Abstract
Background
The impact of pulmonary embolism response teams (PERTs) on treatment choice and outcomes of patients with acute pulmonary embolism (PE) is still uncertain.
Objective
To determine the effect of PERTs in the management and outcomes of patients with PE.
Methods
PubMed, Embase, Web of Science, CINAHL, WorldWideScience and MedRxiv were searched for original articles reporting PERT patient outcomes from 2009. Data were analysed using a random effects model.
Results
16 studies comprising 3827 PERT patients and 3967 controls met inclusion criteria. The PERT group had more patients with intermediate and high-risk PE (66.2%) compared to the control group (48.5%). Meta-analysis demonstrated an increased risk of catheter-directed interventions, systemic thrombolysis and surgical embolectomy (odds ratio (OR) 2.10, 95% confidence interval (CI) 1.74–2.53; p<0.01), similar bleeding complications (OR 1.10, 95% CI 0.88–1.37) and decreased utilisation of inferior vena cava (IVC) filters (OR 0.71, 95% CI 0.58–0.88; p<0.01) in the PERT group. Furthermore, there was a nonsignificant trend towards decreased mortality (OR 0.87, 95% CI 0.71–1.07; p=0.19) with PERTs.
Conclusions
The PERT group showed an increased use of advanced therapies and a decreased utilisation of IVC filters. This was not associated with increased bleeding. Despite comprising more severe PE patients, there was a trend towards lower mortality in the PERT group.
Short abstract
Pulmonary embolism response teams increase use of advanced therapies for pulmonary embolism without increasing bleeding complications https://bit.ly/39NJZuw
Introduction
Acute pulmonary embolism (PE) is a significant cause of morbidity and is the third most common cause of death in hospitalised patients [1]. The therapies available for PE have grown substantially over the last decade, from anticoagulation or systemic thrombolysis, to interventions such as catheter-directed thrombolysis (CDT) [2], surgical and percutaneous thrombectomy [3], and extracorporeal membrane oxygenation (ECMO).
Deciding which subgroup of patients with PE may benefit from advanced reperfusion therapies continues to be an area of active investigation. Since PE patients represent a heterogeneous group, there cannot be a simple one size fits all reperfusion strategy. Given the lack of clinical trials and the variation in societal clinical guidelines [4], many institutions have implemented pulmonary embolism response teams (PERTs) to guide the management of these patients. These multidisciplinary PERTs are aimed to coordinate care and determine interventions for patients with acute PE. For this review, a PERT is defined as a multidisciplinary team with at least two specialties that provide a group consultation regarding PE cases and that refers to itself as a PERT in a publication.
Recently some studies about PERT outcomes have demonstrated benefits in mortality and time to anticoagulation [5], others have not been able to replicate these findings [6]. An assessment of PERTs’ impact on outcomes is necessary to understand their utility. To accomplish this, we conducted a systematic review and meta-analysis of PERT-related outcomes in PE patients.
Methods
Study design, selection criteria and search strategy
We followed the reporting checklist for systematic reviews of the PRISMA 2020 statement [7]. We included articles that reported PERT patient outcomes that had samples of ≥25 adult patients (age ≥18 years). We excluded non-English literature, PE patient outcomes without PERT involvement, secondary sources (e.g. editorials, opinion papers), abstracts and studies focused on the use of a specific device or therapy for the treatment of PE.
The review team (D.F.S., P.R., A.L.L., S.B.B.) and the medical librarian (S.R.) developed a detailed search strategies for each database to identify studies for this systemic review. The search was developed for PubMed (NLM) and was translated to Embase (Elsevier), Web of Science (Clarivate Analytics) and CINAHL (EbscoHost) using a combination of keywords and subject headings. A grey literature search included WorldWideScience and MedRxiv. The search was restricted to human studies from 2009 to the present. The final search was completed on 9 November 2020. The full search details are provided in the supplementary material. The search included results from: PubMed (420 results), Embase (748 results), Web of Science (503 results) and CINAHL (123 results). Given that the first PERT was established in 2012 and we wanted to include all potential literature, we extended our start date back to 2009 [8]. Duplicate studies were omitted using Endnote X7 and Rayyan. Studies were screened by title and abstract by two blinded and independent reviewers using Rayyan (P.R. and S.B.B.). In the case of a tie, a third reviewer served as the tiebreaker (D.F.S.). This process was repeated for full-text article screening and selection. The search strategy was registered in the Temple University Scholar database [9].
Outcomes, data collection and bias assessment
The purpose of our review is to determine if PERT-directed PE care has an impact on clinical outcomes and therapy selection. The outcomes of interest included therapeutic strategy (utilisation of catheter-directed interventions (CDIs), including CDT or catheter-directed embolectomy, systemic thrombolysis, inferior vena cava (IVC) filters, anticoagulation, ECMO, surgical embolectomy) as well as clinical outcomes (bleeding complications, mortality, length of stay (LOS) and readmission). We collected data regarding the composition of the PERT group, country, year of publication, duration of the study, and subtype of PE. If available, the same data were collected for the control group. All variables were obtained from the articles and appendices by D.F.S. and A.L.L. independently. If a particular variable was not reported in a study, that study was excluded for the analysis of that outcome. Comparison groups for the analysis were the PERT group and a control group. The PERT group included patients that were reported under PERT evaluation or PERT era. The control group included patients from the pre-PERT era or a comparison group as defined in each study. Group definitions were done to match the definitions in the individual studies. Group allocation and data were verified by D.F.S. and A.L.L.
Bias assessment was performed by A.L.L. and D.F.S. using the Institute of Health Economics (IHE) case series assessment [10] for and Newcastle Ottawa scale [11] when appropriate. All bias assessments were completed independently. A third independent reviewer (P.R.) was used for any disagreements. The details of the bias assessment are reported in the supplementary material.
Data analysis
Nominal variables are reported as frequency and percentage. Continuous variables are reported as mean and standard deviation (sd). The odds ratio (OR) (95% confidence interval (CI)) was calculated for each dichotomous variable and used in the comparison of the PERT and control groups. We conducted meta-analysis using a fixed-effects model and conducted statistical tests for each subgroup and the overall treatment effects between the PERT and control groups. Forest plots were elaborated as means of reporting the outcomes of the studies. We assessed heterogeneity between studies by estimation of the I2 statistic and by a formal statistical test to indicate statistically significant heterogeneity. We examined the publication bias based on a funnel plot with the regression-based Egger test for small-study effects. p-values of less than 0.05 were considered to indicate statistical significance. OR with 95% CI was reported when appropriate and if did not include 1 was considered statistically significant. All data analyses were performed using Stata 17.0 (StataCorp LLC., College Station, TX, USA).
Results
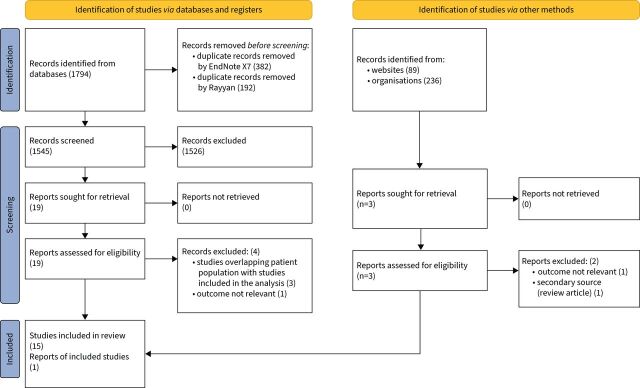
The searches yielded 2119 citations, 1794 from databases and 325 from grey literature search citations. After duplicates were removed, 1545 studies underwent title and abstract review, after which 22 studies remained [5, 6, 12–31]. Of these studies, six were excluded after reviewing the full-text manuscript. Three studies were excluded because of overlapping patients with other included cohorts, two for assessing outcomes that we were not analysing, and another had an alternative study design [26–31] (figure 1).
FIGURE 1.
PRISMA 2020 flow diagram of identified, screened and included manuscripts in the meta-analysis and systemic review. Modified from [7]; for more information visit http://prisma-statement.org/
Descriptive review and study characteristics
We included 16 studies [5, 6, 12–25] published between 2016 and 2020 and all were single-centre studies from academic institutions. They incorporated patients who were evaluated and treated from August 2012 to April 2020. The included studies, study design, definition of control cohort and the risk of bias assessment are shown in table 1.
TABLE 1.
Description of included studies and risk of bias assessment
| Study/year/n | PERT group number | Control group number | Location | Study design/control definition | Types of PE included in PERT | Risk of bias score |
| Kabrhel et al. 2016 [14] (n=314) | 314 | NA | USA | Case series/NA | All PE | 15; IHE# |
| Sista et al. 2017 [20] (n=87) | 87 | NA | USA | Case series/NA | All PE | 15; IHE |
| Mahar et al. 2018 [17] (n=118) | 118 | NA | USA | Case series/NA | All PE | 13; IHE |
| Chaudhury et al. 2019 [5] (n=769) | 426 | 343 | USA | Cohort/historical cohort | All PE | 7; Newcastle¶ |
| Jen et al. 2019 [13] (n=321) | 167 | 154 | Singapore | Cohort/historical cohort | All PE | 7; Newcastle |
| Wright et al. 2019 [24] (n=305) | 146 | 159 | USA | Cohort/historical cohort | Intermediate/high risk | 7; Newcastle |
| Xenos et al. 2019 [25] (n=1069) | 77 | 992 | USA | Cohort/historical cohort | All PE | 8; Newcastle |
| Annabathula et al. 2020 [12] (n=530) | 304 | 226 | USA | Cohort/historical cohort | All PE | 7; Newcastle |
| Carroll et al. 2020 [6] (n=2042) | 1158 | 884 | USA | Cohort/historical cohort | All PE | 9; Newcastle |
| Khaing et al. 2020 [15] (n=52) | 52 | NA | USA | Case series/NA | Intermediate/high risk | 17; IHE |
| Kwok et al. 2020 [16] (n=141) | 60 | 81 | USA | Cohort/no PERT activation | All PE | 7; Newcastle |
| Melamed et al. 2020 [21] (n=1105) | 411 | 694 | USA | Cohort/historical cohort with treatment algorithm | All PE | 6; Newcastle |
| Myc et al. 2020 [18] (n=554) | 120 | 434 | USA | Cohort/historical cohort and no PERT activation | All PE | 7; Newcastle |
| Romano et al. 2020 [19] (n=128) | 128 | NA | Canada | Case series/NA | Intermediate/high risk | 15; IHE |
| Sławek-Szmyt et al. 2020 [22] (n=80) | 80 | NA | Poland | Prospective case series | All PE | 15; IHE |
| Wiske et al. 2020 [23] (n=179) | 179 | NA | USA | Case series/NA | Intermediate/high risk | 13; IHE |
NA: not applicable; PE: pulmonary embolism; PERT: pulmonary embolism response team. #: Institute of Health Economics (IHE) for case series studies; maximum score is 20; higher score, less risk of bias and higher quality. ¶: Newcastle Ottawa for cohort studies; maximum score is 9; higher score, less risk of bias and higher quality.
The pooled sample of patients included a total of 7794 patients, of which 3827 (49.10%) corresponded to the PERT group and 3967 (50.89%) to the control group. The range of patients per study was 52–2042, with a mean of 487.13. The mean±sd age for the PERT group was 61.18±2.57 years and 49.73% were males. The average age of the control group was 60.89 years with sd ±1.81 years and 47.6% males.
In regard to the focus of the PERT group by PE severity, 75% focused on all severities [5, 6, 12–14, 16, 17, 19–22, 25], while the remaining 25% focused only on intermediate and high-risk PE [15, 18, 23, 24]. Severity of PE was inconsistently reported. From the reported numbers, the PERT group included more patients with intermediate or high-risk PE (66.2% versus 48.5%). The distribution of patients by PE severity is reported in table A in the supplementary material.
The composition of PERTs varied based on the study. Pulmonary and critical care medicine was universally present in all PERTs. Other specialties included interventional radiology (92%), cardiac surgery (92%), cardiology (85%) and haematology (23%). Of the 11 studies, that reported timing of PE consultation, 91% included a real-time PE consult [13–19, 21–23]. The rates of outcome reporting across the studies are listed in table 2. Mortality and bleeding complications were the most reported outcomes in the studies, 93.75% and 75%, respectively. Other reported outcomes including LOS (56.25%), assessment of intracerebral haemorrhage (43.75%), intensive care unit (ICU) LOS (37.5%) and readmission rates (18.75%) were inconsistently reported across the studies. All-cause mortality of the PERT group was 7.55% versus 9.22% in the control group (table 2).
TABLE 2.
Outcomes reported in the different studies
| Variable | PERT group | Control group | ||||
| Studies with outcome (n=16) | Number of patients | Number with outcome | Studies with outcome in control (n=9) | Number of patients | Number with outcome | |
| Mortality# | 15 | 3681 | 278 | 8 | 3808 | 351 |
| Bleeding complications | 12 | 2889 | 302 | 6 | 2122 | 184 |
| LOS | 9 | 2495 | NA | 6 | 3031 | NA |
| ICU LOS | 6 | 1098 | NA | 4 | 2066 | NA |
| Readmission | 3 | 1522 | 259 | 2 | 965 | 126 |
| ICH | 7 | 2235 | 27 | 2 | 1227 | 17 |
| IVC filter | 11 | 2689 | 308 | 5 | 1901 | 166 |
The table provides the total number of patients by variable and by group. Numbers will be higher than those used in figure 2, which includes only studies that reported the outcome in the pulmonary embolism response team (PERT) and control groups.
IVC: inferior vena cava; ICH: intracerebral haemorrhage; ICU: intensive care unit; LOS: length of stay; NA: not applicable.
#: Wright et al. [24] does not report mortality.
Risk of bias assessment
The Newcastle Ottawa scores ranged from 6 to 9 (maximum 9) for the nine cohort studies included in the analysis, with higher scores meaning lower risk of bias. Of these, 66.7% had a score of 8 or above and were considered low risk of bias [6, 12, 13, 18, 24, 25]. The IHE case series assessment scores ranged from 13 to 16 (maximum 20). Details of the scores can be found in the supplementary material.
Meta-analysis
There was a nonsignificant trend towards decreased in-hospital mortality in the PERT group compared to the control group (OR 0.87, 95% CI 0.71–1.07; p=0.19) (figure 2). There was also no difference in bleeding complications between the pre- and post-PERT cohorts (OR 1.10, 95% CI 0.88–1.37; p=0.39).
FIGURE 2.
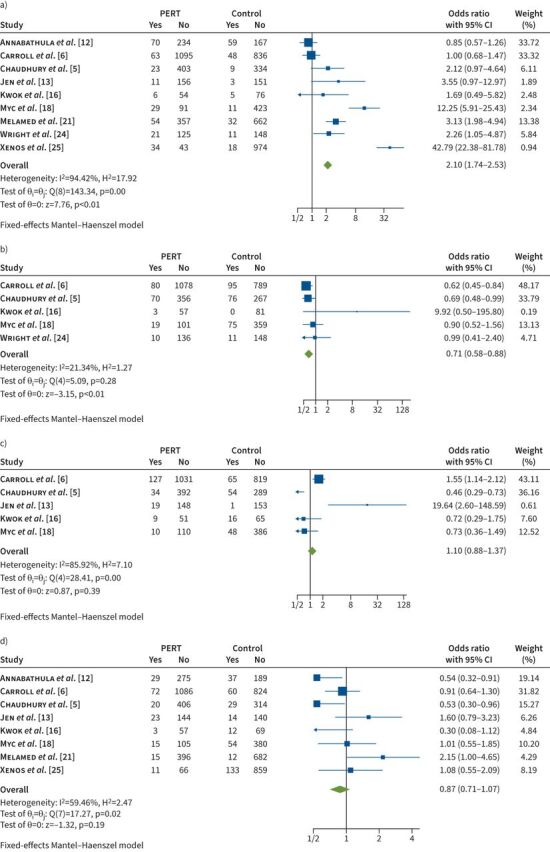
Forest plots for the pulmonary embolism response team (PERT) versus control groups. a) Advanced therapies (embolectomy, catheter-directed intervention and systemic thrombolysis). b) Inferior vena cava filter. c) Bleeding complications. d) Mortality.
Data comparing the PERT and control cohorts demonstrated more use of advanced treatment strategies in the PERT cohort. A combined outcome that included embolectomies (surgical and catheter-assisted) and thrombolysis strategies (systemic thrombolysis or CDT) had an OR 2.10 (95% CI 1.74–2.53; p<0.01) (figure 2). When analysing each of these individually, the same trend was maintained. There was a significant increase in the utilisation of CDT (OR 2.76, 95% CI 2.08–3.66) and catheter-assisted embolectomy (OR 2.38, 95% CI 1.10–5.17; p=0.03). There was no difference between groups in the use of systemic thrombolysis (OR 1.58, 95% CI 0.76–3.28; p=0.22) (table 3) or surgical embolectomy (OR 1.09, 95% CI 0.44–2.71; p=0.86). The utilisation of IVC filters (OR 0.71, 95% CI 0.58–0.88; p<0.01) was decreased in the PERT group (figure 2). The use of ECMO (OR 1.51, 95% CI 0.91–2.52; p=0.11) was similar between the two groups (table 3).
TABLE 3.
Advanced treatment strategies by group
| Treatment |
Total
(n=7794) |
PERT group
(n=3827) |
Control group
(n=3967) |
OR# | 95% CI | p-value |
| CD interventions | 390 (5%) | 314 (8%) | 94 (2.37%) | 2.63 | 2.00–3.45 | <0.01 |
| Systemic thrombolysis | 284 (3.6%) | 190 (5%) | 94 (2.37%) | 1.58 | 0.76–3.28 | 0.22 |
| ECMO | 62 (0.8%) | 43 (0.9%) | 36 (0.91%) | 1.51 | 0.91–2.52 | 0.11 |
| Embolectomy ¶ | 32 (0.41%) | 25 (0.63%) | 7 (0.2%) | 1.09 | 0.44–2.71 | 0.85 |
| IVC filter | 656 (7.25%) | 308 (8%) | 257 (6.47%) | 0.71 | 0.58–0.88 | <0.01 |
| Total + | 1343 (17.23%) | 890 (23.26%) | 440 (11.09%) |
CD: catheter-directed; ECMO: extracorporeal membrane oxygenation; IVC: inferior vena cava; OR: odds ratio; PERT: pulmonary embolism response team.
#: OR corresponds to the subset included in meta-analysis.
¶: excluding Annabathula et al. [12], which combines surgical and catheter-assisted embolectomies.
+: total interventions under PERT and control.
Discussion
Initially established 9 years ago [32], PERTs have been heavily adopted and are now active in over 100 institutions worldwide [33]. Multiple institutions have outlined their single-centre experiences following adoption of the PERT model. Our meta-analysis sought to aggregate the data, and by doing so, assess how the creation of PERTs has affected interventions and patient outcomes.
There was a significant increase in the use of utilisations of advanced therapies in the PERT cohort (figure 2). This finding likely has a multifactorial explanation. Since the PERT group was found to have more patients with high-risk and intermediate risk PE compared to the control group, this patient population is more likely to benefit from advanced PE interventions [34–36]. It is also worth noting that the rise of improved risk stratification tools and advanced interventions (implementation and availability) coincides temporally with the formation of a PERT. Therefore, working as a facilitator of PE care, multidisciplinary PERTs can increase the use of advance treatment strategies among patients who are in the higher risk categories. Despite the increased use of invasive PE therapies in the PERT cohort, institutional adoption of PERTs was associated with a significantly lower use of IVC filter placement. This may be because PERT members are more likely to be aware of the relatively low rates of IVC filter retrieval and the potential for IVC filter-related complications [37].
Even though the PERT group had patients with significantly higher PE severity compared with the control group, there was a trend towards lower mortality among PERT patients (OR 0.87, 95% CI 0.71–1.07; p=0.19). From the included studies, Chaudhury et al. [5] and Myc et al. [18] observed a mortality benefit with PERT implementation. Myc et al. [18] observed a lower hospital readmission rate for PERT group patients when compared to a historical cohort (control group), even though their cohort was more acutely ill. Chaudhury et al. [5] noted that the benefit in mortality was more significant in intermediate and high-risk PE. Given the reported data in the studies we do not have the ability to control for PE severity. However, while not proven, these data still leave the question open as to whether PERTs can affect mortality surrounding higher risk PEs.
Our pooled data (which included 12 of 16 studies) showed that bleeding rates were similar between the groups. This was observed despite the PERT group utilising advanced thrombolytic therapies and embolectomy therapies more often. Prior studies of CDT have reported more bleeding complications compared to anticoagulation alone [2], though fewer bleeding complications when compared to systemic thrombolysis [38]. Our finding of similar bleeding rates in the pre- and post-PERT era may be due to more use of CDI than systemic thrombolysis after PERT implementation. Future reports of PERT outcomes should report bleeding complications in greater detail.
We were unable to perform a meta-analysis on LOS given inconsistent reporting across studies. Nonetheless, descriptive analysis of individual studies demonstrated that PERT creation often resulted in decreased ICU and hospital LOSs. This is likely related to the inclusion of multiple subspecialists in the management of PE patients through PERTs. This may have led to more guideline-driven care and therefore shorter LOSs. There was relative consistency across the subspecialties included in PERTs. Pulmonary and critical care was part of all PERTs. Cardiology, interventional radiology and surgery comprised the next most represented specialties, which is consistent with prior studies [39]. Advanced therapies are often guided or performed by the listed disciplines and cross-institution variability may account for the differences in the team compositions.
Study limitations and future research consideration
There are limitations to our study. First, our meta-analysis relies on the standardised reporting of PERT composition, outcomes and complications. Our review found significant heterogeneity in the reporting of PERT utilisation, which is partially reflected in the high I2 in figure 2. PE therapies inherently increase bleeding risk, yet only 12 of the 16 included studies reported bleeding complications and the details included in the reports were highly variable. Many studies did not fully differentiate between major and minor bleeding complications in their cohort or report the rate of intracerebral haemorrhage, an important adverse event in prior studies of advanced therapies for PE. Furthermore, almost all included studies in our meta-analysis were single-centre retrospective series. Further publications of PERT data should focus on multi-centre prospective cohorts or clinical trials with standardised reports of team composition, choice of therapy, time to treatment, outcomes, and complications. The financial impacts of PERT implementation are also unclear. Another significant limitation of our study was the inclusion of low-risk PE in multiple studies. While the activation of a PERT for these patients may signal higher risk than captured by our study, these patients are traditionally at lower risk for mortality. The inclusion of these patients in the cohorts may have dampened the effect of the PERT on mortality or utilisation of advanced therapies as these patients are more likely to survive to hospital discharge and only receive anticoagulation. It is possible that given the rapidly advancing care for patients with PE, our observations could be linked to evolving practices over time rather that the implementation of a PERT itself. However, in this regard it can be argued that PERT implementation is part of evolving practices, particularly in the USA where most of the studies included in the study were done. Our findings show an association between PERTs and the outcomes mentioned. Future research may focus only on intermediate risk and high-risk PE to assess the true impact of PERTs on the subset of higher-risk PE. It is in this group that an advance therapy might be required and consultation with a multidisciplinary group could have the largest impact.
Lastly, since conducting our literature search and meta-analysis, an additional study has been published on the impact of PERTs on PE outcomes and treatment [40]. While we could not include this study in our analysis, the results demonstrated improved 6-month mortality in the PERT cohort, a trend towards more catheter-based procedures and no difference in major bleeding events, findings in line with our meta-analysis. Additionally, a prior publication from the same lead author and medical centre involved a significant portion of this cohort (188 patients of a total 231) and is included in our current meta-analysis [24].
Questions for future research
Currently, no randomised controlled trials exist to determine the impact of PERT implementation on outcomes in patients with PE, in particular the subset of patients with intermediate and high-risk PE, that we hypothesise benefit from PERTs. These studies might help elucidate the benefit of PERT implementation on mortality and other outcomes. Lastly, further studies on the financial impact of PERTs are necessary.
Conclusion
PERTs were associated with an increased use of advanced therapies (CDI and surgical embolectomy) without a significant increase in bleeding complications. PERTs were also associated with a decreased use of IVC filters. While our systemic analysis showed a trend towards lower mortality with PERT implementation, these results must be interpreted within the context of our inability to control for PE severity. In addition, given the nature of the studies that are the basis for this meta-analysis, the results are hypothesis-generating without proven causality. More thorough reporting of PERT outcomes will be necessary to monitor the impact of these teams on the outcomes of patients with PE, and ideally a randomised clinical trial should be performed to assess the impact of a PERT.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0023-2022.SUPPLEMENT (644.7KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: R. Bashir has patents planned, issued or pending with Thrombolex Inc., and stock or stock options in Thrombolex Inc., outside the submitted work. J. Horowitz has received consulting fees from Inari Medical and Penumbra, outside the submitted work. P. Rali has an unpaid leadership or fiduciary role in other board, society, committee or advocacy group: Chair, Protocol Committee National PERT consortium, outside the submitted work. All other authors have no conflicts to disclose.
References
- 1.Bikdeli B, Wang Y, Jimenez D, et al. Pulmonary embolism hospitalization, readmission, and mortality rates in US older adults, 1999–2015. JAMA 2019; 322: 574–576. doi: 10.1001/jama.2019.8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furfaro D, Stephens RS, Streiff MB, et al. Catheter-directed thrombolysis for intermediate-risk pulmonary embolism . Ann Am Thorac Soc 2018; 15: 134–144. doi: 10.1513/AnnalsATS.201706-467FR [DOI] [PubMed] [Google Scholar]
- 3.Lee T, Itagaki S, Chiang YP, et al. Survival and recurrence after acute pulmonary embolism treated with pulmonary embolectomy or thrombolysis in New York State, 1999 to 2013. J Thorac Cardiovasc Surg 2018; 155: 1084–1090.e12. doi: 10.1016/j.jtcvs.2017.07.074 [DOI] [PubMed] [Google Scholar]
- 4.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020; 41: 543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 5.Chaudhury P, Gadre SK, Schneider E, et al. Impact of multidisciplinary pulmonary embolism response team availability on management and outcomes. Am J Cardiol 2019; 124: 1465–1469. doi: 10.1016/j.amjcard.2019.07.043 [DOI] [PubMed] [Google Scholar]
- 6.Carroll BJ, Beyer SE, Mehegan T, et al. Changes in care for acute pulmonary embolism through a multidisciplinary pulmonary embolism response team. Am J Med 2020; 133: 1313–1321.e6. doi: 10.1016/j.amjmed.2020.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosovsky R, Zhao K, Sista A, et al. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost 2019; 3: 315–330. doi: 10.1002/rth2.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth S, Parth R, Brosnahan S, et al. Search strategies for a systematic review of PERT based outcomes for patients with pulmonary embolism. Temple University Health Sciences Libraries, Systematic Review Service, 2020. 10.34944/dspace/4472doi: 10.34944/dspace/4472 [DOI] [Google Scholar]
- 10.Institute of Heath Economics . IHE quality appraisal checklist for case series studies. Date last updated: 3 March 2016. www.ihe.ca/advanced-search/ihe-quality-appraisal-checklist-for-case-series-studies
- 11.Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa scale. World J Meta-Analysis 2017; 5: 80–84. doi: 10.13105/wjma.v5.i4.80 [DOI] [Google Scholar]
- 12.Annabathula R, Dugan A, Bhalla V, et al. Value-based assessment of implementing a Pulmonary Embolism Response Team (PERT). J Thromb Thrombolysis 2021; 51: 217–225. doi: 10.1007/s11239-020-02188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jen WY, Kristanto W, Teo L, et al. Assessing the impact of a pulmonary embolism response team and treatment protocol on patients presenting with acute pulmonary embolism . Heart Lung Circ 2019; 29: 345–353. doi: 10.1016/j.hlc.2019.02.190 [DOI] [PubMed] [Google Scholar]
- 14.Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team (PERT): initial 30-month experience with a novel approach to delivery of care to patients with sub-massive and massive PE. Acad Emerg Med 2016; 23: S244. [DOI] [PubMed] [Google Scholar]
- 15.Khaing P, Paruchuri A, Eisenbrey JR, et al. First year experience of a pulmonary embolism response team with comparisons of outcomes between catheter directed therapy versus standard anticoagulation. Hosp Pract 2020; 48: 23–28. doi: 10.1080/21548331.2020.1706315 [DOI] [PubMed] [Google Scholar]
- 16.Kwok B, Brosnahan SB, Amoroso NE, et al. Pulmonary embolism response team activation during the COVID-19 pandemic in a New York City academic hospital: a retrospective cohort analysis. J Thromb Thrombolysis 2021; 51: 330–338. doi: 10.1007/s11239-020-02264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahar JH, Haddadin I, Sadana D,et al. A pulmonary embolism response team (PERT) approach: initial experience from the Cleveland Clinic. J Thromb Thrombolysis 2018; 46: 186–192. doi: 10.1007/s11239-018-1686-2 [DOI] [PubMed] [Google Scholar]
- 18.Myc LA, Solanki JN, Barros AJ, et al. Adoption of a dedicated multidisciplinary team is associated with improved survival in acute pulmonary embolism. Respir Res 2020; 21: 159. doi: 10.1186/s12931-020-01422-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romano KR, Cory JM, Ronco JJ, et al. Vancouver General Hospital Pulmonary Embolism Response Team (VGH PERT): initial three-year experience. Can J Anaesth 2020; 67: 1806–1813. doi: 10.1007/s12630-020-01790-6 [DOI] [PubMed] [Google Scholar]
- 20.Sista AK, Friedman OA, Dou E, et al. A pulmonary embolism response team's initial 20 month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med 2018; 23: 65–71. doi: 10.1177/1358863X17730430 [DOI] [PubMed] [Google Scholar]
- 21.Melamed R, St. Hill CA, Engstrom BI,et al. Effects of a consensus-based pulmonary embolism treatment algorithm and response team on treatment modality choices, outcomes, and complications. Clin Appl Thrombosis/Hemostasis 2020; 26: 1076029620928420. doi: 10.1177/1076029620928420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sławek-Szmyt S, Jankiewicz S, Smukowska-Gorynia A, et al. Implementation of a regional multidisciplinary pulmonary embolism response team: PERT -POZ initial 1-year experience. Polish Heart J 2020; 78: 300–310. doi: 10.33963/KP.15230 [DOI] [PubMed] [Google Scholar]
- 23.Wiske CP, Shen C, Amoroso N, et al. Evaluating time to treatment and in-hospital outcomes of pulmonary embolism response teams. J Vasc Surg Venous Lymphat Disord 2020; 8: 717–724. doi: 10.1016/j.jvsv.2019.12.077 [DOI] [PubMed] [Google Scholar]
- 24.Wright C, Elbadawi A, Chen YL, et al. The impact of a pulmonary embolism response team on the efficiency of patient care in the emergency department. J Thromb Thrombolysis 2019; 48: 331–335. doi: 10.1007/s11239-019-01875-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xenos ES, Davis GA, He Q, et al. The implementation of a pulmonary embolism response team in the management of intermediate- or high-risk pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2019; 7: 493–500. doi: 10.1016/j.jvsv.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 26.Carroll BJ, Pemberton H, Bauer KA, et al. Initiation of a multidisciplinary, rapid response team to massive and submassive pulmonary embolism. Am J Cardiol 2017; 120: 1393–1398. doi: 10.1016/j.amjcard.2017.07.033 [DOI] [PubMed] [Google Scholar]
- 27.Deadmon EK, Giordano NJ, Rosenfield K, et al. Comparison of emergency department patients to inpatients receiving a pulmonary embolism response team (PERT) activation. Acad Emerg Med 2017; 24: 814–821. doi: 10.1111/acem.13199 [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Nie SP, Wang X, et al. Role of pulmonary embolism response team in patients with intermediate- and high-risk pulmonary embolism: a concise review and preliminary experience from China. J Geriatr Cardiol 2020; 17: 510–518. doi: 10.11909/j.issn.1671-5411.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roik M, Wretowski D, Łabyk A, et al. Initial experience of pulmonary embolism response team with percutaneous embolectomy in intermediate-high- and high-risk acute pulmonary embolism. Kardiol Pol 2018; 77: 228–231. doi: 10.5603/KP.a2018.0239 [DOI] [PubMed] [Google Scholar]
- 30.Rosovsky R, Chang Y, Rosenfield K, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis 2019; 47: 31–40. doi: 10.1007/s11239-018-1737-8 [DOI] [PubMed] [Google Scholar]
- 31.Schultz J, Giordano N, Zheng H, et al. EXPRESS: a multidisciplinary pulmonary embolism response team (PERT) – experience from a national multicenter consortium. Pulm Circ 2019; 9: 2045894018824563. doi: 10.1177/2045894018824563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract 2014; 42: 31–37. doi: 10.3810/hp.2014.02.1089 [DOI] [PubMed] [Google Scholar]
- 33.Rivera-Lebron B, McDaniel M, Ahrar K, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT consortium. Clin Appl Thromb Hemost 2019; 25: 1076029619853037. doi: 10.1177/1076029619853037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129: 479–486. doi: 10.1161/CIRCULATIONAHA.113.005544 [DOI] [PubMed] [Google Scholar]
- 35.Pei DT, Liu J, Yaqoob M, et al. Meta-analysis of catheter directed ultrasound-assisted thrombolysis in pulmonary embolism. Am J Cardiol 2019; 124: 1470–1477. doi: 10.1016/j.amjcard.2019.07.040 [DOI] [PubMed] [Google Scholar]
- 36.Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015; 8: 1382–1392. doi: 10.1016/j.jcin.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 37.Marron RM, Rali P, Hountras P, et al. Inferior vena cava filters: past, present, and future . Chest 2020; 158: 2579–2589. doi: 10.1016/j.chest.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 38.Beyer SE, Shanafelt C, Pinto DS, et al. Utilization and outcomes of thrombolytic therapy for acute pulmonary embolism: a nationwide cohort study. Chest 2020; 157: 645–653. doi: 10.1016/j.chest.2019.10.049 [DOI] [PubMed] [Google Scholar]
- 39.Barnes GD, Kabrhel C, Courtney DM, et al. Diversity in the pulmonary embolism response team model: an organizational survey of the national PERT consortium members. Chest 2016; 150: 1414–1417. doi: 10.1016/j.chest.2016.09.034 [DOI] [PubMed] [Google Scholar]
- 40.Wright C, Goldenberg I, Schleede S, et al. Effect of a multidisciplinary pulmonary embolism response team on patient mortality. Am J Cardiol 2021; 161: 102–107. doi: 10.1016/j.amjcard.2021.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0023-2022.SUPPLEMENT (644.7KB, pdf)