Abstract
Respiratory virus infection can cause severe illnesses capable of inducing acute respiratory failure that can progress rapidly to acute respiratory distress syndrome (ARDS). ARDS is related to poor outcomes, especially in individuals with a higher risk of infection, such as the elderly and those with comorbidities, i.e. obesity, asthma, diabetes mellitus and chronic respiratory or cardiovascular disease. Despite this, effective antiviral treatments available for severe viral lung infections are scarce. The coronavirus disease 2019 (COVID-19) pandemic demonstrated that there is also a need to understand the role of airborne transmission of respiratory viruses. Robust evidence supporting this exists, but better comprehension could help implement adequate measures to mitigate respiratory viral infections. In severe viral lung infections, early diagnosis, risk stratification and prognosis are essential in managing patients. Biomarkers can provide reliable, timely and accessible information possibly helpful for clinicians in managing severe lung viral infections. Although respiratory viruses highly impact global health, more research is needed to improve care and prognosis of severe lung viral infections. In this review, we discuss the epidemiology, diagnosis, clinical characteristics, management and prognosis of patients with severe infections due to respiratory viruses.
Short abstract
Respiratory viruses can cause severe disease with poor outcomes, especially in individuals with a higher risk of infection. Early identification, epidemiological tracing, and preventative and therapeutic measures are crucial to limiting their spread. https://bit.ly/3mpuFat
Introduction
In April 2022, the World Health Organisation (WHO) reported almost 504 million coronavirus disease 2019 (COVID-19) cases, more than 6.2 million COVID-19-related deaths and more than 11.3 billion vaccine doses administered globally [1].
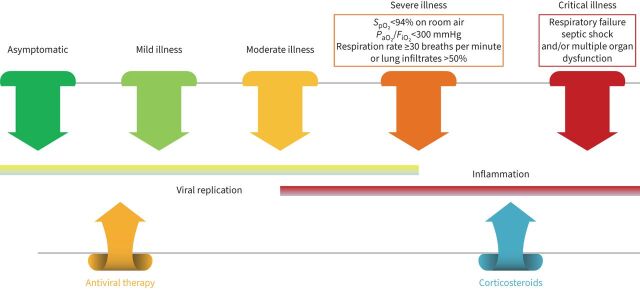
The clinical severity of COVID-19 infection ranges widely, from asymptomatic or mild disease of the upper airways to pneumonia and acute respiratory distress syndrome (ARDS) (figure 1). Several studies have described cases as being 70% mild, 20% severe and 10% critically ill [4–6]. For patients treated in intensive care units (ICUs), mortality falls between 40–50% and is higher in patients with ARDS [7, 8]. However, this clinical picture changed as different waves of COVID-19 took place. Patients who were elderly, fragile or with chronic comorbidities comprised the population most affected in the first and second waves worldwide [9, 10]. In the subsequent waves, a surge of COVID-19 cases was more prevalent in younger unvaccinated adults [11, 12]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic showed us how respiratory viruses affect individuals and continue to be underestimated, even though lower respiratory viral infections (LRVIs) are a significant cause of morbidity and mortality. The pandemic also highlighted the advances made in molecular diagnosis techniques that facilitated early identification in SARS-CoV-2 infection.
FIGURE 1.
Coronavirus disease 2019 (COVID-19) spectrum. COVID-19 has various clinical manifestations, with the spectrum of the disease ranging from asymptomatic to critical illness. Managing patients with COVID-19 will depend on disease severity. Patients with risk factors for severe disease and those with moderate disease should be monitored closely. The presence of respiratory rate ≥30 breaths per minute, oxygen saturation <94%; arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FIO2) <300 mmHg and lung infiltrates >50% are indicators of severe disease. Critical illness is related to respiratory failure, septic shock and/or multiple organ dysfunction. Viral replication is higher before or soon after symptom onset. Inflammation presents later in the disease. SpO2: oxygen saturation measured by pulse oximetry, peripheral oxygen saturation. Information from [2, 3, 132].
This review focuses on the primary viruses responsible for severe respiratory infections. We summarise their role and importance in global health.
The impact of LRVIs
Influenza A virus, influenza B virus, respiratory syncytial virus (RSV), rhinovirus, adenovirus and parainfluenza virus are the most frequent respiratory viruses that cause acute LRVI [13–21]. However, progress in molecular diagnostic techniques has facilitated the identification of other viruses like metapneumoviruses, bocavirus and coronaviruses, with a reported rise occurring in the general population [5, 22–24]. Importantly, in immunocompromised individuals, viruses such as cytomegalovirus, herpes simplex virus or varicella zoster virus constitute the pathogens often responsible for LRVI [25–29].
Approximately 11.5% of all lower respiratory tract infection (LRTI) episodes occurring in 2017 were attributable to the influenza virus This corresponds to about 54 million episodes, including 9.4 million hospitalisations [30]. Also, influenza LRTI was reported as being responsible for an estimated 145 000 (95% confidence interval (CI) 99 000–200 000) deaths among all ages that year. Adults aged >70 years have the highest mortality rate (16.4 deaths per 100 000 (95% CI 11.6–21.9)).
In the case of RSV, 2015 data from industrialised countries showed that about 1.5 million episodes of RSV-causing acute respiratory infections (ARI) occurred in older adults (aged ≥65 years). This study also reported that approximately 14% of the population needed hospitalisation and an estimated 14 000 persons died due to RSV-ARI [31]. Interestingly, a recent systematic review and meta-analysis in industrialised countries reported the annual incidence of RSV-ARI in adults with any comorbidity as 37.6 per 1000 person per year [32]. In the case of children aged <5 years, 33 million RSV-ARI episodes were reported to result in 3.2 million hospital admissions and 59 000 in-hospital deaths [33].
Recently, a prospective European primary care study evaluated 2957 adults presenting acute cough and/or suspected LRTI. A polymerase chain reaction (PCR) test was performed to test six respiratory viruses. The study authors reported that testing was positive in 46% (n=1354) of patients, with rhinovirus (40%; n=537) being the most frequently detected virus, followed by influenza viruses (20%; n=276) and coronavirus (13%; n=174) [34].
Respiratory viruses are also involved in causing chronic obstructive pulmonary disease (COPD) exacerbations. Several studies have reported that 50% of COPD exacerbations are due to respiratory viruses. For instance, in a systematic review that included 19 studies where PCR was used in sputum samples of 1728 patients with COPD exacerbations, the authors reported that rhinovirus/enterovirus (16%), RSV (10%) and influenza viruses (8%) were the most frequent viruses, whereas adenovirus (2%), human metapneumovirus (3%) and bocavirus (0.56%) constituted a rare cause of such exacerbations [35]. Furthermore, a recently published retrospective study of 262 cases of severe COPD exacerbations showed that a respiratory virus was detected in 41% of cases, with rhinovirus/enterovirus (27.5%), influenza virus (23%), RSV (13%), parainfluenza virus (13%) and coronavirus (13%) being the most frequently detected viruses. The authors reported that patients with viral exacerbations faced higher severity [36].
In the case of pneumonia, respiratory viruses comprise 10–20% of adult community-acquired pneumonia (CAP) cases with an established microbiologic diagnosis [37–40]. Respiratory viruses are identifiable in 15–45% of severe CAP cases [21, 41–45]. With respect to hospital-acquired pneumonia (HAP), respiratory viruses account for 10–30% of cases in adults [46, 47]. A retrospective case-control study by Micek et al. [48] including 174 nonventilated (NV) HAP cases and 696 controls reported a prevalence rate of 24% of respiratory viruses in NV-HAP. Similarly, a secondary analysis of a prospective cohort by Shorr et al. [49] detected respiratory viruses in 22% of patients with NV-HAP. In order of frequency, the most common viruses were rhinoviruses, influenza, parainfluenza, coronaviruses, metapneumoviruses and RSV [48, 50].
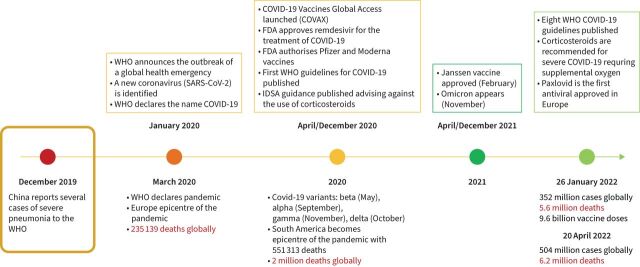
With the arrival of the COVID-19 pandemic (figure 2), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the role of respiratory viruses in causing severe lung infections like pneumonia has become ever more important. Indeed, as observed in other epidemics, e.g. severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and the H1N1 strain of influenza virus A, these types of viruses can lead to complications such as ARDS [22, 38, 51, 52].
FIGURE 2.
Evolution of the coronavirus disease 2019 (COVID-19) pandemic. The first cases of unknown pneumonia were reported in Wuhan, China in December 2019. Over the following 2 years, more than 504 million cases were confirmed worldwide, including 6.2 million deaths. FDA: Food and Drug Administration; IDSA: Infectious Diseases Society of America; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; WHO: World Health Organization.
Respiratory virus transmission and transmissibility
Aerosol (fine, <5 µm, short and long range), droplet spray (large >100 µm), direct contact (physical) and indirect contact (fomite) are the four main modes of respiratory virus transmission. During an acute respiratory viral infection, an infected person may shed the virus during expiratory activities (examples include breathing, speaking, exhalation, sneezing and coughing) in the form of droplets and aerosols [53, 54]. Droplets can travel less than 2 m and, due to their size (>100 µm), fall to the ground or surfaces, resulting in contaminated areas (hands, clothing, tables, etc.). This can then facilitate direct (handshake with contaminated skin) and indirect contact transmission (touching a table with fomites). Furthermore, aerosols (<5 µm) can travel within and beyond a metre. These aerosols can also float in the air for hours, allowing transmission over short and long distances [55].
During the COVID-19 pandemic, research on the role of airborne transmission of respiratory viruses, especially that of SARS-CoV-2, accelerated. This mode of transmission is currently under re-evaluation in respiratory viral infections. The sites of respiratory aerosol formation (oral, laryngeal, bronchial, bronchiolar and alveolar), the process involved in aerosol formation (fluid lining the airways), host characteristics (children and adults) and environment play an important role in the size and number of aerosols produced, as well as the viral content of such aerosols and transmission. For example, during coughing, sneezing, breathing and talking, individuals produce small aerosols (1 µm). Despite their size, they can carry respiratory viruses like influenza virus (100 nm–1 µm) or rhinovirus (15–30 nm). An experimental study by Yan et al. [56] analysed nasopharyngeal and 30 min breath samples (coarse >5 µm and fine ≤5 µm fractions) on days one to three after symptom onset in 355 symptomatic volunteers with acute respiratory illness, where confirmed influenza infection was reported in 142 volunteers. The authors assessed viral RNA copy number for all samples and cultured nasopharyngeal samples and fine aerosols (≤5 µm). They recovered infected virus from 89% of the nasopharyngeal samples (n=150) and 39% from fine aerosols (n=52). They reported RNA copy numbers as follows: 3.8×104 in the 30 min fine sample, 1.2×104 in the 30 min coarse aerosol sample and 8.2×108 in the nasopharyngeal sample. The significance of the study comes from the finding that in the small aerosols produced during natural breathing of infected individuals, recovering influenza viral RNA is possible. Another point of interest obtained from this study was the possible role of asymptomatic individuals in spreading viruses; its relationship to the rapid spread of SARS-CoV-2 is worth considering [57].
A prospective proof-of-concept study by Malik et al. [58] detected a mean viral load of 2.47×103 copies per 20 times exhaled breath samples from patients with COVID-19. Interestingly, an experimental study by Liu et al. [59] measuring viral RNA in aerosols from different areas of two Wuhan hospitals that treated patients with COVID-19 found that higher concentrations of SARS-CoV-2 RNA were detected in patient bathrooms (19 copies·m−3) and healthcare personnel areas (meeting room: 18 copies·m−3; male staff changing room: 20 copies·m−3; female staff changing room: 11). Lower levels of SARS-CoV-2 RNA were detected in isolation wards and ventilated patient rooms. The authors reported that detection of SARS-CoV-2 occurs most commonly in aerosols in the submicron (0.25–1.0 μm) and supermicron (>2.5 μm) ranges. The authors also reported finding deposited samples in the ICU with a range of deposition between 31 and 113 copies·m−2·h−1; these samples were detected approximately 1–3 m away from patients’ beds.
A study by Fears et al. [60] compared the dynamic aerosol efficiency of SARS-CoV-2 with that of SARS-CoV and Middle Eastern respiratory syndrome coronavirus (MERS-CoV), and quantified long-term persistence and the ability to maintain infectivity when suspended in aerosols. The authors reported that, unlike other beta-coronaviruses, SARS-CoV-2 maintained its infectivity when transmitted through the air over short distances. In addition, aerosols containing SARS-CoV-2 kept their morphology, size and aspect ratios for up to 16 h. All these data suggest that an infected person with SARS-CoV-2 is able to produce possibly infectious aerosols via human shedding and airborne transport for a long period, and that SARS-CoV-2 is very resistant in aerosol form, even over lengthy periods.
Airborne transmission was demonstrated in respiratory viruses such as influenza virus, RSV, human rhinovirus, adenovirus, enterovirus, SARS-CoV, SARS-CoV-2 and MERS-CoV. This evidence, therefore, supports the need to continue investigating this mode of transmission and to implement precautionary measures against infections and outbreaks [61–71].
Transmissibility
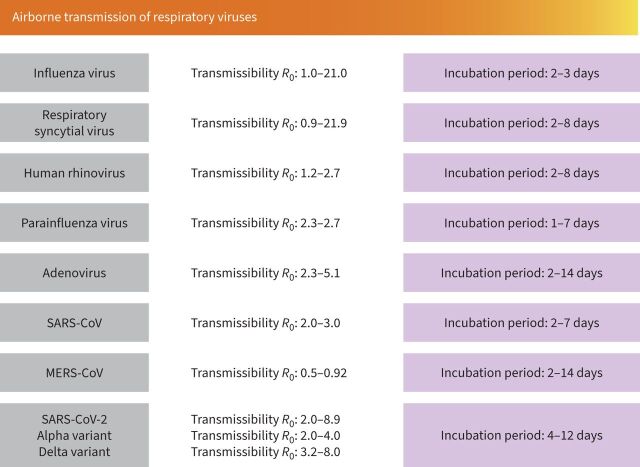
To determine the transmissibility of a pathogen, we should consider several factors, including infectivity of the micro-organism, host susceptibility, contact patterns between micro-organism and host, and environmental characteristics during transmission [72]. Determining the ability of a pathogen to spread is important for applying preventive measures for infections and outbreaks. The basic reproductive number (R0) measures the average number of new cases generated per infectious case; the higher the R0 number, the greater the risk for transmissibility. Figure 3 shows the R0 number of the main respiratory viruses.
FIGURE 3.
Airborne transmission of respiratory viruses. Data about the incubation period for various respiratory viruses and their basic reproduction number (R0).
Host risk factors for severe lung viral infection
Age, sex, previous comorbidities (e.g., obesity, diabetes mellitus, hypertension, cardiovascular disease, cerebrovascular disease, chronic pulmonary disease, liver disease) and immune impairments (e.g., pregnancy, presence of malignancies, cytotoxic therapies, autoimmune diseases, advanced HIV infection) are the main host factors related to severe outcomes (ICU admission and short- and long-term mortality) in lung viral infections (table 1) [73–75].
TABLE 1.
Patient-related risk factors for severe viral lung infections
|
Chronic comorbidities
Diabetes mellitus Hypertension Cardiovascular disease Cerebrovascular disease Chronic pulmonary disease Liver disease Immune impairments |
There is a strong association between the number of underlying medical conditions and the severity of lung viral disease. |
| Age | A decline in immune function or immunosenescence increased patients’ susceptibility to infection and the risk of serious complications due to deterioration of function in both the acquired and innate immune system. Ageing and chronic comorbidities induce chronic endothelial dysfunction, which is related to vasoconstriction, inflammation and coagulation. The latter three physiologic processes may be related to the pathologic process occurring in severe lung infections such as influenza A virus, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle Eastern respiratory syndrome coronavirus and SARS-CoV-2 infection. |
| Male sex | Influence of sex hormones on the immunologic response |
| Obesity | Diminished antibody response, immune deregulation due to adiposity and dysfunction of the immune adaptive immune system. Altered pulmonary mechanics and physiology. |
| Pregnancy | Immunologic alteration during pregnancy. Interplay between sex hormones that influence immune response. |
A decline in immune function or immunosenescence in older adults increases susceptibility to infections and the risk of serious complications. This occurs as a result of a deterioration in function in both the acquired and innate immune systems. In addition, ageing and chronic comorbidities (e.g., hypertension, diabetes and cardiovascular diseases) induce chronic endothelial dysfunction, which is also related to vasoconstriction, inflammation and coagulation. Endothelial dysfunction has been suggested to be related to the pathologic process in severe lung infections due to pathogens like influenza A virus, SARS-CoV, MERS-CoV and SARS-CoV-2 [76–78]. It is important to recognise that immunosenescence and chronic comorbidities impair the capacity of the endothelial progenitor cells to regenerate after endothelial cell injury [79, 80].
In a retrospective observational study by Cillóniz et al. [21], investigators analysed data from 2760 hospitalised patients with CAP, finding that 8% presented viral CAP. The most frequently identified viruses in these cases were influenza virus A (52%), rhinovirus (13%) and RSV (10%). Amongst the patients with viral CAP, 61% presented sepsis; male sex and age ≥65 years were independent risk factors for viral sepsis. Although the pathogenesis of COVID-19 has not been fully investigated, reports from several global studies provide enough information to consider severe COVID-19 as viral sepsis. This hypothesis finds its basis in observations made of the systemic inflammatory response, immunosuppression, damage or failure of multiple systems and organs, and chronic damage in some patients. There is a similarity to the pathogenesis of bacterial sepsis [81, 82].
Conditions such as obesity are risk factors due to diminished antibody response, immune deregulation due to adiposity and deficient adaptive responses. Results from a large cross-sectional study in the US including data from 540 667 adults hospitalised with COVID-19 (1 March 2020–31 March 2021) showed that chronic comorbidities increased the risk of severe COVID-19. Obesity had the strongest association with death [83].
In cases of pregnancy, risk is related to both the tolerant immunologic state and the influence of sex steroid on the immune response. During the influenza pandemic in 1918, pregnant women were at a particularly high risk of infection and poor outcomes. Approximately 50% of pregnant women infected during that time died [84]. Similar findings were reported during the influenza A(H1N1)pdm09, in which pregnant women faced a high risk of severe disease, required intensive care, had complications and experienced mortality more than nonpregnant women [85]. In a repeated cross-sectional study done in the US over nine influenza seasons (2010–2019) [86], which included data of 9652 hospitalised women with influenza infection, investigators reported that 2690 (28%) were pregnant and had a median age of 28 years. Similarly, 62% of these women were in their third trimester, with 42% presenting at least one comorbidity. The authors report that 5% of pregnant women required ICU admission, 2% needed mechanical ventilation and 0.3% died (n=8). Cases in which influenza A H1N1 was identified had more severe outcomes than those in which influenza A H3N2 was the causative pathogen. A propensity score-matched analysis (COV19Mx) analysed data from pregnant and nonpregnant women (n=5183 in each group) hospitalised due to COVID-19. The authors reported that pregnant women had a higher risk of death (odds ratio (OR) 1.84, 95% CI 1.26–2.69), pneumonia (OR 1.86, 95% CI 1.60–2.16) and ICU admission (OR 1.86, 95% CI 1.41–2.45) than non-pregnant women [87]. An interesting systematic review and meta-analysis [88] revealed the outcomes of 19 studies that included data from 79 pregnant women (41 with COVID-19, 12 with MERS and 26 with SARS). 90% of hospitalised pregnant women with coronavirus infections (MERS, SARS, COVID-19) presented pneumonia, with fever, cough and lymphopenia being the most frequent symptoms. These women also presented a higher rate of pre-term birth, pre-eclampsia, caesarean and perinatal death. Interestingly, ICU admission (9%) and mechanical ventilation (5%) were less common in pregnant women with COVID-19 compared to those with either MERS (44% and 40%, respectively) or SARS (53% and 40%, respectively). There was no case of maternal death due to COVID-19 infection; however, the pooled mortality of MERS and SARS infections ranged from 25% to 30%. Finally, a multinational cohort study [89] including data from 706 pregnant women with a COVID-19 diagnosis and 1424 pregnant women without a COVID-19 diagnosis reported that the former group were at a higher risk of pre-eclampsia/eclampsia (relative risk 1.76, 95% CI 1.27–2.43), severe infections (relative risk 3.38; 95% CI 1.63–7.01), ICU admission (relative risk 5.04; 95% CI 3.13–8.10), maternal mortality (relative risk 22.3, 95% CI 2.88–172), pre-term birth (relative risk 1.59, 95% CI 1.30–1.94), medically indicated pre-term birth (relative risk 1.97, 95% CI 1.56–2.51), severe neonatal morbidity index (relative risk 2.66, 95% CI 1.69–4.18) and severe perinatal morbidity and mortality index (relative risk 2.14, 95% CI 1.66–2.75). The most frequent presentation was pneumonia (91.8%), whilst the most common symptoms were fever (82.6%), cough (57.1%) and dyspnoea (27.0%). For all coronavirus infections, the pooled proportion of miscarriage was 64.7%. Eighty percent of pregnant women underwent a caesarean delivery. The pooled proportion of perinatal death was 11.1% and 57.2% of newborns needed intensive care. The pooled maternal death was 12.3%. Finally, a systematic review and meta-analysis including 192 studies and data from more than 67 000 pregnant women reported that those with COVID-19 were more likely to deliver pre-term. Similarly, this same group faced an increased risk of maternal death and needed intensive care. Their babies would also be more likely to need intensive care [90]. All of these data emphasise the importance of influenza and COVID-19 vaccination in this specific population.
The role of biomarkers in severe lung viral infections
The first line of defence against virus infection and limiting viral disease is the innate immune response. However, an excessive and uncontrolled innate immune response can cause damage to the host. This, in turn, can cause excessive tissue infiltration by immune cells and possibly lead to tissue destruction. Indeed, this was the case for the influenza pandemic in 1918, where an exaggerated pro-inflammatory immune response was thought to have played an important role in the high level of morbidity and mortality [91]. During the influenza A H1N1(pdm)09 pandemic in 2009, an exuberant inflammatory cytokine response was also reported in severe cases related to ARDS complication. Increased levels of interleukins (IL) 1, 8, 10, 6 and tumour necrosis factor α (TNF-α) were observed in severe cases of influenza AH1N1 [92]. In patients with MERS infection, serum levels of cytokines (IL-10, IL-15, TGF-β and epidermal growth factor) correlated with disease severity [93]. Similarly, in patients who developed ARDS due to SARS-CoV infection, cytokines such as IL-1, IL-6, IL-8 and TNF-α were detected at higher levels in plasma. In patients with severe COVID-19, many cytokines such as interferon-γ, TNF, granulocyte-colony stimulating factor, IL-1, IL-6, IL-18 and IL-33 were also detected at higher levels. In some cases, these laboratory findings were related to severe complications such as ARDS or multiple organ dysfunction [94, 95]. Interestingly, in most severe COVID-19 cases, lymphopenia was reported. This may explain how SARS-CoV-2 has been able to evade the immune system [96–98]. Lymphocytes play a key role in the immune homeostasis and inflammatory response. Until now, the mechanism responsible for lymphopenia has yet to be fully understood. However, some studies have suggested that lymphocyte apoptosis due to direct SARS-CoV-2 infection is possible [96]. Another possible mechanism that could induce lymphopenia may be the higher levels of cytokines and their synergic actions [96, 99].
Considering the aforementioned studies, the importance of biomarkers for the prognosis of severe lung viral infection is evident, especially in critically ill patients who need prompt intensive care.
Inflammatory biomarkers
Inflammatory biomarkers such as C-reactive protein (CRP), procalcitonin (PCT), IL-6, IL-8, IL-10, lactate dehydrogenase (LDH), ferritin and D-dimer increase greatly during a systemic inflammatory response and are correlated to disease severity. Higher levels of CRP were associated with disease severity in SARS, MERS, influenza AH1N1 and SARS-CoV-2 infection. There are data that indicate that higher levels of CRP are associated with the severity and prognosis of excessive inflammatory responses [100, 101]. Elevated values of PCT would reflect bacterial co-infection, which is related to severe lung viral disease. Especially useful in severe disease, consistent measurements of PCT levels in critically ill patients may provide an important value in predicting co-infections and helping guide antibiotic therapy [102, 103]. In severe viral lung infection, co-infection and superinfection with bacteria (Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa) or fungi (Aspergillus spp.) should always be considered, especially in critically ill patients. Recent reports showed a higher incidence of co-infections and superinfection in patients with COVID-19, especially in severe cases requiring mechanical ventilation [104–109].
Patients with ARDS and sepsis presented elevated levels of PCT. Such patient characteristics could facilitate early identification and treatment. PCT has been suggested to be a mediator of inflammation; partly because higher levels of PCT are a result of a complex interaction between cytokines such as IL-6 and TNF-α. Two meta-analyses reported an association between higher levels of PCT and COVID-19 severity. In the first meta-analysis, which included data from four studies, the authors reported that an increase in PCT values was associated with a nearly fivefold higher risk of severe COVID-19 (OR 4.76, 95% CI 2.74–8.29) [110]. The second large meta-analysis included 32 studies with data from 10 491 COVID-19 cases and reported an association between elevated PCT values and poor outcomes (OR 6.99, 95% CI 4.76–10.27) [111].
Haematological and biochemical biomarkers
Leukocyte, neutrophil, lymphocyte and platelet counts, as well as the neutrophil-to-lymphocyte ratio, are easily available biomarkers and related to disease severity in severe lung viral infection.
Leukocytosis, neutrophilia, lymphopenia and thrombocytopenia are correlated with viral lung infection severity. These patterns have been commonly observed in severe cases of influenza AH1N1 (2009 pandemic), SARS, MERS and COVID-19. There is no clear explanation for lymphopenia; however, a dysregulated immune response to the viral infection is believed to cause lymphocyte apoptosis or result from direct lymphocyte infection.
The continuous infiltration of neutrophils at the site of viral infection and their degranulation and release of neutrophil extracellular traps induce the production of pro-inflammatory cytokines. This elevation in cytokines caused by the dysregulation of the immune response could also contribute to endothelial injury resulting from ARDS and sepsis [112].
Neutrophils and lymphocytes rapidly respond to viral infection. Neutrophil count increases dramatically and then these cells migrate to the area of infection. Conversely, lymphocyte count decreases due to immunosuppression. This explains the relationship between an increase in neutrophil to lymphocyte ratio and severe lung viral disease: the ratio reflects the balance between systemic inflammation and immunity [112].
Thrombocytopenia was reported in severe cases of influenza A/H1N1 (2009 pandemic), SARS, MERS and COVID-19 [92, 113]. Platelets are mediators of inflammation. Viral infections cause a systemic inflammatory response which can cause a dysregulation between pro-coagulant and anticoagulant homeostatic mechanisms. An elevated level of D-dimer reflects a state of hypercoagulability and fibrinolysis and may indicate thrombotic risk. A systematic review and meta-analysis of 100 studies included data from 38 310 patients with COVID-19 and reported that a higher D-dimer level served as a good predictive prognostic marker of disease severity (area under the curve (AUC) 0.69) and mortality (AUC 0.79) [114]. This finding was also observed in severe cases of SARS, MERS and influenza AH1N1 (2009 pandemic) [113, 115].
A dysfunctional immune response is associated with severe lung viral infections and an elevation in biomarkers such as ferritin, LDH and IL-6. Therefore, biomarkers provide reliable, timely and accessible information that could help clinicians in managing severe lung viral infections.
Microbiologic diagnosis
An accurate microbiologic diagnosis of respiratory viruses might help to avoid unnecessary antibiotic treatment and accurately prescribe specific antiviral treatment when indicated. Nasopharyngeal swabs, sputum samples, endotracheal aspirate and bronchoalveolar lavage are recommended to detect respiratory viruses. In mild cases, nasopharyngeal swabs are optimal samples for detecting respiratory viruses due to the higher viral replication. In severe cases, lower respiratory samples (sputum, endotracheal aspirate or bronchoalveolar lavage samples) are recommended. Virus replication in the lower respiratory tract may be detectable for longer periods than in the upper respiratory tract and nasopharyngeal swabs can give false negatives. Two review articles provided general overviews of the microbiologic diagnosis of pneumonia, including important data about microbiologic diagnostic testing for respiratory viruses that cause pneumonia [116, 117]. A recent review by Colagrossi et al. [118] provided an overview of the rapid microbiologic detection of the main respiratory viruses.
Identifying the causative respiratory viruses of severe lung infection is vital for the management and prevention of hospital transmission. There are different diagnostic approaches in cases of severe pulmonary viral infection. In the last few years, the use of molecular techniques has contributed to an increase in the reported rate of respiratory viruses causing severe lung infections. Due to its high sensitivity and specificity, nucleic acid amplification testing is the method of choice for the microbiological diagnosis of virus infection. In any cases, due to the risk of co-infection or superinfection with other viruses or bacteria, it is necessary to use multiplex PCR techniques [119, 120].
Antiviral therapy
Oseltamivir, a neuraminidase inhibitor (NAI), is the most frequently used antiviral to treat severe influenza lung infection [121]. Oseltamivir is active against influenza A and B viruses, but not against other respiratory viruses [121]. Furthermore, oseltamivir is currently recommended as a first-line treatment for severe influenza infection and in patients with a risk of complications [122, 123]. Timing of treatment initiation is a critical aspect when using oseltamivir. Initiating treatment within 2 days of symptom onset or hospitalisation appears to be more efficacious, although benefits have also been reported up to within 4 or 5 days of symptom onset [122]. A meta-analysis that included data from 29 234 patients with influenza virus A (H1N1), of whom 5103 were critically ill adults, reported a significant reduction in mortality risk in patients receiving early NAI (≤2 days of symptom onset) [124]. Another observational study reported that early therapy (<48 h) was associated with reduced mortality (OR 0.69, 95% CI 0.51–0.95) in 2124 critically ill patients with influenza virus lung infection [125]. Early oseltamivir therapy was associated with a 33% reduction in ICU mortality when compared with late therapy. Early oseltamivir therapy was also associated with both a shorter ICU length of stay and duration of mechanical ventilation. Additionally, a cohort study reported that early therapy was associated with a significantly lower mortality and shorter ICU length of stay in critically ill patients with severe influenza A/H3N2 lung infection [126]. However, in cases caused by either influenza A/H1N1 or B viruses, this effect was not observed.
Another NAI is peramivir. A recent randomised controlled study by Chen et al. [127] evaluated the efficacy of peramivir and oseltamivir in severe influenza pneumonia. In terms of virus positivity duration and time of symptom remission, the authors reported no significant differences between patients who received oseltamivir and those who received peramivir. Nevertheless, the study population was small, with 20 cases in each group. Further evidence is needed to evaluate the efficacy and safety of NAIs other than oseltamivir in treating severe influenza lung infections.
In a human airway epithelium model, baloxavir, a new antiviral that inhibits the endonuclease function of the polymerase acidic protein, was investigated in vitro in combination with an NAI in treating severe influenza infections. A synergism was demonstrated between these two antivirals in the model [128]. The FLAGSTONE study compared the efficacy of the combination of baloxavir and NAI (241 cases) with the use of neuraminidase alone (125 cases) in treating severe influenza infections [129].
The combination of baloxavir and NAI was not superior to NAI alone in terms of reduction of median time to clinical improvement (97.5 versus 100.2 h, p=0.467). Interestingly, patients in the baloxavir–NAI group cleared infectious virus from the upper respiratory tract sooner than patients in the NAI-alone group did (23.9 h versus 63.7 h; p<0.0001). Despite that result, this study did not show superiority in the use of combination therapy in treating severe influenza cases. It is important to remark that the reduction in viral load shown with the combination therapy of baloxavir–NAI, together with the fact that the combination therapy targeted different sites of the virus, could reduce the possibility of resistance. However, more research is needed in the use of such therapies for severe influenza infections.
Antivirals used to treat severe and high-risk RSV infections include ribavirin (blocks viral polymerase) and palivizumab (a monoclonal antibody that prevents membrane fusion by binding to the viral envelope fusion protein) [130, 131]. Specific antivirals for the treatment of severe viral lung infections caused by non-influenza viruses are scarce.
In cases of COVID-19, we can distinguish five stages of severity (figure 1): 1) asymptomatic stage, when an individual tests positive for SARS-CoV-2 but does not present symptoms of infection; 2) mild disease stage, when an individual develops mild symptoms (e.g., fever, cough, taste/smell changes but does not present dyspnoea); 3) moderate disease stage, characterised by an oxygen saturation ≥94% and lower respiratory tract disease; 4) severe disease stage, characterised by an oxygen saturation <94%, respiratory rate >30 per min and lung infiltrates >50%; and 5) critical disease stage, characterised by the presence of respiratory failure, shock, multi-organ dysfunction or failure [132]. In the first stages of COVID-19 disease, viral replication is higher, whereas in the moderate to severe stages, inflammation is prominent. In the severe and critically ill disease stages, hypercoagulability has been reported. The current recommendation for treating COVID-19 cases is based on these principles. The effectiveness of antiviral therapy is higher in the early stages of COVID-19 and the effectiveness of anti-inflammatory agents is key during the severe and critical disease stages [133–135]. In hospitalised patients with COVID-19 requiring supplementary oxygen therapy, recommendations include using remdesivir or dexamethasone or a combination of both [133]. In hospitalised patients with COVID-19 requiring oxygen through a high-flow device or noninvasive ventilation, the recommendation is to use dexamethasone alone or in combination with remdesivir. For those patients with systemic inflammation or whose oxygen needs are rapidly increasing, it is advisable to add baricitinib or tocilizumab. Patients requiring extracorporeal membrane oxygenation or invasive mechanical ventilation should receive dexamethasone alone or dexamethasone plus tocilizumab [133].
Interestingly, a recently published prospective controlled nonrandomised study [136] investigated the effectiveness of the combination remdesivir–dexamethasone (76 cases received remdesivir–dexamethasone) compared with dexamethasone alone (75 cases received dexamethasone alone) in cases of severe COVID-19 requiring supplemental oxygen therapy. It showed a significant reduction in mortality and length of hospitalisation and faster SARS-CoV-2 clearance in patients who received remdesivir–dexamethasone compared to patients who received dexamethasone alone.
Despite the higher burden of severe lung viral infections, available effective antiviral treatments are generally scarce.
Corticosteroids: benefit or harm
There are strong data that show a relationship between the administration of corticosteroids and higher mortality rates in patients with severe influenza infection [117, 137, 138] (table 2). In a meta-analysis that included data from 6548 patients with influenza pneumonia, the authors reported that the mortality risk ratio was 1.75 for the group of patients who received corticosteroids [139]. Similar results were reported when only patients with influenza A (H1N1) virus were analysed (relative risk 1.61). In this report, patients who received corticosteroids had a longer ICU length of stay compared to those patients who did not. Another systematic review and meta-analysis that included data of 6427 patients with severe pneumonia and ARDS reported that patients who received corticosteroids had a higher mortality (OR 1.53) and incidence of nosocomial infections (OR 3.15) [140]. A retrospective observational study published in 2021 that used propensity score analysis reported that 25% of the study population received systemic corticosteroids within 7 days of hospital admission. The author found an increased risk of mortality in patients with severe influenza pneumonia, even if corticosteroids were started within this period [141]. The current recommendations from the American Thoracic Society and Infectious Diseases Society of America are against the use of corticosteroids in adults with severe influenza pneumonia (this is a conditional recommendation with low-quality evidence) [122].
TABLE 2.
Experience with corticosteroids: severe influenza virus infection and coronavirus disease 2019 (COVID-19)
| Influenza study/country | Population | Control | Outcomes |
| Cao et al. 2016 [ 137 ]/China | 204 patients with influenza A (H7N9) virus pneumonia | 84 patients with influenza A (H7N9) virus pneumonia did not receive corticosteroids | High-dose corticosteroids were associated with increased mortality and longer viral shedding. |
| Moreno et al. 2018 [ 138 ]/USA | 604 patients with severe influenza pneumonia received corticosteroids | 1242 patients with severe influenza pneumonia did not receive corticosteroids | Corticosteroids associated with increased ICU mortality. |
| Ni et al. 2019 [ 139 ]/China | 2564 patients with influenza pneumonia received corticosteroids | 3984 patients with influenza pneumonia did not receive corticosteroids | Corticosteroids increased mortality, ICU LOS, and the rate of secondary infection. However, it did not influence MV days. |
| Zhou et al. 2020 [ 140 ]/China | 2675 patients with influenza pneumonia or ARDS received corticosteroids | 3962 patients with influenza pneumonia did not receive corticosteroids | The use of corticosteroids increased mortality and incidence of nosocomial infection. However, it did not influence LOS. |
| Okuno et al. 2021 [ 141 ]/Japan | 875 patients with influenza pneumonia and respiratory failure received corticosteroids | 2644 patients with influenza pneumonia and respiratory failure did not receive corticosteroids | In-hospital mortality rate was higher in the group receiving corticosteroids. |
| COVID-19 study/country | Population | Control | Outcomes |
| RECOVERY Collaborative Group et al. 2021 [ 142 ]/UK | 2104 hospitalised patients with COVID-19 received dexamethasone | 4321 hospitalised patients with COVID-19 received usual care | The use of 6 mg of dexamethasone per day for 10 days in patients with COVID-19 requiring oxygen therapy resulted in a reduction in all-cause, 28-day mortality (p<0.001). In patients not requiring oxygen, no benefit was observed: 28-day mortality rates were 17.8% and 14% for the dexamethasone and routine care groups, respectively. |
| WHO Rapid Evidence Appraisal for COVID-19 Therapies (react) Working Group et al. 2020 [ 143 ]/12 countries | 678 critically ill COVID-19 patients received receive systemic dexamethasone, hydrocortisone or methylprednisolone | 1025 critically ill COVID-19 patients received usual care or placebo | The administration of corticosteroids was associated with lower all-cause, 28-day mortality, compared with routine care or placebo. |
| Liu et al. 2020 [ 144 ]/China | 409 patients with severe COVID-19 related to ARDS received corticosteroids | 365 patients with severe COVID-19 related to ARDS did not receive corticosteroids | Corticosteroid use was associated with a higher 28-day mortality rate and a delay in SARS-CoV-2 RNA clearance. |
| van Paassen et al. 2020 [ 146 ]/The Netherlands | A systemic review and meta-analysis 20 197 patients with COVID-19 requiring either oxygen therapy or mechanical ventilation | NA | A beneficial effect of corticosteroids on short-term mortality and a reduction in need for mechanical ventilation were reported. |
| Fadel et al. 2020 [ 147 ] /USA | 132 patients with moderate to severe COVID-19 received early corticosteroids | 81 patients with moderate to severe COVID-19 received standard care | An early short course of methylprednisolone in moderate to severe COVID-19 showed a reduction in escalation of care and improved clinical outcomes. |
| Monedero et al. 2021 [ 148 ]/Spain | 485 critically ill patients with COVID-19 received early corticosteroids | 397 critically ill patients received non-early corticosteroids | Early use of corticosteroids in critically ill patients with COVID-19 was associated with lower mortality than delayed use. |
ARDS: acute respiratory distress syndrome; ICU: intensive care unit; LOS: length of stay; MV: mechanical ventilation; NA: not applicable; WHO: World Health Organization; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
On the other hand, at the beginning of the COVID-19 pandemic, the systemic inflammation observed in patients with severe cases of COVID-19 suggested that the use of corticosteroids may be a good option to modulate the immune response to the viral infection. However, there was not enough clinical evidence about their use (table 2). The results from the RECOVERY trial reported that the use of 6 mg dexamethasone per day for 10 days in patients with COVID-19 requiring oxygen therapy showed a reduction in all-cause, 28-day mortality (p<0.001) [142]. The results of a prospective meta-analysis of clinical trials including critically ill patients with COVID-19 carried out by the WHO showed that the use of systemic corticosteroids compared with routine care or placebo was associated with a reduction in all-cause, 28-day mortality [143]. These findings contribute the necessary clinical evidence to recommend the use of corticosteroids in patients with severe COVID-19 requiring oxygen therapy. Currently, there are more data suggesting that the use of corticosteroids in an early stage of infection could be harmful for the patient, suppress host antiviral activity and cause cytopathic damage to the alveolar epithelial cells [144]. However, the use of corticosteroids in patients whose immune system has controlled viral replication could prove beneficial: these drugs could help reduce pro-inflammatory cytokines, enhance anti-inflammatory cytokines, decrease lung vascular permeability, improve epithelial barrier integrity and promote alveolar oedema fluid clearance [145–148].
The European Respiratory Society living guidelines on COVID-19 recommend the use of corticosteroids only for patients with hypoxemic respiratory failure requiring oxygen administration [149]. The recommendation set in the National Institutes of Health guidelines for COVID-19 therapy recommend the use of systemic corticosteroids in patients requiring supplemental oxygen [150].
At present, there are some remaining questions that warrant further investigations of the use of corticosteroids in severe COVID-19 (including determining the required dosage) in order to understand the effect of corticosteroids on viral clearance and replication and to elucidate any possible long-term benefits in pulmonary sequelae.
Prevention
Influenza vaccination is particularly important for people who are at a higher risk of severe influenza infection (the elderly, pregnant women and those with immunosuppression or chronic medical conditions). Yearly influenza vaccination is also recommended for healthcare workers, inpatient and outpatient settings, and long-term care facilities [151]. Despite the fact that RSV infection can cause severe lung infection (especially in immunocompromised patients), there is no licensed vaccine for RSV. To date, five COVID-19 vaccines (BioNTech-Pfizer, Moderna, AstraZeneca, Johnson & Johnson and Novavax) have been authorised by the European Medicines Agency [152]. These vaccines have demonstrated efficacy and effectiveness [153]; however, more investigation is needed, especially in specific populations such as pregnant women and immunocompromised patients. The type and dose of vaccine and the timing of the primary and booster vaccinations depend on the characteristics of each individual and possible chronic comorbidities. International organisations and scientific societies regularly update clinical considerations for the use of COVID-19 vaccines.
Following the COVID-19 pandemic, different strategies to prevent the transmission of SARS-CoV-2 virus were established. Today, there is an overwhelming amount of scientific evidence about the possible airborne transmission of SARS-CoV-2 and other respiratory viruses. Important strategies to prevent the transmission of respiratory viruses include quarantine in infected people [154], wearing masks [155–157], washing hands, disinfecting surfaces, social distancing [158] and ventilating spaces [159].
The application of these simple measures during the COVID-19 pandemic considerably reduced the transmission of influenza and other respiratory viruses (RSV, metapneumovirus, enterovirus, adenovirus, parainfluenza virus types 1–3 and rhinovirus) in the winter season of the southern hemisphere during 2020 [160, 161]. These observations showed the importance of such measures in reducing the impact of viral infections.
Conclusion
Since COVID-19 first emerged in December 2019, we have witnessed an unprecedented collaborative research effort between the global scientific and medical communities. Such actions have afforded us a better understanding of this disease, including its mode of transmission, symptoms, diagnosis, evaluation, management, prevention and short- and long-term sequelae. Similarly, continually updated knowledge about COVID-19 has facilitated the development of rapid diagnostic techniques, contributing to improved management and treatment of people with a higher risk of severe disease. We are strengthening our comprehension of the relationship at play between our immune systems and SARS-CoV-2, with numerous biomarkers already being investigated to help predict disease severity. Indeed, it has even become possible to develop effective antiviral drugs against SARS-CoV-2 and conceive effective vaccines. However, much remains to be understood about SARS-CoV-2 and the aftermath of the COVID-19 pandemic. The scientific advances made thus far have set the bar high for scientists and clinicians; we must apply this same degree of commitment to other respiratory viruses related to severe lung infection for which high morbidity and mortality pose a real risk for certain patients. There is a critical need to optimise care as it relates to the diagnosis, evaluation, management and therapy of those patients with severe viral lung infections.
Acknowledgments
Thank you to Anthony Armenta, ECHAlliance (European Connected Health Alliance), Spain, for providing language editing assistance for this article.
This article has an editorial commentary: https://doi.org/10.1183/16000617.0150-2022
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Kumar K, Daley CL, Griffith DE, et al. Management of Mycobacterium avium complex and Mycobacterium abscessus pulmonary disease: therapeutic advances and emerging treatments. Eur Respir Rev 2022; 31: 210212.
Number 2 in the Series “Respiratory infections” Edited by Antoni Torres and Michael S. Niederman
Author contributions: All authors participated in the conception, design, critical revision for important intellectual content and final approval of the submitted version.
Conflicts of interest: A. Torres reports he is a current Editorial board member for the European Respiratory Review, and reports participation on Advisory Boards or lectures for Pfizer, MSD, Biomerieux, Biotest and Jansen. The remaining authors have nothing to disclose.
Support statement: C. Cilloniz received a grant from the Fondo de Investigación Sanitaria (PI19/00207).
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int Date last accessed: 20 April 2022.
- 2.Gandhi RT. The multidimensional challenge of treating coronavirus disease 2019 (COVID-19): remdesivir is a foot in the door. Clin Infect Dis 2021; 73: e4175–e4178. 10.1093/cid/ciaa1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Emergency Physicians. ACEP COVID-19 Field Guide. https://www.acep.org/corona/covid-19-field-guide/diagnosis/diagnosis-when-there-is-no-testing Date last updated: 13 July 2021.
- 4.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 9.Rebora P, Focà E, Salvatori A, et al. The effect of frailty on in-hospital and medium-term mortality of patients with coronavirus disease-19: the FRACOVID study. Panminerva Med 2021; 64: 24–30. [DOI] [PubMed] [Google Scholar]
- 10.Águila-Gordo D, Martínez-Del Río J, Mazoteras-Muñoz V, et al. Mortality and associated prognostic factors in elderly and very elderly hospitalized patients with respiratory disease COVID-19. Rev Esp Geriatr Gerontol 2021; 56: 259–267. doi: 10.1016/j.regg.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano JB, Villagrasa JR, Ancochea J. COVID-19 in youth and the fifth wave. Arch Bronconeumol 2022; 58: T213–T214. doi: 10.1016/j.arbres.2021.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee T, Cheng MP, Vinh DC, et al. Organ dysfunction and death in patients admitted to hospital with COVID-19 in pandemic waves 1 to 3 in British Columbia, Ontario and Quebec, Canada: a cohort study. Can Med Assoc Open Access J 2022; 10: E379–E389. doi: 10.9778/cmajo.20210216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pneumonia Etiology Research for Child Health (PERCH) Study Group . Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019; 394: 757–779. doi: 10.1016/S0140-6736(19)30721-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med 2015; 373: 415–427. doi: 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019; 7: e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5 [DOI] [PubMed] [Google Scholar]
- 16.Kodama F, Nace DA, Jump RLP. Respiratory syncytial virus and other noninfluenza respiratory viruses in older adults. Infect Dis Clin North Am 2017; 31: 767–790. doi: 10.1016/j.idc.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burk M, El-Kersh K, Saad M, et al. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur Respir Rev 2016; 25: 178–188. doi: 10.1183/16000617.0076-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piralla A, Mariani B, Rovida F, et al. Frequency of respiratory viruses among patients admitted to 26 intensive care units in seven consecutive winter-spring seasons (2009–2016) in Northern Italy. J Clin Virol 2017; 92: 48–51. doi: 10.1016/j.jcv.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alimi Y, Lim WS, Lansbury L, et al. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. J Clin Virol 2017; 95: 26–35. doi: 10.1016/j.jcv.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Wang Y, Liu Y, et al. Disease severity and clinical outcomes of community acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from CAP-China Network. Eur Respir J 2019; 54: 1802406. doi: 10.1183/13993003.02406-2018 [DOI] [PubMed] [Google Scholar]
- 21.Cillóniz C, Dominedò C, Magdaleno D, et al. Pure viral sepsis secondary to community-acquired pneumonia in adults: risk and prognostic factors. J Infect Dis 2019; 220: 1166–1171. doi: 10.1093/infdis/jiz257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018; 23: 130–137. doi: 10.1111/resp.13196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Li Y, Deloria-Knoll M, et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health 2021; 9: e33–e43. doi: 10.1016/S2214-109X(20)30393-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Tawfiq JA, Zumla A, Gautret P, et al. Surveillance for emerging respiratory viruses. Lancet Infect Dis 2014; 14: 992–1000. doi: 10.1016/S1473-3099(14)70840-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Pasquale MF, Sotgiu G, Gramegna A, et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis 2019; 68: 1482–1493. doi: 10.1093/cid/ciy723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez JA, Musher DM, Evans SE, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest 2020; 158: 1802–1803. doi: 10.1016/j.chest.2020.05.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luyt C-E, Combes A, Deback C, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med 2007; 175: 935–942. doi: 10.1164/rccm.200609-1322OC [DOI] [PubMed] [Google Scholar]
- 28.Coisel Y, Bousbia S, Forel J-M, et al. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS ONE 2012; 7: e51340. doi: 10.1371/journal.pone.0051340 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Vergara A, Cilloniz C, Luque N, et al. Detection of human cytomegalovirus in bronchoalveolar lavage of intensive care unit patients. Eur Respir J 2018; 51: 1701332. doi: 10.1183/13993003.01332-2017 [DOI] [PubMed] [Google Scholar]
- 30.GBD 2017 Influenza Collaborators . Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2019; 7: 69–89. doi: 10.1016/S2213-2600(18)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222: S577–S583. doi: 10.1093/infdis/jiz059 [DOI] [PubMed] [Google Scholar]
- 32.Shi T, Vennard S, Jasiewicz F, et al. Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis 2022; 226; Suppl. 1, S17–S21. [DOI] [PubMed] [Google Scholar]
- 33.Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946–958. doi: 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos LM, Bruyndonckx R, Zuithoff NPA, et al. Lower respiratory tract infection in the community: associations between viral aetiology and illness course. Clin Microbiol Infect 2021; 27: 96–104. doi: 10.1016/j.cmi.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwaans WAR, Mallia P, van Winden MEC, et al. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol 2014; 61: 181–188. doi: 10.1016/j.jcv.2014.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang JG, Ahn JH, Jin HJ. Incidence and prognostic factors of respiratory viral infections in severe acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2021; 16: 1265–1273. doi: 10.2147/COPD.S306916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianchini S, Silvestri E, Argentiero A, et al. Role of respiratory syncytial virus in pediatric pneumonia. Microorganisms 2020; 8: 2048. doi: 10.3390/microorganisms8122048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers 2021; 7: 25. doi: 10.1038/s41572-021-00259-0 [DOI] [PubMed] [Google Scholar]
- 39.Luna CM, Famiglietti A, Absi R, et al. Community-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in Argentina. Chest 2000; 118: 1344–1354. doi: 10.1378/chest.118.5.1344 [DOI] [PubMed] [Google Scholar]
- 40.Cillóniz C, Ewig S, Polverino E, et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 2011; 66: 340–346. doi: 10.1136/thx.2010.143982 [DOI] [PubMed] [Google Scholar]
- 41.Karhu J, Ala-Kokko TI, Vuorinen T, et al. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis 2014; 59: 62–70. doi: 10.1093/cid/ciu237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen C, Kaku S, Tutera D, et al. Viral respiratory infections of adults in the Intensive Care Unit. J Intensive Care Med 2016; 31: 427–441. doi: 10.1177/0885066615585944 [DOI] [PubMed] [Google Scholar]
- 43.Choi S-H, Hong S-B, Ko G-B, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 2012; 186: 325–332. doi: 10.1164/rccm.201112-2240OC [DOI] [PubMed] [Google Scholar]
- 44.Hasvold J, Sjoding M, Pohl K, et al. The role of human metapneumovirus in the critically ill adult patient. J Crit Care 2016; 31: 233–237. doi: 10.1016/j.jcrc.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garg S, Jain S, Dawood FS, et al. Pneumonia among adults hospitalized with laboratory-confirmed seasonal influenza virus infection—United States, 2005–2008. BMC Infect Dis 2015; 15: 369. doi: 10.1186/s12879-015-1004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanhems P, Bénet T, Munier-Marion E. Nosocomial influenza: encouraging insights and future challenges. Curr Opin Infect Dis 2016; 29: 366–372. doi: 10.1097/QCO.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 47.Loubet P, Voiriot G, Houhou-Fidouh N, et al. Impact of respiratory viruses in hospital-acquired pneumonia in the intensive care unit: a single-center retrospective study. J Clin Virol 2017; 91: 52–57. doi: 10.1016/j.jcv.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Micek ST, Chew B, Hampton N, et al. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest 2016; 150: 1008–1014. doi: 10.1016/j.chest.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shorr AF, Fisher K, Micek ST, et al. The burden of viruses in pneumonia associated with acute respiratory failure: an underappreciated issue. Chest 2018; 154: 84–90. doi: 10.1016/j.chest.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 50.Chow EJ, Mermel LA. Hospital-acquired respiratory viral infections: incidence, morbidity, and mortality in pediatric and adult patients. Open Forum Infect Dis 2017; 4: ofx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finelli L, Gupta V, Petigara T, et al. Mortality among US patients hospitalized with SARS-CoV-2 infection in 2020. JAMA Netw Open 2021; 4: e216556. doi: 10.1001/jamanetworkopen.2021.6556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clementi N, Ghosh S, De Santis M, et al. Viral respiratory pathogens and lung injury. Clin Microbiol Rev 2021; 34: e00103-20. doi: 10.1128/CMR.00103-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asadi S, Wexler AS, Cappa CD, et al. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep 2019; 9: 2348. doi: 10.1038/s41598-019-38808-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregson FKA, Watson NA, Orton CM, et al. Comparing aerosol concentrations and particle size distributions generated by singing, speaking and breathing. Aerosol Sci Technol 2021; 55: 681–691. doi: 10.1080/02786826.2021.1883544 [DOI] [Google Scholar]
- 55.Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med 2020; 8: 914–924. doi: 10.1016/S2213-2600(20)30323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan J, Grantham M, Pantelic J, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA 2018; 115: 1081–1086. doi: 10.1073/pnas.1716561115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med 2020; 382: 2158–2160. doi: 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malik M, Kunze A-C, Bahmer T, et al. SARS-CoV-2: viral loads of exhaled breath and oronasopharyngeal specimens in hospitalized patients with COVID-19. Int J Infect Dis 2021; 110: 105–110. doi: 10.1016/j.ijid.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020; 582: 557–560. doi: 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 60.Fears AC, Klimstra WB, Duprex P, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis 2020; 26: 2168–2171. doi: 10.3201/eid2609.201806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cowling BJ, Ip DKM, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun 2013; 4: 1935. doi: 10.1038/ncomms2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tellier R, Li Y, Cowling BJ, et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 2019; 19: 101. doi: 10.1186/s12879-019-3707-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lv J, Gao J, Wu B, et al. Aerosol transmission of coronavirus and influenza virus of animal origin. Front Vet Sci 2021; 8: 572012. doi: 10.3389/fvets.2021.572012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science 2021; 373: eabd9149. doi: 10.1126/science.abd9149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulkarni H, Smith CM, Lee DDH, et al. Evidence of respiratory syncytial virus spread by aerosol. Time to revisit infection control strategies? Am J Respir Crit Care Med 2016; 194: 308–316. doi: 10.1164/rccm.201509-1833OC [DOI] [PubMed] [Google Scholar]
- 66.Dick EC, Jennings LC, Mink KA, et al. Aerosol transmission of rhinovirus colds. J Infect Dis 1987; 156: 442–448. doi: 10.1093/infdis/156.3.442 [DOI] [PubMed] [Google Scholar]
- 67.Yu ITS, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 2004; 350: 1731–1739. doi: 10.1056/NEJMoa032867 [DOI] [PubMed] [Google Scholar]
- 68.Kim S-H, Chang SY, Sung M, et al. Extensive viable middle east respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis 2016; 63: 363–369. doi: 10.1093/cid/ciw239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kutter JS, de Meulder D, Bestebroer TM, et al. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nat Commun 2021; 12: 1653. doi: 10.1038/s41467-021-21918-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lednicky JA, Lauzard M, Fan ZH, et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis 2020; 100: 476–482. doi: 10.1016/j.ijid.2020.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lednicky JA, Lauzardo M, Alam MM, et al. Isolation of SARS-CoV-2 from the air in a car driven by a COVID patient with mild illness. Int J Infect Dis 2021; 108: 212–216. doi: 10.1016/j.ijid.2021.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol 2021; 19: 528–545. doi: 10.1038/s41579-021-00535-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou Q, Zheng S, Wang X, et al. Influenza A-associated severe pneumonia in hospitalized patients: Risk factors and NAI treatments. Int J Infect Dis 2020; 92: 208–213. doi: 10.1016/j.ijid.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 74.Flerlage T, Boyd DF, Meliopoulos V, et al. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat Rev Microbiol 2021; 19: 425–441. doi: 10.1038/s41579-021-00542-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180: 1345–1355. doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goeijenbier M, van Wissen M, van de Weg C, et al. Review: Viral infections and mechanisms of thrombosis and bleeding. J Med Virol 2012; 84: 1680–1696. doi: 10.1002/jmv.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavriilaki E, Anyfanti P, Gavriilaki M, et al. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr Hypertens Rep 2020; 22: 63. doi: 10.1007/s11906-020-01078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Armstrong SM, Darwish I, Lee WL. Endothelial activation and dysfunction in the pathogenesis of influenza A virus infection. Virulence 2013; 4: 537–542. doi: 10.4161/viru.25779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williamson KA, Hamilton A, Reynolds JA, et al. Age-related impairment of endothelial progenitor cell migration correlates with structural alterations of heparan sulfate proteoglycans. Aging Cell 2013; 12: 139–147. doi: 10.1111/acel.12031 [DOI] [PubMed] [Google Scholar]
- 80.Morrone D, Picoi MEL, Felice F, et al. Endothelial progenitor cells: an appraisal of relevant data from bench to bedside. Int J Mol Sci 2021; 22: 12874. doi: 10.3390/ijms222312874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 2020; 395: 1517–1520. doi: 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin H-Y. Why does the sepsis induced by severe COVID-19 have different clinical features from sepsis induced by CrKP? Chin J Traumatol 2021; 25: 25–26. doi: 10.1016/j.cjtee.2021.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020-March 2021. Prev Chronic Dis 2021; 18: E66. doi: 10.5888/pcd18.210123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reid A. The effects of the 1918–1919 influenza pandemic on infant and child health in Derbyshire. Med Hist 2005; 49: 29–54. doi: 10.1017/S0025727300008279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol 2012; 207: S3–S8. doi: 10.1016/j.ajog.2012.06.068 [DOI] [PubMed] [Google Scholar]
- 86.Holstein R, Dawood FS, O'Halloran A, et al. Characteristics and outcomes of hospitalized pregnant women with influenza, 2010 to 2019: a repeated cross-sectional study. Ann Intern Med 2021; 175: 149–158. doi: 10.7326/M21-3668 [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Portilla RJ, Sotiriadis A, Chatzakis C, et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx). Ultrasound Obstet Gynecol 2021; 57: 224–231. doi: 10.1002/uog.23575 [DOI] [PubMed] [Google Scholar]
- 88.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstetrics Gynecol 2020; 2: 100107. doi: 10.1016/j.ajogmf.2020.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021; 175: 817–826. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020; 370: m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loo Y-M, Gale M. Fatal immunity and the 1918 virus. Nature 2007; 445: 267–268. doi: 10.1038/445267a [DOI] [PubMed] [Google Scholar]
- 92.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza . Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362: 1708–1719. doi: 10.1056/NEJMra1000449 [DOI] [PubMed] [Google Scholar]
- 93.Min C-K, Cheon S, Ha N-Y, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6: 25359. doi: 10.1038/srep25359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med 2020; 180: 1152–1154. doi: 10.1001/jamainternmed.2020.3313 [DOI] [PubMed] [Google Scholar]
- 96.Tavakolpour S, Rakhshandehroo T, Wei EX, et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett 2020; 225: 31–32. doi: 10.1016/j.imlet.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rahimmanesh I, Kouhpayeh S, Azizi Y, et al. Conceptual framework for SARS-CoV-2–related lymphopenia. Adv Biomed Res 2022; 11: 16. doi: 10.4103/abr.abr_303_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiang Q, Feng Z, Diao B, et al. SARS-CoV-2 induces lymphocytopenia by promoting inflammation and decimates secondary lymphoid organs. Front Immunol 2021; 12: 661052. doi: 10.3389/fimmu.2021.661052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo Z, Zhang Z, Prajapati M, et al. Lymphopenia caused by virus infections and the mechanisms beyond. Viruses 2021; 13: 1876. doi: 10.3390/v13091876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luan Y, Yin C, Yao Y. Update advances on C-reactive protein in COVID-19 and other viral infections. Front Immunol 2021; 12: 720363. doi: 10.3389/fimmu.2021.720363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020; 58: 1021–1028. doi: 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 102.Richards O, Pallmann P, King C, et al. Procalcitonin increase is associated with the development of critical care-acquired infections in COVID-19 ARDS. Antibiotics 2021; 10: 1425. doi: 10.3390/antibiotics10111425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chalmers S, Khawaja A, Wieruszewski PM, et al. Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: the role of inflammatory biomarkers. World J Crit Care Med 2019; 8: 59–71. doi: 10.5492/wjccm.v8.i5.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.da Costa RL, Lamas CdC, Simvoulidis LFN, et al. Secondary infections in a cohort of patients with COVID-19 admitted to an intensive care unit: impact of Gram-negative bacterial resistance. Rev Inst Med Trop Sao Paulo 2022; 64: e6. doi: 10.1590/s1678-9946202264006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jamnani AN, Montazeri M, Mirzakhani M, et al. Evaluation of bacterial coinfection and antibiotic resistance in patients with COVID-19 under mechanical ventilation. SN Compr Clin Med 2022; 4: 19. doi: 10.1007/s42399-021-01114-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohen R, Babushkin F, Finn T, et al. High rates of bacterial pulmonary co-infections and superinfections identified by multiplex PCR among critically ill COVID-19 patients. Microorganisms 2021; 9: 2483. doi: 10.3390/microorganisms9122483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pickens CO, Gao CA, Cuttica MJ, et al. Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med 2021; 204: 921–932. doi: 10.1164/rccm.202106-1354OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rafat Z, Ramandi A, Khaki PA, et al. Fungal and bacterial co-infections of the respiratory tract among patients with COVID-19 hospitalized in intensive care units. Gene Rep 2022; 27: 101588. doi: 10.1016/j.genrep.2022.101588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreno-García E, Puerta-Alcalde P, Letona L, et al. Bacterial co-infection at hospital admission in patients with COVID-19. Int J Infect Dis 2022; 118: 197–202. doi: 10.1016/j.ijid.2022.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta 2020; 505: 190–191. doi: 10.1016/j.cca.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malik P, Patel U, Mehta D, et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med 2021; 26: 107–108. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li C-H, Chiou H-YC, Lin M-H, et al. Immunological map in COVID-19. J Microbiol Immunol Infect 2021; 54: 547–556. doi: 10.1016/j.jmii.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 2020; 127: 104362. doi: 10.1016/j.jcv.2020.104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varikasuvu SR, Varshney S, Dutt N, et al. D-dimer, disease severity, and deaths (3D-study) in patients with COVID-19: a systematic review and meta-analysis of 100 studies. Sci Rep 2021; 11: 21888. doi: 10.1038/s41598-021-01462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davey RT, Lynfield R, Dwyer DE, et al. The association between serum biomarkers and disease outcome in influenza A(H1N1)pdm09 virus infection: results of two international observational cohort studies. PLoS ONE 2013; 8: e57121. doi: 10.1371/journal.pone.0057121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Torres A, Lee N, Cilloniz C, et al. Laboratory diagnosis of pneumonia in the molecular age. Eur Respir J 2016; 48: 1764–1778. doi: 10.1183/13993003.01144-2016 [DOI] [PubMed] [Google Scholar]
- 117.Cillóniz C, Torres A, Niederman MS. Management of pneumonia in critically ill patients. BMJ 2021; 375: e065871. doi: 10.1136/bmj-2021-065871 [DOI] [PubMed] [Google Scholar]
- 118.Colagrossi L, Mattana G, Piccioni L, et al. Viral respiratory infections: new tools for a rapid diagnosis. Semin Respir Crit Care Med 2021; 42: 747–758. doi: 10.1055/s-0041-1739306 [DOI] [PubMed] [Google Scholar]
- 119.Vos LM, Bruning AHL, Reitsma JB, et al. Rapid molecular tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review of diagnostic accuracy and clinical impact studies. Clin Infect Dis 2019; 69: 1243–1253. doi: 10.1093/cid/ciz056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gadsby NJ, Russell CD, McHugh MP, et al. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis 2016; 62: 817–823. doi: 10.1093/cid/civ1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sur M, Lopez MJ, Baker MB. Oseltamivir. In: StatPearls. Treasure Island, FL, StatPearls Publishing; 2021. Available from: www.ncbi.nlm.nih.gov/books/NBK539909/. [PubMed] [Google Scholar]
- 122.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019; 68: e1–e47. doi: 10.1093/cid/ciy866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2: 395–404. doi: 10.1016/S2213-2600(14)70041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moreno G, Rodríguez A, Sole-Violán J, et al. Early oseltamivir treatment improves survival in critically ill patients with influenza pneumonia. ERJ Open Res 2021; 7: 00888-2020. doi: 10.1183/23120541.00888-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lytras T, Mouratidou E, Andreopoulou A, et al. Effect of early oseltamivir treatment on mortality in critically ill patients with different types of influenza: a multiseason cohort study. Clin Infect Dis 2019; 69: 1896–1902. doi: 10.1093/cid/ciz101 [DOI] [PubMed] [Google Scholar]
- 127.Chen H-D, Wang X, Yu S-L, et al. Clinical effectiveness of intravenous peramivir compared with oseltamivir in patients with severe influenza a with primary viral pneumonia: a randomized controlled study. Open Forum Infect Dis 2020; 8: ofaa562. doi: 10.1093/ofid/ofaa562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Checkmahomed L, Padey B, Pizzorno A, et al. In vitro combinations of baloxavir acid and other inhibitors against seasonal influenza A viruses. Viruses 2020; 12: E1139. doi: 10.3390/v12101139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar D, Ison MG, Mira J-P, et al. Combining baloxavir marboxil with standard-of-care neuraminidase inhibitor in patients hospitalised with severe influenza (FLAGSTONE): a randomised, parallel-group, double-blind, placebo-controlled, superiority trial. Lancet Infect Dis 2022; 22: 718–730. doi: 10.1016/S1473-3099(21)00469-2 [DOI] [PubMed] [Google Scholar]
- 130.Fragkou PC, Moschopoulos CD, Karofylakis E, et al. Update in viral infections in the intensive care unit. Front Med 2021; 8; 575580. doi: 10.3389/fmed.2021.575580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dandachi D, Rodriguez-Barradas MC. Viral pneumonia: etiologies and treatment. J Investig Med 2018; 66: 957–965. doi: 10.1136/jim-2018-000712 [DOI] [PubMed] [Google Scholar]
- 132.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020; 383: 1757–1766. doi: 10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 133.Agarwal A, Rochwerg B, Lamontagne F, et al. Update to living WHO guideline on drugs for covid-19. BMJ 2021; 372: n860. doi: 10.1136/bmj.n860 [DOI] [PubMed] [Google Scholar]
- 134.Information on COVID-19 treatment, prevention and research. COVID-19 treatment guidelines. www.covid19treatmentguidelines.nih.gov Date last accessed: 28 March 2021.
- 135.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 2020; in press [ 10.1093/cid/ciaa478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marrone A, Nevola R, Sellitto A, et al. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of COVID-19 patients requiring supplemental O2 therapy: a prospective controlled non-randomized study. Clin Infect Dis 2022; 75: e403–e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao B, Gao H, Zhou B, et al. Adjuvant corticosteroid treatment in adults with Influenza A (H7N9) viral pneumonia. Crit Care Med 2016; 44: e318–e328. doi: 10.1097/CCM.0000000000001616 [DOI] [PubMed] [Google Scholar]
- 138.Moreno G, Rodríguez A, Reyes LF, et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 2018; 44: 1470–1482. doi: 10.1007/s00134-018-5332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ni Y-N, Chen G, Sun J, et al. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 2019; 23: 99. doi: 10.1186/s13054-019-2395-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou Y, Fu X, Liu X, et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci Rep 2020; 10: 3044. doi: 10.1038/s41598-020-59732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Okuno D, Kido T, Muramatsu K, et al. Impact of corticosteroid administration within 7 days of the hospitalization for influenza pneumonia with respiratory failure: a propensity score analysis using a nationwide administrative database. J Clin Med 2021; 10: 494. doi: 10.3390/jcm10030494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group , Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu J, Zhang S, Dong X, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest 2020; 130: 6417–6428. doi: 10.1172/JCI140617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matthay MA, Wick KD. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J Clin Invest 2020; 130: 6218–6221. doi: 10.1172/JCI143331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Paassen J, Vos JS, Hoekstra EM, et al. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care 2020; 24: 696. doi: 10.1186/s13054-020-03400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fadel R, Morrison AR, Vahia A, et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020; 71: 2114–2120. doi: 10.1093/cid/ciaa601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Monedero P, Gea A, Castro P, et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care 2021; 25: 2. doi: 10.1186/s13054-020-03422-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chalmers JD, Crichton ML, Goeminne PC, et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J 2021; 57: 2100048. doi: 10.1183/13993003.00048-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Information on COVID-19 treatment, prevention and research. COVID-19 treatment guidelines. www.covid19treatmentguidelines.nih.gov Date last accessed: 28 December 2021.
- 151.Grohskopf LA. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2021–22 Influenza Season. MMWR Recomm Rep 2021; 70: 1–28. doi: 10.15585/mmwr.rr7005a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.COVID-19 vaccines. www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines Date last accessed: 15 December 2022
- 153.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA 2021; 325: 1318–1320. doi: 10.1001/jama.2021.3199 [DOI] [PubMed] [Google Scholar]
- 154.Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev 2020; 9: CD013574. doi: 10.1002/14651858.CD013574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eikenberry SE, Mancuso M, Iboi E, et al. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model 2020; 5: 293–308. doi: 10.1016/j.idm.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liang M, Gao L, Cheng C, et al. Efficacy of face mask in preventing respiratory virus transmission: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 36: 101751. doi: 10.1016/j.tmaid.2020.101751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Metersky ML, Aliberti S, Feldman C, et al. Never let a good crisis go to waste. Chest 2021; 159: 917–919. doi: 10.1016/j.chest.2020.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miller SL, Nazaroff WW, Jimenez JL, et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2021; 31: 314–323. doi: 10.1111/ina.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Somsen GA, van Rijn C, Kooij S, et al. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir Med 2020; 8: 658–659. doi: 10.1016/S2213-2600(20)30245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID-19 Pandemic – United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1305–1309. doi: 10.15585/mmwr.mm6937a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun 2021; 12: 1001. doi: 10.1038/s41467-021-21157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]