Abstract
Background
Functional incomplete revascularisation (IR) is associated with a higher risk of major adverse cardiac events (MACE) during long-term follow-up in patients with ST-segment elevation myocardial infarction (STEMI) undergoing percutaneous coronary intervention (PCI).
Aims
This study aimed to investigate the prognostic ability of quantitative flow ratio (QFR)-guided residual functional SYNTAX score (Q-rFSS) and functional IR in STEMI patients undergoing PCI.
Methods
In total, 354 consecutive STEMI patients who successfully underwent PCI were included. Q-rFSS was defined as residual SYNTAX score (rSS) measured only in vessels with QFR ≤0.8. The primary outcome was MACE (a composite of all-cause mortality, myocardial infarction, and ischaemia-driven revascularisation) at 2 years.
Results
At two-year follow-up, functional IR (Q-rFSS ≥1) showed significantly higher risk for MACE than functional complete revascularisation (CR) (Q-rFSS=0) (functional IR vs CR, 22.0% vs 7.4%; hazard ratio [HR] 3.21; 95% confidence interval [Cl]: 1.74 to 5.91; p<0.001). The area under the curve (AUC) of Q-rFSS (0.738, 95% CI: 0.659 to 0.817) was significantly greater than that of rSS (0.648, 95% CI: 0.547 to 0.749). The C-statistic for MACE also increased after the addition of Q-rFSS to the clinical risk factors. Q-rFSS significantly improved risk classification compared with rSS (net reclassification improvement 0.439, 95% CI: 0.201 to 0.548; p<0.001).
Conclusions
Functional IR is associated with higher risk of MACE during long-term follow-up in STEMI patients undergoing PCI. Q-rFSS has a better prognostic ability for the risk of MACE.
Introduction
In patients presenting with ST-segment elevation myocardial infarction (STEMI), about 50% have multivessel disease1. Patients with angiographically incomplete revascularisation (IR) have been reported to have worse clinical outcomes than those with complete revascularisation (CR)2,3. However, CR is often limited when treating complex lesions with a potentially higher risk for occurrence of complications and is not always achievable in STEMI patients. The strategy of performing percutaneous coronary intervention (PCI) in non-infarct-related coronary artery lesions remains controversial.
It is important to know whether reasonable IR would contribute to poor prognosis and confirm the acceptable degree of IR in real-world clinical practice. In previous trials, the decision to perform PCI for these non-infarct-related lesions was based mainly on angiographic characteristics, regardless of whether the lesions were causing ischaemia3,4. The residual SYNTAX score (rSS) was introduced to quantify the degree of residual stenosis by recalculating the SYNTAX score (SS)5,6. However, the discrepancy between anatomic lesion severity and coronary physiology by means of fractional flow reserve (FFR) is well established7,8. Thus, the concept of the functional SS recalculating the SS after counting only ischaemia-producing lesions with FFR ≤0.8 was developed. Functional SS was proved to have a better prognostic value compared with classic SS9.
To expand the use of physiological lesion evaluation further, quantitative flow ratio (QFR), a reliable and fast approach for calculation of functional parameters to detect haemodynamically significant lesions based on three-dimensional quantitative coronary angiography, could be an attractive solution for daily clinical practice. Previous studies have reported that QFR has good agreement with FFR measurements10,11,12.
The aim of this study was to determine whether QFR-guided residual functional SYNTAX score (Q-rFSS), defined as a recalculated SS counting only ischaemia-related lesions assessed by QFR ≤0.8, is a better predictor of two-year clinical outcomes in STEMI patients undergoing PCI.
Methods
STUDY POPULATION AND STUDY DESIGN
This was a retrospective analysis of consecutive STEMI patients prospectively enrolled who had undergone PCI at Tongji Hospital, Tongji University, Shanghai, China, in the period from 2014 to 2016. STEMI was diagnosed if a patient had chest pain for >30 minutes and <24 hours, and persistent ST-segment elevation >2 mm in at least two contiguous precordial electrocardiography leads or >1 mm in at least two contiguous limb electrocardiography leads or a newly developed left bundle branch block. Exclusion criteria included significant left main disease, previous coronary artery bypass graft (CABG) surgery, cardiogenic shock and those who underwent planned CABG. All patients were managed according to usual practice. Angiography views were preceded by administration of intracoronary nitrate.
After successful treatment of the culprit lesion in the infarct-related artery (defined as a Thrombolysis In Myocardial Infarction [TIMI] flow of 3 and residual stenosis <30%) with drug-eluting stents, the decision for PCI to lesions located in the non-infarct-related arteries was at the discretion of the operators. Images from staged procedures were used for QFR computation if second procedures were performed. The study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board or ethics committee approved the study protocol, and all patients provided written informed consent before enrolment.
OFF-LINE QFR ASSESSMENT
Details of the off-line QFR assessment are described in Supplementary Appendix 110,11,12.
CALCULATION OF THE Q-rFSS AND rSS
The Q-rFSS and rSS were calculated from post-procedural angiograms by two interventional cardiologists who were blinded to other information, including patient characteristics, therapies, and clinical outcomes. Each coronary lesion producing 50% diameter stenosis in vessels >1.5 mm by visual estimation was scored separately using the SS score algorithm from its website, and individual scores were added to provide the overall rSS. The classic SS takes into account the presence of lesions in very small vessels (>1.5 mm), which are almost always functionally insignificant and in which the benefit of revascularisation is uncertain. Thus, when we calculated the Q-rFSS value, we simplified this score and only took into account the lesions in vessels >2.0 mm. The Q-rFSS was defined as modified rSS (diameter stenosis >50% in vessels >2.0 mm) measured only in lesions with QFR ≤0.8.
DATA COLLECTION AND FOLLOW-UP
All data were prospectively collected and entered into a central database. Clinical data were obtained at outpatient clinic visits or by telephone contact. An independent clinical events committee, whose members were unaware of clinical, angiographic, and physiologic data, adjudicated all events. Clinical follow-up information about the endpoints was obtained after 24 months (interquartile range, 19 to 26). Follow-up was completed in all patients. The primary endpoint was major adverse cardiac events (MACE), defined as a composite of all-cause mortality, myocardial infarction, or ischaemia-driven revascularisation. Elective revascularisations performed within 45 days after the primary intervention were not counted as events. All clinical outcomes were defined according to the Academic Research Consortium. Ischaemia-driven revascularisation was defined as a revascularisation procedure with at least one of the following: (i) recurrence of angina, (ii) positive non-invasive test, and (iii) positive invasive physiologic test.
Results
PATIENT CHARACTERISTICS
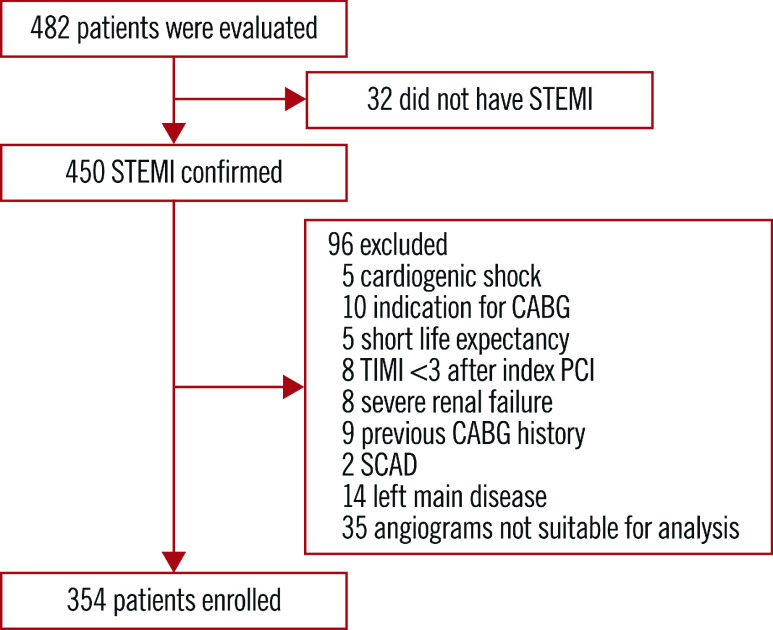
The flow of this study is shown in Figure 1. Three hundred and fifty-four consecutive STEMI patients (median age 63 years) undergoing PCI were enrolled in our study between 2014 and 2016. According to the Q-rFSS value, 204 patients (57.6%) were classified into the functional CR group (Q-rFSS=0) and 150 patients (42.4%) were classified into the functional IR group (Q-rFSS ≥1). The baseline characteristics of the study population are summarised in Supplementary Table 1. Patients with functional IR were older and had a higher rate of Killip class >1, a higher prevalence of three-vessel disease and non-infarct-related artery >70%, a lower prevalence of two-vessel disease, a lower total number of implanted stents and lower estimated glomerular filtration rate (eGFR) value compared with functional CR.
Figure 1.
Study inclusion flow chart. CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention; SCAD: spontaneous coronary artery dissection; STEMI: ST-segment elevation myocardial infarction
Planned staged procedures were performed in 35 patients during index admission or after discharge within one month. We calculated the QFR values of 52 non-culprit lesions available from index and staged angiography. The correlation between QFR values at index and staged procedures was r=0.98 (Supplementary Figure 1). Bland-Altman plot analysis showed a mean difference of −0.003 (−0.045 to 0.040) (Supplementary Figure 2).
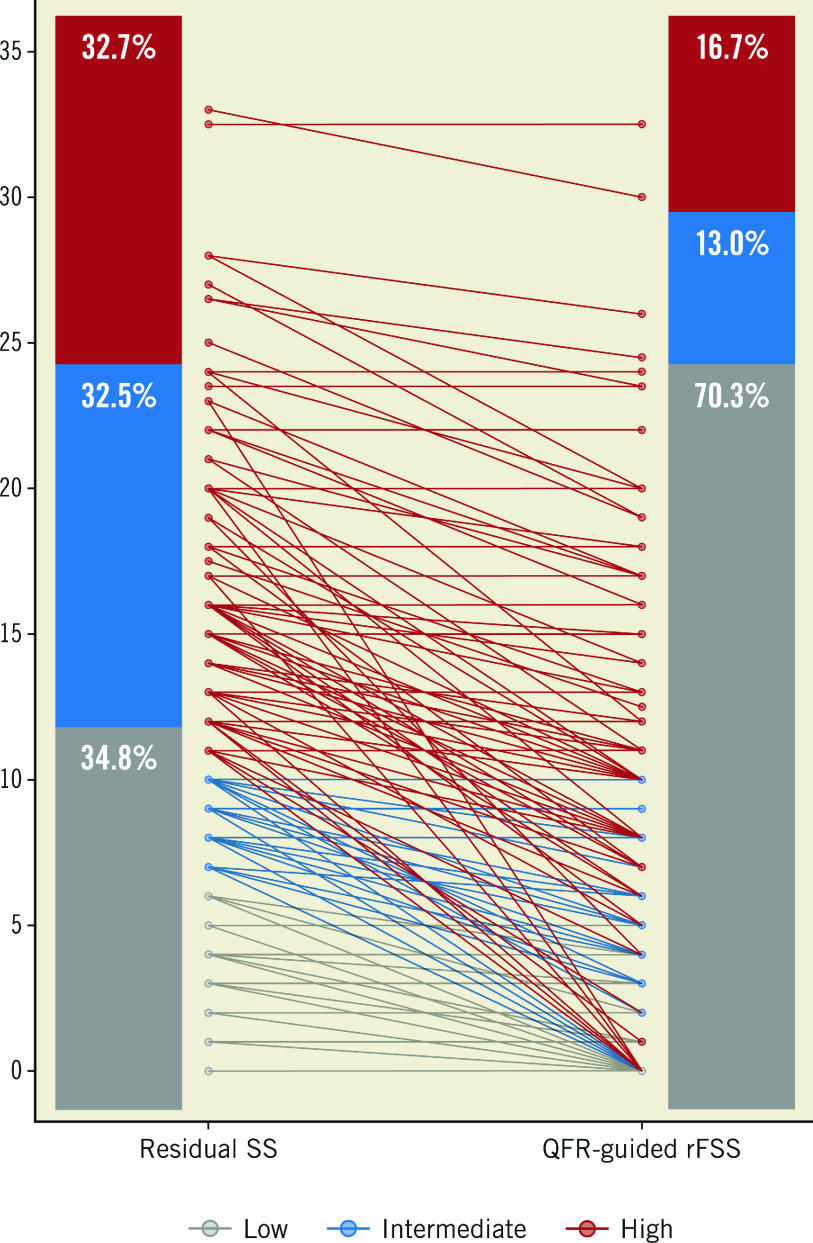
Three hundred and fifty-four patients were divided into tertiles of risk based on the rSS, namely low-risk (<7), intermediate-risk (7 to 11) and high-risk (>11) groups (34.8%, n=123; 32.5%, n=115; and 32.7%, n=116, respectively). After calculation of Q-rFSS, 35.5% of patients were reclassified from the high- or intermediate-risk group into the low-risk group (Figure 2).
Figure 2.
Reclassification by QFR-guided residual functional SYNTAX score. QFR: quantitative flow ratio; rFSS: residual functional SYNTAX score
CLINICAL OUTCOMES
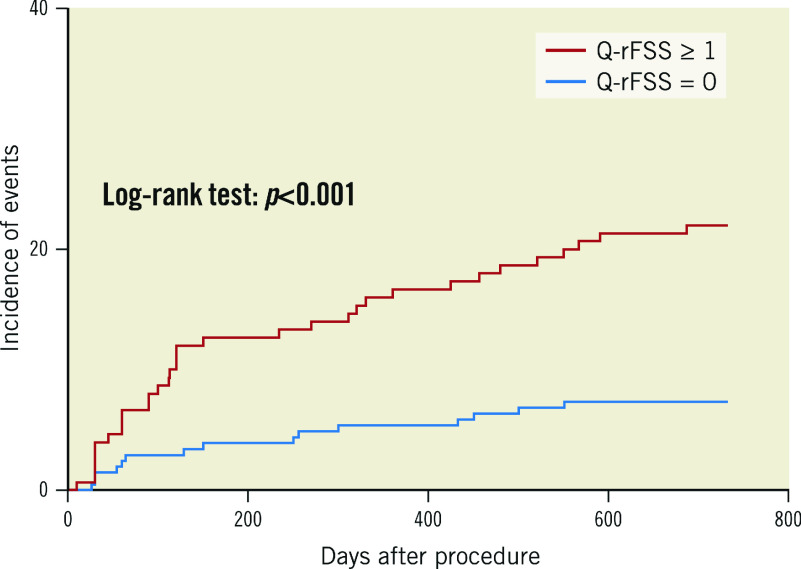
During a median follow-up of 24 months, 48 patients (13.6%) reached the combined endpoints of MACE. Compared with the functional CR group, the functional IR group showed a higher incidence of MACE (22% vs 7.4%; hazard ratio [HR] 3.21, 95% CI: 1.74 to 5.91; p<0.001), non-infarct-related ischaemia-driven revascularisation (18% vs 3.9%; HR 4.97, 95% CI: 2.26 to 9.7; p<0.001) and ischaemia-driven revascularisation (19.3% vs 4.4%; HR 4.74, 95% CI: 2.24 to 9.9; p<0.001) (Supplementary Table 2). The log-rank test based on the Kaplan-Meier curves showed a significant association between functional IR and MACE (p<0.001) (Figure 3).
Figure 3.
Kaplan-Meier curves of major adverse cardiac events (MACE) at two-year follow-up according to quantitative flow ratio-guided residual functional SYNTAX score (Q-rFSS) value.
Survival analysis using the Cox regression model showed that, after adjusted variables, Q-rFSS was an independent predictor of two-year MACE (HR 1.092, 95% CI: 1.054 to 1.113; p<0.001) (Table 1). Compared with the model consisting of conventional risk factors, the C-statistic for MACE increased from 0.656 (0.582 to 0.729) to 0.767 (0.626 to 0.778) after the addition of Q-rFSS value (Supplementary Table 3). Category-free reclassification analysis was used as described by Pencina et al16. Q-rFSS significantly improved risk classification compared with rSS (net reclassification improvement [NRI] 0.439, 95% CI: 0.201 to 0.548; p<0.001). The extended model with further inclusion of Q-rFSS value could significantly improve NRI. After inclusion of Q-rFSS to the reference model, integrated discrimination improvement (IDI) for MACE (IDI 0.102, 95% CI: 0.038 to 0.169; p<0.001) also improved significantly (Supplementary Table 3).
Table 1. Multivariable Cox regression analyses for MACE (n=48).
| HR (95% Cl) | p-value | |
| Age | 1.011 (0.985-1.038) | 0.40 |
| Previous MI | 2.742 (1.138-5.780) | 0.03 |
| Anterior MI | 1.935 (1.074-3.567) | 0.03 |
| Peak TnI | 1.009 (0.999-1.020) | 0.07 |
| 3-vessel disease | 1.681 (0.892-3.331) | 0.11 |
| Q-rFSS | 1.092 (1.054-1.113) | <0.001 |
| Models after LASSO variable selection procedure. CI: confidence interval; HR: hazard ratio; MACE: major adverse cardiac events; MI: myocardial infarction; Q-rFSS: quantitative flow ratio-guided residual functional SYNTAX score; SYNTAX: Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery; TnI: troponin I | ||
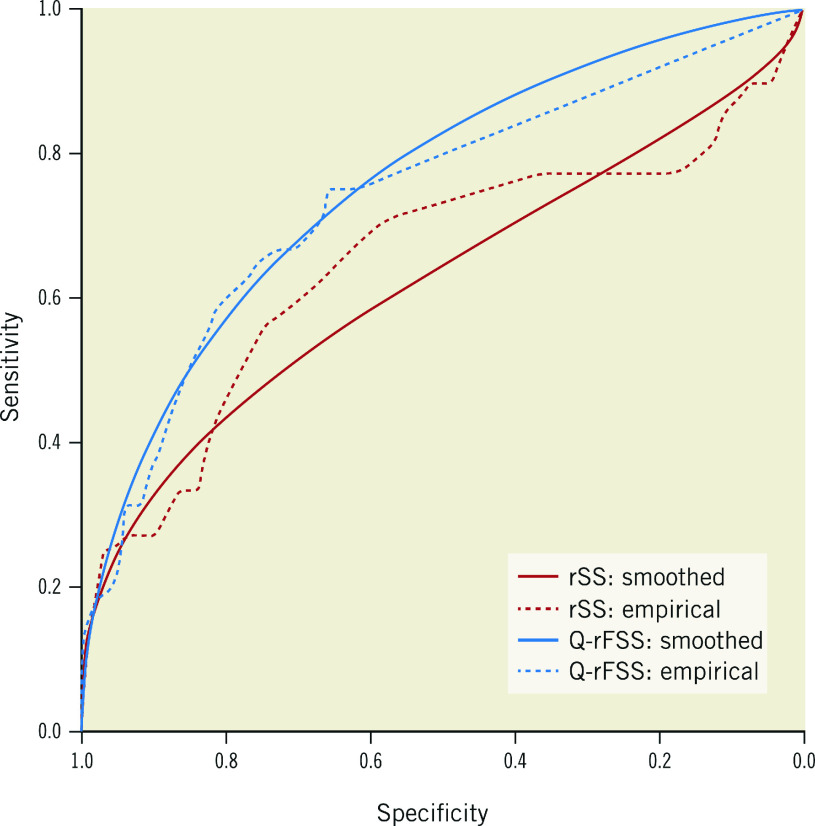
Receiver operating characteristic (ROC) curves were plotted for the Q-rFSS and rSS. The area under the curve (AUC) of Q-rFSS was significantly greater than that of anatomical rSS (AUC 0.738, 95% CI: 0.659 to 0.817 vs AUC 0.648, 95% CI: 0.547 to 0.749; p<0.001) (Figure 4). The AUC of Q-rFSS added to clinical factors (AUC 0.798, 95% CI: 0.732 to 0.864) was also significantly higher than AUC of rSS added to clinical factors (AUC 0.720, 95% CI: 0.636 to 0.805) and clinical factors alone (AUC 0.671, 95% CI: 0.591 to 0.750) (Supplementary Figure 3).
Figure 4.
Receiver operating curves for discrimination of MACE. The blue line is Q-rFSS (AUC 0.738, 95% CI: 0.659 to 0.817); the red line is rSS (AUC 0.648, 95% CI: 0.547 to 0.749). AUC: area under the curve; CI: confidence interval; MACE: major adverse cardiac events. Q-rFSS: quantitative flow ratio-guided residual functional SYNTAX score; rSS: residual SYNTAX score
SUBGROUP AND SENSITIVITY ANALYSIS
During the follow-up period, higher Q-rFSS levels were consistently associated with higher risks of MACE in various subpopulations. There was no significant interaction in the risk of MACE among pre-specified subgroups (all p-values for interaction >0.05) (Supplementary Figure 4).
Discussion
This present study is the first to evaluate the prognostic role of QFR-guided residual functional SYNTAX score in patients with STEMI after successful PCI. The principal findings of the present study are as follows: 1) the MACE rate was significantly higher in patients with functional IR than in those with functional CR; 2) a progressive Q-rFSS was shown to be a surrogate marker of increasing clinical outcomes in a multivariate-adjusted model; and 3) when Q-rFSS and rSS were added to clinical factors, the model with Q-rFSS showed higher discrimination ability for MACE.
Previous major studies have demonstrated that IR was associated with higher risk of adverse clinical outcomes and confirmed the prognostic clinical impact of IR17,18. They mainly used anatomical features as diagnostic criteria of CR and the definitions were various in those studies. The SS is the most accepted objective computational tool to grade the anatomic complexity of coronary artery disease and improve clinical outcome by established evidence-based guidelines for determining the most appropriate revascularisation strategy19,20. Although the rSS, which is a marker of the residual ischaemia burden, was introduced to predict outcomes, rSS also just concerned the anatomic severity. Large randomised studies have proved that FFR is superior to angiographic assessment for the detection of haemodynamically important coronary obstruction and that coronary revascularisation improves clinical outcomes using FFR guidance21. Performing PCI to a functionally non-significant coronary lesion has been proved to be of no benefit to patients, either from a prognostic or from a symptomatic point of view22. For this reason, this study focused on the prognostic role of functional IR. In our study, functional IR determined by QFR was associated with a higher risk of MACE compared with functional CR up to two years and could provide important prognostic information for STEMI patients after PCI, in line with previous studies.
FFR-guided PCI in multivessel disease was associated with improved long-term clinical outcomes compared with PCI guided by angiography in the FAME study23. Nam et al9 first presented the concept of the functional SYNTAX score to estimate the functional severity of lesions, incorporating both the anatomic and functional significance of lesions in pre-PCI evaluation. Kobayashi et al24 have demonstrated that residual angiographic disease is not associated with subsequent ischaemic events in patients with acute coronary syndrome (ACS) based on the rSS after CR of functionally significant stenosis determined by FFR. Furthermore, the improved discriminant ability of the residual functional SS guided by FFR for clinical outcomes was identified compared with anatomic assessment alone25. These observations are in line with earlier non-invasive studies by single-photon emission computed tomography (SPECT) in large numbers of patients, which indicated that the most important prognostic factor in patients with coronary artery disease is the presence and extent of inducible ischaemia26. PCI with the guidance of functional examination such as FFR seems to be more reasonable than with the anatomic characteristics.
Two large randomised trials demonstrated the superiority of FFR-guided revascularisation of non-culprit lesions performed in the early phase of STEMI when compared with culprit lesion PCI alone27,28. However, the clinical use of FFR is still infrequent in the real-world setting, especially in STEMI patients. There are many reasons for this, including equipment and drug costs, physician preferences, and the risk of related complications. Progress in angiography-derived FFR such as QFR can reduce these limitations by calculation of functional parameters in a simple and rapid way. Recently, a study including 110 STEMI patients has demonstrated that the QFR computation of non-culprit lesions is feasible in the STEMI setting and found that functional IR identified by QFR was associated with adverse clinical outcomes in the long-term follow-up29. However, the prognostic ability was not further studied in this study. A post hoc substudy of the SYNTAX II trial has also demonstrated the feasibility of measuring and calculating a QFR-based functional SS in predicting the clinical outcomes of CAD patients30. Our study expanded on identified clinical potentials and findings of the application of this scoring system and investigated the prognostic ability of rSS combined with functional assessment guided by QFR in STEMI patients.
Another potential limitation of the classic SYNTAX score is that it takes into account the presence of lesions in very small vessels (1.5 mm), which are almost always functionally insignificant and in which the benefit of revascularisation is uncertain. Therefore, it is inappropriate to adopt the scoring system with limited applicability in the acute setting such as performing PCI in STEMI patients. To make the scoring system more feasible for real-world clinical practice, we modified the score by taking into account the presence of lesions in vessels whose diameters were >2 mm. We found an improved discriminant capability of the Q-rFSS for clinical outcome in comparison with anatomic rSS. Our results demonstrate that Q-rFSS, guided by a safer and quicker method combining both anatomic and functional information on the residual disease burden, can predict better the risk of STEMI patients after PCI than anatomic assessments alone. The favourable outcomes of STEMI patients who had untreated non-culprit lesions in the functional CR group support the idea that deferral of treatment of non-ischaemia-producing lesions is safe.
Limitations
This study has several limitations. First, the study population was relatively small. Our study was not powered to detect differences in low-frequency events, such as death, reinfarction and other adverse clinical events. Second, the study was not a randomised study, and the decision for non-infarcted artery revascularisation at index procedure was left to the operator’s discretion. Therefore, the optimal treatment strategy for patients with functional IR could not be evaluated. Third, patients were not randomised to IR and CR. Therefore, we had to risk-adjust the data to take into account the fact that IR patients tended to be older and had more severe comorbidities than CR patients. Fourth, since patients with left main disease and patients scheduled for CABG were excluded from the present analysis, our findings cannot be extrapolated to these patients.
Conclusions
Functional IR is associated with a higher risk of MACE during long-term follow-up in STEMI patients undergoing PCI. Q-rFSS has a better prognostic ability for the risk of adverse clinical outcomes in STEMI patients after PCI.
Impact on daily practice
This present study is the first to evaluate the prognostic role of QFR-guided residual functional SYNTAX score and IR in patients with STEMI after successful PCI. Functional IR and rFSS guided by QFR could be fast and feasible risk stratification systems for daily clinical practice treating non-infarct-related coronary artery lesions in STEMI patients. Validation of this concept is warranted in order to find the optimal revascularisation strategy for STEMI patients with multivessel disease using QFR.
Supplementary data
Off-line QFR assessment.
Statistical analysis.
Correlation between QFR values at index and staged procedures.
Bland-Altman plot of QFR values at index and staged procedures.
Comparison of predictive models with rSS, and Q-rFSS in addition to clinical model.
Hazard ratio and 95% confidence interval of Q-rFSS value for major adverse cardiac events in subgroup analyses.
Demographics and baseline clinical characteristics between the Q-rFSS subgroups.
Clinical outcomes.
Discrimination and reclassification performance of the addition of rSS and Q-rFSS in predicting MACE based on C-statistics, NRI, and IDI.
Acknowledgments
Funding
This study was supported by a grant from Shanghai Science and Technology Committee (No.18411950300).
Conflict of interest statement
S. Tu has received research support from Medis Medical Imaging and Pulse Medical Imaging. X.B. Liu reports grants from Shanghai Science and Technology Committee during the conduct of the study. The other authors have no conflicts of interest to declare.
Abbreviations
- ACS
acute coronary syndrome
- AUC
area under the curve
- CI
confidence interval
- CR
complete revascularisation
- FFR
fractional flow reserve
- IDI
integrated discrimination improvement
- IR
incomplete revascularisation
- MACE
major adverse cardiac events
- NRI
net reclassification improvement
- PCI
percutaneous coronary intervention
- Q-rFSS
quantitative flow ratio-guided residual functional SYNTAX score
- QFR
quantitative flow ratio
- ROC
receiver operating characteristic
- rSS
residual SYNTAX score
- SPECT
single-photon emission computed tomography
- STEMI
ST-segment elevation myocardial infarction
Contributor Information
Jiani Tang, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Yan Lai, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Shengxian Tu, Biomedical Instrument Institute, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China.
Fei Chen, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Yian Yao, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Zi Ye, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Jianyun Gu, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Yanhua Gao, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Chunyu Guan, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Jiapeng Chu, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
Cheng Yang, Department of Cardiac Surgery, Zhongshan hospital, Fudan University, Shanghai, China.
Xuebo Liu, Department of Cardiology, Tongji Hospital, Tongji University, Shanghai, China.
References
- Kelbaek H, Terkelsen CJ, Helqvist S, Lassen JF, Clemmensen P, Kløvgaard L, Kaltoft A, Engstrøm T, Bøtker HE, Saunamäki K, Krusell LR, Jørgensen E, Hansen HH, Christiansen EH, Ravkilde J, Køber L, Kofoed KF, Thuesen L. Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention: the drug elution and distal protection in ST-elevation myocardial infarction (DEDICATION) trial. J Am Coll Cardiol. 2008;51:899–905. doi: 10.1016/j.jacc.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Garcia S, Sandoval Y, Roukoz H, Adabag S, Canoniero M, Yannopoulos D, Brilakis ES. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: A meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62:1421–31. doi: 10.1016/j.jacc.2013.05.033. [DOI] [PubMed] [Google Scholar]
- Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–72. doi: 10.1016/j.jacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–23. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- Farooq V, Serruys PW, Bourantas CV, Zhang Y, Muramatsu T, Feldman T, Holmes DR, Mack M, Morice MC, Stahle E, Colombo A, de Vries T, Morel MA, Dawkins KD, Kappetein AP, Mohr FW. Quantification of incomplete revascularization and its association with five-year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation. 2013;128:141–51. doi: 10.1161/CIRCULATIONAHA.113.001803. [DOI] [PubMed] [Google Scholar]
- Farooq V, Girasis C, Magro M, Onuma Y, Morel MA, Heo JH, Garcia-Garcia H, Kappetein AP, van den Brand M, Holmes DR, Mack M, Feldman T, Colombo A, Stahle E, James S, Carrié D, Fournial G, van Es GA, Dawkins KD, Mohr FW, Morice MC, Serruys PW. The CABG SYNTAX Score - an angiographic tool to grade the complexity of coronary disease following coronary artery bypass graft surgery: from the SYNTAX Left Main Angiographic (SYNTAX-LE MANS) substudy. EuroIntervention. 2013;8:1277–85. doi: 10.4244/EIJV8I11A196. [DOI] [PubMed] [Google Scholar]
- Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, MacCarthy PA, Van’t Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–21. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yamagishi M, Ueno T, Hara K, Ishiwata S, Itoh T, Hamanaka I, Wakatsuki T, Sugano T, Kawai K, Akasaka T, Tanaka N, Kimura T. Prevalence of visual-functional mismatch regarding coronary artery stenosis in the CVIT-DEFER registry. Cardiovasc Interv Ther. 2014;29:300–8. doi: 10.1007/s12928-014-0259-3. [DOI] [PubMed] [Google Scholar]
- Nam CW, Mangiacapra F, Entjes R, Chung IS, Sels JW, Tonino PA, De Bruyne B, Pijls NH, Fearon WF FAME Study Investigators. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011;58:1211–8. doi: 10.1016/j.jacc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, Nef H, Tebaldi M, Murasato Y, Lansky A, Barbato E, van der Heijden LC, Reiber JH, Holm NR, Wijns W FAVOR Pilot Trial Study Group. Diagnostic Accuracy of Fast Computational Approach to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc Interv. 2016;19:2024–35. doi: 10.1016/j.jcin.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, Guo L, Sun Z, Li Z, Tian F, Fang W, Chen J, Li W, Guan C, Holm NR, Wijns W, Hu S. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio for Online Assessment of Coronary Stenosis. J Am Coll Cardiol. 2017;70:3077–87. doi: 10.1016/j.jacc.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Westra J, Andersen BK, Campo G, Matsuo H, Koltowski L, Eftekhari A, Liu T, Di Serafino L, Di Girolamo D, Escaned J, Nef H, Naber C, Barbierato M, Tu S, Neghabat O, Madsen M, Tebaldi M, Tanigaki T, Kochman J, Somi S, Esposito G, Mercone G, Mejia-Renteria H, Ronco F, Bøtker HE, Wijns W, Christiansen EH, Holm NR. Diagnostic Performance of In-Procedure Angiography-Derived Quantitative Flow Reserve Compared to Pressure-Derived Fractional Flow Reserve: The FAVOR II Europe-Japan Study. J Am Heart Assoc. 18;7(14) doi: 10.1161/JAHA.118.009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–95. doi: 10.1002/(SICI)1097-0258(19970228)16:4<385::AID-SIM380>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. doi: 10.1093/biomet/80.1.27. [DOI] [Google Scholar]
- Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–17. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan EL, Racz M, Holmes DR, King SB, Walford G, Ambrose JA, Sharma S, Katz S, Clark LT, Jones RH. Impact of completeness of percutaneous coronary intervention revascularization on long-term outcomes in the stent era. Circulation. 2006;113:2406–12. doi: 10.1161/CIRCULATIONAHA.106.612267. [DOI] [PubMed] [Google Scholar]
- Wu C, Dyer AM, King SB 3rd, Walford G, Holmes DR Jr, Stamato NJ, Venditti FJ, Sharma SK, Fergus I, Jacobs AK, Hannan EL. Impact of incomplete revascularization on long-term mortality after coronary stenting. Circ Cardiovasc Interv. 2011;4:413–21. doi: 10.1161/CIRCINTERVENTIONS.111.963058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capodanno D. Risk stratification for percutaneous coronary intervention. Interv Cardiol Clin. 2016;5:249–57. doi: 10.1016/j.iccl.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–42. doi: 10.1161/01.CIR.92.8.2333. [DOI] [PubMed] [Google Scholar]
- Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–8. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, Van’t Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, De Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–11. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, Van’t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B FAME Study Investigators. Fractional flow reserve versus angiography for guiding PCI in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–84. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Nam CW, Tonino PA, Kimura T, De Bruyne B, Pijls NH, Fearon WF FAME Study Investigators. The Prognostic Value of Residual Coronary Stenosis After Functionally Complete Revascularization. J Am Coll Cardiol. 2016;67:1701–11. doi: 10.1016/j.jacc.2016.01.056. [DOI] [PubMed] [Google Scholar]
- Choi KH, Lee JM, Koo BK, Nam CW, Shin ES, Doh JH, Rhee TM, Hwang D, Park J, Zhang J, Kim KJ, Hu X, Wang J, Ye F, Chen S, Yang J, Chen J, Tanaka N, Yokoi H, Matsuo H, Takashima H, Shiono Y, Akasaka T. Prognostic Implication of Functional Incomplete Revascularization and Residual Functional SYNTAX Score in Patients With Coronary Artery Disease. JACC Cardiovasc Interv. 2018;11:237–45. doi: 10.1016/j.jcin.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Shaw LS, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11:171–85. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aarøe J, Jensen SE, Raungaard B, Køber L DANAMI-3—PRIMULTI Investigators. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–71. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angerås O, Richardt G, Omerovic E Compare-Acute Investigators. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376:1234–44. doi: 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- Spitaleri G, Tebaldi M, Biscaglia S, Westra J, Brugaletta S, Erriquez A, Passarini G, Brieda A, Leone AM, Picchi A, Ielasi A, Girolamo DD, Trani C, Ferrari R, Reiber JHC, Valgimigli M, Sabate M, Campo G. Quantitative Flow Ratio Identifies Nonculprit Coronary Lesions Requiring Revascularization in Patients With ST-Segment-Elevation Myocardial Infarction and Multivessel Disease. Circ Cardiovasc Interv. 2018;11:e006023. doi: 10.1161/CIRCINTERVENTIONS.117.006023. [DOI] [PubMed] [Google Scholar]
- Asano T, Katagiri Y, Chang CC, Kogame N, Chichareon P, Takahashi K, Modolo R, Tenekecioglu E, Collet C, Jonker H, Appleby C, Zaman A, van Mieghem N, Uren N, Zueco J, Piek JJ, Reiber JHC, Farooq V, Escaned J, Banning AP, Serruys PW, Onuma Y. Angiography-Derived Fractional Flow Reserve in the SYNTAX II Trial: Feasibility, Diagnostic Performance of Quantitative Flow Ratio, and Clinical Prognostic Value of Functional SYNTAX Score Derived From Quantitative Flow Ratio in Patients With 3-Vessel Disease. JACC Cardiovasc Interv. 2019;12:259–70. doi: 10.1016/j.jcin.2018.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Off-line QFR assessment.
Statistical analysis.
Correlation between QFR values at index and staged procedures.
Bland-Altman plot of QFR values at index and staged procedures.
Comparison of predictive models with rSS, and Q-rFSS in addition to clinical model.
Hazard ratio and 95% confidence interval of Q-rFSS value for major adverse cardiac events in subgroup analyses.
Demographics and baseline clinical characteristics between the Q-rFSS subgroups.
Clinical outcomes.
Discrimination and reclassification performance of the addition of rSS and Q-rFSS in predicting MACE based on C-statistics, NRI, and IDI.