitis, two (20%) bronchial stent infections and two (20%) invasive pulmonary infections. Median time from transplantation to the first non-Aspergillus mould infection was 420 days, and the most common causative agents included Purpureocillium lilacinum (40%) and Scedosporium apiospermum (40%). Of note, four of the 10 patients (40%) were co-infected with Aspergillus species and three (30%) had a bronchial stent. Although the overall incidence of non-Aspergillus IMIs was relatively low, related mortality was 60%, reaching 100% in pulmonary forms.
Patients with viral infections
Respiratory viruses disrupt local innate immunity and harm mucociliary activity and bronchial mucosa, favouring fungal invasion [32–34]. The reported association between invasive aspergillosis and patients with severe influenza requiring intensive care unit (ICU) admission has constituted the prime example of the aforementioned relationship [35–37]. More recently, severe coronavirus disease 2019 (COVID-19) cases associated with pulmonary aspergillosis have also been reported with varying incidence rates [38]. Information on non-Aspergillus moulds associated with both influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is scarce and basically restricted to cases of mucormycosis. A review published at the beginning of 2021 reported eight and seven cases of mucormycosis related to influenza and COVID-19, respectively [39]. Most cases occurred in patients with underlying conditions and either severe influenza or COVID-19. Also, common risk factors included corticosteroid therapy and uncontrolled diabetes. The only difference found regarding the predisposing viral infection was a higher presence of rhino-orbito-cerebral mucormycosis in patients with severe COVID-19. As the pandemic has advanced, and particularly spread further into India, more case series of COVID-19-associated mucormycosis have been reported [40–43]. Again, corticosteroid therapy, uncontrolled diabetes and haematological malignancies comprised the most frequent risk factors. Reported mortality due to COVID-19-associated mucormycosis stands at ∼50%, making it mandatory to undertake a prompt antifungal treatment and surgical approach [44]. Of note, an Iranian study focused on COVID-19-associated pulmonary mould infections in patients requiring mechanical ventilation for ≥4 days [45]. As many as 40 (38%) patients were reported to have an associated pulmonary IMI. Although invasive aspergillosis was the most common infection, Fusarium spp. was isolated in six out of 29 (21%) of the culture-positive samples. Finally, as already known, local damage and immune alteration is probably common to all respiratory viruses. In this sense, we believe that the described association for influenza and SARS-CoV-2 may be extrapolated to other respiratory viruses, as long as they present in a sufficiently severe form.
Other at-risk populations
Some other groups of immunosuppressed patients are also at risk of pulmonary IMIs. These include those with solid tumours [46], autoimmune diseases and immunosuppressants [47, 48] or chronic granulomatous disease [49]. Also, IFD frequency appears to be on the rise in patients admitted to the ICU. However, it is important to bear in mind that IMIs due to non-Aspergillus moulds can also present in otherwise healthy patients. In the Australia and New Zealand Mycoses Interest Group (ANZMIG) cohort [5], 15.4% of patients with a non-Aspergillus IMI had neither underlying immunosuppression nor comorbidity. Nonetheless, it should be noted that European Organisation for Research and Treatment of Cancer/Mycoses Study Group host criteria [6] were not required in the ANZMIG study due to their low applicability outside of the haematological and transplant setting. Eliminating host criteria while maintaining (and probably restricting) clinical and microbiological criteria would likely result in a better depiction of the real-world landscape of invasive fungal infections.
Diagnostic challenges in non-Aspergillus lung IMIs
There are several challenges in diagnosing non-Aspergillus IMIs. First, it is extremely difficult to clinically differentiate invasive aspergillosis from other IMIs. Although some characteristics are typically associated with a particular mould (e.g. cutaneous lesions and fusariosis or rhino-sinus compromise and mucormycosis), these are largely unspecific and any mould could be the causative agent. Second, although the presence of macronodules and the halo sign are commonly associated with invasive aspergillosis [50], these findings are again non-specific and could also be found in infections caused by other moulds. A work conducted at the MD Anderson Cancer Center (Houston, TX, USA) comparing radiological findings between pulmonary mucormycosis and pulmonary aspergillosis showed that the presence of multiple (≥10) nodules and pleural effusion were both associated with pulmonary mucormycosis [51]. However, no differences were noted with respect to the frequency of other computed tomography findings such as masses, cavities, halo sign or air crescent sign. Third, there are no specific fungal biomarkers for mould infections other than Aspergillus. Serum or bronchoalveolar galactomannan is a useful marker for the diagnosis and treatment monitoring of invasive aspergillosis. Although galactomannan can be elevated in some patients with invasive fusariosis or other fungi [52, 53], a positive result will be logically interpreted to indicate invasive aspergillosis unless a culture for another mould tests positive. 1,3-β-d-glucan is a component of the cell wall of many fungi that may be detectable in the serum of patients with non-Aspergillus IMIs, excluding mucormycosis. However, its use is limited due to its complete lack of specificity. In this complex scenario, direct visualisation and/or culture of these moulds remains essential. This is also challenging, given that the yield of cultures is often <50% for non-Aspergillus moulds, and invasive sample collection (e.g. lung biopsy) is typically constrained due to patient instability and/or profound thrombocytopenia. Once a mould is detected in histopathology or culture, molecular methods can confirm the infection and identify the causative strains [54]. In the last few years, quantitative PCR (qPCR) assays to detect fungal DNA have been investigated. These techniques, ranging from pan-fungal to species-specific, may be used in histopathological samples, but also in blood and urine [55], and have a fast turnaround time (∼3 h), moderate sensitivity and high specificity [56]. In fact, some studies showed that specific Mucorales qPCR may diagnose mucormycosis earlier than conventional mycological methods or imaging in susceptible patients [57–60].
The most frequent non-Aspergillus IMIs and available antifungal treatments
Table 2 shows the main characteristics and treatment recommendations for the most frequent non-Aspergillus moulds causing lung infections.
TABLE 2.
Main characteristics and treatment recommendations for the most frequent non-Aspergillus moulds causing lung infections
| Fungal agent | Main species | Microbiological diagnosis [130 ]# | Treatment¶ | Comments¶ |
| Mucorales [71] | Rhizopus spp., Mucor spp., Rhizomucor spp., Cunninghamella spp., Lichtheimia sp., Apophysomyces spp. | Thick, ribbonlike, non-septate hyphae; no available biomarkers; molecular diagnosis (pan-fungal or quantitative species-specific PCR) | Liposomal amphotericin B 5–10 mg·kg−1 per day (first choice) or isavuconazole (second choice); posaconazole (second line); liposomal amphotericin B combined with echinocandins or posaconazole in severe cases (low evidence) | CT scan showing reverse halo sign, multiple nodes (>10) or pleural effusion; perform surgery whenever feasible; AST is recommended; rigorous glycaemic control; strategies to restore immunity; consider deferasirox in diabetic ketoacidosis; consider hyperbaric oxygen |
| Fusarium spp. [94] | F. solani species complex, F. oxysporum species complex, F. verticillioides, F. fujikuroi | Narrow, septate hyphae with acute angle branching (like Aspergillus spp.); canoe-shaped macroconidia; conidiophores with single or clustered conidia, with potential reniform adventitious conidia; galactomannan and 1,3-β-d-glucan may be positive; positive blood cultures in ∼50% of cases (adventitious sporulation); molecular diagnosis | Liposomal amphotericin B or voriconazole; consider initial combination of both liposomal amphotericin B and voriconazole; adding an echinocandin or terbinafine may be considered in severe cases (very low evidence) | Typically disseminated skin lesions; AST is recommended; perform surgery whenever feasible; strategies to restore immunity; importance of neutropenia recovery |
| Scedosporium spp. [94] | S. apiospermum, S. aurantiacum, S. boydii, S. dehoogii | Narrow, septate hyphae with acute angle branching (like Aspergillus spp.); lateral branching off at 60–70° angle may be observed; annellides with a swollen base and elongated neck; oval conidia with truncated base; distinctive coremia or an ascocarp may be seen; molecular diagnosis | Voriconazole (first line); echinocandins (second line); voriconazole combined with echinocandins or terbinafine in severe cases (low evidence) | Consider performing MRI; resistant to amphotericin B; AST is recommended; perform surgery whenever feasible; strategies to restore immunity |
| Lomentospora prolificans (formerly known as Scedosporium prolificans) [94] | Narrow, septate hyphae with acute angle branching (like Aspergillus spp.); black colour colonies; flask-shaped and annellated conidiogenous cells with a swollen base and elongated neck; smooth olive conidia cluster at the apex; molecular diagnosis | Voriconazole (first line); consider voriconazole combined with terbinafine or/and echinocandins (very low evidence); consider the use of olorofim (current lack of evidence and not commercialised) | Commonly resistant to all available antifungals; AST is recommended; consider performing cranial MRI; perform surgery whenever feasible; strategies to restore immunity | |
| Other hyaline moulds [94] | Paecilomyces spp., Acremonium spp., Rasamsonia spp., Penicillium spp., Trichoderma spp. | Narrow, septate hyphae with acute angle branching (like Aspergillus spp.); some differences between species; molecular diagnosis | Optimal antifungal therapy not established and depends on the isolated species | Importance of molecular diagnosis and AST; perform surgery whenever feasible; strategies to restore immunity |
| Phaeohyphomycetes [94] | Alternaria spp., Exophiala spp., Curvularia spp., Cladosporium spp., Ochroconis spp., Bipolaris spp. | Septate hyphae with dark-pigmented colonies (melanin production); some differences between species; 1,3-β-d-glucan may be positive; molecular diagnosis | Optimal antifungal therapy not established and depends on the isolated species | AST is recommended; perform surgery whenever feasible; strategies to restore immunity |
CT: computed tomography; AST: antifungal susceptibility testing; MRI: magnetic resonance imaging. #: microscopic morphology description is based on direct examination; ¶: due to the very low quality of evidence available, some of these recommendations are based on the personal experience of the authors.
Mucormycosis
Invasive mucormycosis is caused by Mucorales, ubiquitous fungi commonly found in decaying organic matter. Mucorales cause severe infections with frequent angioinvasion, tissue infarction and necrosis [61, 62]. The most frequently involved species include Rhizopus, Mucor, Rhizomucor, Cunninghamella, Lichtheimia and Apophysomyces. Poor glycaemic control has been shown to diminish neutrophil activity against Mucorales, thus resulting in its classical recognition as an important risk factor [63–65]. Other common risk factors include haematological malignancies, HSCT, prolonged and profound neutropenia, and trauma [64, 66, 67]. Although rhino-sinus and orbital involvement is characteristic, the lung is the most commonly involved site, especially in immunosuppressed patients [3].
With the current and widespread use of antifungal prophylaxis, reporting of both primary and breakthrough mucormycosis has been on the rise [26, 68–70]. The microbiological diagnosis is particularly challenging in mucormycosis due to low culture susceptibility and a lack of useful biomarkers. In this setting, specific antifungal treatment should be started as soon as there is clinical suspicion, since delayed treatment has been associated with significantly increased mortality [62].
Amphotericin B-based therapies form the basis of mucormycosis treatment, with liposomal amphotericin B being associated with the lowest mortality [67, 71]. Doses between 5 and 10 mg·kg−1 per day are usually recommended, with the highest dose associated with increased nephrotoxicity and often kept for central nervous system involvement [71]. Isavuconazole has been licensed as a first-line treatment for mucormycosis on the basis of similar efficacy to an external matched control group treated with amphotericin B formulations [72]. Posaconazole is another first-line treatment option, especially since the development of intravenous formulations and delayed-release tablets. Importantly, Mucorales exhibit a wide range of intraspecies minimum inhibitory concentration distribution for both posaconazole and isavuconazole [4]. In this setting, antifungal susceptibility testing should be conducted whenever possible. While echinocandins display no antifungal activity against Mucorales, they may act synergistically with amphotericin B due to both the unmasking of β-d-glucan and neutrophil activation [73]. Combined therapy with liposomal amphotericin B plus posaconazole has also been proposed. However, drug combination studies have failed to show any benefit [74, 75]. Further multicentre clinical trials are needed to define the best treatment options.
Fusariosis
Fusarium spp. are ubiquitous environmental moulds and major plant pathogens that cause infections especially among patients with haematological malignancies and HSCT [76, 77]. Around 80% of human infections are caused by Fusarium solani species complex and Fusarium oxysporum species complex [78, 79]. Profound neutropenia and T-cell impairment have been identified as the main associated risk factors [80, 81]. Invasive fusariosis commonly presents as sinus, pulmonary or disseminated infections, characteristically producing multiple skin lesions in the setting of haematogenous spread [77, 80, 82, 83]. In the setting of disseminated infections, there have been reports of mortality reaching as high as 80% [80, 84]. As already presented, both β-d-glucan and galactomannan may be useful in diagnosing and monitoring fusariosis.
Amphotericin B-based formulations or voriconazole comprise the two treatments of choice for invasive fusariosis, with no clear superiority of one over the other. Some in vitro and murine models have displayed potential synergism between different antifungals for fusariosis [85–90]. Considering the high treatment failure, intrinsic resistance to most antifungals and devastating mortality rates [80, 84, 91–93], combination therapies are commonly undertaken [94]. However, clinical results of such an approach have been controversial to date [92, 95]. In fact, in the setting of invasive fusariosis, immune reconstitution endeavours, such as neutropenia recovery and withdrawal of immunosuppressant therapy, are the only factors commonly associated with improved survival [4, 82, 96]. Nonetheless, primary combination therapy, with a potential early step down to monotherapy later (once antifungal susceptibility testing results are available), is recommended by international guidelines [94].
Scedosporiosis
Scedosporium spp. and Lomentospora (formerly Scedosporium) prolificans are ubiquitous filamentous fungi frequently found in soil, sewage and polluted waters. These moulds cause infection mainly among immunosuppressed patients presenting with subcutaneous infections, pneumonia, fungaemia and brain abscesses [97, 98]. Scedosporium spp., mainly represented by S. apiospermum, S. boydii and S. aurantiacum, are commonly resistant to amphotericin B, while susceptible to voriconazole and posaconazole [98]. The activity of isavuconazole or itraconazole seems marginal [99]. Voriconazole-based regimens are recommended [94].
In turn, L. prolificans is usually resistant to all available antifungals [100–102], with voriconazole being the most active agent in vitro once again [103]. Both moulds show a particular neurotropism, making low permeability of the blood–brain barrier an additional difficulty when facing treatment. For all of these reasons, it is not unexpected that mortality rates up to 90% are reported [104, 105]. In this scenario, combination therapies are often considered, with in vitro synergism found for combinations of echinocandins and triazoles, and voriconazole or itraconazole and terbinafine [106–108]. However, a recent study analysing all published studies of scedosporiosis showed no benefits in terms of mortality when comparing the combination of voriconazole and terbinafine against voriconazole alone [98]. Despite this, current European Confederation of Medical Mycology (ECMM) guidelines for rare moulds support voriconazole-based combination antifungal therapy for infections caused by L. prolificans, particularly voriconazole plus terbinafine, even considering the addition of other antifungal agents [94].
Olorofim is a novel antifungal agent. It belongs to a new class of drugs, the orotomides, which target dihydroorotate dehydrogenase, an important enzyme for pyrimidine biosynthesis [109, 110]. This new antifungal has shown potent in vitro activity against both Scedosporium spp. and L. prolificans strains [111–113]; however, clinical experience is still lacking.
Others
There are several other non-Aspergillus moulds that cause lung infections. The hyalohyphomycetes or hyaline moulds (including Fusarium spp. and Scedosporium spp.) are filamentous fungi characterised by a lack of pigmentation, with branching septate hyphae similar to Aspergillus. Some examples of other hyaline moulds include Paecilomyces spp., Acremonium spp., Rasamsonia spp., Penicillium spp. and Trichoderma spp. Hyaline moulds are ubiquitous in the environment, and may cause pulmonary and/or disseminated infection, especially in immunosuppressed patients [3, 114]. Other moulds that cause lung infections include the phaeohyphomycetes or dematiaceous fungi, which produce melanin that results in the dark pigmentation of their colonies. These moulds are also ubiquitous and may cause superficial infection in immunocompetent hosts, but pulmonary or disseminated infections in the immunosuppressed [3, 114]. Information on clinical characteristics or the best antifungal treatment for both groups of moulds is scarce. In this sense, the recent ECMM guidelines provided recommendations on the treatment of some of these fungi [94], although the quality of evidence is very low. Molecular techniques for species identification as well as antifungal susceptibility testing are recommended.
Other treatment strategies
Surgery
Surgery may prove useful in pulmonary IMIs for source control, to prevent massive haemoptysis when infection affects pulmonary arteries, and to reduce fungal burden and decrease the risk of later relapse. Some case series reported a possible benefit conferred by surgery on patients with IMIs, even with decreased mortality [67, 115, 116]. However, most studies focused on aspergillosis, were conducted before the introduction of new antifungals and were subject to significant publication bias. Additionally, surgery is commonly not feasible in many patients who are clinically unstable and/or display multiple pulmonary lesions or disseminated infections [68]. Despite the lack of scientific evidence, aggressive and prompt surgery is still recommended, especially in cases of mucormycosis [71], whenever feasible.
Restoration and/or enhancement of the host immune response
As already presented, immunosuppression is commonly the main risk factor underlying a lung IFD due to non-Aspergillus moulds. For this reason, strategies to restore the host immune response could be appealing.
Administering granulocyte–macrophage or granulocyte colony-stimulating factor is used in some cases to promote neutropenia recovery. Additionally, granulocyte–macrophage colony-stimulating factor alone or in combination with interferon-γ has been shown to enhance the fungicidal activity of innate phagocytic cells [117–119]. However, benefits in patients with non-Aspergillus IMIs have not been demonstrated.
Some studies have evaluated the use of white blood cell (granulocyte) transfusions in neutropenic patients. Two recent Cochrane reviews concluded that there was low quality of evidence to support the use of granulocyte transfusion in prophylaxis [120] and not enough evidence to make a recommendation on treatment [121]. In this scenario, granulocyte transfusion may still be considered on a case-by-case basis.
Some pre-clinical studies showed that vaccination could improve the response to fungal infection [122]. Its use might be appliable prophylactically in some high-risk patients such as those receiving lung transplants. However, there is concern about the efficacy of this strategy in highly immunocompromised patients.
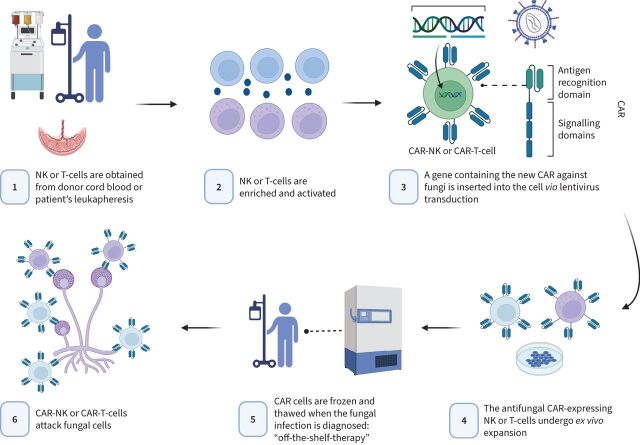
Adoptive T-cell therapy is a promising new discipline wherein immune cells (usually T-lymphocytes) are isolated and expanded ex vivo in an antigen-specific manner, and later infused into the patient. This can be done via either the adoptive transfer of antimould T-cells or the engineering of immune cells (natural killer or T-cells) with chimeric antigen receptors (CARs). Both approaches have shown some encouraging results in murine models [123–126]. Moreover, freezing and thawing protocols are being established so that these therapies could conceptually be used together with antifungals as an “off-the-shelf” option when a severe IMI is documented. Figure 1 displays the simplified process and mechanism of action of a potential antifungal CAR therapy.
FIGURE 1.
A simplified production process and mechanism of action of a potential antifungal chimeric antigen receptor (CAR) therapy. NK: natural killer. Figure created with BioRender.com.
Other therapeutic strategies
Iron overload has been related to an increased risk of IMIs, as this element is essential for fungal survival and growth. The iron chelator deferasirox demonstrated direct fungicidal activity against Mucorales in vitro and in animal models via iron starvation [127]. Additionally, it was even shown to be synergistic with liposomal amphotericin B. The role of deferasirox was evaluated against placebo (with both arms receiving liposomal amphotericin B) in 20 proven or probable cases of mucormycosis [128]. Patients in the deferasirox arm had higher mortality; however, they also randomly had an overrepresentation of some poor prognostic factors such as active malignancy, neutropenia and corticosteroid therapy. Regardless, deferasirox may play a role in diabetic ketoacidosis, in which there is an excess of free iron in tissues [4].
The use of hyperbaric oxygen has been suggested as an adjunctive therapeutic modality in IMIs due to its capacity to revert hypoxia in necrotic tissues. Some studies suggest that adjunctive hyperbaric oxygen may be useful in patients with diabetes, but its benefit in immunosuppressed patients is unclear [129]. Moreover, its use has been tested primarily in patients with rhino-sinus infections and not in those with lung infections.
Future research
Large prospective studies evaluating the current epidemiology of non-Aspergillus IMIs are needed.
Improvement in microbiological techniques is needed. There is a need for the development of new fungal biomarkers. Species-specific molecular diagnosis might be helpful.
Novel antifungal treatments are under development and could improve the prognosis of these entities.
Various promising strategies to enhance immune response against fungi are being investigated, with special interest in the use of antifungal CAR cells.
Conclusions
Non-Aspergillus filamentous fungi causing IMIs have increased over recent years, especially in patients with haematological malignancies, and in HSCT and SOT recipients. Although the overall incidence appears low, these infections are probably underrecognised. At the same time, however, they are associated with exceedingly high mortality. Diagnosing non-Aspergillus IMIs is extremely challenging due to a lack of definitive symptoms, radiological findings and specific biomarkers, together with the low yield of cultures. A high degree of suspicion in conjunction with thorough knowledge of local epidemiology and different mould characteristics is essential. The future scenario of this currently devastating entity may improve, nonetheless, due to the advent of molecular diagnostic techniques, revolutionary strategies to restore or even enhance the immune response and novel antifungal drugs.
Acknowledgements
Figure 1 was created with BioRender.com (license agreement MP245FHH4Q). We would like to thank Anthony Armenta (Barcelona, Spain) for providing medical editing assistance.
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Kumar K, Daley CL, Griffith DE, et al. Management of Mycobacterium avium complex and Mycobacterium abscessus pulmonary disease: therapeutic advances and emerging treatments. Eur Respir Rev 2022; 31: 210212. No. 2: Cilloniz C, Luna CM, Hurtado JC, et al. Respiratory viruses: their importance and lessons learned from COVID-19. Eur Respir Rev 2022; 31: 220051. No. 3: Cavallazzi R, Ramirez JA. How and when to manage respiratory infections out of hospital. Eur Respir Rev 2022; 31: 220092. No. 4: Reynolds D, Burnham JP, Vazquez Guillamet C, et al. The threat of multidrugresistant/extensively drug-resistant Gram-negative respiratory infections: another pandemic. Eur Respir Rev 2022; 31: 220068.
Number 5 in the Series “Respiratory infections” Edited by Antoni Torres and Michael S. Niederman
This article has an editorial commentary: https://doi.org/10.1183/16000617.0150-2022
Conflict of interest: P. Puerta-Alcalde has received honoraria for talks on behalf of Gilead Science, MSD, ViiV Healthcare and Lilly.
Conflict of interest: C. Garcia-Vidal has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Janssen, Lilly and GSK, as well as grants from Gilead Science, Pfizer and MSD.
Support statement: P. Puerta-Alcalde (grants: JR20/00012 and PI21/00498) and C. Garcia-Vidal (grant: PI21/01640) have received research grants funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union. The funder had no role in the current manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Bongomin F, Gago S, Oladele RO, et al. Global and multi-national prevalence of fungal diseases – estimate precision. J Fungi 2017; 3: 57. doi: 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puerta-Alcalde P, Garcia-Vidal C. Changing epidemiology of invasive fungal disease in allogeneic hematopoietic stem cell transplantation. J Fungi 2021; 7: 848. doi: 10.3390/jof7100848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas AP, Chen SCA, Slavin MA. Emerging infections caused by non-Aspergillus filamentous fungi. Clin Microbiol Infect 2016; 22: 670–680. doi: 10.1016/j.cmi.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 4.Lamoth F, Kontoyiannis DP. Therapeutic challenges of non-Aspergillus invasive mold infections in immunosuppressed patients. Antimicrob Agents Chemother 2019; 63: e01244-19. doi: 10.1128/AAC.01244-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavin M, van Hal S, Sorrell TC, et al. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect 2015; 21: 490.e1–490.e10. doi: 10.1016/j.cmi.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 6.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–1821. doi: 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71: 1367–1376. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HJ, Cho SY, Lee DG, et al. Characteristics and risk factors for mortality of invasive non-Aspergillus mould infections in patients with haematologic diseases: a single-centre 7-year cohort study. Mycoses 2020; 63: 257–264. doi: 10.1111/myc.13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 2010; 50: 1091–1100. doi: 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 10.Nucci M, Garnica M, Gloria AB, et al. Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin Microbiol Infect 2013; 19: 745–751. doi: 10.1111/1469-0691.12002 [DOI] [PubMed] [Google Scholar]
- 11.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009; 48: 265–273. doi: 10.1086/595846 [DOI] [PubMed] [Google Scholar]
- 12.Fox ML, Barba P, Heras I, et al. A registry-based study of non-Aspergillus mould infections in recipients of allogeneic haematopoietic cell transplantation. Clin Microbiol Infect 2015; 21: e1–e3. doi: 10.1016/j.cmi.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peghin M, Monforte V, Martin-Gomez MT, et al. Epidemiology of invasive respiratory disease caused by emerging non-Aspergillus molds in lung transplant recipients. Transpl Infect Dis 2016; 18: 70–78. doi: 10.1111/tid.12492 [DOI] [PubMed] [Google Scholar]
- 14.Hsiue HC, Ruan SY, Kuo YL, et al. Invasive infections caused by non-Aspergillus moulds identified by sequencing analysis at a tertiary care hospital in Taiwan, 2000–2008. Clin Microbiol Infect 2010; 16: 1204–1206. doi: 10.1111/j.1469-0691.2009.03103.x [DOI] [PubMed] [Google Scholar]
- 15.Girmenia C, Raiola AM, Piciocchi A, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014; 20: 872–880. doi: 10.1016/j.bbmt.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 16.Douglas AP, Slavin MA. Risk factors and prophylaxis against invasive fungal disease for haematology and stem cell transplant recipients: an evolving field. Expert Rev Anti Infect Ther 2016; 14: 1165–1177. doi: 10.1080/14787210.2016.1245613 [DOI] [PubMed] [Google Scholar]
- 17.Pagano L, Akova M, Dimopoulos G, et al. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J Antimicrob Chemother 2011; 66: Suppl. 1, i5–i14. doi: 10.1093/jac/dkq437 [DOI] [PubMed] [Google Scholar]
- 18.Anastasopoulou A, DiPippo AJ, Kontoyiannis DP. Non-Aspergillus invasive mould infections in patients treated with ibrutinib. Mycoses 2020; 63: 787–793. doi: 10.1111/myc.13120 [DOI] [PubMed] [Google Scholar]
- 19.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation – a prospective, randomized, double-blind study. J Infect Dis 1995; 171: 1545–1552. doi: 10.1093/infdis/171.6.1545 [DOI] [PubMed] [Google Scholar]
- 20.Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 2010; 326: 845–851. doi: 10.1056/NEJM199203263261301 [DOI] [PubMed] [Google Scholar]
- 21.Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 2000; 96: 2055–2061. doi: 10.1182/blood.V96.6.2055 [DOI] [PubMed] [Google Scholar]
- 22.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356: 348–359. doi: 10.1056/NEJMoa061094 [DOI] [PubMed] [Google Scholar]
- 23.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335–347. doi: 10.1056/NEJMoa061098 [DOI] [PubMed] [Google Scholar]
- 24.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116: 5111–5118. doi: 10.1182/blood-2010-02-268151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamoth F, Chung SJ, Damonti L, et al. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin Infect Dis 2017; 64: 1619–1621. doi: 10.1093/cid/cix130 [DOI] [PubMed] [Google Scholar]
- 26.Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis 2011; 17: 1855–1864. doi: 10.3201/eid1710.110087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanternier F, Sun HY, Ribaud P, et al. Mucormycosis in organ and stem cell transplant recipients. Clin Infect Dis 2012; 54: 1629–1636. doi: 10.1093/cid/cis195 [DOI] [Google Scholar]
- 28.Sun HY, Aguado JM, Bonatti H, et al. Pulmonary zygomycosis in solid organ transplant recipients in the current era. Am J Transplant 2009; 9: 2166–2171. doi: 10.1111/j.1600-6143.2009.02754.x [DOI] [PubMed] [Google Scholar]
- 29.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev 2005; 18: 44–69. doi: 10.1128/CMR.18.1.44-69.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silveira FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol 2007; 45: 305–320. doi: 10.1080/13693780701200372 [DOI] [PubMed] [Google Scholar]
- 31.Hosseini-Moghaddam SM, Ouédraogo A, Naylor KL, et al. Incidence and outcomes of invasive fungal infection among solid organ transplant recipients: a population-based cohort study. Transpl Infect Dis 2020; 22: e13250. doi: 10.1111/tid.13250 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Vidal C, Barba P, Arnan M, et al. Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin Infect Dis 2011; 53: e16–e19. doi: 10.1093/cid/cir485 [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Vidal C, Royo-Cebrecos C, Peghin M, et al. Environmental variables associated with an increased risk of invasive aspergillosis. Clin Microbiol Infect 2014; 20: O939–O945. doi: 10.1111/1469-0691.12650 [DOI] [PubMed] [Google Scholar]
- 34.Magira EE, Chemaly RF, Jiang Y, et al. Outcomes in invasive pulmonary aspergillosis infections complicated by respiratory viral infections in patients with hematologic malignancies: a case-control study. Open Forum Infect Dis 2019; 6: ofz247. doi: 10.1093/ofid/ofz247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wauters J, Baar I, Meersseman P, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med 2012; 38: 1761–1768. doi: 10.1007/s00134-012-2673-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 2018; 6: 782–792. doi: 10.1016/S2213-2600(18)30274-1 [DOI] [PubMed] [Google Scholar]
- 37.Lat A, Bhadelia N, Miko B, et al. Invasive aspergillosis after pandemic (H1N1) 2009. Emerg Infect Dis 2010; 16: 971–973. doi: 10.3201/eid1606.100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamoth F, Lewis RE, Walsh TJ, et al. Navigating the uncertainties of COVID-19-associated aspergillosis: a comparison with influenza-associated aspergillosis. J Infect Dis 2021; 224: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadikia K, Hashemi SJ, Khodavaisy S, et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021; 64: 798–808. doi: 10.1111/myc.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia 2021; 186: 289–298. doi: 10.1007/s11046-021-00528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revannavar SM, Supriya P, Samaga L, et al. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep 2021; 14: e241663. doi: 10.1136/bcr-2021-241663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi 2021; 7: 298. doi: 10.3390/jof7040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selarka L, Sharma S, Saini D, et al. Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses 2021; 64: 1253–1260. doi: 10.1111/myc.13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basile K, Halliday C, Kok J, et al. Fungal infections other than invasive aspergillosis in COVID-19 patients. J Fungi 2022; 8: 58. doi: 10.3390/jof8010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghazanfari M, Arastehfar A, Davoodi L, et al. Pervasive but neglected: a perspective on COVID-19-associated pulmonary mold infections among mechanically ventilated COVID-19 patients. Front Med 2021; 8: 649675. doi: 10.3389/fmed.2021.649675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dib RW, Khalil M, Fares J, et al. Invasive pulmonary aspergillosis: comparative analysis in cancer patients with underlying haematologic malignancies versus solid tumours. J Hosp Infect 2020; 104: 358–364. doi: 10.1016/j.jhin.2019.09.020 [DOI] [PubMed] [Google Scholar]
- 47.Chen HS, Tsai WP, Leu HS, et al. Invasive fungal infection in systemic lupus erythematosus: an analysis of 15 cases and a literature review. Rheumatology 2007; 46: 539–544. doi: 10.1093/rheumatology/kel343 [DOI] [PubMed] [Google Scholar]
- 48.Lao M, Wang X, Ding M, et al. Invasive fungal disease in patients with systemic lupus erythematosus from Southern China: a retrospective study. Lupus 2019; 28: 77–85. doi: 10.1177/0961203318817118 [DOI] [PubMed] [Google Scholar]
- 49.Dotis J, Pana ZD, Roilides E. Non-Aspergillus fungal infections in chronic granulomatous disease. Mycoses 2013; 56: 449–462. doi: 10.1111/myc.12049 [DOI] [PubMed] [Google Scholar]
- 50.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 2007; 44: 373–379. doi: 10.1086/509917 [DOI] [PubMed] [Google Scholar]
- 51.Chamilos G, Marom EM, Lewis RE, et al. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis 2005; 41: 60–66. doi: 10.1086/430710 [DOI] [PubMed] [Google Scholar]
- 52.Nucci M, Carlesse F, Cappellano P, et al. Earlier diagnosis of invasive fusariosis with Aspergillus serum galactomannan testing. PLoS One 2014; 9: e87784. doi: 10.1371/journal.pone.0087784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tortorano AM, Esposto MC, Prigitano A, et al. Cross-reactivity of Fusarium spp. in the Aspergillus galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol 2012; 50: 1051–1053. doi: 10.1128/JCM.05946-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi 2020; 6: 265. doi: 10.3390/jof6040265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lackner N, Posch W, Lass-Flörl C. Microbiological and molecular diagnosis of mucormycosis: from old to new. Microorganisms 2021; 9: 1518. doi: 10.3390/microorganisms9071518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seo H, Kim JY, Son HJ, et al. Diagnostic performance of real-time polymerase chain reaction assay on blood for invasive aspergillosis and mucormycosis. Mycoses 2021; 64: 1554–1562. doi: 10.1111/myc.13319 [DOI] [PubMed] [Google Scholar]
- 57.Millon L, Larosa F, Lepiller Q, et al. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis 2013; 56: e95–e101. doi: 10.1093/cid/cit094 [DOI] [PubMed] [Google Scholar]
- 58.Millon L, Herbrecht R, Grenouillet F, et al. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect 2016; 22: 810.e1. doi: 10.1016/j.cmi.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 59.Springer J, Lackner M, Ensinger C, et al. Clinical evaluation of a Mucorales-specific real-time PCR assay in tissue and serum samples. J Med Microbiol 2016; 65: 1414–1421. doi: 10.1099/jmm.0.000375 [DOI] [PubMed] [Google Scholar]
- 60.Millon L, Caillot D, Berceanu A, et al. Evaluation of serum Mucorales polymerase chain reaction (PCR) for the diagnosis of mucormycoses: the MODIMUCOR prospective trial. Clin Infect Dis 2022; 75: 777–785. doi: 10.1093/cid/ciab1066 [DOI] [PubMed] [Google Scholar]
- 61.Kontoyiannis DP, Wessel VC, Bodey GP, et al. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 2000; 30: 851–856. doi: 10.1086/313803 [DOI] [PubMed] [Google Scholar]
- 62.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2008; 47: 503–509. doi: 10.1086/590004 [DOI] [PubMed] [Google Scholar]
- 63.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005; 18: 556–569. doi: 10.1128/CMR.18.3.556-569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41: 634–653. doi: 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 65.Kontoyiannis DP, Lewis RE. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am 2006; 20: 581–607. doi: 10.1016/j.idc.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 66.Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012; 54: Suppl. 1, S23–S34. doi: 10.1093/cid/cir866 [DOI] [PubMed] [Google Scholar]
- 67.Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 2011; 17: 1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x [DOI] [PubMed] [Google Scholar]
- 68.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis 2005; 191: 1350–1360. doi: 10.1086/428780 [DOI] [PubMed] [Google Scholar]
- 69.Trifilio SM, Bennett CL, Yarnold PR, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant 2007; 39: 425–429. doi: 10.1038/sj.bmt.1705614 [DOI] [PubMed] [Google Scholar]
- 70.Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis 2009; 15: 1395–1401. doi: 10.3201/eid1509.090334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019; 19: e405–e421. doi: 10.1016/S1473-3099(19)30312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016; 16: 828–837. doi: 10.1016/S1473-3099(16)00071-2 [DOI] [PubMed] [Google Scholar]
- 73.Lamaris GA, Lewis RE, Chamilos G, et al. Caspofungin-mediated β-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis 2008; 198: 186–192. doi: 10.1086/589305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abidi MZ, Sohail MR, Cummins N, et al. Stability in the cumulative incidence, severity and mortality of 101 cases of invasive mucormycosis in high-risk patients from 1995 to 2011: a comparison of eras immediately before and after the availability of voriconazole and echinocandin-amphotericin combination therapies. Mycoses 2014; 57: 687–698. doi: 10.1111/myc.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kyvernitakis A, Torres HA, Jiang Y, et al. Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin Microbiol Infect 2016; 22: 811.e1. doi: 10.1016/j.cmi.2016.03.029 [DOI] [PubMed] [Google Scholar]
- 76.Fleming R V, Walsh TJ, Anaissie EJ. Emerging and less common fungal pathogens. Infect Dis Clin North Am 2002; 16: 915–933. doi: 10.1016/S0891-5520(02)00041-7 [DOI] [PubMed] [Google Scholar]
- 77.Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34: 909–917. doi: 10.1086/339202 [DOI] [PubMed] [Google Scholar]
- 78.Tortorano AM, Prigitano A, Esposto MC, et al. European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur J Clin Microbiol Infect Dis 2014; 33: 1623–1630. doi: 10.1007/s10096-014-2111-1 [DOI] [PubMed] [Google Scholar]
- 79.Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev 2007; 20: 695–704. doi: 10.1128/CMR.00014-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campo M, Lewis RE, Kontoyiannis DP. Invasive fusariosis in patients with hematologic malignancies at a cancer center: 1998–2009. J Infect 2010; 60: 331–337. doi: 10.1016/j.jinf.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 81.Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood 1997; 90: 999–1008. doi: 10.1182/blood.V90.3.999.999_999_1008 [DOI] [PubMed] [Google Scholar]
- 82.Kontoyiannis DP, Bodey GP, Hanna H, et al. Outcome determinants of fusariosis in a tertiary care cancer center: the impact of neutrophil recovery. Leuk Lymphoma 2004; 45: 139–141. doi: 10.1080/1042819031000149386 [DOI] [PubMed] [Google Scholar]
- 83.Nucci M, Marr KA, Queiroz-Telles F, et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis 2004; 38: 1237–1242. doi: 10.1086/383319 [DOI] [PubMed] [Google Scholar]
- 84.Nucci M, Anaissie EJ, Queiroz-Telles F, et al. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer 2003; 98: 315–319. doi: 10.1002/cncr.11510 [DOI] [PubMed] [Google Scholar]
- 85.Heyn K, Tredup A, Salvenmoser S, et al. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob Agents Chemother 2005; 49: 5157–5159. doi: 10.1128/AAC.49.12.5157-5159.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arikan S, Lozano-Chiu M, Paetznick V, et al. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob Agents Chemother 2002; 46: 245–247. doi: 10.1128/AAC.46.1.245-247.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Córdoba S, Rodero L, Vivot W, et al. In vitro interactions of antifungal agents against clinical isolates of Fusarium spp. Int J Antimicrob Agents 2008; 31: 171–174. doi: 10.1016/j.ijantimicag.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 88.Ruiz-Cendoya M, Marine M, Guarro J. Combined therapy in treatment of murine infection by Fusarium solani. J Antimicrob Chemother 2008; 62: 543–546. doi: 10.1093/jac/dkn215 [DOI] [PubMed] [Google Scholar]
- 89.Ruiz-Cendoya M, Marine M, Rodriguez MM, et al. Interactions between triazoles and amphotericin B in treatment of disseminated murine infection by Fusarium oxysporum. Antimicrob Agents Chemother 2009; 53: 1705–1708. doi: 10.1128/AAC.01606-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruíz-Cendoya M, Pastor FJ, Capilla J, et al. Treatment of murine Fusarium verticillioides infection with liposomal amphotericin B plus terbinafine. Int J Antimicrob Agents 2011; 37: 58–61. doi: 10.1016/j.ijantimicag.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 91.Perfect JR. Treatment of non-Aspergillus moulds in immunocompromised patients, with amphotericin B lipid complex. Clin Infect Dis 2005; 40: Suppl. 6, S401–S408. doi: 10.1086/429331 [DOI] [PubMed] [Google Scholar]
- 92.Lortholary O, Obenga G, Biswas P, et al. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob Agents Chemother 2010; 54: 4446–4450. doi: 10.1128/AAC.00286-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raad II, Hachem RY, Herbrecht R, et al. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis 2006; 42: 1398–1403. doi: 10.1086/503425 [DOI] [PubMed] [Google Scholar]
- 94.Hoenigl M, Salmanton-García J, Walsh TJ, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 2021; 21: e246–e257. doi: 10.1016/S1473-3099(20)30784-2 [DOI] [PubMed] [Google Scholar]
- 95.Liu J-Y, Chen W-T, Ko B-S, et al. Combination antifungal therapy for disseminated fusariosis in immunocompromised patients: a case report and literature review. Med Mycol 2011: 49: 872–878. doi: 10.3109/13693786.2011.567304 [DOI] [PubMed] [Google Scholar]
- 96.Nucci M, Marr KA, Vehreschild MJGT, et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect 2014; 20: 580–585. doi: 10.1111/1469-0691.12409 [DOI] [PubMed] [Google Scholar]
- 97.Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev 2008; 21: 157–197. doi: 10.1128/CMR.00039-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seidel D, Meißner A, Lackner M, et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope. Crit Rev Microbiol 2019; 45: 1–21. doi: 10.1080/1040841X.2018.1514366 [DOI] [PubMed] [Google Scholar]
- 99.Lackner M, De Hoog GS, Verweij PE, et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 2012; 56: 2635–2642. doi: 10.1128/AAC.05910-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cuenca-Estrella M, Ruiz-Díez B, Martínez-Suárez JV, et al. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother 1999; 43: 149–151. doi: 10.1093/jac/43.1.149 [DOI] [PubMed] [Google Scholar]
- 101.Carrillo AJ, Guarro J. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob Agents Chemother 2001; 45: 2151–2153. doi: 10.1128/AAC.45.7.2151-2153.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boucher HW, Groll AH, Chiou CC, et al. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs 2004; 64: 1997–2020. doi: 10.2165/00003495-200464180-00001 [DOI] [PubMed] [Google Scholar]
- 103.Meletiadis J, Meis JFGM, Mouton JW, et al. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob Agents Chemother 2002; 46: 62–68. doi: 10.1128/AAC.46.1.62-68.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lamaris GA, Chamilos G, Lewis RE, et al. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989–2006. Clin Infect Dis 2006; 43: 1580–1584. doi: 10.1086/509579 [DOI] [PubMed] [Google Scholar]
- 105.Blyth CC, Gilroy NM, Guy SD, et al. Consensus guidelines for the treatment of invasive mould infections in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J 2014; 44: 1333–1349. doi: 10.1111/imj.12598 [DOI] [PubMed] [Google Scholar]
- 106.Lackner M, Fernández-Silva F, Guarro J, et al. Assessing micafungin/triazole combinations for the treatment of invasive scedosporiosis due to Scedosporium apiospermum and Scedosporium boydii. J Antimicrob Chemother 2014; 69: 3027–3032. doi: 10.1093/jac/dku224 [DOI] [PubMed] [Google Scholar]
- 107.Cuenca-Estrella M, Alastruey-Izquierdo A, Alcazar-Fuoli L, et al. In vitro activities of 35 double combinations of antifungal agents against Scedosporium apiospermum and Scedosporium prolificans. Antimicrob Agents Chemother 2008; 52: 1136–1139. doi: 10.1128/AAC.01160-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meletiadis J, Mouton JW, Meis JFGM, et al. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob Agents Chemother 2003; 47: 106–117. doi: 10.1128/AAC.47.1.106-117.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoenigl M, Sprute R, Egger M, et al. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 2021; 81: 1703–1729. doi: 10.1007/s40265-021-01611-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCarty TP, Pappas PG. Antifungal pipeline. Front Cell Infect Microbiol 2021; 11: 811. doi: 10.3389/fcimb.2021.732223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiederhold NP, Law D, Birch M. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother 2017; 72: 1977–1980. doi: 10.1093/jac/dkx065 [DOI] [PubMed] [Google Scholar]
- 112.Biswas C, Law D, Birch M, et al. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol 2018; 56: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 113.Kirchhoff L, Dittmer S, Buer J, et al. In vitro activity of olorofim (F901318) against fungi of the genus, Scedosporium and Rasamsonia as well as against Lomentospora prolificans, Exophiala dermatitidis and azole-resistant Aspergillus fumigatus. Int J Antimicrob Agents 2020; 56: 106105. doi: 10.1016/j.ijantimicag.2020.106105 [DOI] [PubMed] [Google Scholar]
- 114.Jacobs SE, Wengenack NL, Walsh TJ. Non-Aspergillus hyaline molds: emerging causes of sino-pulmonary fungal infections and other invasive mycoses. Semin Respir Crit Care Med 2020; 41: 115–130. doi: 10.1055/s-0039-3401989 [DOI] [PubMed] [Google Scholar]
- 115.Yeghen T, Kibbler CC, Prentice HG, et al. Management of invasive pulmonary aspergillosis in hematology patients: a review of 87 consecutive cases at a single institution. Clin Infect Dis 2000; 31: 859–868. doi: 10.1086/318133 [DOI] [PubMed] [Google Scholar]
- 116.Caillot D, Mannone L, Cuisenier B, et al. Role of early diagnosis and aggressive surgery in the management of invasive pulmonary aspergillosis in neutropenic patients. Clin Microbiol Infect 2001; 7: Suppl. 2, 54–61. doi: 10.1111/j.1469-0691.2001.tb00010.x [DOI] [PubMed] [Google Scholar]
- 117.Roilides E, Blake C, Holmes A, et al. Granulocyte-macrophage colony-stimulating factor and interferon-gamma prevent dexamethasone-induced immunosuppression of antifungal monocyte activity against Aspergillus fumigatus hyphae. J Med Vet Mycol 1996; 34: 63–69. doi: 10.1080/02681219680000101 [DOI] [PubMed] [Google Scholar]
- 118.Roilides E, Holmes A, Blake C, et al. Antifungal activity of elutriated human monocytes against Aspergillus fumigatus hyphae: enhancement by granulocyte-macrophage colony-stimulating factor and interferon-gamma. J Infect Dis 1994; 170: 894–899. doi: 10.1093/infdis/170.4.894 [DOI] [PubMed] [Google Scholar]
- 119.Safdar A, Rodriguez G, Zuniga J, et al. Granulocyte macrophage colony-stimulating factor in 66 patients with myeloid or lymphoid neoplasms and recipients of hematopoietic stem cell transplantation with invasive fungal disease. Acta Haematol 2013; 129: 26–34. doi: 10.1159/000342121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Estcourt LJ, Stanworth S, Doree C, et al. Granulocyte transfusions for preventing infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev 2015; 6: CD005341. doi: 10.1002/14651858.CD005341.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Estcourt LJ, Stanworth SJ, Hopewell S, et al. Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev 2016; 4: CD005339. doi: 10.1002/14651858.CD005339.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ito JI, Lyons JM, Hong TB, et al. Vaccinations with recombinant variants of Aspergillus fumigatus allergen Asp f 3 protect mice against invasive aspergillosis. Infect Immun 2006; 74: 5075–5084. doi: 10.1128/IAI.00815-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grazziutti M, Przepiorka D, Rex JH, et al. Dendritic cell-mediated stimulation of the in vitro lymphocyte response to Aspergillus. Bone Marrow Transplant 2001; 27: 647–652. doi: 10.1038/sj.bmt.1702832 [DOI] [PubMed] [Google Scholar]
- 124.Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood 2005; 106: 4397–4406. doi: 10.1182/blood-2005-05-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beck O, Topp MS, Koehl U, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood 2006; 107: 2562–2569. doi: 10.1182/blood-2005-04-1660 [DOI] [PubMed] [Google Scholar]
- 126.Kumaresan PR, Manuri PR, Albert ND, et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci USA 2014; 111: 10660–10665. doi: 10.1073/pnas.1312789111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood 2011; 118: 1216–1224. doi: 10.1182/blood-2011-03-316430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox–AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother 2012; 67: 715–722. doi: 10.1093/jac/dkr375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect 2005; 11: 515–517. doi: 10.1111/j.1469-0691.2005.01170.x [DOI] [PubMed] [Google Scholar]
- 130.Walsh TJ, Hayden RT, Larone DH. Larone's Medically Important Fungi. Washington, ASM Press, 2018. [Google Scholar]