Abstract
Aims
We aimed to understand the association between stent length and clinical outcomes after percutaneous coronary intervention (PCI) using newer-generation drug-eluting stents (DES).
Methods and results
We analysed 9,217 patients who underwent stenting for a single lesion from the GRAND-DES registry, a patient-level pooled registry including five Korean multicentre DES registries. The median follow-up duration was 730 days (interquartile range 708 to 752 days). A total of 8,035 patients were classified into the short stenting group (≤40 mm), and 1,182 into the long stenting group (>40 mm). The primary endpoint was target lesion failure (TLF). Long stenting (>40 mm) was significantly associated with higher TLF (IPTW adjusted HR 1.88, 95% CI: 1.67-2.13; p<0.001), and definite or probable stent thrombosis (IPTW adjusted HR 2.20, 95% CI: 1.51-3.20; p<0.001). In the landmark analysis, the incidence of TLF was significantly higher with long stenting during the first 30 days after PCI (log-rank p=0.001) and also after 30 days (log-rank p<0.001). Long stenting was associated with a higher risk of early stent thrombosis (log-rank p=0.001), but not with that of late stent thrombosis (log-rank p=0.887).
Conclusions
In the contemporary second-generation DES era, stenting longer than 40 mm continues to be associated with less favourable clinical outcomes such as TLF and stent thrombosis.
Introduction
The rate of angiographic restenosis and the need for repeat revascularisation after percutaneous coronary intervention (PCI) has reduced significantly1. At the beginning of the drug-eluting stent (DES) era, implanting a stent covering the entire length of the lesion was widely adopted, based on confidence in the performance of DESs2. However, this strategy was soon discontinued, as the risk of stent thrombosis (ST) was well recognised in first-generation DES3. Moreover, the risk of target lesion revascularisation (TLR) was found to be associated with stent length4. To overcome these shortcomings, second-generation DESs, which had a thinner strut thickness, advanced polymer technology (biocompatible or biodegradable), and adjusted drug potency, were developed5,6,7. With these second-generation DESs, clinical outcomes after PCI such as TLR and ST were significantly improved8. However, it is still not clear whether the total stent length affects the clinical outcomes in the second-generation DES era. Only a few small-scale studies have reported on this topic with controversial results2,9,10,11. We aimed to assess the efficacy and safety of long coronary stenting using a large-scale pooled registry, and to arrive at the optimal cut-off value for total stent length predicting adverse outcomes in the second-generation DES era.
Methods
An extended description of the methods is presented in Supplementary Appendix 1.
STUDY POPULATION
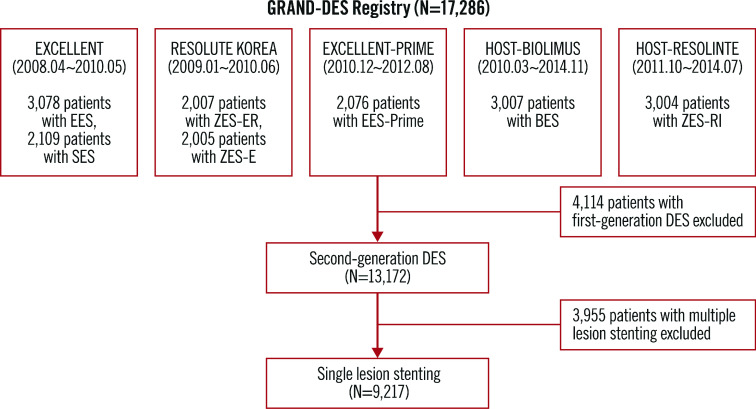
The GRAND-DES registry is a patient-level pooled registry consisting of 17,286 patients from five Korean multicentre DES registries (Figure 1, Supplementary Appendix 1). There were no exclusion criteria in any of the five registries except the patient’s withdrawal of consent. To avoid any potential confounding effect on clinical outcomes due to multiple lesions, and to reveal the singular impact of the stent length per lesion, we included only 9,217 patients who had a single target lesion.
Figure 1.
Study flow chart.
Each trial included in this analysis complied with the provisions of the Declaration of Helsinki, and the study protocols were approved by the institutional review board at each participating centre. All patients provided written informed consent for participation in each study.
ENDPOINTS AND DEFINITIONS
The endpoints and definitions of this study are presented in Supplementary Appendix 2.
STATISTICAL ANALYSIS
The statistical analysis for this study is described in Supplementary Appendix 3.
Results
BASELINE CHARACTERISTICS
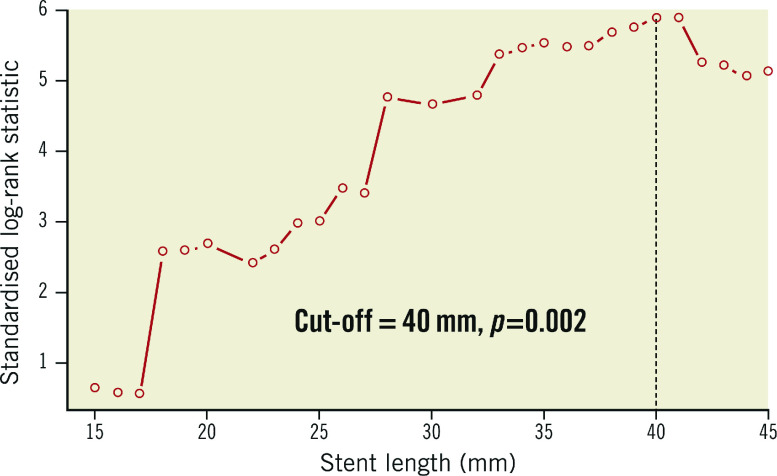
Maximally selected rank statistics revealed that the optimal total stent length cut-off for TLF as the primary endpoint was 40 mm (p=0.002) (Figure 2). Thus, a total stent length >40 mm was defined as long stenting. In a total of 9,217 patients, 8,035 patients (87.2%) belonged to the short stenting group, and 1,182 patients (12.8%) to the long stenting group. Baseline clinical characteristics are shown in Table 1. Patients in the long stenting group were older and had a higher prevalence of diabetes, hypertension, chronic kidney disease, and previous history of coronary artery bypass graft, congestive heart failure (CHF), or cerebrovascular attack compared to those who received short stents. In contrast, the short stenting group had a higher prevalence of current smoking and previous PCI, compared to the long stenting group. More patients in the long stenting group were prescribed angiotensin receptor blockers and beta-blockers, but more in the short stenting group took statins. The percentage of patients who were prescribed dual antiplatelet therapy (DAPT) at discharge and each follow-up was not significantly different between the two groups (Supplementary Table 1).
Figure 2.
Maximally selected rank statistics to determine the optimal cut-off for TLF.
Table 1. Baseline patient characteristics.
| Variables | Total stent length (mm) | Overall population | p-value | |
| ≤40 (N=8,035) | >40 (N=1,182) | (N=9,217) | ||
| Age, years | 63.3±11.1 | 65.5±10.8 | 63.6±11.1 | <0.001 |
| Gender, male | 5,631 (70.1%) | 839 (71.0%) | 6,470 (70.2%) | 0.527 |
| Diabetes mellitus | 2,699 (33.6%) | 463 (39.2%) | 3,162 (34.3%) | <0.001 |
| Hypertension | 4,814 (59.9%) | 751 (63.5%) | 5,565 (60.4%) | 0.017 |
| Dyslipidaemia | 3,274 (40.7%) | 486 (41.1%) | 3,760 (40.8%) | 0.809 |
| PVD | 141 (1.8%) | 29 (2.5%) | 170 (1.8%) | 0.096 |
| CKD | 2,670 (35.4%) | 453 (40.7%) | 3,123 (36.0%) | <0.001 |
| Current smoking | 2,426 (30.2%) | 307 (26.0%) | 2,733 (29.7%) | 0.003 |
| Previous PCI | 1,375 (17.1%) | 173 (14.6%) | 1,548 (16.8%) | 0.033 |
| Previous CABG | 160 (2.0%) | 35 (3.0%) | 195 (2.1%) | 0.031 |
| Previous MI | 476 (5.9%) | 72 (6.1%) | 548 (5.9%) | 0.820 |
| Previous CHF | 162 (2.0%) | 38 (3.2%) | 200 (2.2%) | 0.008 |
| Previous CVA | 589 (7.3%) | 112 (9.5%) | 701 (7.6%) | 0.009 |
| Diagnosis | ||||
| Stable angina | 2,622 (32.6%) | 396 (33.5%) | 3,018 (32.7%) | |
| Unstable angina | 2,689 (33.5%) | 370 (31.3%) | 3,059 (33.2%) | |
| NSTEMI | 1,078 (13.4%) | 166 (14.0%) | 1,244 (13.5%) | |
| STEMI | 1,300 (16.2%) | 177 (15.0%) | 1,477 (16.0%) | |
| LVEF (%) | 58.5±14.2 | 56.5±11.9 | 58.2±13.9 | <0.001 |
| LV dysfunction (EF <40%) | 1,659 (20.6%) | 243 (20.6%) | 1,902 (20.6%) | 0.944 |
| Medications | ||||
| Aspirin | 7,969 (99.3%) | 1,171 (99.2%) | 9,140 (99.3%) | 0.804 |
| Clopidogrel | 7,845 (97.8%) | 1,161 (98.4%) | 9,006 (97.8%) | 0.170 |
| DAPT | 7,952 (99.0%) | 1,170 (99.0%) | 9,122 (99.0%) | 0.955 |
| Statin | 7,070 (88.0%) | 1,012 (85.6%) | 8,082 (87.7%) | 0.020 |
| ACE inhibitors | 2,579 (32.1%) | 355 (30.0%) | 2,394 (31.8%) | 0.155 |
| ARBs | 2,540 (31.6%) | 424 (35.9%) | 2,964 (32.2%) | 0.003 |
| Beta-blockers | 4,973 (61.9%) | 772 (65.3%) | 5,745 (62.3%) | 0.023 |
| CCB | 2,035 (25.3%) | 301 (25.5%) | 2,336 (25.3%) | 0.919 |
| Warfarin | 158 (2.0%) | 28 (2.4%) | 186 (2.0%) | 0.358 |
| Continuous variables expressed as mean±SD. ACE: angiotensin-converting enzyme; ARB: aldosterone receptor blocker; CABG: coronary artery bypass graft; CCB: calcium channel blocker; CHF: congestive heart failure; CKD: chronic kidney disease; CVA: cerebrovascular accident; DAPT: dual antiplatelet therapy; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; PVD: peripheral vascular disease; STEMI: ST-elevation myocardial infarction | ||||
Baseline angiographic and procedural characteristics are presented in Table 2. Total stent length was 23.3±6.7 mm versus 56.2±13.7 mm, stent diameter was 3.1±0.4 versus 3.0±0.3 mm, and the number of stents was 1.0±0.2 versus 2.2±0.4 for the short and long stenting groups, respectively. The long stenting group had higher rates of left main artery disease, B2 or C lesions, calcified lesions, tortuous lesions, multivessel disease, and bifurcations. The lengths of stent available in our country are provided in Supplementary Table 2.
Table 2. Baseline target lesion and procedural characteristics.
| Variables | Total stent length (mm) | Overall population (N=9,217) | p-value | ||
| ≤40 (N=8,035) | >40 (N=1,182) | ||||
| Total stent length, mm | 23.3±6.7 | 56.2±13.7 | 27.5±13.6 | <0.001 | |
| Stent diameter, mm | 3.1±0.4 | 3.0±0.3 | 3.1±0.6 | <0.001 | |
| Stents per lesion | 1.0±0.2 | 2.2±0.4 | 1.18±0.46 | <0.001 | |
| Number of stents | 1 | 7,728 (96.2%) | 7,728 (83.8%) | ||
| 2 | 286 (3.6%) | 1,012 (85.6%) | 1,298 (14.1%) | ||
| 3 | 21 (0.3%) | 145 (12.3%) | 166 (1.8%) | ||
| 4 | 25 (2.1%) | 25 (0.3%) | |||
| Left main disease | 344 (4.3%) | 85 (7.2%) | 429 (4.7%) | 0.003 | |
| Type B2 or C | 5,031 (62.6%) | 1,005 (85.0%) | 6,036 (65.5%) | <0.001 | |
| Calcified lesion | 497 (6.2%) | 149 (12.6%) | 646 (7.0%) | <0.001 | |
| Tortuous lesion | 1,485 (18.5%) | 275 (23.5%) | 1,763 (19.1%) | 0.002 | |
| Thrombus | 892 (11.1%) | 129 (10.9%) | 1,021 (11.1%) | 0.848 | |
| Previously treated lesion | 654 (8.1%) | 85 (7.2%) | 739 (8.0%) | 0.262 | |
| Bifurcation | 1,682 (20.0%) | 355 (30.0%) | 2,037 (22.1%) | <0.001 | |
| Target vessel | LAD | 4,107 (51.1%) | 637 (53.9%) | 4,744 (51.5%) | |
| LCX | 1,425 (17.7%) | 108 (9.1%) | 1,533 (16.6%) | ||
| RCA | 2,141 (26.6%) | 347 (29.4%) | 2,488 (27.0%) | ||
| LM | 344 (4.3%) | 85 (7.2%) | 429 (4.7%) | ||
| Type of stent | EES | 2,827 (37.7%) | 749 (43.7%) | 3,576 (38.8%) | |
| ZES | 2,729 (36.4%) | 760 (44.3%) | 3,489 (37.9%) | ||
| BES | 1,946 (25.9%) | 206 (12.0%) | 2,152 (23.3%) | ||
| GP IIb/IIIa inhibitors | 250 (3.1%) | 49 (4.1%) | 299 (3.2%) | 0.061 | |
| IVUS | 2,785 (34.7%) | 554 (46.9%) | 3,339 (36.2%) | <0.001 | |
| Emergency CABG | 3 (0.0%) | 0 (0.0%) | 3 (0.0%) | 0.506 | |
| Cardiogenic shock | 33 (0.4%) | 11 (0.9%) | 44 (0.5%) | 0.015 | |
| BES: biolimus-eluting stent; DAPT: dual antiplatelet therapy; EES: everolimus-eluting stent; IVUS: intravascular ultrasound; ZES: zotarolimus-eluting stent | |||||
CLINICAL OUTCOMES
The median follow-up duration was 730 days (interquartile range 708 to 752 days).
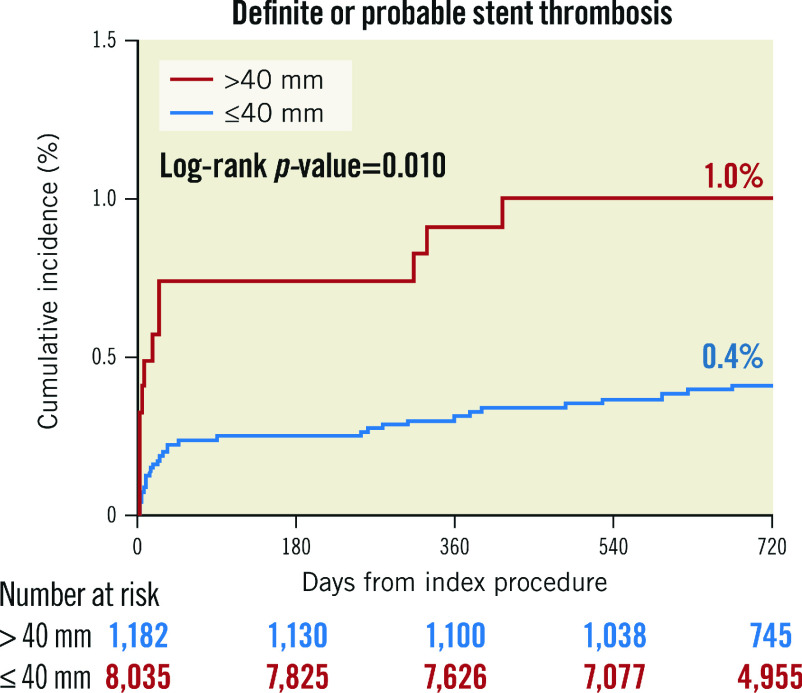
The Kaplan-Meier analysis revealed poor primary and secondary outcomes in the long stenting group (Figure 3, Figure 4). TLF (8.1% vs 4.5%, log-rank p-value <0.001) as well as ST (1.0% vs 0.4%, log-rank p-value=0.010) occurred more frequently in the long stenting group. After adjustment for potential confounders, the risk of all clinical outcomes was still significantly higher in the long stenting group (Table 3). These included TLF (inverse probability of treatment weighting [IPTW] adjusted HR 1.88, 95% CI: 1.67 to 2.13; p<0.001), cardiac death (IPTW adjusted HR 1.43, 95% CI: 1.20 to 1.70; p<0.001), target vessel myocardial infarction (MI) (IPTW adjusted HR 2.10, 95% CI: 1.38 to 3.22; p<0.001), clinically driven TLR (IPTW adjusted HR 2.54, 95% CI: 2.14 to 3.01; p<0.001), patient-oriented composite outcomes (POCO) (IPTW adjusted HR 1.48, 95% CI: 1.36 to 1.61; p<0.001), and definite or probable ST (IPTW adjusted HR 2.20, 95% CI: 1.51 to 3.20; p<0.001). Other independent predictors of TLF were age, diabetes, peripheral vascular disease, chronic kidney disease, previous history of MI, CHF or cerebrovascular disease, acute MI, left main artery disease, previously treated lesion, and left ventricular (LV) dysfunction (Supplementary Table 3). Other independent predictors of definite or probable ST were hypertension, acute MI, previously treated lesion (in-stent restenosis [ISR]), and LV dysfunction (Supplementary Table 4). The difference in stents used did not impact significantly on TLF and definite or probable ST (Supplementary Figure 1, Supplementary Figure 2).
Figure 3.
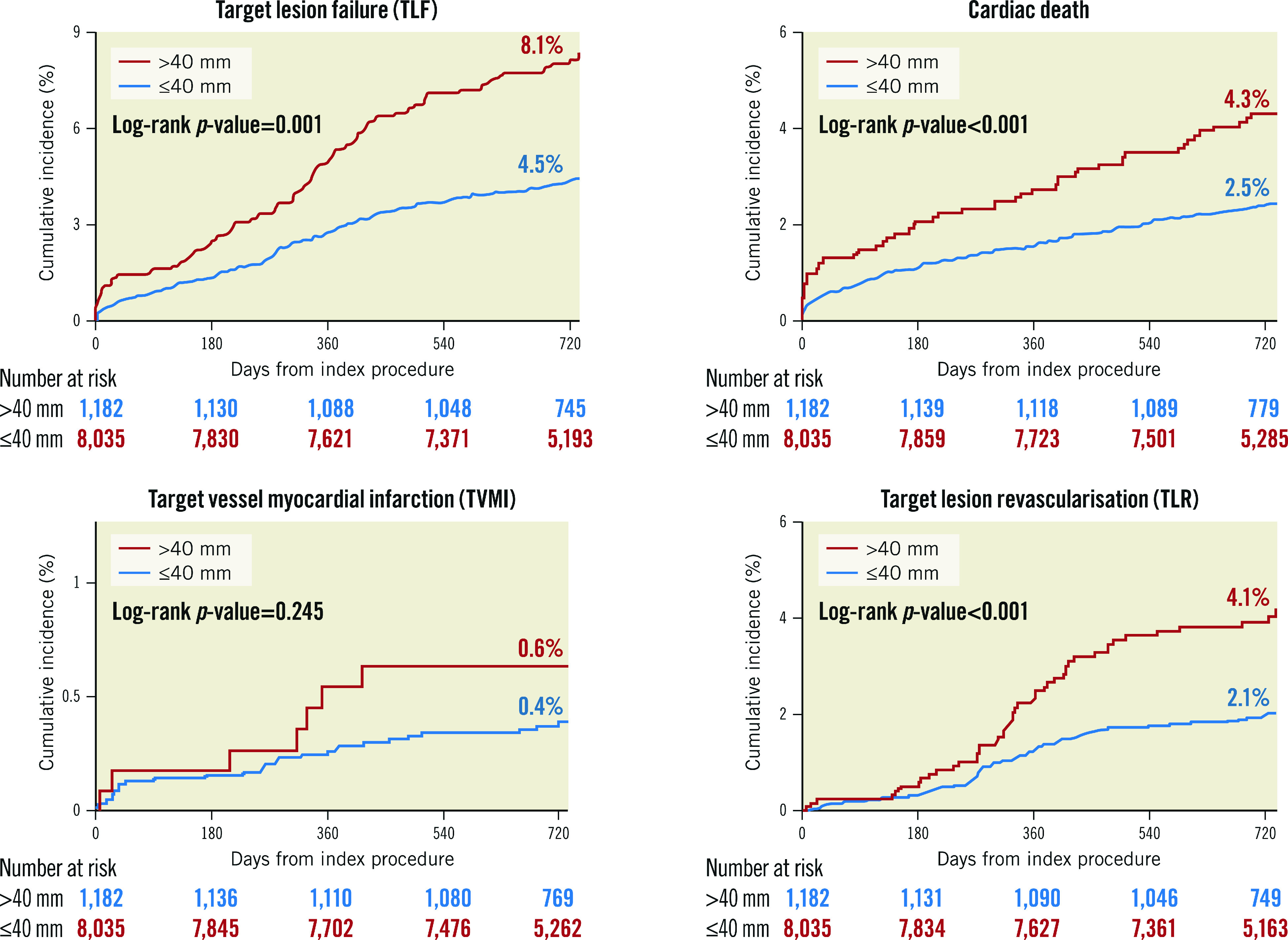
Kaplan-Meier curves of clinical endpoints including target lesion failure (TLF), cardiac death, target vessel myocardial infarction (TVMI), and clinically driven target lesion revascularisation (TLR).
Figure 4.
Kaplan-Meier curves of definite or probable stent thrombosis (ST).
Table 3. Clinical outcomes by total stent length.
| Variables | Total stent length (mm) | Unadjusted | Multivariate adjusted | Adjusted by inverse probability of treatment weights (IPTW) | |||||
| ≤40 (N=8,035) | >40 (N=1,182) | HR | p-value | HR | p-value | HR | p-value | ||
| Target lesion failure | 369 (4.6%) | 102 (8.6%) | 1.92 (1.54-2.39) | <0.001 | 1.76 (1.40-2.21) | <0.001 | 1.88 (1.67-2.13) | <0.001 | |
| Death | Cardiac death | 207 (2.6%) | 54 (4.6%) | 1.81 (1.34-2.44) | <0.001 | 1.61 (1.17-2.20) | 0.003 | 1.43 (1.20-1.70) | <0.001 |
| Non-cardiac death | 131 (1.6%) | 33 (2.8%) | 1.75 (1.19-2.56) | 0.004 | 1.44 (0.95-2.17) | 0.085 | 1.35 (1.08-1.69) | 0.009 | |
| Myocardial infarction | All MI | 61 (0.8%) | 14 (1.2%) | 1.53 (0.80-2.94) | 0.200 | 1.38 (0.71-2.68) | 0.350 | 1.36 (0.99-1.86) | 0.060 |
| Target vessel MI | 32 (0.4%) | 9 (0.8%) | 1.95 (0.93-4.09) | 0.076 | 1.79 (0.81-3.97) | 0.149 | 2.10 (1.38-3.22) | <0.001 | |
| Non-target vessel MI | 29 (0.4%) | 5 (0.4%) | 1.53 (0.52-4.53) | 0.440 | 1.26 (0.55-2.91) | 0.583 | 0.74 (0.45-1.23) | 0.244 | |
| Revascularisation | Any revascularisation | 493 (6.1%) | 111 (9.4%) | 1.58 (1.29-1.94) | <0.001 | 1.47 (1.18-1.83) | 0.001 | 1.58 (1.41-1.76) | <0.001 |
| Clinically driven TLR | 171 (2.1%) | 52 (4.4%) | 2.13 (1.56-2.91) | <0.001 | 2.03 (1.47-2.80) | <0.001 | 2.54 (2.14-3.01) | <0.001 | |
| Clinically driven TVR | 238 (3.0%) | 64 (5.4%) | 1.89 (1.43-2.49) | <0.001 | 1.82 (1.36-2.42) | <0.001 | 2.21 (1.91-2.57) | <0.001 | |
| POCO | 823 (10.2%) | 193 (16.3%) | 1.65 (1.41-1.93) | <0.001 | 1.45 (1.23-1.71) | <0.001 | 1.48 (1.36-1.61) | <0.001 | |
| Definite or probable stent thrombosis | 36 (0.4%) | 12 (1.0%) | 2.30 (1.19-4.41) | 0.013 | 2.17 (1.10-4.28) | 0.026 | 2.20 (1.51-3.20) | <0.001 | |
| Adjusted for the following covariates: age, DM, hypertension, dyslipidaemia, PVD, CKD, current smoker, AMI, previous MI, previous CHF, previous CVA, left main disease, bifurcation lesion, tortuous lesion, calcification lesion, previously treated lesion, and left ventricular dysfunction (EF <40%). AMI: acute myocardial infarction; CHF: congestive heart failure; CKD: chronic kidney disease; CVA: cerebrovascular accident; DM: diabetes mellitus; EF: ejection fraction; MI: myocardial infarction; POCO: patient-oriented composite outcome; PVD: peripheral vascular disease; TLR: target lesion revascularisation; TVR: target vessel revascularisation | |||||||||
When the clinical outcomes were analysed according to the stratified total stent length, length >40 mm was significantly associated with a higher incidence of TLF (4.3%, 4.8%, and 8.6%, for total stent length <20 mm, 20-40 mm, and >40 mm, respectively), cardiac death (2.2%, 2.7%, and 4.6%), TLR (2.2%, 2.1%, and 4.4%), and ST (0.4%, 0.5%, and 1.0%), demonstrating that total stent length of 40 mm has a significant clinical relevance (Figure 5).
Figure 5.
Clinical outcomes according to the total stent length divided into three groups. # p<0.05 between ≤20 mm and >40 mm group; * p<0.05 between 20-40 mm and >40 mm group. CD: cardiac death; ST: definite or probable stent thrombosis; TLF: target lesion failure; TLR: clinically driven target lesion revascularisation; TVMI: target vessel myocardial infarction
30-DAY LANDMARK ANALYSIS FOR TLF AND DEFINITE OR PROBABLE STENT THROMBOSIS
The landmark analysis revealed that the incidence of TLF was significantly higher in the long stenting group during the first 30 days after PCI (log-rank p-value=0.001) as well as beyond 30 days (log-rank p-value <0.001) (Figure 6). Interestingly, the incidence of definite or probable ST was also higher in the long stenting group in the early period (first 30 days) (log-rank p-value=0.001) but was not significantly different between the two groups in the late period (beyond 30 days) (log-rank p-value=0.887).
Figure 6.
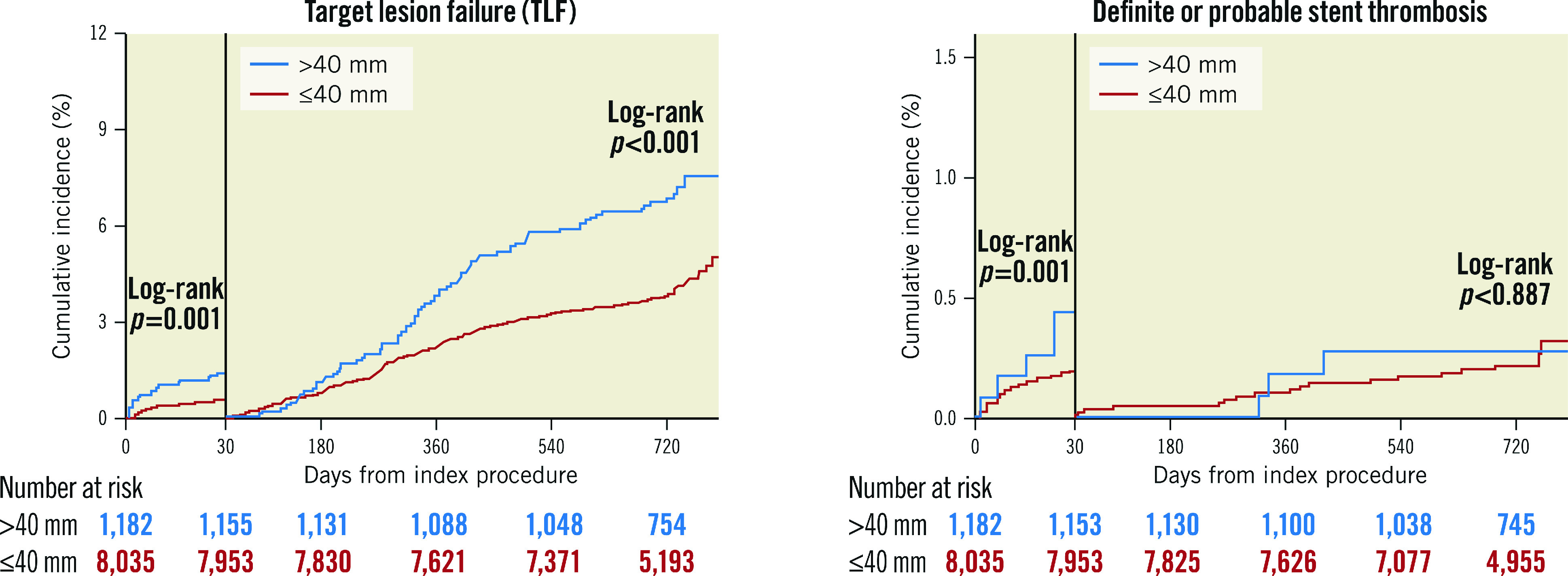
30-day landmark analysis for target lesion failure (TLF) and definite or probable stent thrombosis (ST).
IMPACT OF OVERLAPPING STENTS ON CLINICAL OUTCOMES
Stent overlapping rather than total stent length might affect the outcomes after PCI. The overlapping group (total stent length 50.9±16.5 mm) showed worse clinical outcomes compared with the non-overlapping group (total stent length 23.0±6.5 mm) in the overall population (Supplementary Table 5). However, long stenting is closely related with stent overlapping. Stenting longer than 40 mm requires overlapping of more than two stents. Because this raises a multicollinearity issue, we could not include the variable of overlapping into multivariate analysis in the overall population to determine which factor is more important between long stenting and stent overlapping. Instead, we analysed data of the short stenting group to understand the singular role of stent overlapping. The results showed that the overlapping was not an independent predictor for any clinical outcomes (Supplementary Table 6).
Discussion
The main findings of our study are as follows: (1) the total stent length predicting adverse clinical outcomes was above 40 mm; (2) even in the second-generation DES era, long length of stenting (>40 mm) was associated with a higher incidence of TLF and ST; (3) stent implantation up to 40 mm was relatively safe and effective.
In the second-generation DES era, a few small-scale studies have been published regarding the impact of total stent length on clinical outcome. Choi et al suggested ≥32 mm as a definition of long stenting based on receiver operating characteristic (ROC) curve analysis in the entire study population including patients receiving first- or second-generation DESs to predict TVR9. They found that long stenting (≥32 mm) was not associated with the three-year incidence of TVR or ST in the second-generation DES group. However, the sample size for the second-generation DES of this study was quite limited: 1,733 for <32 mm, and 378 for ≥32 mm. Honda et al arbitrarily defined long stenting as >50 mm2. The study reported long stenting (>50 mm) to be a predictor of TLR, but not of ST (median 23-month follow-up). However, this study also included only a small number of subjects: 1,292 for <20 mm, 1,212 for 20-50, and 259 for >50 mm. Konishi et al randomly defined long stenting as >32 mm10. The authors showed that long stenting (>32 mm) was not associated with major adverse cardiac events (MACE), defined as a composite of all-cause death, acute coronary syndrome, and TVR (median 3.6 years of follow-up). The sample size was again quite small: 186 for ≤32 mm and 110 for >32 mm. Furthermore, because the endpoint was broadly defined including all-cause death and TVR, the stent length-specific outcomes could not be well elucidated in this study. Hiromasa et al also arbitrarily defined long stenting as >28 mm12. They reported a higher incidence of three-year TLR in the long stenting (>28 mm) group, but not of ST. Not many subjects were included in the study: 486 for <18 mm, 475 for 18-28 mm, 421 for >28 mm. Recently, in the WIN-DES substudy, Chandrasekhar et al analysed the patient-level pooled data from 14 randomised trials in women undergoing PCI with second-generation DESs13. The results showed that the arbitrarily selected total stent length of ≥27 mm (n=1,474) was associated with an increased risk for three-year MACE (a composite of all-cause death, MI, or TLR) and MI, but not for cardiac death, TLR or ST.
Our large-scale study of second-generation DESs indicated that long stenting still resulted in a higher incidence not only of TLR, but also of ST. Interestingly, the previous studies suggested that long stenting was not associated with an increased risk of ST in the second-generation DES era, although the studies comprised small sample sizes and showed controversial outcomes regarding TLR and MACE2,9,12,13. Our study revealed that the incidence of early ST was negatively affected by total stent length, but late ST was not. Mechanically, early ST is mainly attributed to procedural factors14. We surmise that the characteristics of lesions requiring long stents or the long stenting procedure itself might make stent optimisation difficult, resulting in suboptimal stent implantation such as underexpansion and malapposition. In contrast, stent factors which are usually related to late ST such as incomplete endothelialisation and delayed healing are improved in the second-generation DESs. This might have resulted in no relationship being seen between late ST and long stenting in our study.
Choi et al reported that IVUS-guided PCI was associated with a lower risk of cardiac death and adverse cardiac events compared with angiographic guidance in patients with complex lesions including long lesions (implanted stent length ≥38 mm)15. In addition, Zhang et al recently demonstrated that IVUS-guided DES implantation significantly improved clinical outcome in the randomised all-comers ULTIMATE trial16. In our study, the long stenting group showed worse clinical outcomes even though IVUS was adopted more frequently (34.7% vs 46.9%, short vs long stenting group, p-value <0.001) (Table 2). However, our study did not compare the outcomes according to the use of IVUS guidance in the long stenting group. Our best interpretation of the data is that the long stenting group showed worse outcomes in spite of a higher rate of IVUS guidance.
Theoretically, thinner strut thickness and advanced polymer technology of second-generation DESs might reduce the risk of ST while restenosis is adequately inhibited5. Despite this suggested efficacy of second-generation DESs, long stenting was still a major prognostic determinant of poor outcomes in our study. Some plausible causes might be surmised. First, the lesions which need long stenting are usually more complex, such as type B2 or C lesions including left main disease, bifurcation, tortuosity, and calcification. Moreover, they have a higher plaque burden. These lesion characteristics might be related to worse outcomes17. However, long stenting was an important prognostic factor of clinical outcomes after adjustment of these lesion characteristics. Second, the risk of vascular injury is higher during implantation of long stents. Subsequently, these technical characteristics might result in more clinical events. Third, the patients with long lesions have a worse cardiovascular risk profile such as old age, diabetes, hypertension, and chronic kidney disease, as our study showed. These risk factors are well known to cause endothelial dysfunction and atherosclerosis and contribute to worse outcomes11. However, long stenting was still associated with a higher risk of TLF and ST even after these confounding patient characteristics were statistically adjusted in our study. Fourth, stent overlapping might be linked to clinical events. Because the longest length of a single stent is shorter than 40 mm in most commercialised DESs, stenting longer than 40 mm inevitably requires overlapping of two stents or more. Räber et al reported that stent overlapping was associated with a higher risk of TLR and poor clinical outcomes including death or MI in the first-generation DESs (n=1,012 including 134 overlapping cases)18. In contrast, Sgueglia et al showed that stent overlapping was not a determinant of adverse outcomes such as MACE, cardiac death, TVR, and ST in the second-generation DES (n=203 including 79 overlapping cases)19. O’Sullivan et al also reported that stent overlapping was not associated with worse clinical outcomes including death, MI, and TVR in second-generation DESs (n=1,601 including 580 overlapping cases)20. In our study (n=8,035 for the short stenting group, including 307 overlapping cases), overlapping itself was not associated with worse clinical outcomes (Supplementary Table 6). Fifth, the improved performance of second-generation DESs might not be adequate to avoid clinical events in stenting longer than 40 mm for now. Further advances regarding strut thickness, stent material, polymer technology, drug elution and more might be necessary for successful long stenting.
Limitations
There are some limitations in this study. First, our study has the intrinsic limitations of non-randomised comparisons such as allocation bias, different distribution of clinical risk factors and lesion characteristics, and the possibility of influences from unmeasured confounding factors, although we used Cox regression analysis with IPTW to overcome this intrinsic limitation. Second, our study did not analyse the impact of lesion length, but that of stenting length. Therefore, careful attention is necessary when interpreting this study. This study does not convey that a shorter stenting strategy is better than a long stenting one for lesions longer than 40 mm. It only indicates that careful decision making and meticulous follow-up are mandatory for lesions requiring stenting longer than 40 mm. Third, the median follow-up duration of this study was two years. To understand the longer-term clinical relevance of long stenting, further studies are warranted. Fourth, current guidelines preferentially recommend more potent P2Y12 inhibitors such as ticagrelor and prasugrel in acute coronary syndrome (ACS). However, most of the patients with ACS in our study were prescribed clopidogrel. Our results might not reflect the current practice in ACS patients well.
Conclusions
In the contemporary second-generation DES era, long stenting (>40 mm) continues to be associated with poor clinical outcomes such as TLF, cardiac death, TLR, and stent thrombosis. In a situation requiring stenting longer than 40 mm, understanding the potential future risk should be carefully considered.
Impact on daily practice
In the era of second-generation DESs with thinner strut thickness and advanced polymer technology, the worse clinical outcomes in long stenting could be questioned. Our large-scale study revealed that stenting longer than 40 mm was still associated with poor outcomes in the contemporary DES era. Thus, for lesions requiring stenting longer than 40 mm, interventionists should keep in mind the possibility of future adverse events after stenting and should judge the treatment plan carefully.
Appendix. Study collaborators
Young Jin Choi, MD, PhD; Division of Cardiology, Department of Internal Medicine, Sejong General Hospital, Bucheon, Republic of Korea. Eun-Seok Shin, MD, PhD; Department of Cardiology, Ulsan Medical Center, Ulsan Hospital, Ulsan, Republic of Korea. Jang-Whan Bae, MD, PhD; Division of Cardiology, Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Republic of Korea. Kook-Jin Chun, MD, PhD; Division of Cardiology, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea. Doo-Il Kim, MD, PhD; Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea. Seung-Woon Rha, MD, PhD; Cardiovascular Center, Korea University Guro Hospital, Seoul, Republic of Korea. Sung Yun Lee, MD, PhD; Division of Cardiology, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Republic of Korea. Jay Young Rhew, MD, PhD; Department of Internal Medicine and Cardiovascular Center, Presbyterian Medical Center, Jeonju, Republic of Korea. Seong-Ill Woo, MD, PhD; Division of Cardiology, Department of Internal Medicine, Inha University Hospital, Incheon, Republic of Korea. Han Cheol Lee, MD, PhD; Division of Cardiology, Department of Internal Medicine, Pusan National University Hospital, Busan, Republic of Korea. Jin-Ok Jeong, MD, PhD; Division of Cardiology, Department of Internal Medicine, Chungnam National University School of Medicine, Daejon, Republic of Korea.
Supplementary data
Full names of the registries.
Endpoints and definitions.
Statistical analysis.
Kaplan-Meier curves of target lesion failure (TLF) according to stent type.
Kaplan-Meier curves of definite or probable stent thrombosis (ST) according to stent type.
Use of dual antiplatelet therapy.
Length profile of stents.
Independent predictors of target lesion failure (TLF).
Independent predictors of definite or probable stent thrombosis.
Clinical outcomes by overlapping.
Clinical outcomes by overlapping in patients with a total stent length ≤40 mm.
Acknowledgments
Funding
This study was supported by SNUH (Grant no. 06-2011-3680, 06-2011-3280, 06-2010-1560, 06-2008-2020, 06-2009-2340).
Conflict of interest statement
The authors/study collaborators have no conflicts of interest to declare.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- POCO
patient-oriented composite outcomes
- TLF
target lesion failure
- TLR
target lesion revascularisation
Contributor Information
Min Kong, Cardiovascular Center, Seoul National University Hospital, Seoul, Republic of Korea.
Jung-Kyu Han, Cardiovascular Center, Seoul National University Hospital, Seoul, Republic of Korea.
Hyun-Jae Kang, Cardiovascular Center, Seoul National University Hospital, Seoul, Republic of Korea.
Bon-Kwon Koo, Cardiovascular Center, Seoul National University Hospital, Seoul, Republic of Korea.
In-Ho Chae, Cardiovascular Center, Seoul National University Bundang Hospital, Seongnam-si, Republic of Korea.
Hyo-Soo Kim, Cardiovascular Center, Seoul National University Hospital, Seoul, Republic of Korea.
References
- Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- Honda Y, Muramatsu T, Ito Y, Sakai T, Hirano K, Yamawaki M, Araki M, Kobayashi N, Takimura H, Sakamoto Y, Mouri S, Tsutumi M, Takama T, Takafuji H, Tokuda T, Makino K. Impact of ultra-long second-generation drug-eluting stent implantation. Catheter Cardiovasc Interv. 2016;87:E44–53. doi: 10.1002/ccd.26010. [DOI] [PubMed] [Google Scholar]
- Moreno R, Fernández C, Hernandez R, Alfonso F, Angiolillo DJ, Sabate M, Escaned J, Banuelos C, Fernández-Ortiz A, Macaya C. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005;45:954–9. doi: 10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- Habara S, Mitsudo K, Goto T, Kadota K, Fujii S, Yamamoto H, Kato H, Takenaka S, Fuku Y, Hosogi S, Hirono A, Yamamoto K, Tanaka H, Hasegawa D, Nakamura Y, Tasaka H, Otsuru S, Okamoto Y, Yamada C, Miyamoto M, Inoue K. The impact of lesion length and vessel size on outcomes after sirolimus-eluting stent implantation for in-stent restenosis. Heart. 2008;94:1162–5. doi: 10.1136/hrt.2007.128595. [DOI] [PubMed] [Google Scholar]
- Kedhi E, Joesoef KS, McFadden E, Wassing J, Van Mieghem C, Goedhart D and Smits PC. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–9. doi: 10.1016/S0140-6736(09)62127-9. [DOI] [PubMed] [Google Scholar]
- Udipi K, Melder RJ, Chen M, Cheng P, Hezi-Yamit A, Sullivan C, Wong J, Wilcox J. The next generation Endeavor Resolute Stent: role of the BioLinx Polymer System. EuroIntervention. 2007;3:137–9. [PubMed] [Google Scholar]
- Chevalier B, Silber S, Park SJ, Garcia E, Schuler G, Suryapranata H, Koolen J, Hauptmann KE, Wijns W, Morice MC, Carrié D, van Es GA, Nagai H, Detiege D, Paunovic D, Serruys PW NOBORI 1 Clinical Investigators. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial--Phase 2. Circ Cardiovasc Interv. 2009;2:188–95. doi: 10.1161/CIRCINTERVENTIONS.108.823443. [DOI] [PubMed] [Google Scholar]
- Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ SPIRIT IV Investigators. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663–74. doi: 10.1056/NEJMoa0910496. [DOI] [PubMed] [Google Scholar]
- Choi IJ, Koh YS, Lim S, Kim JJ, Chang M, Kang M, Hwang BH, Kim CJ, Kim TH, Seo SM, Shin DI, Park MW, Choi YS, Park HJ, Her SH, Kim DB, Kim PJ, Lee JM, Park CS, Moon KW, Chang K, Kim HY, Yoo KD, Jeon DS, Chung WS, Seung KB. Impact of the stent length on long-term clinical outcomes following newer-generation drug-eluting stent implantation. Am J Cardiol. 2014;113:457–64. doi: 10.1016/j.amjcard.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Konishi H, Miyauchi K, Dohi T, Tsuboi S, Ogita M, Naito R, Kasai T, Tamura H, Okazaki S, Isoda K, Daida H. Impact of stent length on clinical outcomes of first-generation and new-generation drug-eluting stents. Cardiovasc Interv Ther. 2016;31:114–21. doi: 10.1007/s12928-015-0362-0. [DOI] [PubMed] [Google Scholar]
- Bouras G, Jhamnani S, Vivian G, Haimi I, Mao V, Deible R, Cao S, Krishnakutty S, Lansky AJ. Clinical outcomes after PCI treatment of very long lesions with the XIENCE V everolimus eluting stent; Pooled analysis from the SPIRIT and XIENCE V USA prospective multicenter trials. Catheter Cardiovasc Interv. 2017;89:984–91. doi: 10.1002/ccd.26711. [DOI] [PubMed] [Google Scholar]
- Hiromasa T, Kuramitsu S, Shinozaki T, Jinnouchi H, Morinaga T, Kobayashi Y, Domei T, Soga Y, Shirai S, Ando K. Impact of total stent length after cobalt chromium everolimus-eluting stent implantation on 3-year clinical outcomes. Catheter Cardiovasc Interv. 2017;89:207–16. doi: 10.1002/ccd.26455. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar J, Baber U, Sartori S, Stefanini GG, Sarin M, Vogel B, Farhan S, Camenzind E, Leon MB, Stone GW, Serruys PW, Wijns W, Steg PG, Weisz G, Chieffo A, Kastrati A, Windecker S, Morice MC, Smits PC, von Birgelen C, Mikhail GW, Itchhaporia D, Mehta L, Kim HS, Valgimigli M, Jeger RV, Kimura T, Galatius S, Kandzari D, Dangas G, Mehran R. Effect of Increasing Stent Length on 3-Year Clinical Outcomes in Women Undergoing Percutaneous Coronary Intervention With New-Generation Drug-Eluting Stents: Patient-Level Pooled Analysis of Randomized Trials From the WIN-DES Initiative. JACC Cardiovasc Interv. 2018;11:53–65. doi: 10.1016/j.jcin.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7:1081–92. doi: 10.1016/j.jcin.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Choi KH, Song YB, Lee JM, Lee SY, Park TK, Yang JH, Choi JH, Choi SH, Gwon HC, Hahn JY. Impact of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention on Long-Term Clinical Outcomes in Patients Undergoing Complex Procedures. JACC Cardiovasc Interv. 2019;12:607–20. doi: 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, Li Q, Liu Z, Chen Y, Qian X, Wang J, Chai D, Chen C, Li X, Gogas BD, Pan T, Shan S, Ye F, Chen SL. Intravascular Ultrasound-Guided Versus Angiography-Guided Implantation of Drug-Eluting Stent in All-Comers: The ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126–37. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Caputo RP, Goel A, Pencina M, Cohen DJ, Kleiman NS, Yen CH, Waksman R, Tolerico P, Dhar G, Gordon P, Bach RG, Lopez JJ. Impact of drug eluting stent length on outcomes of percutaneous coronary intervention (from the EVENT registry). Am J Cardiol. 2012;110:350–5. doi: 10.1016/j.amjcard.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Räber L, Jüni P, Löffel L, Wandel S, Cook S, Wenaweser P, Togni M, Vogel R, Seiler C, Eberli F, Lüscher T, Meier B, Windecker S. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55:1178–88. doi: 10.1016/j.jacc.2009.11.052. [DOI] [PubMed] [Google Scholar]
- Sgueglia GA, Belloni F, Summaria F, Conte M, Cortese B, Silva PL, Ricci R, Lioy E, Pucci E, Gaspardone A. One-year follow-up of patients treated with new-generation polymer-based 38 mm everolimus-eluting stent: the P38 study. Catheter Cardiovasc Interv. 2015;85:218–24. doi: 10.1002/ccd.25542. [DOI] [PubMed] [Google Scholar]
- O’Sullivan CJ, Stefanini GG, Räber L, Heg D, Taniwaki M, Kalesan B, Pilgrim T, Zanchin T, Moschovitis A, Büllesfeld L, Khattab AA, Meier B, Wenaweser P, Jüni P, Windecker S. Impact of stent overlap on long-term clinical outcomes in patients treated with newer-generation drug-eluting stents. EuroIntervention. 2014;9:1076–84. doi: 10.4244/EIJV9I9A182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full names of the registries.
Endpoints and definitions.
Statistical analysis.
Kaplan-Meier curves of target lesion failure (TLF) according to stent type.
Kaplan-Meier curves of definite or probable stent thrombosis (ST) according to stent type.
Use of dual antiplatelet therapy.
Length profile of stents.
Independent predictors of target lesion failure (TLF).
Independent predictors of definite or probable stent thrombosis.
Clinical outcomes by overlapping.
Clinical outcomes by overlapping in patients with a total stent length ≤40 mm.