Abstract
Background
As mortality from coronavirus disease 2019 (COVID-19) is strongly age-dependent, we aimed to identify population subgroups at an elevated risk for adverse outcomes from COVID-19 using age-/gender-adjusted data from European cohort studies with the aim to identify populations that could potentially benefit from booster vaccinations.
Methods
We performed a systematic literature review and meta-analysis to investigate the role of underlying medical conditions as prognostic factors for adverse outcomes due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including death, hospitalisation, intensive care unit (ICU) admission and mechanical ventilation within three separate settings (community, hospital and ICU). Cohort studies that reported at least age and gender-adjusted data from Europe were identified through a search of peer-reviewed articles published until 11 June 2021 in Ovid Medline and Embase. Results are presented as odds ratios with 95% confidence intervals and absolute risk differences in deaths per 1000 COVID-19 patients.
Findings
We included 88 cohort studies with age-/gender-adjusted data from 6 653 207 SARS-CoV-2 patients from Europe. Hospital-based mortality was associated with high and moderate certainty evidence for solid organ tumours, diabetes mellitus, renal disease, arrhythmia, ischemic heart disease, liver disease and obesity, while a higher risk, albeit with low certainty, was noted for chronic obstructive pulmonary disease and heart failure. Community-based mortality was associated with a history of heart failure, stroke, diabetes and end-stage renal disease. Evidence of high/moderate certainty revealed a strong association between hospitalisation for COVID-19 and solid organ transplant recipients, sleep apnoea, diabetes, stroke and liver disease.
Interpretation
The results confirmed the strong association between specific prognostic factors and mortality and hospital admission. Prioritisation of booster vaccinations and the implementation of nonpharmaceutical protective measures for these populations may contribute to a reduction in COVID-19 mortality, ICU and hospital admissions.
Short abstract
There is a strong association between specific prognostic factors and mortality and hospital admission due to SARS-CoV-2, including, but not limited to, diabetes, cardiovascular diseases and respiratory diseases. https://bit.ly/3Qo4zCc
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to detrimental consequences for society, the global economy and public health. Healthcare systems worldwide continue to face high pressure, particularly at peaks of transmission waves. After a period of decrease during the European summer months of 2021, increases in the number of cases and hospital and intensive care unit (ICU) admissions have been noted from October 2021 in most of the European Union and European Economic Area (EU/EEA) countries [1]. According to the European Centre for Disease Prevention and Control (ECDC)'s most recent risk assessment, this rise was mainly attributed to the newly emerging variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), such as the Delta variant (B.1.617.2) and the Omicron variant (B.1.1.529), the loosening of nonpharmaceutical interventions (NPIs) across Europe and inadequate vaccination coverage [2].
In an effort to stratify patients with SARS-CoV-2 for better management of economic and human resources, particular attention has been paid to the determination of risk factors that predispose to adverse outcomes related to COVID-19, including hospitalisation, ICU admission, the need for ventilatory support or death. Consistent evidence demonstrates a high risk of severe COVID-19 manifestations in older individuals, especially in combination with pre-existing medical conditions [3–5]. Population-level data have indicated that older COVID-19 patients with comorbidities such as chronic cardiac, nonasthmatic chronic pulmonary, chronic kidney and liver diseases, and obesity have higher mortality in hospitals [6–8]. Although the European Surveillance System has noted through the use of population data that COVID-19 patients with cardiac disorders (25.7%), diabetes (15.5%) and cancers (9.9%) have the highest case-fatality rates in the European population [9], and while previous systematic reviews have separately assessed several clinical indicators or comorbidities, they lack age-/gender-adjusted analyses and stratification by patient setting [4].
As mortality from COVID-19 is strongly age-dependent, a meta-analysis of pooled age-adjusted estimates from available cohort studies is needed to determine which comorbidities should classify patients into high-risk groups for adverse COVID-19 outcomes. Additionally, as new variants of SARS-CoV-2 emerge, most updated scientific evidence should be regularly assessed regarding the risk factors for adverse COVID-19 outcomes. For example, the newest variant called Omicron (B1.1.529), which was declared as a variant of concern by the World Health Organisation (WHO) on 26 November 2021 [10], has caused severe pneumonia in young patients even without profound risk factors [11]. Such evidence would be of interest to clinicians to better manage patient flow and to policymakers when planning forthcoming public health measures, such as booster vaccination strategies in coming phases of the pandemic.
Methods
The systematic review as conducted adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [12] and MOOSE (Meta-analyses Of Observational Studies in Epidemiology) guidelines [13]. The protocol of this systematic review was pre-reviewed by the ECDC. The protocol was not pre-registered in any database for systematic reviews.
Only cohort studies conducted in Europe, including EU/EEA countries, the United Kingdom (UK) and Switzerland were considered eligible provided 1) they evaluated patients with clinically diagnosed or laboratory-confirmed COVID-19 in one of three settings (community, hospital or ICU) and 2) they assessed the associations between underlying clinical conditions (as risk factors) and primary adverse outcomes of COVID-19. All underlying clinical conditions (risk factors) that were described in the original studies were considered acceptable for data extraction as long as the results were at least age- and gender-adjusted. All other study designs (i.e. case control, cross-sectional), were excluded, as were cohorts that provided only unadjusted data.
The primary outcomes of our meta-analyses were mortality, hospital admission and ICU admission. Supplementary outcomes included, where available, the use of mechanical ventilatory support and a composite outcome comprising admission to the ICU and/or death and/or hospice care (e.g. “death or ICU admission”). The latter secondary outcome was considered crucial to account for the bias introduced by excluding patients from higher levels of care due to their poor baseline status or severe comorbidities.
Relevant peer-reviewed studies published in English were identified within Medline (Ovid) and Embase (Ovid) until 11 June 2021. Subject headings relating to COVID-19 and epidemiological study design terms were used to develop a comprehensive search strategy, presented in Appendix 1. Reference lists of all included studies and identified reviews were also screened to identify additional relevant studies. Full texts of potentially eligible studies were evaluated independently by two reviewers. Disagreements or uncertainties in screening stages were resolved through discussion and consensus.
An ad hoc designed structured form was used for the adjusted data from each eligible study, including details on the study design, baseline characteristics of the participants, clinical conditions (risk factors) and outcome data (mortality, hospitalisation, ICU admission). For accuracy, each study's data were extracted by one reviewer, with each study cross-checked by a second. Adequate information was extracted to allow us to identify overlapping populations across studies. In the case of overlapping populations, we prioritised including data from the study with the larger population and a more rigorously described methodology.
The methodological quality of each included study was evaluated independently by two reviewers using the Joanna Briggs Institute (JBI) standardised critical appraisal tool for the appropriate design [8]. Disagreements were resolved with discussion and, when necessary, adjudication by a third reviewer.
The certainty of the evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used for evaluating the certainty in the body of evidence for each meta-analysis [14]. In line with GRADE recommendations for the assessment of evidence about prognostic factors, we initially ascribed high certainty for all our findings which were subsequently rated down in cases of study limitations of the included studies, inconsistency, indirectness or imprecision of the results, or evidence of publication bias. Moreover, certainty was rated up in cases of large observed effects. Results that were of high or moderate certainty are primarily reported in the text of the current article.
Statistical analysis
In anticipation of significant clinical and methodological heterogeneity in the meta-analyses, we fitted logistic regression models with random effects. All outcome data were adjusted at least for gender and age. All variables analysed were dichotomous and analysed as odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs). Heterogeneity was quantified using the I2 statistics. We considered values above 75% to represent considerable heterogeneity. To facilitate interpretability of the results and in line with recommendations by GRADE [14], we also present the absolute risk differences (RDs) per 1000 COVID-19 patients with corresponding 95% CI. The median prevalence of the evaluated risk factors and the median incidence rate of each adverse outcome in each recruitment setting were used for calculating the absolute RDs.
Results
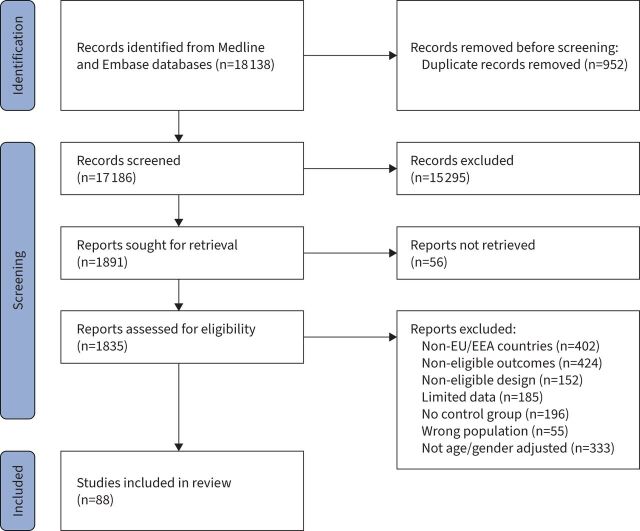
We included 88 studies, involving 6 653 207 cases with COVID-19 in the review (PRISMA flowchart: figure 1). Recruitment dates ranged from 1 January 2020 to 30 April 2021, but were primarily based in 2020. There were 69 cohort studies that assessed 6 029 126 patients in the hospital setting [15–83], 13 cohort studies involving 621 792 patients in the community setting – of which 10 were based solely in the community [84–93] while three combined community/hospital recruitment [94–96], and six cohort studies involving 2289 patients based in the ICU setting [97–102]. Details on the characteristics of the included cohort studies are presented in Appendix 2, while the assessment of the risk of bias according to the JBI critical appraisal tool is provided in Appendix 3.
FIGURE 1.
PRISMA flowchart of the included studies.
As per our inclusion criteria, all cohort studies were based in Europe and included 23 studies from Spain [16, 18, 19, 24, 30, 31, 34, 35, 37, 52, 56, 59–61, 66, 69, 72, 82, 91, 94, 98, 99, 103], 22 studies from Italy [15, 21, 23, 25, 27, 32, 40, 41, 47, 49, 50, 57, 58, 63, 64, 70, 73, 79, 81, 92, 93, 95], 15 studies from France [22, 26, 29, 33, 42, 43, 45, 55, 62, 65, 67, 76, 78, 88, 101], 10 studies from the UK [28, 36, 46, 51, 54, 68, 74, 77, 89, 100], four included patients from more than one country [71, 75, 90, 96], three from Germany [48, 53, 80], two studies from Sweden [38, 102], two from Denmark [44, 85] and one study each from Belgium [83], Finland [5], the Netherlands [20], Norway [87] and Poland [39]. The diagnosis of COVID-19 was made mainly with a PCR test, except for in five studies in which the diagnosis was made with the International Classification of Diseases [104 22, 26, 29, 44, 86].
Underlying medical conditions as prognostic factors of mortality due to COVID-19
While all meta-analyses findings are presented with both adjusted OR (aOR) and absolute RDs in figures 2a–b, below, we primarily present key results supported by evidence of moderate or high certainty as assessed using the GRADE approach.
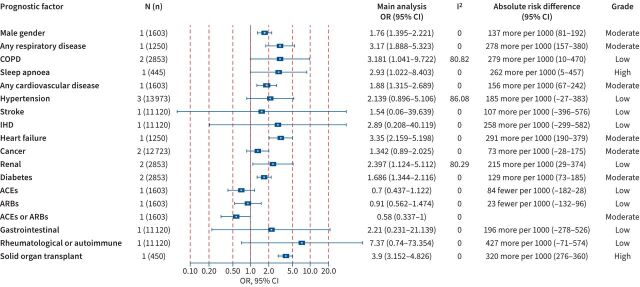
FIGURE 2.
Association between prognostic factors and mortality among coronavirus disease 2019 (COVID-19) cases within a) community, b) hospital and c) intensive care unit (ICU) settings. Odds ratios (ORs) (95% confidence intervals (CIs)), I2 test for heterogeneity, absolute risk differences (95% CIs) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment are presented. COPD: chronic obstructive pulmonary disease; IHD: ischaemic heart disease; N (n): number of studies (number of population); VTE: venous thromboembolism
The presence of any cardiovascular disease posed an increased risk for mortality in the hospital setting (51 (95% CI 17–85) more deaths per 1000 cases; high certainty). Additionally, stroke (35 (95% CI 3–75) more deaths per 1000 cases; high certainty) and heart failure (57 (95% CI 13–144) more deaths per 1000 cases; moderate certainty) were associated with increased mortality risk in the community setting, while ischaemic heart disease (IHD) was estimated to increase the risk of death in hospitalised patients with COVID-19 by 187 (95% CI 11–385) more deaths per 1000 cases (moderate certainty). Diabetes mellitus was associated with increased mortality in the hospital setting as 57 (95% CI 31–84) (high certainty) additional deaths were observed for every 1000 patients recruited with COVID-19 and diabetes mellitus. Arrythmia was associated with increased mortality in the hospital setting (174 (95% CI 28–338); moderate certainty). A history of solid organ transplant receipt was associated with increased mortality in the community setting (163 (95% CI 124–206) more deaths per 1000 cases; high certainty). Patients with neurological disease were also at an elevated risk of death within both the hospital (133 (95% CI 17–264) more deaths per 1000 cases; moderate certainty) and the community setting (34 (95% CI 7–67) more deaths per 1000 cases; moderate certainty). Similarly, COVID-19 patients with any respiratory disease were found to have increased mortality risk in the community (142 (95% CI 16–339) more deaths per 1000 cases; moderate certainty) and in the ICU (94 (95% CI 13–176) more deaths per 1000 cases; moderate certainty) setting.
Other factors associated with an increased mortality in the hospital setting were renal disease (91 (95% CI 25–163); moderate certainty); dementia (99 (95% CI 4–209); moderate certainty); cancer (121 (95% CI 78–166); high certainty); liver disease (239 (95% CI 53–441); moderate certainty); and male gender (68 (95% CI 50–86); high certainty). Male gender was found to be associated with increased death also in the ICU setting (96 (95% CI 22–166); moderate certainty). Data regarding the presence of other comorbid diseases with COVID-19 mortality were less consistent or of lower certainty.
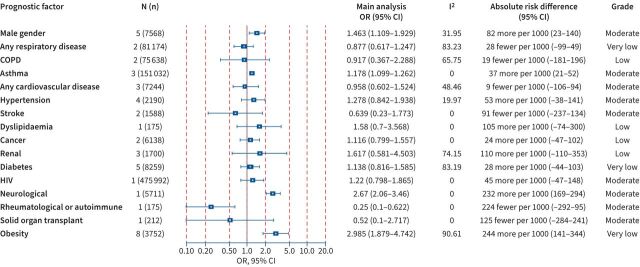
Hospital and ICU admission
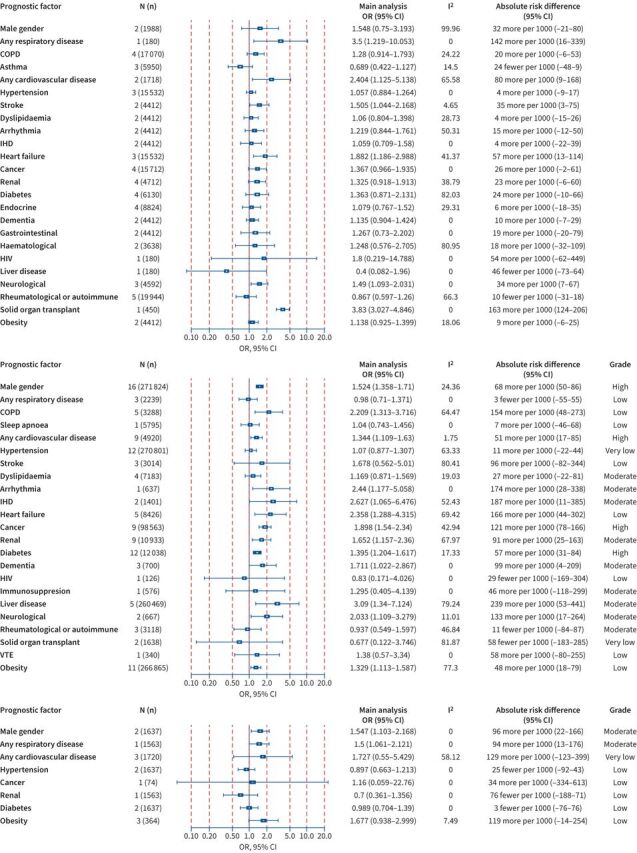
Figure 3 presents a forest plot that summarises the available evidence presented as aOR and absolute RDs regarding the association between COVID-19 and hospital admission within the community setting. Increased hospitalisation per 1000 COVID-19 patients was noted for the history of solid organ transplant (320; 95% CI 276–360) and sleep apnoea (262; 95% CI 5–457), supported by evidence of high certainty. Moreover, other prognostic factors were associated with hospital admission with evidence of moderate certainty and included any cardiovascular disease (156; 95% CI 67–242), heart failure (291; 95% CI 190–379), any respiratory disease (278; 95% CI 157–380), diabetes (129; 95% CI 73–185) and male gender (137; 95% CI 81–192). Figure 4 presents the available evidence of the association between prognostic factors and ICU admission within the hospital setting due to COVID-19. Supported by evidence of moderate certainty, the presence of asthma and neurological diseases was significantly associated with ICU admission, other factors were supported by evidence of lower certainty or indicated nonstatistically significant results.
FIGURE 3.
Association between prognostic factors and hospital admission among coronavirus disease 2019 (COVID-19) within the community setting. Odds ratios (ORs) (95% confidence intervals (CIs)), I2 test for heterogeneity, absolute risk differences (95% CIs) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment are presented for all studies. ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; COPD: chronic obstructive pulmonary disease; IHD: ischaemic heart disease; N (n): number of studies (number of population).
FIGURE 4.
Association between prognostic factors and intensive care unit (ICU) admission among coronavirus disease 2019 (COVID-19) cases within the hospital setting. Odds ratios (ORs) (95% confidence intervals (CIs)), I2 test for heterogeneity, absolute risk differences (95% CIs) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment are presented for all studies. COPD: chronic obstructive pulmonary disease; N (n): number of studies (number of population).
Composite outcome of death or ICU admission
As a supplementary analysis, we assessed the prognostic factors related to the composite adverse outcome of combined death and/or ICU admission in Appendix 4 for community (Appendix 4a) and hospital settings (Appendix 4b). Among hospitalised patients, the presence of chronic obstructive pulmonary disease (COPD) (252; 95% CI 115–383); dyslipidaemia (95; 95% CI 4–190); hypertension (91; 95% CI 27–153); diabetes (71; 95% CI 47–95) and any cardiovascular disease (76; 95% CI 1–153) was associated with this composite outcome supported by evidence of high certainty. Moreover, obesity (143; 95% CI 94–1920, heart failure (141; 95% CI 39–246) and male gender (144; 95% CI 98–188) were significantly associated with this composite outcome in the hospital setting, supported by evidence of moderate certainty. Diabetes (33; 95% CI 4–66) and male gender (33; 95% CI 12–54) were also estimated to increase the risk for ICU admission or death in the community setting with evidence of moderate certainty.
Mechanical ventilation
Appendix 5 presents the assessment of the need for mechanical ventilation, as a secondary outcome, for both the hospital setting (Appendix 5a) and the ICU setting (Appendix 5b). Obesity was associated with an increased need for mechanical ventilation in both the hospital (169; 95% CI 162–176; high certainty) and ICU setting (128; 95% CI 67–176; low certainty). Moreover, data of high certainty indicated an increased risk for mechanical ventilation in hospitalised COVID-19 patients with asthma (18; 95% CI 2–35), while male gender was associated with a higher odds of mechanical ventilation need in both hospital (108; 95% CI 48–164; high certainty) and ICU settings (172; 95% CI 3–321; moderate certainty).
Discussion
Based on quantitative age- and gender-adjusted data extracted from 88 cohort studies conducted in Europe, reporting on 6 653 207 patients with COVID-19, this systematic review and meta-analyses strengthen the understanding of prognostic factors for adverse outcomes of infection that would set the base for the identification of high-risk populations in order to support public health decision-making regarding booster vaccinations and the application of personal NPIs as societies reopen across Europe.
Extensive research has confirmed the strong association of age and gender with mortality and hospital admissions [104–108], hence it is important to take these factors into account in meta-analyses, particularly given the strong association of COVID-19 outcomes with age, which likely leads to an overestimation of the risk posed by ageing-associated diseases, such as congestive heart disease, COPD and dementia. Hence, in contrast to previous meta-analyses, our approach evaluated the weight of evidence for factors that were at least age-/gender-adjusted to overcome the issue. Notably, male gender was identified as a strong predisposing factor across multiple analyses even after adjusting for patient age.
Hospital and ICU mortality
In our age-/gender-adjusted analyses, increased hospital mortality was noted among patients with COPD, arrhythmia, IHD, heart failure, cancer, renal disease, liver disease, obesity and diabetes. Previous systematic reviews had identified COPD, cardiovascular disease, hypertension, diabetes, chronic renal disease and chronic liver disease as factors associated with mortality in the hospital setting; however, within these reviews, substantial heterogeneity was noted and their results were unadjusted [108–111]. With regards to ICU mortality, our meta-analysis of adjusted data for ICU mortality due to COVID-19 indicated that male gender and the presence of respiratory disease was associated with mortality. However, unadjusted meta-analyses showed associations with several comorbidities, such as COPD and renal disease – results also identified in a recent meta-analysis of unadjusted data on ICU patients which identified hypertension, COPD and cardiovascular disease as prognostic factors of mortality [112]. Whilst obesity has been identified in previous meta-analyses to be strongly associated with mortality [113], within our meta-analyses, the data available for obesity were heterogeneous due to different classifications used within each study. Obesity was associated with overall hospital mortality, supported however only by very low quality of evidence.
Hospital and ICU admission
Our meta-analysis focusing only on European data and evaluating ICU admission indicated that in age-/gender-adjusted analyses, asthma, neurological diseases and autoimmune diseases were associated with an increased likelihood of the need for ICU admission. Furthermore, the need for mechanical ventilation was higher among patients with asthma, liver disease and obesity. These results are different from other ICU cohort data, which may be attributable to our analyses being age-/gender-adjusted [114]; although previous research has also indicated that ICU outcomes may be better predicted by frailty than either age or comorbidity [115]. Notably, our analyses noted that several comorbidities associated with an increased risk of death or hospital admission were not typically associated with an increased incidence of ICU admission or mechanical ventilation. This is not unexpected since patients with pre-existing severe comorbidities may not be offered ICU admission or mechanical ventilation as therapeutic options during times of unprecedently high pressure on healthcare systems, leading to strict triage criteria [116, 117]. COVID-19, during the first wave of the pandemic, was managed heterogeneously worldwide, especially in resource-constrained countries, where the health systems often had less capacity and fewer resources to face the unfolding pandemic [118]. However, even in the selected high-income countries, ICUs were under unprecedented pressure and strict criteria for selecting patients were applied based on the patients' short-term prognosis [119].
The above results indicate populations of high risk for adverse outcomes and could possibly benefit from potential booster vaccinations as a preventive measure to reduce their elevated risk for adverse COVID-19 outcomes. According to the WHO statement in May 2022, a review of studies on an additional booster dose of mRNA vaccine, which were conducted during the worldwide predominant circulation of Omicron strain, showed benefits in health workers, those over 60 years of age or those with immunocompromising conditions [120]. Moreover, our analyses can further assist to inform planning of patient flows in healthcare settings. Notably, outcomes and prognostic factors in different settings were often different, a factor which may be attributable to several factors. It is possible that the fact that the majority of those contracting SARS-CoV-2 experience a mild or asymptomatic disease, and only a minority progresses to the development of pneumonitis, which may warrant hospital admission [121]. Another potential explanation may be the variability of the impact of different comorbidities on disease progression or clinician bias to the presence of specific comorbidities among patients triaged and on the threshold of hospital admission (e.g. a patient with COVID-19 and concomitant COPD is more likely to be admitted than a patient without COPD), indicating a type of selection bias by the clinicians during triage.
Strengths and limitations
Several strengths of our meta-analysis increase our confidence in our findings. The inclusion of only age-/gender-adjusted cohort study data allowed us to control for the primary factor associated with adverse outcomes, which is the patient's age, hence allowing us to assess the independent effect of each prognostic factor [107]. Moreover, we performed separate analyses by clinical setting that allowed us to quantify the incidence of adverse outcomes as separate end-points, which may assist in managing patient flows and triaging. As we applied the GRADE methodology for assessing the evidence about prognostic factors, we rigorously evaluated the certainty of the evidence behind each meta-analysis. Some limitations of our study should also be acknowledged as our results reflect the status quo in Europe during the first waves of the pandemic when the Alpha and Beta variants were the most dominant in the largely unvaccinated EU population, hence further research is needed to assess how subsequent vaccination impacts patient mortality. Furthermore, the research identified within this review reflects the Alpha and Beta variants and may not reflect necessarily the risk factors within the post-Omicron strain (B.1.1.529) context. We restricted our inclusion criteria to Europe to enhance the comparability of the results and utility for European clinicians and policymakers, as they have similar healthcare resources, acknowledging that our results may not be generalisable to other areas of the globe. However, similar results have been noted in different geographical areas [108]. Furthermore, there was evidence of heterogeneity as the definition of specific prognostic factors, such as hypertension, obesity and dyslipidaemia, were ambiguous in some of the included studies and variability in the definitions may have also contributed to the observed variability. Given that a direct comparison among different risk factors was not performed and similar effect sizes were found, no conclusions can be drawn on the comparative ranking or cumulative/synergistic effect of specific prognostic factors leading to COVID-19-related adverse outcomes. Nevertheless, conclusions on the individual impact of specific comorbidities can be drawn.
Points for clinical practice
In this systematic review and meta-analysis, only age-/gender-adjusted European cohort study data were used, stratified by clinical setting to control for the primary factor associated with adverse outcomes, which is the patient's age, hence allowing us to assess the independent effect of each prognostic factor. The results of this meta-analysis indicated that COPD, arrhythmia, IHD, heart failure, cancer, renal disease, liver disease, obesity and diabetes were also associated with hospital mortality in age-/gender-adjusted data, while male gender and respiratory diseases were associated with ICU mortality. Additionally, COPD, dyslipidaemia, hypertension, diabetes, cardiovascular disease, obesity, heart failure and male gender were associated with the composite outcome of death and/or ICU admission. This data could be of great significance for European policymakers in terms of prioritisation of preventive public health measures, such as potential booster vaccinations and use of personal NPIs, which could be further refined by accounting for underlining pre-existing conditions in the current as well as future epidemics.
Conclusion
The results of this systematic literature review and meta-analysis of European cohort age-/gender-adjusted data indicate that COPD, arrhythmia, IHD, heart failure, cancer, renal disease, liver disease, obesity and diabetes were associated with hospital mortality while male gender and respiratory diseases were associated with ICU mortality. COPD, dyslipidaemia, hypertension, diabetes, cardiovascular disease, obesity, heart failure and male gender were associated with the composite outcome of death and/or ICU admission. With regards to patient management, factors that were associated with hospital admission included solid organ transplant, sleep apnoea, cardiovascular disease, diabetes, heart failure, respiratory disease and male gender while obesity, asthma and male gender were associated with the need for mechanical ventilation. Taking the above into account, the prioritisation of preventive public health measures, such as potential booster vaccinations and use of personal NPIs, could be further refined by accounting for underlining pre-existing conditions as societies and healthcare systems across Europe continue to face pressure from COVID-19.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0098-2022.SUPPLEMENT (318.4KB, pdf)
Acknowledgements
We would like to thank Katerina Papathanasaki (University of Crete), Chrysa Chatzopoulou (University of Crete) and Konstantinos Skouloudakis (University of Crete) for their assistance in data archiving. We acknowledge the bravery and dedication of healthcare professionals everywhere during these most challenging times.
Provenance: Submitted article, peer reviewed.
Author contributions: C.I. Vardavas, J. Leonardi-Bee, R. Phalkey and J.E. Suk designed the study. K. Nikitara, D. Carnicer-Pont and A.G. Mathioudakis undertook the literature review and extracted the data. J. Leonardi-Bee and R. Phalkey developed the search code. A.G. Mathioudakis, J. Vestbo, K. Stamatelopoulos and K. Nikitara analysed and interpreted the data. E. Fernandez and G. Georgiopoulos participated in data evaluation and interpretation along with: C.I. Vardavas, J. Leonardi-Bee, R. Phalkey, J.E. Suk, K. Nikitara, K. Stamatelopoulos, and A.G. Mathioudakis. C.I. Vardavas wrote the first draft of the manuscript with input from all authors. J.C. Semenza, C. Deogan, P. Kramarz, F. Lamb and P. Penttinen along with all other authors reviewed and revised subsequent drafts.
Conflicts of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Support statement: This report was commissioned by the European Centre for Disease Prevention and Control (ECDC) to the PREP-EU Consortium, coordinated by C.I. Vardavas (School of Medicine, University of Crete) under specific contract No. 10 ECD.11843 within Framework contract ECDC/2019/001 Lot 1B. Role of the funding agency: the funder contributed to the definition of the scope of the review and commented on the protocol and draft manuscript and was not involved in the conduct of the systematic review. ECDC provided intellectual input throughout the project and arranged for an internal peer review of the final manuscript prior to submission. The lead author has full access to the data and has the responsibility for submission. A.G. Mathioudakis and J. Vestbo are supported by the NIHR Manchester Biomedical Research Centre (NIHR Manchester BRC). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.European Centre for Disease Prevention and Control . COVID-19 situation update worldwide. www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases Date last accessed: 2 December 2020. Date last updated: 21 June 2022.
- 2.European Centre for Disease Prevention and Control . Latest risk assessment: further spread and potential impact of the SARS-CoV-2 Omicron variant of concern in the EU/EEA, 27 January 2022. www.ecdc.europa.eu/en/current-risk-assessment-novel-coronavirus-situation Date last accessed: 28 January 2022.
- 3.Wolff D, Nee S, Hickey NS, et al. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection 2021; 49: 15–28. doi: 10.1007/s15010-020-01509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Huang DQ, Zou B, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol 2021; 93: 1449–1458. doi: 10.1002/jmv.26424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadehsani R, Alizadeh Sani Z, Behjati M, et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J Med Virol 2021; 93: 2307–2320. doi: 10.1002/jmv.26699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC COVID-19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. Morb Mort Wkly Rep 2020; 69: 382–386. doi: 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty AB, Harrison EM, Green CA, et al. Features of 20133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369: m1985. doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control . The European Surveillance System (TESSy). www.ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy Date last updated: 22 July 2022.
- 10.World Health Organization . Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern 2021. www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern Date last updated: 26 November 2021.
- 11.Ito N, Kitahara Y, Miwata K, et al. Can the Omicron variant of COVID-19 cause pneumonia in young patients without risk factors? Clin Case Rep 2022; 10: e05684. doi: 10.1002/ccr3.5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283: 2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14.Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med 2013; 6: 50–54. doi: 10.1111/jebm.12018 [DOI] [PubMed] [Google Scholar]
- 15.Ponziani FR, Del Zompo F, Nesci A, et al. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther 2020; 52: 1060–1068. doi: 10.1111/apt.15996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fresan U, Guevara M, Trobajo-Sanmartin C, et al. Hypertension and related comorbidities as potential risk factors for COVID-19 hospitalization and severity: a prospective population-based cohort study. J Clin Med 2021; 10: 1194. doi: 10.3390/jcm10061194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirim AB, Demir E, Yadigar S, et al. COVID-19 in chronic kidney disease: a retrospective, propensity score-matched cohort study. Int Urol Nephrol 2021; 53: 2117–2125. doi: 10.1007/s11255-021-02783-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020; 79: 1544–1549. doi: 10.1136/annrheumdis-2020-218296 [DOI] [PubMed] [Google Scholar]
- 19.Diez C, Del Romero-Raposo J, Mican R, et al. COVID-19 in hospitalized HIV-positive and HIV-negative patients: a matched study. HIV Med 2021; 22: 867–876. doi: 10.1111/hiv.13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blomaard LC, van der Linden CMJ, van der Bol JM, et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing 2021; 50: 631–640. doi: 10.1093/ageing/afab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biscarini S, Colaneri M, Ludovisi S, et al. The obesity paradox: analysis from the SMAtteo COvid-19 REgistry (SMACORE) cohort. Nutr Metab Cardiovasc Dis 2020; 30: 1920–1925. doi: 10.1016/j.numecd.2020.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fond G, Pauly V, Leone M, et al. Disparities in intensive care unit admission and mortality among patients with schizophrenia and COVID-19: a national cohort study. Schizophr Bull 2021; 47: 624–634. doi: 10.1093/schbul/sbaa158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galiero R, Pafundi PC, Simeon V, et al. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: findings from COVOCA study. PLoS One 2020; 15: e0243700. doi: 10.1371/journal.pone.0243700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabezon Villalba G, Amat-Santos IJ, Duenas C, et al. Impact of the presence of heart disease, cardiovascular medications and cardiac events on outcome in COVID-19. Cardiol J 2021; 28: 360–368. doi: 10.5603/CJ.a2021.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciardullo S, Zerbini F, Perra S, et al. Impact of diabetes on COVID-19-related in-hospital mortality: a retrospective study from Northern Italy. J Endocrinol Invest 2021; 44: 843–850. doi: 10.1007/s40618-020-01382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard A, Cottenet J, Bonniaud P, et al. Comparison of cancer patients to non-cancer patients among COVID-19 inpatients at a national level. Cancers 2021; 13: 1436. doi: 10.3390/cancers13061436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract 2020; 168: 108374. doi: 10.1016/j.diabres.2020.108374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGurnaghan SJ, Weir A, Bishop J, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol 2021; 9: 82–93. doi: 10.1016/S2213-8587(20)30405-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallet V, Beeker N, Bouam S, et al. Prognosis of French COVID-19 patients with chronic liver disease: a national retrospective cohort study for 2020. J Hepatol 2021; 75: 848–855. doi: 10.1016/j.jhep.2021.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.San Roman JA, Uribarri A, Amat-Santos IJ, et al. The presence of heart disease worsens prognosis in patients with COVID-19. Rev Esp Cardiol 2020; 73: 773–775. doi: 10.1016/j.recesp.2020.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra Veloz MF, Cordero Ruiz P, Rios-Villegas MJ, et al. Liver manifestations in COVID-19 and the influence of pre-existing liver disease in the course of the infection. Rev Esp Enferm Dig 2021; 113: 103–109. doi: 10.17235/reed.2020.7627/2020 [DOI] [PubMed] [Google Scholar]
- 32.Angeli F, Marazzato J, Verdecchia P, et al. Joint effect of heart failure and coronary artery disease on the risk of death during hospitalization for COVID-19. Eur J Intern Med 2021; 89: 81–86. doi: 10.1016/j.ejim.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavarot N, Gueguen J, Bonnet G, et al. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant 2021; 21: 1285–1294. doi: 10.1111/ajt.16416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belarte-Tornero LC, Valdivielso-More S, Vicente Elcano M, et al. Prognostic implications of chronic heart failure and utility of NT-proBNP levels in heart failure patients with SARS-CoV-2 infection. J Clin Med 2021; 10: 323. doi: 10.3390/jcm10020323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miarons M, Larrosa-Garcia M, Garcia-Garcia S, et al. COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation 2021; 105: 138–150. doi: 10.1097/TP.0000000000003460 [DOI] [PubMed] [Google Scholar]
- 36.Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis 2021; 73: e2095–ee106. doi: 10.1093/cid/ciaa1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarmiento-Monroy JC, Espinosa G, Londono MC, et al. A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. J Autoimmun 2021; 117: 102580. doi: 10.1016/j.jaut.2020.102580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Garcia-Ptacek S, Annetorp M, et al. Acute kidney injury and mortality risk in older adults with COVID-19. J Nephrol 2021; 34: 295–304. doi: 10.1007/s40620-021-01022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann-Podczaska A, Chojnicki M, Karbowski LM, et al. Clinical characteristics and survival analysis in a small sample of older COVID-19 patients with defined 60-day outcome. Int J Environ Res Public Health 2020; 17: 8362. doi: 10.3390/ijerph17228362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddaloni E, D'Onofrio L, Alessandri F, et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II). Cardiovasc Diabetol 2020; 19: 164. doi: 10.1186/s12933-020-01140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellan M, Patti G, Hayden E, et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patients. Sci Rep 2020; 10: 20731. doi: 10.1038/s41598-020-77698-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaeuffer C, Le Hyaric C, Fabacher T, et al. Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Euro Surveill 2020; 25: 2000895. doi: 10.2807/1560-7917.ES.2020.25.48.2000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutter W, Duceau B, Vignac M, et al. Association of diabetes and outcomes in patients with COVID-19: Propensity score-matched analyses from a French retrospective cohort. Diabetes Metab 2021; 47: 101222. doi: 10.1016/j.diabet.2020.101222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kjeldsen J, Nielsen J, Ellingsen T, et al. Outcome of COVID-19 in hospitalized patients with chronic inflammatory diseases. A population based national register study in Denmark. J Autoimmun 2021; 120: 102632. doi: 10.1016/j.jaut.2021.102632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czernichow S, Beeker N, Rives-Lange C, et al. Obesity doubles mortality in patients hospitalized for severe acute respiratory syndrome coronavirus 2 in Paris hospitals, France: a cohort study on 5,795 patients. Obesity 2020; 28: 2282–2289. doi: 10.1002/oby.23014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joharatnam-Hogan N, Hochhauser D, Shiu KK, et al. Outcomes of the 2019 novel coronavirus in patients with or without a history of cancer: a multi-centre North London experience. Ther Adv Med Oncol 2020; 12: 1758835920956803. doi: 10.1177/1758835920956803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Giorgi A, Fabbian F, Greco S, et al. Prediction of in-hospital mortality of patients with SARS-CoV-2 infection by comorbidity indexes: an Italian internal medicine single center study. Eur Rev Med Pharmacol Sci 2020; 24: 10258–10266. doi: 10.26355/eurrev_202010_23250 [DOI] [PubMed] [Google Scholar]
- 48.Siepmann T, Sedghi A, Barlinn J, et al. Association of history of cerebrovascular disease with severity of COVID-19. J Neurol 2021; 268: 773–784. doi: 10.1007/s00415-020-10121-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vena A, Giacobbe DR, Di Biagio A, et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect 2020; 26: 1537–1544. doi: 10.1016/j.cmi.2020.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Rosa FG, Palazzo A, Rosso T, et al. Risk factors for mortality in COVID-19 hospitalized patients in Piedmont, Italy: results from the multicenter, regional, CORACLE registry. J Clin Med 2021; 10: 1951. doi: 10.3390/jcm10091951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khawaja SA, Mohan P, Jabbour R, et al. COVID-19 and its impact on the cardiovascular system. Open Heart 2021; 8: e001472. doi: 10.1136/openhrt-2020-001472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galvez-Barron C, Arroyo-Huidobro M, Minarro A, et al. COVID-19: clinical presentation and prognostic factors of severe disease and mortality in the oldest-old population: a cohort study. Gerontology 2022: 30–43. doi: 10.1159/000515159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anastasiou OE, Korth J, Herbstreit F, et al. Mild versus severe liver injury in SARS-CoV-2 infection. Dig Dis 2020; 39: 52–57. doi: 10.1159/000510758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forlano R, Mullish BH, Mukherjee SK, et al. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One 2020; 15: e0240400. doi: 10.1371/journal.pone.0240400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Salameh A, Lanoix JP, Bennis Y, et al. Characteristics and outcomes of COVID-19 in hospitalized patients with and without diabetes. Diabetes Metab Res Rev 2021; 37: e3388. doi: 10.1002/dmrr.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varela Rodriguez C, Arias Horcajadas F, Martin-Arriscado Arroba C, et al. COVID-19-related neuropsychiatric symptoms in patients with alcohol abuse conditions during the SARS-CoV-2 pandemic: a retrospective cohort study using real world data from electronic health records of a tertiary hospital. Front Neurol 2021; 12: 630566. doi: 10.3389/fneur.2021.630566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orsucci D, Trezzi M, Anichini R, et al. Increased creatine kinase may predict a worse COVID-19 outcome. J Clin Med 2021; 10: 1734. doi: 10.3390/jcm10081734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanni G, Materazzo M, Dauri M, et al. Lymphocytes, interleukin 6 and d-dimer cannot predict clinical outcome in coronavirus cancer patients: LyNC1.20 study. Anticancer Res 2021; 41: 307–316. doi: 10.21873/anticanres.14777 [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Gonzalez CG, Chamorro-de-Vega E, Valerio M, et al. COVID-19 in hospitalised patients in Spain: a cohort study in Madrid. Int J Antimicrob Agents 2021; 57: 106249. doi: 10.1016/j.ijantimicag.2020.106249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linares L, Cofan F, Diekmann F, et al. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS One 2021; 16: e0247251. doi: 10.1371/journal.pone.0247251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rey JR, Caro-Codon J, Rosillo SO, et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail 2020; 22: 2205–2215. doi: 10.1002/ejhf.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanhems P, Gustin MP, Elias C, et al. Factors associated with admission to intensive care units in COVID-19 patients in Lyon-France. PLoS One 2021; 16: e0243709. doi: 10.1371/journal.pone.0243709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Vito A, Geremia N, Fiore V, et al. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia, Italy. Eur Rev Med Pharmacol Sci 2020; 24: 7861–7868. doi: 10.26355/eurrev_202007_22291 [DOI] [PubMed] [Google Scholar]
- 64.Cipriani A, Capone F, Donato F, et al. Cardiac injury and mortality in patients with coronavirus disease 2019 (COVID-19): insights from a mediation analysis. Intern Emerg Med 2021; 16: 419–427. doi: 10.1007/s11739-020-02495-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Salameh A, Lanoix JP, Bennis Y, et al. The association between body mass index class and coronavirus disease 2019 outcomes. Int J Obesity 2021; 45: 700–705. doi: 10.1038/s41366-020-00721-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomez-Belda AB, Fernandez-Garces M, Mateo-Sanchis E, et al. COVID-19 in older adults: what are the differences with younger patients? Geriatr Gerontol Int 2021; 21: 60–65. doi: 10.1111/ggi.14102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smati S, Tramunt B, Wargny M, et al. Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: results from the CORONADO study. Diabetes Obes Metab 2021; 23: 391–403. doi: 10.1111/dom.14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Livingston G, Rostamipour H, Gallagher P, et al. Prevalence, management, and outcomes of SARS-CoV-2 infections in older people and those with dementia in mental health wards in London, UK: a retrospective observational study. Lancet Psychiatry 2020; 7: 1054–1063. doi: 10.1016/S2215-0366(20)30434-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abadia Otero J, Briongos Figuero LS, Gabella Mattin M, et al. The nutritional status of the elderly patient infected with COVID-19: the forgotten risk factor? Curr Med Res Opin 2021; 37: 549–554. doi: 10.1080/03007995.2021.1882414 [DOI] [PubMed] [Google Scholar]
- 70.Marengoni A, Zucchelli A, Grande G, et al. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing 2020; 49: 923–926. doi: 10.1093/ageing/afaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welch C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing 2021; 50: 617–630. doi: 10.1093/ageing/afab026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.García-Guimaraes M, Mojón D, Calvo A, et al. Influence of cardiovascular disease and cardiovascular risk factors in COVID-19 patients. Data from a large prospective Spanish cohort. REC: CardioClinics 2021; 56: 108–117. doi: 10.1016/j.rccl.2020.11.004 [DOI] [Google Scholar]
- 73.Novelli L, Raimondi F, Ghirardi A, et al. At the peak of COVID-19 age and disease severity but not comorbidities are predictors of mortality: COVID-19 burden in Bergamo, Italy. Panminerva Med 2021; 63: 51–61. doi: 10.23736/S0031-0808.20.04063-X [DOI] [PubMed] [Google Scholar]
- 74.Bloom CI, Drake TM, Docherty AB, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med 2021; 9: 699–711. doi: 10.1016/S2213-2600(21)00013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonifazi M, Mei F, Skrami E, et al. Predictors of worse prognosis in young and middle-aged adults hospitalized with COVID-19 pneumonia: a multi-center Italian study (COVID-UNDER50). J Clin Med 2021; 10: PA3651. doi: 10.3390/jcm10061218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grandbastien M, Piotin A, Godet J, et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract 2020; 8: 2600–2607. doi: 10.1016/j.jaip.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolhe NV, Fluck RJ, Selby NM, et al. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med 2020; 17: e1003406. doi: 10.1371/journal.pmed.1003406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maquet J, Lafaurie M, Sommet A, et al. Thrombocytopenia is independently associated with poor outcome in patients hospitalized for COVID-19. Br J Haematol 2020; 190: e276–e279. doi: 10.1111/bjh.16950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spinoni EG, Mennuni M, Rognoni A, et al. Contribution of atrial fibrillation to in-hospital mortality in patients with COVID-19. Circ Arrhythm Electrophysiol 2021; 14: e009375. doi: 10.1161/CIRCEP.120.009375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hugo C, Stecher M, Dolff S, et al. Solid organ transplantation is not a risk factor for COVID-19 disease outcome. Transpl Int 2021; 34: 378–381. doi: 10.1111/tri.13795 [DOI] [PubMed] [Google Scholar]
- 81.Rottoli M, Bernante P, Belvedere A, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur J Endocrinol 2020; 183: 389–397. doi: 10.1530/EJE-20-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.García-Azorín D, Martinez-Pias E, Trigo J, et al. Neurological comorbidity is a predictor of death in Covid-19 disease: a cohort study on 576 patients. Front Neurol 2020; 11: 781. doi: 10.3389/fneur.2020.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moonen H, van Zanten FJL, Driessen L, et al. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: The BIAC-19 study. Clin Nutr 2021; 40: 2328–2336. doi: 10.1016/j.clnu.2020.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oetjens MT, Luo JZ, Chang A, et al. Electronic health record analysis identifies kidney disease as the leading risk factor for hospitalization in confirmed COVID-19 patients. PLoS One 2020; 15: e0242182. doi: 10.1371/journal.pone.0242182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Attauabi M, Seidelin JB, Felding OK, et al. Coronavirus disease 2019, immune-mediated inflammatory diseases and immunosuppressive therapies – a Danish population-based cohort study. J Autoimmun 2021; 118: 102613. doi: 10.1016/j.jaut.2021.102613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strausz S, Kiiskinen T, Broberg M, et al. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir Res 2021; 8: e000845. doi: 10.1136/bmjresp-2020-000845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Telle KE, Grosland M, Helgeland J, et al. Factors associated with hospitalization, invasive mechanical ventilation treatment and death among all confirmed COVID-19 cases in Norway: Prospective cohort study. Scand J Public Health 2021; 49: 41–47. doi: 10.1177/1403494820985172 [DOI] [PubMed] [Google Scholar]
- 88.Yordanov Y, Dinh A, Bleibtreu A, et al. Clinical characteristics and factors associated with hospital admission or death in 43103 adult outpatients with coronavirus disease 2019 managed with the Covidom telesurveillance solution: a prospective cohort study. Clin Microbiol Infect 2021; 27: 1158–1166. doi: 10.1016/j.cmi.2021.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamer M, Gale CR, Kivimaki M, et al. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci USA 2020; 117: 21011–21013. doi: 10.1073/pnas.2011086117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020; 4: 653–661. doi: 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poblador-Plou B, Carmona-Pírez J, Ioakeim-Skoufa I, et al. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID Study in Spain. Int J Environ Res Public Health 2020; 17: 5171. doi: 10.3390/ijerph17145171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deiana G, Azara A, Dettori M, et al. Deaths in SARS-Cov-2 positive patients in Italy: the influence of underlying health conditions on lethality. Int J Environ Resr Public Health 2020; 17: 4450. doi: 10.3390/ijerph17124450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bravi F, Flacco ME, Carradori T, et al. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin ii receptor blockers, in a sample of infected Italian citizens. PLoS One 2020; 15: e0235248. doi: 10.1371/journal.pone.0235248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siso-Almirall A, Kostov B, Mas-Heredia M, et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PLoS One 2020; 15: e0237960. doi: 10.1371/journal.pone.0237960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study. Am J Transplant 2021; 21: 2509–2521. doi: 10.1111/ajt.16428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Waldman M, Soler MJ, Garcia-Carro C, et al. Results from the IRoc-GN international registry of patients with COVID-19 and glomerular disease suggest close monitoring. Kidney Int 2021; 99: 227–237. doi: 10.1016/j.kint.2020.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parikh R, Garcia MA, Rajendran I, et al. ICU outcomes in Covid-19 patients with obesity. Ther Adv Respir Dis 2020; 14: 1753466620971146. doi: 10.1177/1753466620971146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pineiro GJ, Molina-Andujar A, Hermida E, et al. Severe acute kidney injury in critically ill COVID-19 patients. J Nephrol 2021; 34: 285–293. doi: 10.1007/s40620-020-00918-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lobo-Valbuena B, Garcis-Arias M, Pérez RB, et al. Characteristics of critical patients with COVID-19 in a Spanish second-level hospital. Med Intensiva 2021; 45: 56–58. doi: 10.1016/j.medin.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomson RJ, Hunter J, Dutton J, et al. Clinical characteristics and outcomes of critically ill patients with COVID-19 admitted to an intensive care unit in London: a prospective observational cohort study. PLoS One 2020; 15: e0243710. doi: 10.1371/journal.pone.0243710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020; 28: 1195–1199. doi: 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chew MS, Blixt PJ, Ahman R, et al. National outcomes and characteristics of patients admitted to Swedish intensive care units for COVID-19: a registry-based cohort study. Eur J Anaesthesiol 2021; 38: 335–343. doi: 10.1097/EJA.0000000000001459 [DOI] [PubMed] [Google Scholar]
- 103.Fresan U, Guevara M, Elia F, et al. Independent role of severe obesity as a risk factor for COVID-19 hospitalization: a Spanish population-based cohort study. Obesity 2021; 29: 29–37. doi: 10.1002/oby.23029 [DOI] [PubMed] [Google Scholar]
- 104.Pérez-López FR, Tajada M, Savirón-Cornudella R, et al. Coronavirus disease 2019 and gender-related mortality in European countries: a meta-analysis. Maturitas 2020; 141: 59–62. doi: 10.1016/j.maturitas.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open 2021; 11: e044640. doi: 10.1136/bmjopen-2020-044640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peckham H, De Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11: 6317. doi: 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Romero Starke K, Reissig D, Petereit-Haack G, et al. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Health 2021; 6: e006434. doi: 10.1136/bmjgh-2021-006434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One 2021; 16: e0247461. doi: 10.1371/journal.pone.0247461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol 2020; 92: 1875–1883. doi: 10.1002/jmv.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dorjee K, Kim H, Bonomo E, et al. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One 2020; 15: e0243191. doi: 10.1371/journal.pone.0243191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis 2021; 21: 855. doi: 10.1186/s12879-021-06536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang R, Elhusseiny KM, Yeh Y-C, et al. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes–a systematic review and meta-analysis. PLoS One 2021; 16: e0246318. doi: 10.1371/journal.pone.0246318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang Y, Lu Y, Huang YM, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism 2020; 113: 154378. doi: 10.1016/j.metabol.2020.154378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor EH, Marson EJ, Elhadi M, et al. Factors associated with mortality in patients with COVID-19 admitted to intensive care: a systematic review and meta-analysis. Anaesthesia 2021; 76: 1224–1232. doi: 10.1111/anae.15532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020; 5: e444–e451. doi: 10.1016/S2468-2667(20)30146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Truog RD, Mitchell C, Daley GQ. The toughest triage – allocating ventilators in a pandemic. N Engl J Med 2020; 382: 1973–1975. doi: 10.1056/NEJMp2005689 [DOI] [PubMed] [Google Scholar]
- 117.Gristina GR, Piccinni M. COVID-19 pandemic in ICU. Limited resources for many patients: approaches and criteria for triaging. Minerva Anestesiol 2021; 87: 1367–1379. doi: 10.23736/S0375-9393.21.15736-0 [DOI] [PubMed] [Google Scholar]
- 118.Liu X, Li X, Sun T, et al. East–West differences in clinical manifestations of COVID-19 patients: a systematic literature review and meta-analysis. J Med Virol 2021; 93: 2683–2693. doi: 10.1002/jmv.26737 [DOI] [PubMed] [Google Scholar]
- 119.Swiss Academy of Medical Sciences . COVID-19 pandemic: triage for intensive-care treatment under resource scarcity. Swiss Med Wkly 2020; 150: w20229. doi: 10.4414/smw.2020.20229 [DOI] [PubMed] [Google Scholar]
- 120.World Health Organization . Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additional-booster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19 Date last updated: 17 May 2022.
- 121.Polak SB, Van Gool IC, Cohen D, et al. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol 2020; 33: 2128–2138. doi: 10.1038/s41379-020-0603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0098-2022.SUPPLEMENT (318.4KB, pdf)