Abstract
Aims
Reduced coronary flow velocity reserve (CFVR) is associated with adverse cardiovascular outcomes. Whether CFVR and coronary blood flow (CBF) are similar in men and women with chest pain and non-obstructive CAD remains unknown. We hypothesised sex differences in CFVR and CBF.
Methods and results
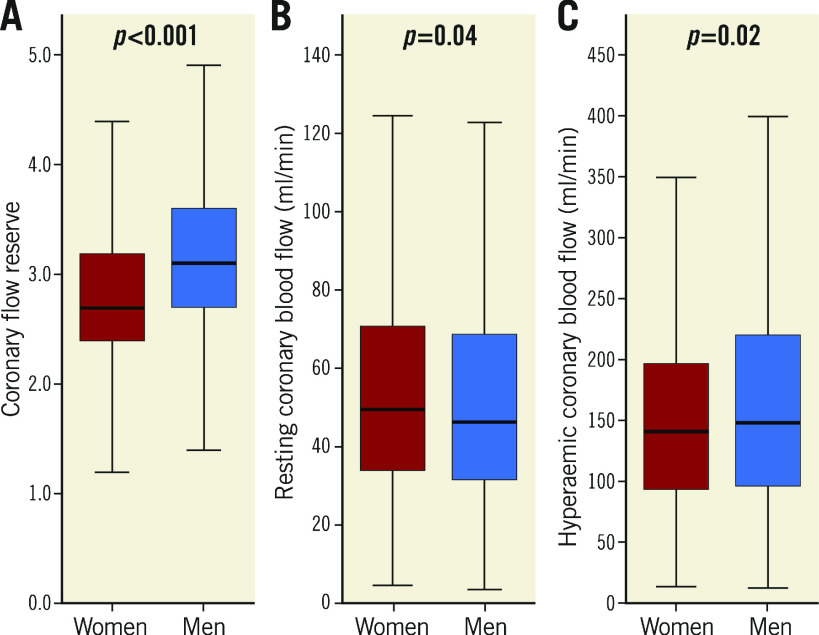
A total of 1,683 patients with signs/symptoms of ischaemia and angiographically unobstructed coronary arteries (<40% angiographic stenosis) underwent coronary vasomotion evaluation. CFVR was measured as hyperaemic/resting average velocity in the LAD. Mid-LAD diameter was measured with quantitative angiography and CBF calculated at rest (rCBF) and hyperaemia (hCBF). Resting microvascular resistance (rMR) was calculated as mean arterial pressure/rCBF. Of the total number of patients, 1,096 (65%) were women, median age 51 [42, 59] years. Compared to men, women had lower median CFVR (2.7 [2.4, 3.2] vs 3.1 [2.7, 3.6], p<0.001), higher rCBF (49.7 [34.0, 71.1] vs 45.9 [31.8, 68.7] ml/min, p=0.04), lower hCBF (139.5 [93.0, 195.2] vs 147.1 [95.7, 218.6] ml/min, p=0.02), but similar rMR (p=0.82). Female sex was an independent predictor of lower CFVR, higher rCBF, and lower hCBF.
Conclusions
Compared to men, women with signs/symptoms of ischaemia and non-obstructive CAD have lower CFVR, higher rCBF, and lower hCBF. Female sex is a predictor of these sex-specific differences. The clinical diagnostic and prognostic implications of sex differences in coronary physiology need further evaluation.
Introduction
The prevalence of non-obstructive coronary artery disease (CAD) in patients undergoing coronary angiography for chest pain is 30-50%1,2. Women with angina are more likely to have non-obstructive CAD on coronary angiography as compared to symptomatic men1,3. More than 60% of patients with non-obstructive CAD have coronary microvascular dysfunction (CMD) on functional invasive coronary testing4. CMD has been associated with angina and increased risk of major cardiovascular events (MACE), including acute coronary syndrome (ACS), myocardial infarction (MI), progressive congestive heart failure, and sudden cardiac death5,6,7,8.
Endothelium-independent CMD is characterised by impaired augmentation of coronary blood flow (CBF) in response to hyperaemia induced by intravenous or intracoronary adenosine infusion. In the absence of obstructive epicardial CAD, coronary flow velocity reserve (CFVR), the ratio of hyperaemic/basal average peak blood velocity, is used for contemporary diagnosis of CMD9. While abnormal CFVR and CMD diagnosis are associated with traditional cardiovascular risk factors10,11,12,13,14,15,16, CFVR remains an independent predictor of mortality and MACE5,17,18,19.
In current practice, a uniform cut-off value for CFVR (either <2.0 or <2.5) is used to diagnose CMD in both men and women9,20. This is based on the assumption that resting coronary blood flow (rCBF) and hyperaemic coronary blood flow (hCBF) are similar in men and women. However, our group previously reported lower CFVR in women21. Similarly, Kobayashi et al22 reported lower invasive CFVR values but suggested similar mean hyperaemic transit time in women as compared to men presenting with angina and non-obstructive CAD. We hypothesised that women, as compared to men, have higher rCBF and lower hCBF, accounting for the lower CFVR observed in women. We also hypothesised that female sex is an independent predictor of rCBF, hCBF and CFVR.
Methods
One thousand six hundred and eighty-three (1,683) consecutive patients with chest pain referred for clinically indicated coronary angiography and coronary vasomotion evaluation, between 1992 and 2015, and who were found to have angiographically normal coronary arteries or mild CAD (<40%) were enrolled in the Mayo Clinic Endothelial Database and included in this study. All patients provided written informed consent and the study was approved by the Mayo Clinic Institutional Review Board. Patients with ACS presentation, MI or cerebrovascular accident within the last six months, previous percutaneous coronary intervention (PCI), use of radiographic contrast agents within 12 hours prior to catheterisation, advanced chronic kidney disease (GFR <30 ml/min/m2), cardiomyopathy (ejection fraction <50%), inflammatory diseases, and pregnant patients were excluded. All patients fasted for at least eight hours and withheld all prescription medications that could affect coronary vasoreactivity for at least 48 hours prior to the study procedure.
The study protocol has been described in detail previously4,23,24,25,26. In brief, patients underwent a diagnostic coronary angiography using standard clinical protocols. Those with no CAD or non-obstructive CAD (angiographic stenosis <40%) received 5,000 intravenous units of heparin and a Doppler guidewire (FloWire®; Philips Volcano, San Diego, CA, USA) was positioned in the mid-left anterior descending coronary artery (LAD). CFVR was measured as the ratio of hyperaemic peak flow velocity in response to intracoronary adenosine (36-72 µg) over resting flow velocity. Mid-LAD diameter was measured by an independent investigator in the segment 5 mm distal to the tip of the Doppler wire using a quantitative coronary angiography program (Medis, Leiden, the Netherlands), as previously described27. Coronary artery cross-sectional area (CSA) was calculated as π×(coronary artery diameter/2)2. Resting and hyperaemic average peak velocities (APV) were derived from the Doppler flow velocity spectra. Resting and hyperaemic CBF were calculated as [π×(coronary artery diameter/2)2×(average peak velocity/2)]28. Resting and hyperaemic CBF were also indexed to body surface area and evaluated between sexes. In this patient population with non-obstructive CAD, mean arterial pressure (MAP) is equivalent to proximal and distal pressures in the LAD and used to calculate resting microvascular resistance (rMR) as a ratio of MAP/rCBF.
STATISTICAL ANALYSIS
Continuous variables are described as mean±standard deviation (SD) if normally distributed or median and interquartile range (IQR) if non-normally distributed, and categorical variables as proportions. The Student’s t-test and chi-square test were used for comparison of means or proportions for continuous and categorical variables, respectively. Non-normally distributed variables were compared using the Mann-Whitney test and were log transformed as required. The associations between those variables were investigated using univariate and multivariate regression analyses. A two-sided p-value of <0.05 was considered statistically significant. The statistical analyses were performed using SPSS, Version 22 (IBM Corp., Armonk, NY, USA).
Results
Table 1 summarises the demographic characteristics, lipids and medication profiles. Among the 1,683 patients, 1,096 (65%) were women and 587 (35%) were men. Median age was 51 (IQR 42, 59) years; 729 (43%) patients had hypertension, 156 (9%) had diabetes, 913 (54%) had hyperlipidaemia and 200 (12%) were current smokers. Women were older (52 [44, 60] vs 49 [39, 57] years), had higher resting MAP (99 [90, 109] vs 96 [88, 105] mmHg), and higher resting heart rate (73 [64, 82] vs 67 [60, 77] beats per minute) (p<0.001 for all). As compared to women, men had a higher prevalence of tobacco smoking (18 vs 9%, p<0.001) and higher median body mass index (BMI) (28.4 [25.5, 32.1] vs 27.6 [23.7, 32.8], p=0.008).
Table 1. Demographics, lipids and medication profiles of the study population stratified by sex.
| All patients (n=1,683) | Women n=1,096 (65%) | Men n=587 (35%) | p-value | |
| Age, years | 51 (42, 59) | 52 (44, 60) | 49 (39, 57) | <0.001 |
| Hypertension, n (%) | 729 (43) | 476 (44) | 253 (43) | 0.80 |
| Diabetes mellitus, n (%) | 156 (9) | 98 (9) | 58 (10) | 0.54 |
| Hyperlipidaemia, n (%) | 913 (54) | 583 (54) | 330 (56) | 0.27 |
| Tobacco smoking, n (%) | 200 (12) | 92 (9) | 108 (18) | <0.001 |
| Height, cm | 168 (162, 175) | 164 (160, 168) | 178 (173, 183) | <0.001 |
| Weight, kg | 81.0 (68.0, 95.2) | 74.0 (64.0, 88.5) | 91.0 (80.0, 103.0) | <0.001 |
| BMI, kg/m2 | 28.1 (24.4, 32.5) | 27.6 (23.7, 32.8) | 28.4 (25.5, 32.1) | 0.008 |
| Resting mean arterial pressure, mmHg | 98 (89, 108) | 99 (90, 109) | 96 (88, 105) | <0.001 |
| Resting heart rate, bpm | 71 (62, 80) | 73 (64, 82) | 67 (60, 77) | <0.001 |
| Lipid profiles | ||||
| Total cholesterol, mg/dl | 184 (157, 214) | 187 (163, 216) | 175 (148, 205) | <0.001 |
| Triglycerides, mg/dl | 110 (77, 167) | 106 (72, 154) | 125 (86, 188) | <0.001 |
| High-density lipoprotein, mg/dl | 51 (42, 63) | 56 (47, 69) | 43 (35, 50) | <0.001 |
| Low-density lipoprotein, mg/dl | 103 (81, 128) | 104 (81, 128) | 102 (78, 129) | 0.43 |
| Vasoactive medications | ||||
| Beta-blockers, n (%) | 479 (28) | 317 (29) | 162 (28) | 0.58 |
| Calcium channel blockers, n (%) | 538 (32) | 352 (32) | 186 (32) | 0.90 |
| Nitrates, n (%) | 626 (37) | 408 (37) | 218 (37) | 1.00 |
| Data presented as n (%) and median (interquartile range). p-value is for comparison between sexes. | ||||
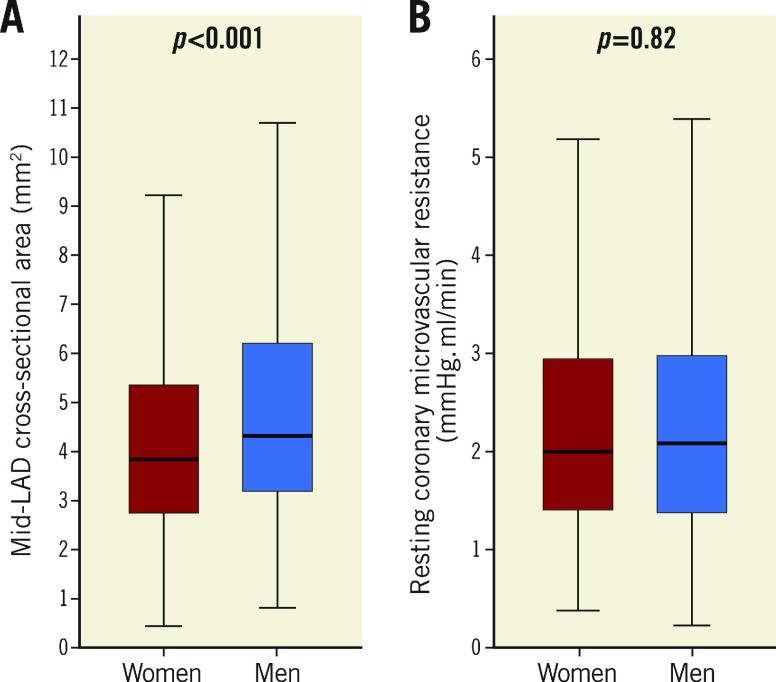
As compared to men, women had significantly lower median CFVR (2.7 [2.4, 3.2] vs 3.1 [2.7, 3.6], p<0.001) (Figure 1A), higher median rCBF (49.7 [34.0, 71.1] vs 45.9 [31.8, 68.7] ml/min, p=0.04)(Figure 1B), lower median hCBF (139.5 [93.0, 195.2] vs 147.1 [95.7, 218.6] ml/min, p=0.02) (Figure 1C), and smaller median mid-LAD CSA (3.80 [2.69, 5.31] vs 4.26 [3.11, 6.15] mm2, p<0.001) (Figure 2A). Median rMR was not different between men and women (2.00 [1.39, 2.94] vs 2.08 [1.36, 2.98] mmHg.min/ml, p=NS) (Figure 2B). Moreover, as compared to men, women had higher median indexed rCBF (27.13 [18.38, 39.10] vs 22.10 [15.00, 33.35] ml/min.m2, p<0.001) and lower median indexed hCBF (69.17 [46.76, 101.74] vs 75.91 [50.66, 107.55], p=0.02), but similar median indexed mid-LAD CSA (2.09 [1.45, 2.86] vs 2.08 [1.42, 2.98], p=0.97).
Figure 1.
Coronary blood flow stratified by sex. A) Coronary flow velocity reserve. B) Resting coronary blood flow. C) Hyperaemic coronary blood flow. Box plots represent medians and interquartile range. Whiskers represent minimum and maximum values.
Figure 2.
Coronary artery cross-sectional area and resting coronary microvascular resistance stratified by sex. A) Mid-left anterior descending coronary artery cross-sectional area. B) Resting coronary microvascular resistance. Box plots represent medians and interquartile range. Whiskers represent minimum and maximum values.
Table 2 summarises the independent predictors of rCBF. Female sex (p<0.001) was a strong independent predictor of higher rCBF after adjusting for age, sex, hypertension, diabetes, hyperlipidaemia, BMI, smoking, baseline heart rate, MAP, CSA, and rMR. Other independent predictors were BMI (p=0.002), resting heart rate (p<0.001), resting MAP (p<0.001), CSA (p<0.001), smoking (p=0.007) and rMR (p<0.001).
Table 2. Multivariable regression analysis for resting coronary blood flow independent predictors.
| Variable | Beta | SE | p-value |
| Age | 0.03 | 0.044 | 0.54 |
| Sex (male) | –3.93 | 1.10 | <0.001 |
| Hypertension | 1.01 | 1.12 | 0.37 |
| Diabetes | 2.32 | 1.75 | 0.19 |
| Hyperlipidaemia | 0.41 | 1.09 | 0.71 |
| Body mass index | –0.26 | 0.09 | 0.002 |
| Tobacco smoking | 1.99 | 0.73 | 0.007 |
| Resting heart rate | 0.25 | 0.04 | <0.001 |
| Resting mean arterial pressure | 0.21 | 0.04 | <0.001 |
| Coronary cross-sectional area | 7.75 | 0.26 | <0.001 |
| Resting microvascular resistance | –6.36 | 0.33 | <0.001 |
| Female sex, body mass index, tobacco smoking, resting heart rate, resting mean arterial pressure, coronary artery cross-sectional area, and microvascular resistance are independent predictors of resting coronary blood flow. | |||
Table 3 summarises the independent predictors of hCBF. Female sex (p=0.01) was a strong independent predictor of lower hCBF after adjusting for age, sex, hypertension, diabetes, hyperlipidaemia, BMI, and smoking. Other independent predictors were age (p<0.001), BMI (p=0.01) and hyperlipidaemia (p=0.03).
Table 3. Multivariable regression analysis for hyperaemic coronary blood flow predictors.
| Variable | Beta | SE | p-value |
| Age | –1.16 | 0.20 | <0.001 |
| Sex (male) | 13.40 | 5.00 | 0.01 |
| Hypertension | 9.62 | 5.12 | 0.06 |
| Diabetes | –2.65 | 8.20 | 0.75 |
| Hyperlipidaemia | –10.90 | 5.08 | 0.03 |
| Body mass index | 1.10 | 0.40 | 0.01 |
| Tobacco smoking | 0.34 | 3.43 | 0.92 |
| Age, sex, hyperlipidaemia, and body mass index are independent predictors of hyperaemic coronary blood flow. | |||
Table 4 summarises independent predictors of CFVR. Female sex (p<0.001) was a strong independent predictor of lower CFVR after adjusting for age, sex, hypertension, diabetes, hyperlipidaemia, BMI, smoking, baseline heart rate, MAP, CSA, rCBF and rMR. Other independent predictors were age (p<0.001), diabetes mellitus (p=0.004), BMI (p<0.001), smoking (p=0.02), resting heart rate (p<0.001), CSA (p<0.001), and rMR (p<0.001).
Table 4. Multivariable regression analysis for coronary flow velocity reserve independent predictors.
| Variable | Beta | SE | p-value |
| Age | –0.02 | 0.001 | <0.001 |
| Sex (male) | 0.20 | 0.04 | <0.001 |
| Hypertension | 0.03 | 0.04 | 0.47 |
| Diabetes | –0.17 | 0.06 | 0.004 |
| Hyperlipidaemia | 0.01 | 0.04 | 0.87 |
| Body mass index | 0.01 | 0.003 | <0.001 |
| Tobacco smoking | –0.06 | 0.03 | 0.02 |
| Resting heart rate | –0.02 | 0.001 | <0.001 |
| Resting mean arterial pressure | –0.001 | 0.001 | 0.49 |
| Coronary cross-sectional area | 0.06 | 0.01 | <0.001 |
| Resting microvascular resistance | 0.10 | 0.01 | <0.001 |
| Age, female sex, diabetes mellitus, body mass index, smoking, resting heart rate, coronary artery cross-sectional area, resting coronary blood flow and resting microvascular resistance are independent predictors of coronary flow velocity reserve. | |||
Discussion
This study demonstrates that women with chest pain and no or mild epicardial CAD have lower CFVR, higher rCBF, lower hCBF, and smaller CSA as compared to men. Moreover, female sex is an independent predictor of lower CFVR, higher rCBF and lower hCBF.
Coronary flow velocity reserve as a marker of CMD is an important prognostic marker. However, the heterogeneity in defining CMD based on a CFVR cut-off value is not only evident in research studies but also in everyday clinical practice. Britten et al have demonstrated increased long-term risk of MACE in patients with CMD (defined as % change of coronary flow index post contrast infusion) and non-obstructive CAD5, Serruys et al demonstrated increased risk of adverse events and recurrent ischaemia with a CFVR cut-off value of 2.5 post revascularisation20, and the Women’s Ischemia Syndrome Evaluation (WISE) study used CFVR <2.24 for CMD diagnosis in women29, demonstrating increased risk of MACE in women with chest pain and impaired CFVR defined as <2.328. Similarly, two recent positron emission tomography (PET) studies demonstrated that impaired CFVR (defined as <2.0) is a strong predictor of cardiovascular mortality30 and that increased risk of MACE in women, relative to men, is associated with severely impaired CFVR (defined as <1.6) rather than obstructive CAD31. Clinically, a CFVR cut-off value of 2.0 has been suggested for diagnosis of CMD in both men and women9. In the absence of obstructive CAD, the assumption that resting (denominator) and hyperaemic (numerator) blood flow, mirroring measured APV in the mathematical equation of CFVR, are the same in men and women has never been challenged before. Moreover, the assumption that all-comer men and women have similar CFVR has also never been challenged before in a large-scale invasive study.
Three small studies have previously suggested that coronary physiology is different between the sexes and that an “ideal” cut-off value for impaired CFVR might be sex-specific. The first is a PET study that showed higher baseline myocardial blood flow in asymptomatic healthy females as compared to healthy males32. The second is a study from our group that suggested that CFVR is lower in women, as compared to men, with chest pain and non-obstructive CAD21. The third is a recent study that demonstrated lower invasive CFVR values in women as compared to men, suggested similar mean hyperaemic transit time in women as compared to men presenting with angina and non-obstructive CAD, and hypothesised that lower CFVR in women was possibly secondary to higher rCBF22. The current study is the first large-scale invasive haemodynamic study investigating differences in coronary physiology between the sexes in symptomatic patients undergoing a clinically indicated angiogram – the patient population in which CFVR is likely to be measured for assessment of microcirculation. Our findings not only confirm that women with chest pain and non-obstructive CAD have lower CFVR as compared to men, but also demonstrate that female sex is an independent predictor of lower CFVR, a finding that our prior study21 failed to show due to its small sample size. Moreover, lower hCBF in women, as demonstrated in this study, is in contrast to similar hyperaemic blood flow in men and women suggested by the aforementioned smaller study by Kobayashi et al22.
While lower CFVR in women is related to both higher rCBF and lower hCBF, the reason for this sex-specific difference in CBF remains unclear. The associated finding of higher resting heart rate in women, as compared to men, suggests that higher resting sympathetic tone in women could possibly explain these findings. Our group has previously demonstrated sex-specific genetic variants associated with coronary epicardial endothelial dysfunction33, but whether sex-specific genetic variants are also associated with differences in sympathetic tone, adenosine resistance and/or endothelium-independent CMD remains unknown and warrants future investigation using invasive catheter-based coronary reactivity testing (Doppler wire-derived CFR or pressure-temperature wire-derived CFR using the thermodilution technique), or non-invasive PET-, magnetic resonance imaging-, or echocardiography-derived CFVR measurements and CMD diagnostic tools.
The findings of this study may have important clinical implications. Diagnosis and treatment of CMD improves angina, quality of life, physical function and mental health in patients with angina and/or signs of ischaemia but no obstructive CAD34,35. Our findings show sex-specific differences in CFVR and CBF in symptomatic patients and are hypothesis-generating as to whether CFVR cut-offs for CMD diagnosis and MACE prediction should be sex-specific. Coronary microvascular dysfunction is currently widely regarded as a “women’s disease” possibly secondary to underdiagnosis in men (where the diagnostic cut-off value of CFVR might be >2.5) and/or overdiagnosis in women (where the diagnostic cut-off might be <2.5). Moreover, whether asymptomatic “healthy” patients have similar sex-specific coronary haemodynamic differences to those observed in this study population remains unknown. While this study design is unable to answer these important questions, future outcome data studies linking sex-specific CFVR values in both symptomatic and asymptomatic patients without obstructive CAD to increased cardiac morbidity, mortality and adverse events will hopefully clarify whether optimal CMD diagnostic CFVR cut-off values are indeed sex-neutral or perhaps sex-specific. Finally, the prognostic value not only of CFVR, but also of rCBF and hCBF in predicting MACE in asymptomatic “healthy” versus symptomatic women and men warrants further evaluation.
Study limitations
First, non-obstructive CAD status was determined angiographically as the visual absence of a focal coronary stenosis >40%, and therefore the presence of diffuse epicardial disease without focal stenosis in some patients, affecting CFVR measurements independent of any coexistent CMD, is possible but unlikely to have been missed. Identification of this small fraction of patients with uniform diffuse epicardial narrowing requires the use of alternative imaging techniques (magnetic resonance angiography, PET, or intravascular imaging tools) which were not available in this study. Second, coronary artery diameter post adenosine infusion was not measured, and therefore computation of hCBF was based on the assumption that resting CSA does not significantly change post adenosine. Importantly, previously published data in both humans36 and animals37 have demonstrated that baseline epicardial coronary diameter and CSA do not significantly change post adenosine and therefore support our assumption and the methodology used to calculate hCBF. Finally, we do not have complete longitudinal outcome data on this patient population yet, but long-term follow-up and evaluation of optimal sex-specific CFVR cut-off values associated with adverse outcomes in men versus women would certainly be complementary to this study and help in the identification of a patient population that needs earlier medical intervention and aggressive risk factor modification. Future outcome studies are warranted to evaluate not only the sex-specific cut-off values of CFVR tied to MACE but also the prognostic values of rCBF and hCBF in men versus women.
Conclusions
Compared to men, women with chest pain and no or mild epicardial CAD have lower CFVR, higher rCBF, lower hCBF and smaller CSA, but similar rMR. Female sex is an independent predictor of lower CFVR, higher rCBF and lower hCBF. Whether CFVR cut-offs for coronary microvascular dysfunction diagnosis and MACE prediction should be sex-specific needs further evaluation.
Impact on daily practice
The clinical diagnostic (microvascular disease diagnosis) and prognostic (MACE outcomes) implications of lower CFVR, higher rCBF, and lower hCBF in women with signs/symptoms of angina and unobstructed CAD need further evaluation.
Acknowledgments
Conflict of interest statement
The authors have no conflicts of interest to declare.
Abbreviations
- APV
average peak velocity
- CFVR
coronary flow velocity reserve
- CMD
coronary microvascular dysfunction
- CSA
coronary artery cross-sectional area
- hCBF
hyperaemic coronary blood flow
- rCBF
resting coronary blood flow
- rMR
resting microvascular resistance
Contributor Information
Michel Corban, Department of Cardiovascular Diseases, Mayo Clinic College of Medicine and Science, Rochester, MN, USA.
Abhiram Prasad, Department of Cardiovascular Diseases, Mayo Clinic College of Medicine and Science, Rochester, MN, USA.
Rajiv Gulati, Department of Cardiovascular Diseases, Mayo Clinic College of Medicine and Science, Rochester, MN, USA.
Lilach Lerman, Department of Cardiovascular Diseases, Mayo Clinic College of Medicine and Science, Rochester, MN, USA; Division of Nephrology and Hypertension, Department of Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN, USA.
Amir Lerman, Department of Cardiovascular Diseases, Mayo Clinic College of Medicine and Science, Rochester, MN, USA.
References
- Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155:375–81. doi: 10.1016/j.ahj.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445–553. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259–64. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–82b. doi: 10.1093/eurheartj/ehs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8:210–20. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern MJ, Samady H. Current concepts of integrated coronary physiology in the catheterization laboratory. J Am Coll Cardiol. 2010;55:173–85. doi: 10.1016/j.jacc.2009.06.062. [DOI] [PubMed] [Google Scholar]
- Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387–93. doi: 10.1016/S0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- Pitkänen OP, Nuutila P, Raitakari OT, Rönnemaa T, Koskinen PJ, Iida H, Lehtimäki TJ, Laine HK, Takala T, Viikari JS, Knuuti J. Coronary flow reserve is reduced in young men with IDDM. Diabetes. 1998;47:248–54. doi: 10.2337/diab.47.2.248. [DOI] [PubMed] [Google Scholar]
- Pitkänen OP, Raitakari OT, Niinikoski H, Nuutila P, Iida H, Voipio-Pulkki LM, Härkönen R, Wegelius U, Rönnemaa T, Viikari J, Knuuti J. Coronary flow reserve is impaired in young men with familial hypercholesterolemia. J Am Coll Cardiol. 1996;28:1705–11. doi: 10.1016/S0735-1097(96)00376-2. [DOI] [PubMed] [Google Scholar]
- Schelbert HR. Coronary circulatory function abnormalities in insulin resistance: insights from positron emission tomography. J Am Coll Cardiol. 2009;53:S3–8. doi: 10.1016/j.jacc.2008.09.053. [DOI] [PubMed] [Google Scholar]
- Yokoyama I, Momomura S, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472–7. doi: 10.1016/S0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- Yokoyama I, Murakami T, Ohtake T, Momomura S, Nishikawa J, Sasaki Y, Omata M. Reduced coronary flow reserve in familial hypercholesterolemia. J Nucl Med. 1996;37:1937–42. [PubMed] [Google Scholar]
- Yokoyama I, Ohtake T, Momomura S, Nishikawa J, Sasaki Y, Omata M. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation. 1996;94:3232–8. doi: 10.1161/01.CIR.94.12.3232. [DOI] [PubMed] [Google Scholar]
- Marks DS, Gudapati S, Prisant LM, Weir B, diDonato-Gonzalez C, Waller JL, Houghton JL. Mortality in patients with microvascular disease. J Clin Hypertens (Greenwich) 2004;6:304–9. doi: 10.1111/j.1524-6175.2004.03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–8. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Verna E, Ford AJ Jr, Peels HO, Danzi G, Muhlberger V, Emanuelsson H, Geschwind H, Voudris V, Haude M, Di Mario C, Fleck E, Hanet C, De Bruyne B, Probst P, Vrints C, Schroeder E, Piek J, Boersma E. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe). Circulation. 1997;96:3369–77. doi: 10.1161/01.CIR.96.10.3369. [DOI] [PubMed] [Google Scholar]
- Han SH, Bae JH, Holmes DR Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–69. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, Lee DP, Stefanick M, Yeung AC, Tremmel JA. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1433–41. doi: 10.1016/j.jcin.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corban MT, Prasad A, Nesbitt L, Loeffler D, Herrmann J, Lerman LO, Lerman A. Local Production of Soluble Urokinase Plasminogen Activator Receptor and Plasminogen Activator Inhibitor-1 in the Coronary Circulation is Associated With Coronary Endothelial Dysfunction in Humans. J Am Heart Assoc. 2018;7:e009881. doi: 10.1161/JAHA.118.009881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasdai D, Cannan CR, Mathew V, Holmes DR Jr, Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;53:203–8. doi: 10.1016/0167-5273(95)02548-0. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Holmes DR Jr, Higano ST, Burnett JC Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc. 1998;73:1133–40. doi: 10.4065/73.12.1133. [DOI] [PubMed] [Google Scholar]
- Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol. 2013;304:H393–7. doi: 10.1152/ajpheart.00765.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Suwaidi J, Higano ST, Holmes DR Jr, Rihal CS, Lerman A. Measuring maximal percent area stenosis poststent placement with intracoronary Doppler and the continuity equation and correlation with intracoronary ultrasound and angiography. Am J Cardiol. 1999;84:650–4. doi: 10.1016/S0002-9149(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–911. doi: 10.1161/01.CIR.85.5.1899. [DOI] [PubMed] [Google Scholar]
- Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, Sopko G, Pepine CJ. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–75. doi: 10.1016/S0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Gupta A, Harrington M, Camici PG, Blankstein R, Bhatt DL, Dorbala S, Rimoldi O, Hainer J, Bibbo CF, Taqueti VR, Vita T, Seidelmann SB, Osborne MT, Murthy VL, Bravo PE, Bajaj NS, van de Hoef TP, Di Carli MF. Integrated Noninvasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients With Stable Coronary Artery Disease. Circulation. 2017;136:2325–36. doi: 10.1161/CIRCULATIONAHA.117.029992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 2017;135:566–77. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50:151–61. doi: 10.1016/S0008-6363(01)00202-4. [DOI] [PubMed] [Google Scholar]
- Yoshino S, Cilluffo R, Prasad M, Best PJ, Atkinson EJ, Aoki T, Cunningham JM, de Andrade M, Lerman LO, Lerman A. Sex-Specific Genetic Variants are Associated With Coronary Endothelial Dysfunction. J Am Heart Assoc. 2016;5:e002544. doi: 10.1161/JAHA.115.002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford TJ, McDade R, Oldroyd KG, Touyz RM, McConnachie A, Rush C, Collison D, Corcoran D, McCartney P, Sidik N, Yii E, McGeoch R, Stanley B, Hood S, Robertson K, Lindsay M, Shaukat A, Eteiba H, Watkins S, McEntegart M, Rocchiccioli P, Good R, Berry C. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–55. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Reriani M, Flammer AJ, Duhe J, Li J, Gulati R, Rihal CS, Lennon R, Tilford JM, Prasad A, Lerman LO, Lerman A. Coronary endothelial function testing may improve long-term quality of life in subjects with microvascular coronary endothelial dysfunction. Open Heart. 2019;6:e000870. doi: 10.1136/openhrt-2018-000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen JD, Quillen JE, Lopez AG, Stenberg RG, Talman CL, Winniford MD. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18:485–91. doi: 10.1016/0735-1097(91)90604-8. [DOI] [PubMed] [Google Scholar]
- Sudhir K, MacGregor JS, Barbant SD, Foster E, Fitzgerald PJ, Chatterjee K, Yock PG. Assessment of coronary conductance and resistance vessel reactivity in response to nitroglycerin, ergonovine and adenosine: in vivo studies with simultaneous intravascular two-dimensional and Doppler ultrasound. J Am Coll Cardiol. 1993;21:1261–8. doi: 10.1016/0735-1097(93)90255-Y. [DOI] [PubMed] [Google Scholar]