Abstract
Lower respiratory infections include acute bronchitis, influenza, community-acquired pneumonia, acute exacerbation of COPD and acute exacerbation of bronchiectasis. They are a major cause of death worldwide and often affect the most vulnerable: children, elderly and the impoverished. In this paper, we review the clinical presentation, diagnosis, severity assessment and treatment of adult outpatients with lower respiratory infections. The paper is divided into sections on specific lower respiratory infections, but we also dedicate a section to COVID-19 given the importance of the ongoing pandemic. Lower respiratory infections are heterogeneous entities, carry different risks for adverse events, and require different management strategies. For instance, while patients with acute bronchitis are rarely admitted to hospital and generally do not require antimicrobials, approximately 40% of patients seen for community-acquired pneumonia require admission. Clinicians caring for patients with lower respiratory infections face several challenges, including an increasing population of patients with immunosuppression, potential need for diagnostic tests that may not be readily available, antibiotic resistance and social aspects that place these patients at higher risk. Management principles for patients with lower respiratory infections include knowledge of local surveillance data, strategic use of diagnostic tests according to surveillance data, and judicious use of antimicrobials.
Short abstract
Management principles for patients with lower respiratory infections include knowledge of local surveillance data, strategic use of diagnostic tests according to surveillance data, and judicious use of antimicrobials. https://bit.ly/3ziuPGS
Introduction
Acute respiratory infections can be divided into upper and lower respiratory infections. The focus of this review is lower respiratory infections in adults. Lower respiratory infections comprise acute bronchitis, influenza, community-acquired pneumonia, acute exacerbation of COPD and acute exacerbation of bronchiectasis [1]. They are responsible for 13% of all deaths in children aged <5 years and 4.4% of all deaths in the whole population. They are the leading cause of death in children aged <5 years old and the sixth cause of death overall. In the adult population, the absolute number of deaths from lower respiratory infections has been increasing in those aged ≥70 years, mostly as a result of an ageing population. Lower respiratory infections markedly affect the vulnerable: children, elderly and the impoverished [2]. In a prospective cohort study that included 587 participants aged 85 years, independent risk factors for development of lower respiratory tract infections included smoking, systemic corticosteroid use, history of COPD, history of stroke, severe cognitive impairment and declined functional status [3]. In another prospective study that included 475 residents in nursing homes, independent risk factors for development of pneumonia included older age, male sex, swallowing difficulty and inability to take oral medications. Independent risk factors for lower respiratory tract infections other than pneumonia included older age and immobility. Vaccination against influenza was protective against pneumonia and other lower respiratory tract infections [4]. The majority of devastating pandemics and outbreaks are due to respiratory viruses and manifest as lower respiratory tract infections [5]. Analysis from the pre-COVID-19 era identified Streptococcus pneumoniae as the leading pathogen responsible for lower respiratory tract infection mortality [2]. The main sections of this paper are divided into specific lower respiratory tract diseases rather than aetiologies, but we dedicate a section to COVID-19 given the importance of the ongoing pandemic.
Acute bronchitis
Definition
Acute bronchitis is defined as an acute illness causing cough and other symptoms and signs suggestive of lower respiratory tract infection. These include sputum production, wheezing, dyspnoea and chest discomfort or pain [1]. The absence of underlying lung disease distinguishes acute bronchitis from acute exacerbation of COPD or bronchiectasis, which will be covered in later sections.
Clinical examination and diagnostic work-up
When assessing a patient with suspected acute bronchitis, it is important to attempt to clinically exclude pneumonia. The latter should be suspected in the presence of tachycardia, tachypnoea, fever, low oxygen saturation or chest pain. Additionally, clues for the presence of undiagnosed underlying lung disease, particularly asthma, should be elicited in the clinical assessment. This is illustrated by a longitudinal cohort study that found that 19% of patients without known underlying lung disease who present with an isolated episode of acute bronchitis are subsequently diagnosed with asthma [6]. Complementary tests such as chest radiography, procalcitonin or sputum culture are not routinely obtained [7]. They can be obtained on a case by case basis if there is suspicion of pneumonia or another underlying lung disease. Testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently indicated for patients with acute respiratory illness, and this applies to all lower respiratory infections discussed. Testing for influenza is also indicated in patients with acute respiratory illness if the result will lead to a change in management or if there are epidemiological reasons for testing. For both SARS-CoV-2 and influenza, a nucleic acid detection test is preferred, but an antigen detection assay test can be used in the absence of a nucleic acid detection test [8].
Microbiology
In one representative study, an aetiologic diagnosis was established in 68% of the patients. The detection rate was 63% for viruses and 26% for bacteria. The most common virus was rhinovirus followed by influenza. The most common bacterium was S. pneumoniae [9].
Treatment
Antibiotics lead to shorter cough duration and reduction in days feeling ill and days with impaired activity. However, there is no difference in clinical improvement between patients who receive antibiotics and those who receive placebo [10]. The recognition that acute bronchitis is commonly self-limited, and that antimicrobial therapy is not indicated in immunocompetent patients, should prompt avoidance of antibiotic prescription in these patients. There is evidence that the majority of the antibiotic prescriptions for patients with acute respiratory tract infection are unnecessary [11]. Additionally, community antimicrobial intake represents 85–95% of the entire antimicrobial intake [12]. Currently, there are no data supporting the use of other therapies such as antitussives, inhaled bronchodilators, inhaled corticosteroids or systemic corticosteroids [7]. In adult outpatients diagnosed or suspected to have influenza, antiviral treatment is indicated for those with severe or progressive illness, high risk of complications (e.g. immunocompromised status or chronic medical conditions), pregnancy or post-partum status within 2 weeks. Treatment for influenza should also be considered for those with an early diagnosis (<2 days), household contacts of at-risk persons (e.g. immunocompromised) and healthcare providers [13]. Treatment for COVID-19 is discussed in a section below.
Community-acquired pneumonia
Epidemiology
The incidence of community-acquired pneumonia (CAP) is higher in men and rises considerably with age [14]. High-deprivation areas have higher incidence of CAP [15]. COPD and heart failure are the underlying diseases most commonly associated with CAP [16–18]. The mortality of outpatients with CAP is <1% [19]. However, for those requiring hospitalisation, the in-hospital and 1-year mortalities are 6.5% and 30.6%, respectively [17]. For those admitted to the intensive care unit (ICU), the in-hospital and 1-year mortalities are 17% and 47%, respectively [20]. Underlying diseases associated with increased mortality include diabetes mellitus, neoplastic disease, neurological disease [21] and congestive heart failure [22]. Increasing deprivation is also associated with increased mortality [15].
Clinical manifestations
Patients with pneumonia often present acutely with a combination of the following: cough, sputum production, dyspnoea, pleuritic chest pain, fever and crackles on lung auscultation [23]. The most common respiratory symptom is cough (86%) followed by dyspnoea (72%), sputum production (64%) and pleuritic chest pain (46%). Most common nonrespiratory symptoms include fatigue (91%), fever (91%) and chills (73%). The most common signs include tachypnoea (49%), tachycardia (41%) and hyperthermia (34%) [24]. The value of specific symptoms and signs for the diagnosis of pneumonia has been evaluated. The following symptoms and signs are independently predictive of radiograph-confirmed pneumonia: temperature >37.8°C (RR 2.6, 95% CI 1.5–4.8), heart rate >100 per minute (RR 1.9. 95% CI 1.1–3.2), crackles on auscultation (RR 1.8, 95% CI 1.1–3.0), and oxygen saturation <95% (RR 1.7, 95% CI 1.0–3.1) [25]. Older patients can have a different presentation. They often do not report pleuritic chest pain or dyspnoea, but tachypnoea is a more commonly seen [24].
Diagnostic work-up
A routine chest radiograph in every patient presenting to a primary care clinic with lower respiratory infection is not warranted. Most of these patients do not have pneumonia. Chest radiography should be reserved for selected patients when the pre-test probability of pneumonia is moderate to high. In the primary care setting, a chest radiograph changed the probability of pneumonia in half of the patients. In most patients, the chest radiograph result decreased the probability of pneumonia, leading to less frequent use of antibiotics and more frequent reassurance of the patients [26]. Chest radiography can also at times provide valuable information about the severity of the pneumonia such as when there is pleural effusion or bilateral infiltrates [27]. It is, however, important to recognise the limitations of chest radiography in pneumonia. In most cases, the pattern seen on the chest radiograph cannot be used to predict the aetiology of pneumonia although at times it can be suggestive (e.g. multiple cavities in a patient with history of intravenous drug use is suggestive of Staphylococcus aureus infection) [28].
Lung ultrasound has emerged as an important imaging tool for the diagnosis of lung diseases, including pneumonia. It has high accuracy for the diagnosis of pneumonia, but most of these studies were performed in the ICU or emergency department [29]. As the technology becomes more omnipresent and clinicians more proficient, we anticipate increasing use of ultrasound in primary care.
Routine sputum culture in patients with CAP is unlikely to yield an aetiologic diagnosis in most cases [30]. In the outpatient setting, we reserve sputum culture for select cases depending on factors such as ease of obtainment, severity of the pneumonia, the pre-test probability of unusual pathogens, and the presence of structural lung disease.
Microbiology
Atypical bacteria, respiratory viruses and typical bacteria are the three major categories of pathogens causing CAP. Atypical pathogens have been identified in 22–31% of patients in cohorts of CAP treated in the outpatient setting. In aggregate, they have been the most common cause of CAP treated in the outpatient setting in some studies [31–33]. Of the atypical pathogens, Mycoplasma pneumoniae is the most commonly identified [31, 32, 34, 35], but in some series Chlamydia pneumoniae was also prevalent [33, 36]. There is a range of prevalences of the respiratory viruses in the different cohorts, which reflects the varying extent of testing for different viruses. In a study in an outpatient population with CAP, the prevalence of viral infection detected by PCR was 12.1% [37]. However, a meta-analysis of studies that included mixed inpatient and outpatient populations with CAP showed a prevalence of viral infection of 22.4% [38]. Typical pathogens have been detected in 9–56% of the cases in different studies [31–36]. S. pneumoniae remains the most common typical pathogen. Other common typical pathogens include Haemophilus influenzae, Moraxella catarrhalis and Enterobacteriaceae (e.g. Klebsiella spp., Escherichia coli, Enterobacter spp., Serratia marcescens, Proteus mirabilis and Morganella morganii) [39]. Anaerobic pathogens are not common causes of CAP even in the setting of aspiration. However, they should be considered in the setting of lung abscess or empyema [40].
Among the organisms usually causing CAP, resistance to antimicrobials has been a concern with S. pneumoniae and H. influenzae. S. pneumoniae resistance to macrolides, which has increased over time [41], is mostly secondary to target alteration promoted by ribosomal methylation and an efflux mechanism [42]. H. influenzae is classified into encapsulated (with six serotypes from a to f) and nonencapsulated or nontypeable. The use of H. influenzae type b conjugate vaccines has led to a marked decrease in invasive disease caused by H. influenzae type b [43], but infection by other serotypes and mainly by nontypeable H. influenzae remains common. Antibiotic exposure has led to an increase in H. influenzae resistance to β-lactam antibiotics, which occurs mainly via expression of β-lactamase enzyme or change in penicillin-binding proteins. In a cohort of 92 patients who developed 95 episodes of CAP with isolates of nontypeable H. influenzae, COPD was the most common underlying disease, present in 28% of the patients. Resistance and intermediate resistance to ampicillin were present in 10.5% and 23.2% of the isolates, respectively [44].
Multidrug-resistant organisms, including Pseudomonas aeruginosa, extended-spectrum β-lactamase-producing Enterobacteriaceae and methicillin-resistant S. aureus (MRSA), have been identified in 3.3–6.0% of patients with CAP requiring hospitalisation [45, 46]. Previously, patients with a set of risk factors for multidrug-resistant organisms (e.g. hospitalisation for 2 or more days within 90 days, nursing home or long-term care facility residence, recent receipt of intravenous antibiotics or chemotherapy, wound care within the last 30 days, or dialysis treatment) were labelled as having health care-associated pneumonia [47]. The recognition that these risk factors are individually weak predictors of multidrug-resistant organisms has led to the abandonment of this categorisation [40]. It is, however, still important to recognise the subset of patients at higher risk of multidrug-resistant organism infection. To this end, a number of studies have developed scores for better prediction [45, 46, 48–50]. As pointed out by the 2019 Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guidelines, patients with CAP and risk factors for P. aeruginosa and MRSA are not commonly managed as outpatients [40].
Immunocompromised status has become increasingly common [51, 52]. At least one risk factor for immunocompromise was present in 18% of patients from the community with pneumonia requiring hospitalisation [53]. Immunocompromised patients develop infection not only from the same pathogens as nonimmunocompromised patients, but they are also susceptible to opportunistic pathogens such as fungi (e.g. Aspergillus species, Pneumocystis jirovecii), viruses from the Herpesviridae family, parasites (e.g. Strongyloides stercoralis), mycobacteria and other multidrug-resistant bacteria [54]. Patients with immunocompromised status are generally managed as inpatients.
Coccidioidomycosis, blastomycosis and histoplasmosis are endemic mycoses that often present as CAP. Unfortunately, diagnosis is often late and commonly suspected when there is no resolution of the pneumonia despite a course of antibiotics. Although immunocompromised patients are particularly susceptible, these endemic mycoses are also often present in nonimmunocompromised patients [55]. Knowledge of the geographical distribution of these endemic mycoses can be valuable for the clinician. Coccidioidomycosis is found mostly in the United States (Southern Arizona and the San Joaquin Valley region in California), Central America (Guatemala and Honduras) and South America (Argentina, Brazil, Colombia, Paraguay and Venezuela). Blastomycosis is found in the United States (mid-west, east, south east and south central), Canada, the African continent and, to a lesser extent, India. Histoplasmosis is endemic in areas of the United States (Ohio and Mississippi River Valleys), Canada (Quebec and Ontario), Central America and South America, but its geographical reach is global in part because of the HIV pandemic and immunosuppressive diseases and medications [56].
Mycobacterium tuberculosis infection is suspected with a more protracted respiratory course, but it can present acutely. A large pneumonia cohort study showed it occurred in 0.9% of patients with CAP requiring hospital admission. The following factors were independently associated with CAP caused by M. tuberculosis: haemoptysis; upper lobe infiltrate localisation; weight loss, or 10% or less of ideal body weight; prior history of tuberculosis (TB) or recent exposure to TB or history of positive purified protein derivative (PPD) test; and night sweats [57].
Burkholderia pseudomallei is a Gram-negative bacterium and the cause of melioidosis, which presents as pneumonia in half of all cases. In 91% of melioidosis pneumonia cases, presentation is acute or subacute. Chronic pneumonia, which resembles TB, occurs in 9% of cases. Mode of acquisition is mainly via percutaneous inoculation or inhalation. Geographical distribution is mainly Southeast Asia and northern Australia [58].
Francisella tularensis is an intracellular Gram-negative coccobacillus and the cause of tularemia. Modes of acquisition include vector-borne transmission (ticks and mosquitoes), direct contact with animals (e.g. hare), ingestion of contaminated food or water, and inhalation of aerosols [59]. The presentation is largely dependent on the mode of transmission. For instance, while the ulcero-glandular form tends to occur after a bite from an infected tick, the pneumonic form often occurs after inhalation of contaminated aerosols [60]. The disease is found in the North American and Eurasian continents [59].
Acinetobacter baumannii is a Gram-negative aerobic bacillus that traditionally causes nosocomial infection. More recently, there have been reported cases of CAP caused by A. baumannii from the United States, Australia, China and Taiwan [61–64]. Community-acquired A. baumannii causes severe disease [63], but community-acquired isolates are less likely to be multidrug-resistant than the nosocomial isolates [64]. Excessive alcohol consumption, smoking, COPD, renal disease and diabetes mellitus are commonly reported in patients with community-acquired A. baumannii [65].
Chlamydia psittaci is an obligate intracellular bacterium that causes the zoonotic disease psittacosis. The most common presentation of psittacosis is CAP. A pooled analysis showed that psittacosis is responsible for approximately 1% of the cases of CAP, but most patients in the analysis were hospitalised [66]. The most common modes of transmission have been exposure to infected birds and their droppings [67, 68] followed by contact with poultry [69].
Assessment of need for admission
Hospitalisation is required in 39–45% of patients with CAP [18, 70]. The decision to admit a patient with CAP can be helped by clinical prediction rules. The two most commonly used clinical prediction rules are CURB-65 and the pneumonia severity index. The CURB-65 was initially developed by the British Thoracic Society and subsequently validated in a modified form. It consists of five variables: confusion, urea >7 mmol·L−1, respiratory rate ≥30·min−1, blood pressure (systolic blood pressure <90 mmHg or diastolic blood pressure ≤60 mmHg) and age ≥65 years. A more simplified version, CRB-65, omits urea. A score is computed by the sum of the variables, each of which is assigned a point of 0 or 1 (if abnormal). In an early cohort study, a CURB-65 score of 0 or 1 was associated with a mortality of 1.5% and these patients were deemed suitable for home treatment; a score of 2 was associated with a mortality of 9.2% and these patients were deemed to require hospital-supervised treatment (short inpatient admission or hospital-supervised outpatient treatment); and a score of 3 or more was associated with a mortality of 22% and these patients were deemed to require hospitalisation [71].
A version of CURB-65 using weighed and continuous variables available through the electronic medical record was tested against the traditional, dichotomous CURB-65 and showed a superior area under the curve to predict mortality [72]. The advantage of CURB-65 and its interactions is the simplicity, but that comes at a cost. For instance, criteria for admission other than the variables present in the CURB-65 were identified in a large retrospective cohort study of patients with CAP. In patients with CURB-65 score of 1, an oxygen tension <60 mmHg was identified in 28.7%, comorbidity in 22.8%, bilateral or multilobe radiographic involvement in 14.1% and pleural effusion in 8.2% [73]. This demonstrates that in patients with low CURB-65 score other physiological and clinical parameters need to be carefully assessed before a decision is made on whether patient can be treated as outpatient.
The pneumonia severity index (PSI) variables include age, comorbidities, physical examination findings and laboratory and radiographic parameters. A point score is computed by the sum of the score assigned to each variable. Patients are then categorised into one of five risk classes according to the total score [19]. PSI is computation intensive and may be more widely used if integrated into the electronic health record [74]. In prospective studies, the use of a critical pathway that included PSI led to a modest decrease in the rate of hospitalisations without affecting clinical outcomes [75, 76]. However, in one study, 5% of patients required late hospitalisations despite the use of PSI [76]. Social aspects such as homelessness, lack of health insurance and health illiteracy should be taken into account in the decision making. In summary, the use of prediction rules can serve as guidance, but they should not override clinical judgement in the decision to admit patients with CAP.
Treatment
The 2019 IDSA/ATS guidelines recommend amoxicillin or doxycycline as first choices for outpatient treatment of CAP in patients without comorbidities. Macrolide monotherapy is an option only when S. pneumoniae resistance to macrolide is less than 25%. In patients with comorbidities, the recommendation is combination therapy of amoxicillin/clavulanate and macrolide, or monotherapy with a respiratory fluoroquinolone [40].
The use of amoxicillin as one of the first-choice antibiotics in the most recent IDSA/ATS guideline is in line with the 2015 annotated version of the British Thoracic Society [77] and the 2011 European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases [1] guidelines and is a change from the 2007 IDSA/ATS guidelines in which macrolide was the first-choice antibiotic for previously healthy patients without risk factors for drug-resistant S. pneumoniae [78] (table 1).
TABLE 1.
Treatment recommendations for outpatient management of community-acquired pneumonia according to different guidelines
| ATS and IDSA guidelines, 2019 [40] | Recommendation | Amoxicillin 1 g three times a day or doxycycline 100 mg twice a day |
| Alternative choices for areas with pneumococcal resistance to macrolide <25% | Azithromycin 500 mg first day followed by 250 mg daily or clarithromycin 500 mg twice a day | |
| Recommendations in patients with comorbidities (chronic heart, lung, liver or renal disease; diabetes mellitus; alcohol use disorder; cancer; and asplenia) | Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily) AND macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin (500 mg twice daily or extended release 1000 mg once daily)) | |
| Respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily) | ||
| BTS guidelines, 2015 [77] | Preferred agent | Amoxicillin 500 mg three times a day |
| Alternative choices | Doxycycline or clarithromycin | |
| ERS/ESCMI guidelines, 2011 [1] | First choice | Amoxicillin or tetracycline |
| Hypersensitivity to penicillin | Tetracycline or macrolide |
ATS: American Thoracic Society; IDSA: Infectious Diseases Society of America; BTS: British Thoracic Society; ERS: European Respiratory Society; ESCMI: European Society for Clinical Microbiology and Infectious Diseases.
The current preference for β-lactam monotherapy, which comes at the expense of not covering atypical bacteria, is based on two premises. On the one hand, there is the concern for high rates of S. pneumoniae resistance to macrolide. Indeed, 54.9% of the S. pneumoniae isolates in the United States were resistant to macrolides in 2018 and 2019 [41]. On the other hand, resistance and intermediate susceptibility to penicillin were 2% and 1.6% [79]. It is important to recognise there is marked geographical variation in the susceptibility profile to both macrolide and penicillin [42]. In Europe, invasive isolates of S. pneumoniae, for which benzylpenicillin has a minimal inhibitory concentration >0.06 mg·L−1, ranged from below 5% in three countries to equal or above 25% in nine countries in 2020. Resistance to macrolides occurred in 16.9% in aggregate, but there was also large variation among countries [80].
In addition, the clinical relevance of atypical bacteria in outcomes of outpatients has been questioned [81]. However, it is well established that Legionella pneumophila causes severe pneumonia [82]. Other atypical pathogens can also cause severe pneumonia [83, 84]. One review estimated that 0.5–2.0% of patients with M. pneumoniae pneumonia present in a fulminant form, and these severe cases occur more often in young patients [85]. In one study, patients with severe M. pneumoniae pneumonia had significantly longer times to initiation of adequate antibiotic therapy [83]. In one cohort of 276 hospitalised patients with CAP, Chlamydia pneumoniae was identified in 24 patients (8.7%). Of these, four had severe pneumonia upon presentation [86].
Our approach has been to treat patients without comorbidities with amoxicillin for appropriate coverage of S. pneumoniae with the addition of a second agent (e.g. macrolide or doxycycline) to cover atypical pathogens. The combination therapy approach is not supported by randomised clinical trials, but is based on epidemiological and clinical data demonstrating the relevance of atypical pathogens in the aetiology of CAP.
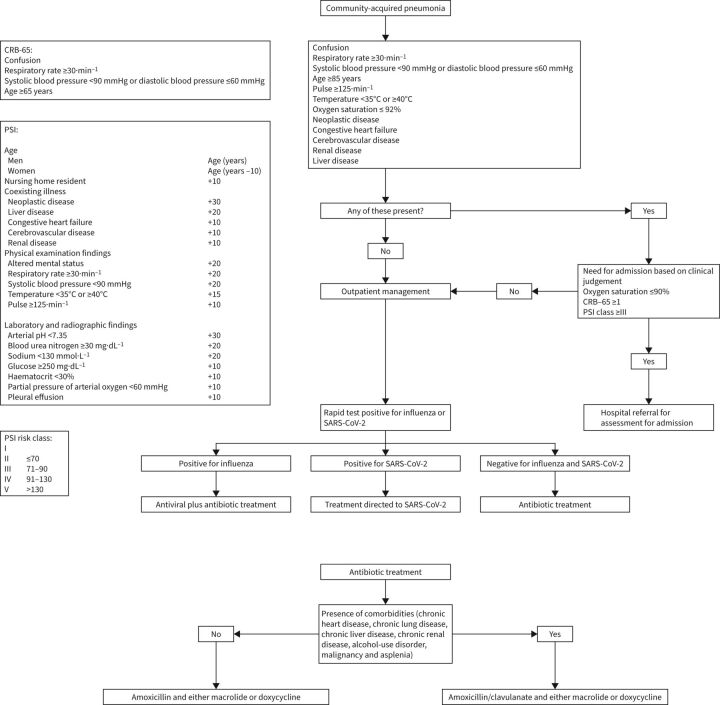
We treat patients with comorbidities with amoxicillin/clavulanate to cover both S. pneumoniae and other bacterial pathogens with the addition of either a macrolide or doxycycline to cover atypical pathogens. If a microbiological work-up is obtained and a pathogen is identified, we generally provide pathogen-directed therapy. Bacterial co-infection has been found in a third of patients diagnosed with influenza pneumonia [87]. Thus, we treat patients diagnosed with influenza pneumonia with both antiviral and antibiotic therapies. We treat patients with pneumonia diagnosed with other respiratory community-acquired viruses with antibiotic therapy because bacterial co-infection is common in this setting as well. In patients diagnosed with SARS-CoV-2 infection, we generally provide targeted therapy only (see section below) since bacterial co-infection is uncommon [88] (figure 1).
FIGURE 1.
Triage and treatment of patients with community-acquired pneumonia. PSI: pneumonia severity index; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
COVID-19 infection
Epidemiology
SARS-CoV-2, the aetiology of COVID-19 pandemic, has emerged as a major cause of death. In the United States, it was the third leading cause of death in 2021, behind only heart disease and cancer [89]. The total number of COVID-19 deaths since the beginning of the pandemic is nearing 1 million in the United States [90]. There have been five waves of the COVID-19 pandemic in the United States. Early in the pandemic, the more densely populated urban areas were predominantly affected. The less densely populated areas were more affected later in the pandemic [91]. While most infected patients have either asymptomatic or mild disease, a subset of patients develop severe disease. Increasing age and comorbidities are risk factors for more severe disease [92].
More than 2 years after the pandemic onset, SARS-CoV-2 infection persists. Because immunity to coronaviruses decreases over time, re-infection occurs. It is thus likely that SARS-CoV-2 will become one of the endemic community-acquired pathogens causing respiratory infections. The endemic phase does not necessarily mean a decreased burden from SARS-CoV-2. Rather, it represents a stable level of infection. The extent of severity of infection in individual patients will depend on the protection generated by immunity, either via prior natural infection or vaccination [93].
Clinical manifestations
In the outpatient setting, the most common symptoms include fatigue (65.7%), cough (58.8%), headache (45.6%), chills (38.2%) and anosmia (27.9%). Symptoms peak at day 10 and recede at day 20 from the onset of clinical manifestations. One characteristic feature that distinguishes COVID-19 from most other viral respiratory infections is that symptoms associated with COVID-19 are often protracted. Some patients have symptoms that last weeks. Those are more often fatigue, cough, dyspnoea and anosmia [94]. In a comprehensive review of the diagnostic accuracy of signs and symptoms, the following were found to be more predictive of COVID-19 infection: headache, myalgia, fever, arthralgia and fatigue [95]. Less severe disease and thus changing clinical presentations are expected with the increasing number of individuals who acquire immunity to SARS-CoV-2 via vaccination or natural infection [96].
Assessment of need for admission
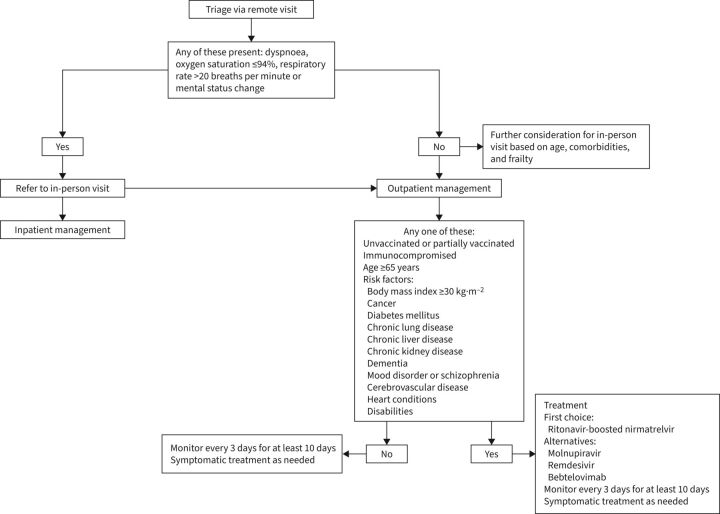
If initial triage of patients with confirmed or suspected COVID-19 is via telehealth, the following should prompt a referral to in-person visit either in the emergency department or in a dedicated COVID-19 clinic: dyspnoea, oxygen saturation ≤94% or mental status change [97]. The use of outpatient oxygen saturation monitoring has received special consideration as a triage tool during the pandemic because of the recognition that some patients with COVID-19 do not experience worsening respiratory symptoms upon development of hypoxia. Patients with hypoxia have higher odds of requiring hospitalisation, and those with hypoxia have worse outcomes [94, 98]. However, the use of a home pulse oximeter requires attention to technical aspects. The device should be a finger oximeter approved by the United States Food and Drug Administration. Smart phone applications are not sufficiently accurate for safe use. Patients should be reminded that the measurement is to be performed at rest, using the index or middle finger and waiting at least 30 s before recording the results. Nail polish and cold extremities can lead to misleading results [99]. Lower thresholds for in-person evaluation should also be used in older people or those with high-risk medical conditions [97]. A clinical trial did not show an improvement in the number of days alive and out of the hospital at 30 days for patients who received monitoring of oxygen saturation via a home pulse oximeter [100]. In this study, however, both the intervention and control groups were being closely monitored by automated text messages and, if needed, callbacks from nurses. It is unclear whether the findings of the trial can be generalised to other practices.
The decision to admit a patient with COVID-19 relies on clinical judgement and is influenced by several factors, including vital signs, oxygen saturation, imaging findings, comorbidities, age, frailty and social aspects. In that decision, one should bear in mind that, for the same degree of illness severity as assessed by PSI or CURB-65, hospitalised patients with COVID-19 pneumonia have a much higher mortality as compared with hospitalised patients with CAP due to other aetiologies [101]. A clinical prediction rule for adverse events in outpatients has been developed and may have a role in the future if integrated with the electronic medical record [102].
Treatment
There are now two oral antiviral agents for which the Federal Drug Administration issued Emergency Use Authorizations: ritonavir-boosted nirmatrelvir and molnupiravir. Nirmatrelvir is an antiviral agent with inhibitory activity against SARS-CoV-2-3CL protease. The latter is an enzyme used by SARS-CoV-2 for replication. A slower metabolism and longer metabolic activity of nirmatrelvir is achieved by adding ritonavir [103]. Molnupiravir is a ribonucleoside that is converted into N-hydroxycytidine, which in turn binds to the viral genome. As a result, deleterious errors occur and SARS-CoV-2 replication is inhibited [104].
Remdesivir is an intravenous antiviral agent that directly inhibits the SARS-CoV-2 RNA-dependent RNA polymerase [105]. Bebtelovimab is an intravenous recombinant neutralising human monoclonal antibody that binds to spike protein of SARS-CoV-2. The Federal Drug Administration issued an Emergency Use Authorization of bebtelovimab for the treatment of mild to moderate COVID-19 based on the results of a phase 2 trial (BLAZE-4) showing safety [106].
Outpatient treatment is more likely to benefit three major categories of patients: immunocompromised, unvaccinated or partially vaccinated (including those who have not received a booster), and vaccinated with risk factors for severe disease (e.g. age ≥65 years or age <65 years with comorbidities or risk factors for severe disease) [107]. The clinical trials testing the above outpatient treatments were conducted predominantly in unvaccinated patients [103–106]. The efficacy of these treatments is thus unclear in vaccinated patients, but we agree that vaccinated patients with risk factors for severe disease should be offered treatment if available.
According to the National Institutes of Health guidelines, the preferred therapy should be ritonavir-boosted nirmatrelvir. Remdesivir is considered second choice. Alternative therapies in the absence of ritonavir-boosted nirmatrelvir or remdesivir include bebtelovimab or molnupiravir [107]. Globally, we believe feasibility favours oral medications. Based on efficacy and ease of administration, ritonavir-boosted nirmatrelvir is our first choice. However, the ritonavir component inhibits the cytochrome P450 (CYP) 3A4, leading to several drug interactions and limiting its use in some occasions. If the interaction is not expected to be clinically relevant, ritonavir-boosted nirmatrelvir can still be administered. If the interaction is expected to be clinically significant, strategies can be used to minimise it. Those include temporarily withholding the concomitant medication or the prescription of an alternative therapy for COVID-19. More information on medications that interact with ritonavir-boosted nirmatrelvir and strategies to minimise drug–drug interactions can be found in the drug–drug interactions information given on the National Institutes of Health's COVID-19 Treatment Guidelines webpage [108]. Ritonavir-boosted nirmatrelvir is currently not recommended in patients with estimated glomerular filtration rates <30 mL·min−1 or with severe hepatic impairment [109]. Molnupiravir is a reasonable alternative despite an efficacy that is not as high by indirect comparison. Molnupiravir should not be administered during pregnancy as data from animal studies indicate it may cause fetal harm. Females of childbearing potential should be assessed for pregnancy and should use an effective method of contraception during treatment and for 4 days after treatment completion. Sexually active males with partners of childbearing potential should use an effective method of contraception during treatment and for at least 3 months after treatment completion [110] (figure 2).
FIGURE 2.
Triage and treatment of patients with COVID-19.
Acute exacerbation of COPD
Definition
Exacerbation of COPD has been defined as “a sustained worsening of the patient's condition, from the stable state and beyond normal day-to-day variations, that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD” [111]. Thus, the distinctive feature of acute exacerbation is the need for additional therapy [112]. Commonly, patients present with one or a combination of the following: purulent sputum or increase sputum amount, increased dyspnoea, wheezing and chest tightness. In mild exacerbations, patients do not need typically require further medical care. In a moderate exacerbation, patients need further medical assistance (e.g. a clinic visit). In a severe exacerbation, patients require hospitalisation [111].
Conditions that commonly mimic COPD exacerbation include heart failure [113, 114] and pulmonary embolism [115]. A potential pitfall is the nonrecognition of asthma features (e.g. asthma-COPD overlap syndrome) in some patients with COPD [116, 117] (table 2).
TABLE 2.
Conditions that are commonly associated with COPD exacerbation
| Heart failure | Heart failure is a common trigger of COPD exacerbation. Additionally, heart failure exacerbation may be misdiagnosed in up to 13% patients with asthma or COPD and acute dyspnoea [113]. Proper heart failure management may reduce the risk of COPD exacerbation [114]. Past medical history of heart failure, paroxysmal nocturnal dyspnoea, third heart sound, jugular venous distension, crackles, pulmonary venous congestion on chest radiography, presence of atrial fibrillation on electrocardiogram, and elevated brain natriuretic peptide are findings more strongly associated with heart failure in patients with dyspnoea [160]. |
| Pulmonary embolism | In patients admitted for acute exacerbation of COPD, the prevalence of pulmonary embolism is 12.9%. Risk factors for pulmonary embolism include recent immobilisation, lower extremity oedema, concomitant deep venous thrombosis and elevated d-dimer [115]. |
| Asthma-COPD overlap syndrome and COPD with peripheral eosinophilia | Features of asthma-COPD overlap syndrome include persistent airflow obstruction, history of at least 10 pack-years of tobacco smoking, bronchodilator response of at least 400 mL in forced expiratory volume in 1 s, and peripheral blood eosinophil count >300 cells·µL-1 [116]. In patients with COPD, higher blood eosinophil counts predict better response to inhaled corticosteroids [117]. |
Epidemiology
The annual frequency of exacerbation increases with the severity of COPD: 0.85 per person for patients with moderate disease, 1.34 for those with severe disease, and 2.00 for those with very severe disease. The prevalence of hospitalisation in a year also increases with severity of disease: 7% for moderate disease, 18% for severe disease and 33% for very severe disease. A prior exacerbation is the most predictive variable for a future exacerbation. Other factors predictive of an exacerbation include worsening lung function, history of reflux or heartburn, increasing white cell count, and increasing health impairment [118].
Airway inflammation
The airways of patients with stable COPD are characterised by an increased number of T-lymphocytes and macrophages in the bronchial mucosa [119]. Patients with an exacerbation phenotype have further airway inflammation at baseline [120]. Colonisation of the airways by bacterial pathogens has been associated with higher levels of interleukin-8 and worse respiratory symptoms [121]. During an exacerbation, the number of inflammatory cells (lymphocytes, neutrophils and eosinophils) and levels of cytokines, eosinophil cationic protein and neutrophil elastase in the sputum increase [122]. Sputum levels of leukotriene B4 [123], tumour necrosis factor-α and interleukin-8 [124], and gene expression epithelial-derived neutrophil attractant-78 [125] increase and appear to play a role in neutrophil recruitment during an exacerbation. The antioxidant capacity is decreased whereas oxidant products are increased in patients with COPD exacerbation [126]. Recently, basic research has shown that altered microbiota induces lung inflammation [127]. Additionally, bacterial load has been shown to correlate with airway inflammation and symptoms in stable state [128].
Diagnostic work-up
Similar to the work-up for patients with CAP, we reserve sputum culture for select cases such as patients with recurrent exacerbation or recent hospitalisation.
Aetiology
Infection is the major trigger of COPD exacerbations, although exposure to pollutants is also an important contributor [129]. Respiratory viruses are responsible for approximately 50% of the infections [130]. Commonly identified viruses include rhinovirus and influenza. Other less common viruses include parainfluenza, adenovirus, coronavirus and human metapneumovirus [131]. Typical bacteria, identified in 24–42% of the infections [130, 132], commonly include M. catarrhalis, H. influenzae and S. pneumoniae. As the severity of COPD increases, patients are at higher risk of infection by P. aeruginosa [16]. Atypical bacteria are variably identified.
Bacterial pathogens can often be identified in the airway of patients with COPD when they are in stable conditions [133]. This finding casts doubt on the pathogenic role of bacteria in exacerbation of COPD. However, molecular analysis shows that exacerbations are often associated with a new strain of a bacterial pathogen, endorsing the pathogenic role of bacteria [134].
Assessment of need for admission
Criteria for hospital admission include a change in mental status, marked increase in dyspnoea, tachypnoea, worsening hypoxia or hypercapnia, inadequate response to outpatient treatment, and lack of social support for safe outpatient treatment [112, 135]. Factors to be considered in the decision to admit a patient include presence of comorbidities, the underlying severity of the COPD, history of prior admissions for exacerbation, need for home oxygen therapy, and adherence to treatment [136].
Treatment
The pharmacological treatment of COPD exacerbation centers on the use of short-acting bronchodilators, systemic glucocorticoids and antibiotics. Antibiotics are more likely to be beneficial in patients with severe exacerbations requiring hospitalisation [137]. In outpatients with exacerbation of mild to moderate severity, antibiotics have a modest effect on the reduction of treatment failure [138]. The core symptoms indicating the need for antibiotics in COPD exacerbation include increase in dyspnoea, sputum volume, and sputum purulence [139]. Accordingly, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend the use of antibiotics in patients with increases in dyspnoea, sputum volume and sputum purulence, or in the presence of increased sputum purulence plus an additional criterion (increase in dyspnoea or sputum volume) [112]. We favour a 5-day course of amoxicillin-clavulanic acid, which led to a higher rate of symptom improvement as compared with placebo in a clinical trial [140].
Treatment with systemic glucocorticoid leads to fewer treatment failures [141] and lower relapse rates [142]. A clinical trial demonstrated noninferiority of a 5-day course compared with a 14-day course of prednisone [143]. Our approach has been to treat with a 5-day course of 40 mg of prednisone daily. Nebulised budesonide has been evaluated as an alternative to systemic glucocorticoids with promising results in hospitalised patients [144, 145], but more studies are needed before routine use.
Acute exacerbation of noncystic fibrosis bronchiectasis
Definition
A consensus statement defined an acute exacerbation of bronchiectasis as a worsening of three of the following symptoms for at least 2 days: cough, sputum volume, sputum purulence, dyspnoea, fatigue and haemoptysis. Additionally, a clinician must establish that a change of treatment is needed [146]. The British Thoracic Society guidelines have highlighted that is in not uncommon for adults with bronchiectasis to have variations in daily symptoms of cough and sputum production. Neither the usual variation in daily symptoms nor the isolated finding of mucopurulent (pale yellow) or purulent (yellow to green) sputum establish an exacerbation. Rather, it is the combination of an increase in respiratory or systemic symptoms (e.g. cough, wheezing, dyspnoea or fatigue), increased volume or change in viscosity of sputum, and increased sputum purulence that indicate an exacerbation [147].
Epidemiology
In a cohort of 1826 patients with bronchiectasis included in a US registry, the mean age was 64 years, 79% were women and 60% were never-smokers. The most common comorbidities were history of pneumonia (68%), gastroesophageal reflux disease (47%), asthma (29%), COPD (20%), rheumatologic disease (8%), primary immunodeficiency (5%), prior TB (4%), primary ciliary dyskinesia (3%) and inflammatory bowel disease (3%). In 63% of the patients, nontuberculous mycobacteria (NTM) was identified or previously reported. Patients with NTM more often had tree-in-bud and mucoid impaction on CT. An exacerbation in the prior 2 years was present in 64% of the patients, and the average number of exacerbations in the prior 2 years was three [148]. Table 3 shows the aetiologies or diagnoses associated with bronchiectasis based on a mutinational study [161].
TABLE 3.
Aetiologies or diagnoses commonly associated with bronchiectasis
| Aetiologies or associated diagnoses [161] | Remarks |
| Post-infectious (20%) | Other aetiologies of bronchiectasis should be excluded. Tuberculosis is the most common cause and is more prevalent in developing countries [162]. |
| COPD (15%) | Prevalence of bronchiectasis of 29% in a cohort of patients with COPD; the most common pattern is tubular [163]. May represent primary bronchiectasis in the setting of COPD or bronchiectasis secondary to COPD. Investigation for aetiology of bronchiectasis may be warranted on a case by case basis [164]. |
| Connective tissue disease (10%) | Bronchiectasis has been present in up 35% of patients with rheumatoid arthritis [165]. Patients with connective tissue disease may also have traction bronchiectasis as a result of fibrotic lung disease [166]. |
| Immunodeficiency (5.8%) | Most frequent immunodeficiency is common variable immune deficiency [167]. As such, different guidelines suggest obtaining serum immunoglobulins as part of the work-up of the aetiology of bronchiectasis [154, 168]. |
| Allergic bronchopulmonary aspergillosis (4.5%) | Suspect in patients with difficult to control asthma or cystic fibrosis, eosinophilia (often >500 cells·mm−3) and elevated total serum IgE levels (often >417 IU·mL−1). On chest CT, bronchiectasis tends to be central and there may be mucus plugs in central bronchi and high attenuation mucus in the bronchi. Other corroborating tests include cutaneous reactivity or specific IgE antibody to filamentous fungal antigen, precipitating antibodies or IgG antibodies against filamentous fungal antigen, and filamentous fungal growth in respiratory cultures [169]. |
| Asthma (3.3%) | In one cohort, bronchiectasis was present in 67.5% of patients with severe asthma. Those with bronchiectasis are more likely to have growth of pathogens in sputum culture and consume more antibiotics. The most common type of bronchiectasis is cylindrical [170]. |
| Inflammatory bowel disease (1.9%) | Most common thoracic manifestation of inflammatory bowel disease. Bronchiectasis is seen more often with ulcerative colitis [171]. |
| Ciliary dysfunction (1.7%) | Primary ciliary dyskinesia is an autosomal recessive disorder that manifests as chronic rhinosinusitis, recurrent respiratory infections, male infertility and bronchiectasis. Half of all patients have defects in organ laterality [172]. |
| Aspiration/oesophageal reflux (0.6%) | Gastrointestinal symptoms are common in patients with bronchiectasis [173]. There is also high prevalence of IgG against Helicobacter pylori in serum of patients with bronchiectasis [174]. Bronchiolitis may also be present with chronic aspiration [175]. |
| α1-antitrypsin deficiency (0.6%) | Clinically significant bronchiectasis has been identified in 27% of patients with α1-antitrypsin deficiency. Bronchiectasis is more often tubular and in the lower lobes. Panlobular emphysema is common [176]. |
| Yellow nail syndrome (0.1%) | Yellow nail syndrome is characterised by lower extremities lymphoedema, bronchiectasis, pleural effusion, and yellow nail discolouration [177]. |
CT: computed tomography.
Airway inflammation
The pathogenesis of bronchiectasis is characterised by a vicious cycle that includes impaired mucus clearance, recurrent infection, dysregulated inflammatory response, and airway dilatation and damage [149]. Patients with bronchiectasis have a predominantly neutrophilic airway inflammation profile. However, it is now established that 20% of patients with bronchiectasis have blood eosinophil count >300 cells·µL-1. Such a peripheral eosinophilic profile may be a risk factor for exacerbation in patients who previously received antibiotics for infection. However, the presence of low blood eosinophil count (<100 cells·µL-1) may indicate higher neutrophilic inflammation and portends a higher mortality in patients with bronchiectasis [150].
The concept that a single pathogen overgrowth causes infection may not fully explain why some patients are more predisposed to recurrent bronchiectasis exacerbation. The interaction between microbes (interactome), rather than the microbe itself, may better predict risk of exacerbations. Interactomes may also explain why antimicrobials that are not effective against the prevailing pathogen may still lead to clinical improvement in some patients [151].
Aetiology
In a cohort study of 186 patients with bronchiectasis exacerbation, a microbiological aetiology was obtained in 98 (68.5%) patients. P. aeruginosa was the most common pathogen (39%) followed by respiratory viruses (26%), S. pneumoniae (16%), H. influenzae (15%), S. aureus (12%) and Enterobacteriaceae (7%), Aspergillus sp. (7%) and NTM (3%) [152].
Diagnostic work-up
Whenever feasible, a sputum culture for routine pathogens, mycobacterium and fungus is obtained. Prior culture results should be reviewed if available.
Assessment of need for admission
Hospitalisation is indicated in the presence of worsening respiratory failure (respiratory rate >25·min−1), substantial decrease in oxygen saturation, temperature >38°C or other signs of sepsis, haemodynamic instability, development of cyanosis, inability to take oral antibiotic, need for intravenous antibiotics in the absence of a home intravenous antibiotics programme and lack of social support [153, 154].
Treatment
Empiric antimicrobials are initiated while waiting for the culture results [154]. A previously isolated pathogen should be covered [155]. When P. aeruginosa has been previously isolated, we suggest oral levofloxacin. Oral ciprofloxacin is an alternative to oral levofloxacin against P. aeruginosa, but we reserve it for pathogen-directed therapy because of its lower antimicrobial activity against S. pneumoniae [156]. In nonsevere exacerbations, and when there is no evidence of prior P. aeruginosa isolation, we suggest oral amoxicillin-clavulanic acid. If the culture reveals a pathogen, empiric antibiotic therapy can be switched to pathogen-directed therapy [154]. In a recent trial of hospitalised patients, antibiotic course based on bacterial load count led to a shorter antibiotic course length (8 days) and, in those colonised with nonpseudomonas organisms, a longer time to another exacerbation as compared with a fixed 14-day antibiotic course [157]. While the trial indicates that an antibiotic course shorter than the traditionally recommended 14-day course is feasible in these patients, the proof-of-concept design and small sample size make the findings preliminary. The length of treatment, thus, remains a contentious topic. A 14-day course of antibiotics is still recommended when treating patients for P. aeruginosa while a shorter course may be applied in patients without P. aeruginosa [158].
Conclusion
Lower respiratory infections encompass a range of diseases that are heterogenous and require different management strategies. Much progress has been made in the field of respiratory infections, including better surveillance systems, development of clinical prediction rules, new molecular diagnostic tests and novel treatment options. Perhaps the greatest scientific breakthrough we have recently witnessed was the development of effective vaccines against COVID-19. Those achievements are to be celebrated, but they should not distract us from the ongoing challenges, including medical misinformation [159], antibiotic resistance [41] and more importantly health disparities and lack of access to healthcare [2].
The outpatient management of patients with respiratory infections is better achieved with comprehensive clinical assessment for precise clinical diagnosis, knowledge of local surveillance data (e.g. the circulating pathogens), strategic use of diagnostic tests according to surveillance data, assessment of illness severity and need for admission using clinical data integration and prediction rules when available, judicious use of antimicrobials, recognition of those at higher risk due to disability, poverty or health illiteracy and patient follow-up.
Questions for future research in respiratory infections
Development and application of clinical pathways for site triage and treatment.
Development and testing of new molecular diagnostic tests for both viral and bacterial pathogens.
Development of new antimicrobial agents, including antiviral agents.
Development and testing of vaccines against respiratory pathogens.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/16000617.0150-2022
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Kumar K, Daley CL, Griffith DE, et al. Management of Mycobacterium avium complex and Mycobacterium abscessus pulmonary disease: therapeutic advances and emerging treatments. Eur Respir Rev 2022; 31: 210212. No. 2: Cilloniz C, Luna CM, Hurtado JC, et al. Respiratory viruses: their importance and lessons learned from COVID-19. Eur Respir Rev 2022; 31: 220051.
Number 3 in the Series “Respiratory infections” Edited by Antoni Torres and Michael S. Niederman
Conflict of interest: R. Cavallazzi has nothing to disclose.
Conflict of interest: J.A. Ramirez reports grants or contracts from Pfizer (institutional payment for research); consulting fees from Dompe (personal payment); and participation on a Data Safety Monitoring Board or Advisory Board for Paratek (personal payment), all outside the submitted work.
References
- 1.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections – full version. Clin Microbiol Infect 2011; 17: Suppl. 6, E1–E59. doi: 10.1111/j.1469-0691.2011.03602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Lower Respiratory Infections Collaborators . Estimates of the global, regional and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018;18: 1191–1210. doi: 10.1016/S1473-3099(18)30310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sliedrecht A, den Elzen WP, Verheij TJ, et al. Incidence and predictive factors of lower respiratory tract infections among the very elderly in the general population. The Leiden 85–plus Study. Thorax 2008; 63: 817–822. doi: 10.1136/thx.2007.093013 [DOI] [PubMed] [Google Scholar]
- 4.Loeb M, McGeer A, McArthur M, et al. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med 1999; 159: 2058–2064. doi: 10.1001/archinte.159.17.2058 [DOI] [PubMed] [Google Scholar]
- 5.Cavallazzi R, Ramirez JA. Influenza and viral pneumonia. Clin Chest Med 2018; 39: 703–721. doi: 10.1016/j.ccm.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jónsson JS, Gíslason T, Gíslason D, et al. Acute bronchitis and clinical outcome three years later: prospective cohort study. BMJ 1998; 317: 1433–1433. doi: 10.1136/bmj.317.7170.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MP, Lown M, Singh S, et al. Acute cough due to acute bronchitis in immunocompetent adult outpatients: CHEST expert panel report. Chest 2020; 157: 1256–1265. doi: 10.1016/j.chest.2020.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . Testing Guidance for Clinicians When SARS-CoV-2 and Influenza Viruses are Co-circulating. www.cdc.gov/flu/professionals/diagnosis/testing-guidance-for-clinicians.htm. Date last updated: February 9, 2022.
- 9.Creer DD, Dilworth JP, Gillespie SH, et al. Aetiological role of viral and bacterial infections in acute adult lower respiratory tract infection (LRTI) in primary care. Thorax 2006; 61: 75–79. doi: 10.1136/thx.2004.027441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SM, Fahey T, Smucny J, et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2017; 6: CD000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott JG, Cohen D, DiCicco-Bloom B, et al. Antibiotic use in acute respiratory infections and the ways patients pressure physicians for a prescription. J Fam Pract 2001; 50: 853–858. [PubMed] [Google Scholar]
- 12.Duffy E, Ritchie S, Metcalfe S, et al. Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J Clin Pharm Ther 2018; 43: 59–64. doi: 10.1111/jcpt.12610 [DOI] [PubMed] [Google Scholar]
- 13.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal Influenzaa. Clin Infect Dis 2019; 68: e1–e47. doi: 10.1093/cid/ciy866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welte T, Köhnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ Network. Semin Respir Crit Care Med 2009; 30: 127–135. doi: 10.1055/s-0029-1202941 [DOI] [PubMed] [Google Scholar]
- 15.Wiemken TL, Carrico RM, Furmanek SP, et al. Socioeconomic position and the incidence, severity, and clinical outcomes of hospitalized patients with community-acquired pneumonia. Public Health Rep 2020; 135: 364–371. doi: 10.1177/0033354920912717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavallazzi R, Ramirez J. Community-acquired pneumonia in chronic obstructive pulmonary disease. Curr Opin Infect Dis 2020; 33: 173–181. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 65: 1806–1812. doi: 10.1093/cid/cix647 [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin JM, Johnson MH, Kagan SA, et al. Clinical and economic burden of community-acquired pneumonia in the Veterans Health Administration, 2011: a retrospective cohort study. Infection 2015; 43: 671–680. doi: 10.1007/s15010-015-0789-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243–250. doi: 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 20.Cavallazzi R, Furmanek S, Arnold FW, et al. The burden of community-acquired pneumonia requiring admission to ICU in the United States. Chest 2020; 158: 1008–1016. doi: 10.1016/j.chest.2020.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community acquired pneumonia. A metaanalysis. JAMA 1996; 275: 134–141. doi: 10.1001/jama.1996.03530260048030 [DOI] [PubMed] [Google Scholar]
- 22.Aliberti S, Amir A, Peyrani P, et al. Incidence, etiology, timing, and risk factors for clinical failure in hospitalized patients with community-acquired pneumonia. Chest 2008; 134: 955–962. doi: 10.1378/chest.08-0334 [DOI] [PubMed] [Google Scholar]
- 23.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001; 163: 1730–1754. doi: 10.1164/ajrccm.163.7.at1010 [DOI] [PubMed] [Google Scholar]
- 24.Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997; 157: 1453–1459. doi: 10.1001/archinte.1997.00440340089009 [DOI] [PubMed] [Google Scholar]
- 25.Moore M, Stuart B, Little P, et al. Predictors of pneumonia in lower respiratory tract infections: 3C prospective cough complication cohort study. Eur Respir J 2017; 50: 1700434. doi: 10.1183/13993003.00434-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speets AM, Hoes AW, van der Graaf Y, et al. Chest radiography and pneumonia in primary care: diagnostic yield and consequences for patient management. Eur Respir J 2006; 28: 933–938. doi: 10.1183/09031936.06.00008306 [DOI] [PubMed] [Google Scholar]
- 27.Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996; 156: 2206–2212. doi: 10.1001/archinte.1996.00440180068008 [DOI] [PubMed] [Google Scholar]
- 28.Wootton D, Feldman C. The diagnosis of pneumonia requires a chest radiograph (X-ray) – yes, no or sometimes? Pneumonia (Nathan) 2014; 5: Suppl. 1, 1–7. doi: 10.15172/pneu.2014.5/464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Accuracy of lung ultrasonography in the diagnosis of pneumonia in adults: systematic review and meta-analysis. Chest 2017; 151: 374–382. doi: 10.1016/j.chest.2016.10.039 [DOI] [PubMed] [Google Scholar]
- 30.García-Vázquez E, Marcos MA, Mensa J, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med 2004; 164: 1807–1811. doi: 10.1001/archinte.164.16.1807 [DOI] [PubMed] [Google Scholar]
- 31.Bochud P-Y, Moser F, Erard P, et al. Community acquired pneumonia. A prospective outpatient study. Medicine 2001; 80: 75–87. doi: 10.1097/00005792-200103000-00001 [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez F, Masiá M, Rodríguez JC, et al. Epidemiology of community-acquired pneumonia in adult patients at the dawn of the 21st century: a prospective study on the Mediterranean coast of Spain. Clin Microbiol Infect 2005; 11: 788–800. doi: 10.1111/j.1469-0691.2005.01226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capelastegui A, España PP, Bilbao A, et al. Etiology of community-acquired pneumonia in a population-based study: link between etiology and patients characteristics, process-of-care, clinical evolution and outcomes. BMC Infect Dis 2012; 12: 134. doi: 10.1186/1471-2334-12-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Li X, Wang W, et al. The prevalence of respiratory pathogens in adults with community-acquired pneumonia in an outpatient cohort. Infect Drug Resist 2019; 12: 2335–2341. doi: 10.2147/IDR.S213296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagerström F, Bader M, Foldevi M, et al. Microbiological etiology in clinically diagnosed community-acquired pneumonia in primary care in Orebro, Sweden. Clin Microbiol Infect 2003; 9: 645–652. doi: 10.1046/j.1469-0691.2003.00602.x [DOI] [PubMed] [Google Scholar]
- 36.Almirall J, Boixeda R, Bolíbar I, et al. Differences in the etiology of community-acquired pneumonia according to site of care: a population-based study. Respir Med 2007; 101: 2168–2175. doi: 10.1016/j.rmed.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 37.Yin YD, Zhao F, Ren LL, et al. Evaluation of the Japanese Respiratory Society guidelines for the identification of Mycoplasma pneumoniae pneumonia. Respirology 2012; 17: 1131–1136. doi: 10.1111/j.1440-1843.2012.02227.x [DOI] [PubMed] [Google Scholar]
- 38.Burk M, El-Kersh K, Saad M, et al. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur Respir Rev 2016; 25: 178–188. doi: 10.1183/16000617.0076-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Baum H, Welte T, Marre R, et al. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J 2010; 35: 598–605. doi: 10.1183/09031936.00091809 [DOI] [PubMed] [Google Scholar]
- 40.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta V, Yu KC, Schranz J, et al. A multicenter evaluation of the US Prevalence and regional variation in macrolide-resistant S. pneumoniae in ambulatory and hospitalized adult patients in the United States. Open Forum Infect Dis 2021; 8: ofab063. doi: 10.1093/ofid/ofab063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinert RR. The antimicrobial resistance profile of Streptococcus pneumoniae. Clin Microbiol Infect 2009; 15: 7–11. doi: 10.1111/j.1469-0691.2009.02724.x [DOI] [PubMed] [Google Scholar]
- 43.Agrawal A, Murphy TF. H. influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 2011; 49: 3728–3732. doi: 10.1128/JCM.05476-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puig C, Calatayud L, Martí S, et al. Molecular epidemiology of nontypeable H. influenzae causing community-acquired pneumonia in adults. PLoS One 2013; 8: e82515. doi: 10.1371/journal.pone.0082515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceccato A, Mendez R, Ewig S, et al. Validation of a prediction score for drug-resistant microorganisms in community-acquired pneumonia. Ann Am Thorac Soc 2021; 18: 257–265. doi: 10.1513/AnnalsATS.202005-558OC [DOI] [PubMed] [Google Scholar]
- 46.Aliberti S, Di Pasquale M, Zanaboni AM, et al. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis 2012; 54: 470–478. doi: 10.1093/cid/cir840 [DOI] [PubMed] [Google Scholar]
- 47.American Thoracic Society; Infectious Diseases Society of America . Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171: 388–416. doi: 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 48.Shorr AF, Zilberberg MD, Micek ST, et al. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med 2008; 168: 2205–2210. doi: 10.1001/archinte.168.20.2205 [DOI] [PubMed] [Google Scholar]
- 49.Webb BJ, Dascomb K, Stenehjem E, et al. Derivation and multicenter validation of the drug resistance in pneumonia clinical prediction score. Antimicrob Agents Chemother 2016; 60: 2652–2663. doi: 10.1128/AAC.03071-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falcone M, Russo A, Giannella M, et al. Individualizing risk of multidrug-resistant pathogens in community-onset pneumonia. PLoS One 2015; 10: e0119528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol 2016; 137: 19–27. doi: 10.1016/j.jaci.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 52.Morelli T, Fujita K, Redelman-Sidi G, et al. Infections due to dysregulated immunity: an emerging complication of cancer immunotherapy. Thorax 2022; 77: 304–311. doi: 10.1136/thoraxjnl-2021-217260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Pasquale MF, Sotgiu G, Gramegna A, et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis 2019; 68: 1482–1493. doi: 10.1093/cid/ciy723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramirez JA, Musher DM, Evans SE, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest 2020; 158: 1896–1911. doi: 10.1016/j.chest.2020.05.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hage CA, Knox KS, Wheat LJ. Endemic mycoses: overlooked causes of community acquired pneumonia. Respir Med 2012; 106: 769–776. doi: 10.1016/j.rmed.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 56.Ashraf N, Kubat RC, Poplin V, et al. Re-drawing the maps for endemic mycoses. Mycopathologia 2020; 185: 843–865. doi: 10.1007/s11046-020-00431-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavallazzi R, Wiemken T, Christensen D, et al. Predicting Mycobacterium tuberculosis in patients with community-acquired pneumonia. Eur Respir J 2014; 43: 178–184. doi: 10.1183/09031936.00017813 [DOI] [PubMed] [Google Scholar]
- 58.Meumann EM, Cheng AC, Ward L, et al. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin Infect Dis 2012; 54: 362–369. doi: 10.1093/cid/cir808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tärnvik A, Berglund L. Tularaemia. Eur Respir J 2003; 21: 361–373. doi: 10.1183/09031936.03.00088903 [DOI] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention . Tularemia. www.cdc.gov/tularemia/clinicians/index.html. Date last accessed: March 19; 2021. Date last updated: July 5, 2022.
- 61.Xu A, Zhu H, Gao B, et al. Diagnosis of severe community-acquired pneumonia caused by Acinetobacter baumannii through next-generation sequencing: a case report. BMC Infect Dis 2020; 20: 45. doi: 10.1186/s12879-019-4733-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serota DP, Sexton ME, Kraft CS, et al. Severe community-acquired pneumonia due to Acinetobacter baumannii in north America: case report and review of the literature. Open Forum Infect Dis 2018; 5: ofy044. doi: 10.1093/ofid/ofy044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis JS, McMillan M, Swaminathan A, et al. A 16-year prospective study of community-onset bacteremic Acinetobacter pneumonia: low mortality with appropriate initial empirical antibiotic protocols. Chest 2014; 146: 1038–1045. doi: 10.1378/chest.13-3065 [DOI] [PubMed] [Google Scholar]
- 64.Chen CT, Wang YC, Kuo SC, et al. Community-acquired bloodstream infections caused by Acinetobacter baumannii: A matched case-control study. J Microbiol Immunol Infect 2018; 51: 629–635. doi: 10.1016/j.jmii.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 65.Falagas ME, Karveli EA, Kelesidis I, et al. Community-acquired Acinetobacter infections. Eur J Clin Microbiol Infect Dis 2007; 26: 857–868. doi: 10.1007/s10096-007-0365-6 [DOI] [PubMed] [Google Scholar]
- 66.Hogerwerf L, De Gier B, Baan B, et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect 2017; 145: 3096–3105. doi: 10.1017/S0950268817002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsui T, Nakashima K, Ohyama T, et al. An outbreak of psittacosis in a bird park in Japan. Epidemiol Infect 2008; 136: 492–495. doi: 10.1017/S0950268807008783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Telfer BL, Moberley SA, Hort KP, et al. Probable psittacosis outbreak linked to wild birds. Emerg Infect Dis 2005; 11: 391–397. doi: 10.3201/eid1103.040601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaede W, Reckling KF, Dresenkamp B, et al. Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health 2008; 55: 184–188. doi: 10.1111/j.1863-2378.2008.01108.x [DOI] [PubMed] [Google Scholar]
- 70.Yu H, Rubin J, Dunning S, et al. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J Am Geriatr Soc 2012; 60: 2137–2143. doi: 10.1111/j.1532-5415.2012.04208.x [DOI] [PubMed] [Google Scholar]
- 71.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–382. doi: 10.1136/thorax.58.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones BE, Jones J, Bewick T, et al. CURB-65 pneumonia severity assessment adapted for electronic decision support. Chest 2011; 140: 156–163. doi: 10.1378/chest.10-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J 2006; 27: 151–157. doi: 10.1183/09031936.06.00062505 [DOI] [PubMed] [Google Scholar]
- 74.Katz MH. Integrating prediction rules into clinical work flow. JAMA Intern Med 2013; 173: 1591. doi: 10.1001/jamainternmed.2013.8971 [DOI] [PubMed] [Google Scholar]
- 75.Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-acquired pneumonia intervention trial assessing levofloxacin. JAMA 2000; 283: 749–755. doi: 10.1001/jama.283.6.749 [DOI] [PubMed] [Google Scholar]
- 76.Atlas SJ, Benzer TI, Borowsky LH, et al. Safely increasing the proportion of patients with community-acquired pneumonia treated as outpatients: an interventional trial. Arch Intern Med 1998; 158: 1350–1356. doi: 10.1001/archinte.158.12.1350 [DOI] [PubMed] [Google Scholar]
- 77.British Thoracic Society . Annotated BTS Guideline for the management of CAP in adults (2009) Summary of recommendations. 2015. Available from: www.brit-thoracic.org.uk/quality-improvement/guidelines/pneumonia-adults/
- 78.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44: Suppl. 2, S27–S72. doi: 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Centers for Disease Control and Prevention . Active Bacterial Core Surveillance (ABCs). www.cdc.gov/abcs/reports-findings/surv-reports.html. Date last updated: July 19, 2022.
- 80.European Centre for Disease Prevention and Control . Antimicrobial Resistance Surveillance in Europe 2022 – 2020 Data. www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data. Date last updated: January 26, 2022.
- 81.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005; 26: 1138–1180. doi: 10.1183/09031936.05.00055705 [DOI] [PubMed] [Google Scholar]
- 82.Arnold F, Summersgill J, Ramirez J. Role of atypical pathogens in the etiology of community-acquired pneumonia. Semin Respir Crit Care Med 2016; 37: 819–828. doi: 10.1055/s-0036-1592121 [DOI] [PubMed] [Google Scholar]
- 83.Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol 2007; 56: 1625–1629. doi: 10.1099/jmm.0.47119-0 [DOI] [PubMed] [Google Scholar]
- 84.Phares CR, Wangroongsarb P, Chantra S, et al. Epidemiology of severe pneumonia caused by Legionella longbeachae, Mycoplasma pneumoniae, and Chlamydia pneumoniae: 1–year, population-based surveillance for severe pneumonia in Thailand. Clin Infect Dis 2007; 45: e147–e155. doi: 10.1086/523003 [DOI] [PubMed] [Google Scholar]
- 85.Izumikawa K. Clinical features of severe or fatal Mycoplasma pneumoniae pneumonia. Front Microbiol 2016; 7: 800. doi: 10.3389/fmicb.2016.00800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reechaipichitkul W, Saelee R, Lulitanond V. Prevalence and clinical features of Chlamydia pneumoniae pneumonia at Srinagarind Hospital, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health 2005; 36: 151–155. [PubMed] [Google Scholar]
- 87.Abelenda-Alonso G, Rombauts A, Gudiol C, et al. Influenza and bacterial coinfection in adults with community-acquired pneumonia admitted to conventional wards: risk factors, clinical features, and outcomes. Open Forum Infect Dis 2020; 7: ofaa066. doi: 10.1093/ofid/ofaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hedberg P, Johansson N, Ternhag A, et al. Bacterial co-infections in community-acquired pneumonia caused by SARS-CoV-2, influenza virus and respiratory syncytial virus. BMC Infect Dis 2022; 22: 108. doi: 10.1186/s12879-022-07089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmad FB, Cisewski JA, Anderson RN. Provisional mortality data – United States, 2021. MMWR Morb Mortal Wkly Rep 2022; 71: 597–600. doi: 10.15585/mmwr.mm7117e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention. COVID-19. www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Date last accessed: March 25, 2022. Date last updated: August 19, 2022.
- 91.Jones B. The Changing Political Geography of COVID-19 Over the Last Two Years. www.pewresearch.org/politics/2022/03/03/the-changing-political-geography-of-covid-19-over-the-last-two-years/. Date last updated: March 2, 2022.
- 92.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 93.Antia R, Halloran ME. Transition to endemicity: understanding COVID-19. Immunity 2021; 54: 2172–2176. doi: 10.1016/j.immuni.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blair PW, Brown DM, Jang M, et al. The clinical course of COVID-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis 2021; 8: ofab007. doi: 10.1093/ofid/ofab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev 2020; 7: CD013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mensah AA, Lacy J, Stowe J, et al. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect 2022; 84: 542–550. doi: 10.1016/j.jinf.2022.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.National Institutes of Health . General Management of Nonhospitalized Patients with Acute COVID-19. www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-patients--general-management/. Date last updated: December 16, 2021.
- 98.Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med 2020; 27: 681–692. doi: 10.1111/acem.14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance. Ann Am Thorac Soc 2020; 17: 1040–1046. doi: 10.1513/AnnalsATS.202005-418FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee KC, Morgan AU, Chaiyachati KH, et al. Pulse oximetry for monitoring patients with COVID-19 at home – a pragmatic, randomized trial. N Engl J Med 2022; 386: 1857–1859. doi: 10.1056/NEJMc2201541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bradley J, Sbaih N, Chandler TR, et al. Pneumonia severity index and CURB-65 score are good predictors of mortality in hospitalized patients with SARS-CoV-2 community-acquired pneumonia. Chest 2022; 161: 927–936. doi: 10.1016/j.chest.2021.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun H, Jain A, Leone MJ, et al. COVID-19 outpatient screening: a prediction score for adverse events. medRxiv 2020; preprint [ 10.1101/2020.06.17.20134262]. [DOI] [Google Scholar]
- 103.Pfizer . Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel Covid-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death. www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results. Date last updated: December 14, 2021.
- 104.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022; 386: 509–520. doi: 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med 2022; 386: 305–315. doi: 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.U.S. Food and Drug Administration . Fact Sheet for Healthcare Providers: Emergency Use Authorization for Bebtelovimab. www.fda.gov/media/156152/download. Date last updated: August 5, 2022.
- 107.National Institutes of Health . Therapeutic Management of Nonhospitalized Adults With COVID-19. www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/#:∼:text=The%20Panel%20recommends%20using%20remdesivir,of%20symptom%20onset%20(BIIa). Date last accessed: April 8, 2022. Date last updated: August 8, 2022.
- 108.National Institutes of Health . Drug–Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Concomitant Medications. www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/. Date last accessed: May 13, 2022.
- 109.National Institutes of Health . Ritonavir-Boosted Nirmatrelvir (Paxlovid). www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/#:∼:text=Ritonavir%2Dboosted%20nirmatrelvir%20may%20be,5%20days%20of%20symptom%20onset. Date last updated: May 13, 2022.
- 110.US Food and Drug Administration . Fact Sheet For Healthcare Providers: Emergency Use Authorization For Lagevrio™ (Molnupiravir) Capsules. https://www.fda.gov/media/155054/download. Date last updated: August, 2022. [Google Scholar]
- 111.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000; 117: 398s–401s. doi: 10.1378/chest.117.5_suppl_2.398S [DOI] [PubMed] [Google Scholar]
- 112.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 Report. 2022. Available from: https://goldcopd.org/2022-gold-reports-2/.
- 113.McCullough PA, Hollander JE, Nowak RM, et al. Uncovering heart failure in patients with a history of pulmonary disease: rationale for the early use of B-type natriuretic peptide in the emergency department. Acad Emerg Med 2003; 10: 198–204. doi: 10.1197/aemj.10.3.198 [DOI] [PubMed] [Google Scholar]
- 114.Axson EL, Bottle A, Cowie MR, et al. Relationship between heart failure and the risk of acute exacerbation of COPD. Thorax 2021; 76: 807–814. doi: 10.1136/thoraxjnl-2020-216390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Ding YM. Prevalence and risk factors of pulmonary embolism in acute exacerbation of chronic obstructive pulmonary disease and its impact on outcomes: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 2021; 25: 2604–2616. [DOI] [PubMed] [Google Scholar]
- 116.Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016; 48: 664–673. doi: 10.1183/13993003.00436-2016 [DOI] [PubMed] [Google Scholar]
- 117.Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med 2019; 7: 745–756. doi: 10.1016/S2213-2600(19)30190-0 [DOI] [PubMed] [Google Scholar]
- 118.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 119.Di Stefano A, Turato G, Maestrelli P, et al. Airflow limitation in chronic bronchitis is associated with T-lymphocyte and macrophage infiltration of the bronchial mucosa. Am J Respir Crit Care Med 1996; 153: 629–632. doi: 10.1164/ajrccm.153.2.8564109 [DOI] [PubMed] [Google Scholar]
- 120.Bhowmik A, Seemungal TA, Sapsford RJ, et al. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000; 55: 114–120. doi: 10.1136/thorax.55.2.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2014; 11: 303–309. doi: 10.1513/AnnalsATS.201310-350OC [DOI] [PubMed] [Google Scholar]
- 122.Fujimoto K, Yasuo M, Urushibata K, et al. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J 2005; 25: 640–646. doi: 10.1183/09031936.05.00047504 [DOI] [PubMed] [Google Scholar]
- 123.Gompertz S, O'Brien C, Bayley DL, et al. Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J 2001; 17: 1112–1119. doi: 10.1183/09031936.01.99114901 [DOI] [PubMed] [Google Scholar]
- 124.Aaron SD, Angel JB, Lunau M, et al. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 349–355. doi: 10.1164/ajrccm.163.2.2003122 [DOI] [PubMed] [Google Scholar]
- 125.Qiu Y, Zhu J, Bandi V, et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 968–975. doi: 10.1164/rccm.200208-794OC [DOI] [PubMed] [Google Scholar]
- 126.Rahman I, Skwarska E, MacNee W. Attenuation of oxidant/antioxidant imbalance during treatment of exacerbations of chronic obstructive pulmonary disease. Thorax 1997; 52: 565–568. doi: 10.1136/thx.52.6.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yadava K, Pattaroni C, Sichelstiel AK, et al. Microbiota promotes chronic pulmonary inflammation by enhancing IL-17A and autoantibodies. Am J Respir Crit Care Med 2016; 193: 975–987. doi: 10.1164/rccm.201504-0779OC [DOI] [PubMed] [Google Scholar]
- 128.Barker BL, Haldar K, Patel H, et al. Association between pathogens detected using quantitative polymerase chain reaction with airway inflammation in COPD at stable state and exacerbations. Chest 2015; 147: 46–55. doi: 10.1378/chest.14-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wedzicha JA. Exacerbations: etiology and pathophysiologic mechanisms. Chest 2002; 121: Suppl. 5, 136s–141s. doi: 10.1378/chest.121.5_suppl.136S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lieberman D, Lieberman D, Ben-Yaakov M, et al. Infectious etiologies in acute exacerbation of COPD. Diagn Microbiol Infect Dis 2001; 40: 95–102. doi: 10.1016/S0732-8893(01)00255-3 [DOI] [PubMed] [Google Scholar]
- 131.Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax 2006; 61: 250–258. doi: 10.1136/thx.2005.041822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perotin JM, Dury S, Renois F, et al. Detection of multiple viral and bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a pilot prospective study. J Med Virol 2013; 85: 866–873. doi: 10.1002/jmv.23495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monsó E, Rosell A, Bonet G, et al. Risk factors for lower airway bacterial colonization in chronic bronchitis. Eur Respir J 1999; 13: 338–342. doi: 10.1034/j.1399-3003.1999.13b20.x [DOI] [PubMed] [Google Scholar]
- 134.Sethi S, Evans N, Grant BJ, et al. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 347: 465–471. doi: 10.1056/NEJMoa012561 [DOI] [PubMed] [Google Scholar]
- 135.Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946. doi: 10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- 136.Garcia-Gutierrez S, Quintana JM, Aguirre U, et al. Explicit criteria for hospital admission in exacerbations of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2011; 15: 680–686. doi: 10.5588/ijtld.10.0408 [DOI] [PubMed] [Google Scholar]
- 137.Puhan MA, Vollenweider D, Latshang T, et al. Exacerbations of chronic obstructive pulmonary disease: when are antibiotics indicated? A systematic review. Respir Res 2007; 8: 30. doi: 10.1186/1465-9921-8-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vollenweider DJ, Frei A, Steurer-Stey CA, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018; 10: CD010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106: 196–204. doi: 10.7326/0003-4819-106-2-196 [DOI] [PubMed] [Google Scholar]
- 140.Allegra L, Blasi F, de Bernardi B, et al. Antibiotic treatment and baseline severity of disease in acute exacerbations of chronic bronchitis: a re-evaluation of previously published data of a placebo-controlled randomized study. Pulm Pharmacol Ther 2001; 14: 149–155. doi: 10.1006/pupt.2001.0289 [DOI] [PubMed] [Google Scholar]
- 141.Thompson WH, Nielson CP, Carvalho P, et al. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996; 154: 407–412. doi: 10.1164/ajrccm.154.2.8756814 [DOI] [PubMed] [Google Scholar]
- 142.Aaron SD, Vandemheen KL, Hebert P, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med 2003; 348: 2618–2625. doi: 10.1056/NEJMoa023161 [DOI] [PubMed] [Google Scholar]
- 143.Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013; 309: 2223–2231. doi: 10.1001/jama.2013.5023 [DOI] [PubMed] [Google Scholar]
- 144.Gunen H, Hacievliyagil SS, Yetkin O, et al. The role of nebulised budesonide in the treatment of exacerbations of COPD. Eur Respir J 2007; 29: 660–667. doi: 10.1183/09031936.00073506 [DOI] [PubMed] [Google Scholar]
- 145.Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2002; 165: 698–703. doi: 10.1164/ajrccm.165.5.2109093 [DOI] [PubMed] [Google Scholar]
- 146.Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49: 1700051. doi: 10.1183/13993003.00051-2017 [DOI] [PubMed] [Google Scholar]
- 147.Pasteur MC, Bilton D, Hill AT. British thoracic society guideline for non-CF bronchiectasis. Thorax 2010; 65: Suppl. 1, i1–58. doi: 10.1136/thx.2010.136119 [DOI] [PubMed] [Google Scholar]
- 148.Aksamit TR, O'Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the us bronchiectasis research registry. Chest 2017; 151: 982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Macfarlane L, Kumar K, Scoones T, et al. Diagnosis and management of non-cystic fibrosis bronchiectasis. Clin Med (Lond) 2021; 21: e571–e577. doi: 10.7861/clinmed.2021-0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shoemark A, Shteinberg M, De Soyza A, et al. Characterisation of eosinophilic bronchiectasis: a European multicohort study. Am J Respir Crit Care Med 2022; 205: 894–902. doi: 10.1164/rccm.202108-1889OC [DOI] [PubMed] [Google Scholar]
- 151.Aogáin MM, Narayana JK, Tiew PY, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med 2021; 27: 688–699. doi: 10.1038/s41591-021-01289-7 [DOI] [PubMed] [Google Scholar]
- 152.Polverino E, Rosales-Mayor E, Benegas M, et al. Pneumonic and non-pneumonic exacerbations in bronchiectasis: clinical and microbiological differences. J Infect 2018; 77: 99–106. doi: 10.1016/j.jinf.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 153.Martínez-García M, Máiz L, Olveira C, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol (Engl Ed) 2018; 54: 88–98. [DOI] [PubMed] [Google Scholar]
- 154.Hill AT, Sullivan AL, Chalmers JD, et al. British thoracic society guideline for bronchiectasis in adults. Thorax 2019; 74: Suppl. 1, 1–69. [DOI] [PubMed] [Google Scholar]
- 155.Polverino E, Rosales-Mayor E, Torres A. Exacerbation of bronchiectasis. Bronchiectasis 2017; Dec 23: 205–222. [Google Scholar]
- 156.Garrison MW. Comparative antimicrobial activity of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J Antimicrob Chemother 2003; 52: 503–506. doi: 10.1093/jac/dkg380 [DOI] [PubMed] [Google Scholar]
- 157.Bedi P, Cartlidge MK, Zhang Y, et al. Feasibility of shortening intravenous antibiotic therapy for bronchiectasis based on bacterial load: a proof-of-concept randomised controlled trial. Eur Respir J 2021; 58: 2004388. doi: 10.1183/13993003.04388-2020 [DOI] [PubMed] [Google Scholar]
- 158.Chalmers JD, Keir HR. Less is more? Antibiotic treatment duration for exacerbations of bronchiectasis. Eur Respir J 2021; 58: 2101416. doi: 10.1183/13993003.01416-2021 [DOI] [PubMed] [Google Scholar]
- 159.Love JS, Blumenberg A, Horowitz Z. The parallel pandemic: medical misinformation and COVID-19. J Gen Intern Med 2020; 35: 2435–2436. doi: 10.1007/s11606-020-05897-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294: 1944–1956. doi: 10.1001/jama.294.15.1944 [DOI] [PubMed] [Google Scholar]
- 161.Lonni S, Chalmers JD, Goeminne PC, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc 2015; 12: 1764–1770. doi: 10.1513/AnnalsATS.201507-472OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martínez-García M. Idiopathic and post-infectious bronchiectasis: take care with the diagnosis! Int J Tuberc Lung Dis 2021; 25: 961–963. doi: 10.5588/ijtld.21.0506 [DOI] [PubMed] [Google Scholar]
- 163.O'Brien C, Guest PJ, Hill SL, et al. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000; 55: 635–642. doi: 10.1136/thorax.55.8.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hurst JR, Elborn JS, Soyza AD. COPD–bronchiectasis overlap syndrome. Eur Respir J 2015; 45: 310–313. doi: 10.1183/09031936.00170014 [DOI] [PubMed] [Google Scholar]
- 165.Wilsher M, Voight L, Milne D, et al. Prevalence of airway and parenchymal abnormalities in newly diagnosed rheumatoid arthritis. Respir Med 2012; 106: 1441–1446. doi: 10.1016/j.rmed.2012.06.020 [DOI] [PubMed] [Google Scholar]
- 166.Walsh SLF, Sverzellati N, Devaraj A, et al. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax 2014; 69: 216–222. doi: 10.1136/thoraxjnl-2013-203843 [DOI] [PubMed] [Google Scholar]
- 167.Goussault H, Salvator H, Catherinot E, et al. Primary immunodeficiency-related bronchiectasis in adults: comparison with bronchiectasis of other etiologies in a French reference center. Respir Res 2019; 20: 275. doi: 10.1186/s12931-019-1242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 169.Asano K, Hebisawa A, Ishiguro T, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol 2021; 147: 1261–1268.e5. doi: 10.1016/j.jaci.2020.08.029 [DOI] [PubMed] [Google Scholar]
- 170.Dimakou K, Gousiou A, Toumbis M, et al. Investigation of bronchiectasis in severe uncontrolled asthma. Clin Respir J 2018; 12: 1212–1218. doi: 10.1111/crj.12653 [DOI] [PubMed] [Google Scholar]
- 171.Betancourt SL, Palacio D, Jimenez CA, et al. Thoracic manifestations of inflammatory bowel disease. AJR Am J Roentgenol 2011; 197: W452–W456. doi: 10.2214/AJR.10.5353 [DOI] [PubMed] [Google Scholar]
- 172.Tilley AE, Walters MS, Shaykhiev R, et al. Cilia dysfunction in lung disease. Annu Rev Physiol 2015; 77: 379–406. doi: 10.1146/annurev-physiol-021014-071931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsang K, Lam W, Kwok E, et al. Helicobacter pylori and upper gastrointestinal symptoms in bronchiectasis. Eur Respir J 1999; 14: 1345–1350. doi: 10.1183/09031936.99.14613459 [DOI] [PubMed] [Google Scholar]
- 174.Tsang KW, Lam SK, Lam WK, et al. High seroprevalence of Helicobacter pylori in active bronchiectasis. Am J Respir Crit Care Med 1998; 158: 1047–1051. doi: 10.1164/ajrccm.158.4.9712104 [DOI] [PubMed] [Google Scholar]
- 175.Franquet T, Giménez A, Rosón N, et al. Aspiration diseases: findings, pitfalls, and differential diagnosis. Radiographics 2000; 20: 673–685. doi: 10.1148/radiographics.20.3.g00ma01673 [DOI] [PubMed] [Google Scholar]
- 176.Parr DG, Guest PG, Reynolds JH, et al. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2007; 176: 1215–1221. doi: 10.1164/rccm.200703-489OC [DOI] [PubMed] [Google Scholar]
- 177.Vignes S, Baran R. Yellow nail syndrome: a review. Orphanet J Rare Dis 2017; 12: 42. doi: 10.1186/s13023-017-0594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]