Abstract
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are a leading cause of hospitalisation and death in COPD patients. In addition to the identification of better strategies to prevent AECOPD, there is an intense focus on discovering novel markers of disease severity that enhance risk stratification on hospital admission for the targeted institution of aggressive versus supportive treatments. In the quest for such biomarkers, an increasing body of evidence suggests that specific indexes derived from routine complete blood counts, i.e. the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), can significantly predict adverse outcomes in AECOPD. This narrative review discusses the current evidence regarding the association between the NLR and the PLR on admission and several clinical end-points (need for invasive ventilation, noninvasive mechanical ventilation failure, admission to an intensive care unit, pulmonary hypertension, length of hospitalisation, and mortality) in AECOPD. Future research directions and potential clinical applications of these haematological indexes in this patient group are also discussed.
Short abstract
A review of the relationship between neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte (PLR) ratios and several adverse outcomes in AECOPD. NLR emerges as a useful predictor of mortality in these patients; the prognostic value of PLR is less clear. https://bit.ly/3Jo6XGs
Introduction
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD), a clinical state that is characterised by the rapid worsening in airway function and respiratory symptoms, represents the leading cause of hospitalisation and death in COPD patients and a significant financial burden to healthcare systems [1, 2]. Between 22–40% and 9–16% of patients with COPD suffer at least one or more than one episode of AECOPD annually, respectively [3]. Although a wide range of infectious and noninfectious factors trigger AECOPD, pharmacological management is standard and includes β2-agonists, glucocorticoids and antibiotics [1, 4]. The lack of objective measures of AECOPD has prompted the search for novel, easily measurable prognostic biomarkers to develop more effective, patient-centred, management strategies [5–7]. Epidemiological studies have reported that circulating biomarkers of inflammation, e.g. C-reactive protein (CRP), fibrinogen, cytokines and white blood cell differentiation, do not significantly enhance risk prediction over clinical assessment, including history of previous AECOPD [8, 9]. Other studies, however, have shown that the combination of high CRP concentrations, neutrophils and laboured breathing is effective in discriminating between AECOPD and stable COPD [10].
Haematological indexes of inflammation, e.g. the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), have been investigated in several disease states, e.g. solid tumours, systemic lupus erythematosus, coronary artery disease, retinal artery occlusion, chronic kidney disease and stable COPD [11–24].
This narrative review critically appraises recent developments in the capacity of the NLR and the PLR on admission to predict several clinical end-points specifically in AECOPD and discusses future research directions and potential clinical applications of these haematological indexes in this group.
Methods
We searched PubMed from inception to May 2022 for articles reporting retrospective or prospective studies investigating the associations between the NLR and/or the PLR assessed within 24–48 h of hospital admission and pre-defined clinical end-points (all-cause mortality, admission to the intensive care unit (ICU), length of hospital stay, noninvasive mechanical ventilation failure, need for invasive ventilation, pulmonary hypertension and composite outcomes) in adult patients hospitalised with a clinical diagnosis of AECOPD. The following data were extracted from each study: study design, sample size, specific in-hospital setting, outcome(s) investigated and relevant odds ratio (OR), area under the curve, cut-off used for NLR and PLR, sensitivity, specificity, and any other between-group comparisons of the NLR and/or the PLR. Plots of estimates weighted by power were also generated to provide an overall quantitative assessment of the association between the NLR and the PLR and clinical outcomes. Analyses were performed using Stata 14 (StataCorp LLC, College Station, TX, USA).
Results
18 studies investigated associations between the NLR and clinical outcomes (table 1) whereas 10 studies investigated associations between the PLR and clinical outcomes (table 2). The following paragraphs describe the main characteristics and results of individual studies.
TABLE 1.
Characteristics of studies investigating the association between neutrophil-to-lymphocyte ratio (NLR) on admission and adverse outcomes in acute exacerbations of chronic obstructive pulmonary disease
| First author, year, country | Study design | Sample size | Setting | Outcome | OR (95% CI) | AUC (95% CI) | Cut-off | Sensitivity (%) | Specificity (%) | Additional findings |
| Kumar, 2017, Australia [25] | R | 181 | ED | Mortality | 0.95# (0.84–1.08) | NR | NR | NR | NR | NLR in survivors versus nonsurvivors: 7±8 versus 13±10, p=0.004 |
| Yao, 2017, China [26] | R | 303 | Ward | Mortality | NR | 0.803 (NR) | 6.24 | 0.81 | 0.69 | NLR in survivors versus nonsurvivors: 7±8 versus 15±10, p<0.001 |
| Rahimirad, 2017, Iran [27] | R | 174 | Ward | Mortality | 3.586# (1.69–7.60) | 0.717 (0.623–0.811) | 4 | 0.87 | 0.4 | NLR in survivors versus nonsurvivors: 8±8 versus 17±18, p<0.001 |
| Aksoy, 2018, Turkey [28] | R | 2727 | Ward | Mortality | 1.13# (0.46–2.78) | NR | NR | NR | NR | NLR¶ in survivors versus nonsurvivors: 7 (4–12) versus 10 (4–19), p=0.07 |
| Ergun, 2018, Turkey [29] | P | 132 | ICU | Mortality | NR | NR | NR | NR | NR | NLR in survivors versus nonsurvivors: 18±23 versus 24±32, p=0.65 |
| Teng, 2018, China [30] | R | 904 | Ward | Mortality | 1.067# (1.039–1.095) | 0.737 (0.661–0.814) | 8.13 | 0.61 | 0.75 | NLR significantly higher in nonsurvivors, p<0.001 |
| Liu, 2019, China [31] | R | 622 | ED | Mortality | 2.05# (1.21–3.48) | 0.742 (0.554–0.881) | 4.19 | 0.71 | 0.74 | NLR in survivors versus nonsurvivors: 3±7 versus 8±10, p<0.001 |
| Yilmaz, 2019, Turkey [32] | R | 171 | ICU | Mortality | 1.902# (1.108–3.266) | NR | 3.18 | 0.71 | 0.72 | NLR in survivors versus nonsurvivors: 2.8±1.4 versus 3.5±1.9, p=0.037 |
| Ardestani, 2020, Iran [33] | R | 829 | NR | Mortality | 1.08# (1.02–1.14) | 0.7 (0.67–0.73) | 6.9 | 0.61 | 0.73 | NLR in survivors versus nonsurvivors: 6±5 versus 11±10, p<0.001 |
| Luo, 2021, China [34] | R | 533 | Ward | Mortality | 3.87# (1.29–10.3) | 0.801 (NR) | 6.74 | 0.83 | 0.71 | NLR in survivors versus nonsurvivors: 8±6 versus 15±13, p<0.001 |
| Yao, 2021, China [35] | R | 146 | Ward | Mortality | 1.01# (0.999–1.022) | 0.83 (0.761–0.899) | 16.83 | 0.69 | 0.65 | NLR¶ in survivors versus nonsurvivors: 12 (6–25) versus 22 (11–40), p<0.001 |
| Karauda, 2021, Poland [36] | R | 275 | Ward | Mortality | NR | 0.96 (0.93–0.99) | 13.2 | 1.00 | 0.93 | NR |
| Teng, 2018, China [30] | R | 906 | Ward | ICU | 1.046# (1.023–1.068) | 0.676 (0.607–0.744) | 8.13 | 0.54 | 0.77 | NLR significantly higher in patients admitted to ICU, p<0.001 |
| Teng, 2018, China [30] | R | 906 | Ward | IMV | 1.042# (1.019–1.066) | 0.732 (0.656–0.807) | 10.345 | 0.54 | 0.85 | NLR significantly higher in patients requiring IMV, p<0.001 |
| Sun, 2021, China [37] | R | 212 | Ward | NIMVF | 10.783# (2.069–56.194) | 0.858 (0.785–0.931) | 8.9 | 0.69 | 0.88 | NLR¶ in NIMV success versus failure: 4 (3–6) versus 14 (7–17), p<0.001 |
| Zuo, 2019, China [38] | R | 185 | NR | PH | 1.161# (0.924–1.458) | 0.701 (0.629–0.766) | 4.659 | 0.81 | 0.6 | NLR¶ in patients without versus with PH: 4 (3–6) versus 6 (5–12), p<0.001 |
| Wang, 2022, China [39] | P | 598 | Ward | LHS | 0.981# (0.963–0.999) | NR | NR | NR | NR | NLR in patients with normal, mildly prolonged and significantly prolonged hospital stay: 4.6±6.0, 6.0±8.1 and 5.5±5.3, p=0.006 |
| Esmaeel, 2017, Egypt [40] | P | 80 | Ward | Death/ICU | 1.2# (0.9–1.5) | 0.642 (0.526–0.746) | 3.4 | 0.89 | 0.49 | NR |
| Gòmez-Rosero, 2021, Colombia [41] | P | 610 | Ward | Death/ICU | 3.0# (1.7–5.4) | NR | NR | NR | NR | NLR¶ in survivors/non-ICU admitted versus nonsurvivors/ICU admitted: 5 (3–10) versus 8 (5–14), p<0.001 |
| Lu, 2021, China [42] | R | 282 | Ward | Death/ICU/IMV | 41.85# (9.57–306.74) | 0.883 (0.771–0.894) | 10.23 | 0.62 | 0.92 | NLR¶ in survivors/non-ICU admitted/not requiring IMV versus nonsurvivors/ICU admitted/requiring IMV: 5 (3–8) versus 11 (8–16), p<0.001 |
AUC: area under the curve; CI: confidence interval; ED: emergency department; ICU: intensive care unit; IMV: invasive mechanical ventilation; LHS: length of hospital stay; OR: odds ratio; NIMV: noninvasive mechanical ventilation; NIMVF: noninvasive mechanical ventilation failure; NR: not reported; P: prospective; PH: pulmonary hypertension; R: retrospective. #: from multivariate analysis. ¶: median and interquartile range.
TABLE 2.
Characteristics of studies investigating the association between platelet-to-lymphocyte ratio (PLR) on admission and adverse outcomes in acute exacerbations of chronic obstructive pulmonary disease
| First author, year, country | Study design | Sample size | Setting | Outcome | OR (95% CI) | AUC (95% CI) | Cut-off | Sensitivity (%) | Specificity (%) | Additional findings |
| Kumar, 2017, Australia [25] | R | 181 | ED | Mortality | 1.15# (1.02–1.30) | 0.695 (0.551–0.840) | 235 | 63 | 74 | PLR in survivors versus nonsurvivors: 208±164 versus 348±228, p=0.01 |
| Yao, 2017, China [26] | R | 303 | Ward | Mortality | NR | 0.639 (NR) | 182.68 | 64.86 | 58.27 | PLR in survivors versus nonsurvivors: 198±141 versus 273±184, p=0.004 |
| Rahimirad, 2017, Iran [27] | R | 174 | Ward | Mortality | NR | 0.576 (0.465–0.686) | 150 | 59 | 46 | PLR in survivors versus nonsurvivors: 207±192 versus 241±224, p=0.15 |
| Aksoy, 2018, Turkey [28] | R | 2,727 | Ward | Mortality | NR | NR | NR | NR | NR | PLR¶ in survivors versus nonsurvivors: 225 (147–354) versus 224 (123–391), p=0.75 |
| Ergun, 2018, Turkey [29] | P | 132 | ICU | Mortality | NR | NR | NR | NR | NR | PLR in survivors versus nonsurvivors: 308±260 versus 405±414, p=0.26 |
| Yilmaz, 2019, Turkey [32] | R | 171 | ICU | Mortality | 1.004# (0.994–1.014) | NR | NR | NR | NR | PLR in survivors versus nonsurvivors: 98±57 versus 128±82, p=0.021 |
| Ardestani, 2020, Iran [33] | R | 829 | NR | Mortality | 1.00# (0.99–1.01) | 0.56 (0.52–0.59) | 213 | 58 | 82 | PLR in survivors versus nonsurvivors: 153±105 versus 196±154, p=0.11 |
| Luo, 2021, China [34] | R | 533 | Ward | Mortality | 3.45# (1.43–12.62) | 0.750 (NR) | 203.60 | 76.86 | 65.27 | PLR in survivors versus nonsurvivors: 212±90 versus 284±132, p<0.001 |
| Yao, 2021, China [35] | R | 146 | Ward | Mortality | NR | NR | NR | NR | NR | PLR¶ in survivors versus nonsurvivors: 257 (160–417) versus 270 (162–408), p=0.47 |
| Zuo, 2019, China [38] | R | 185 | NR | PH | 1.003# (0.993–1.013) | 0.669 (0.596–0.736) | 150 | 77.2 | 53.6 | PLR¶ in patients without versus with PH: 157 (123–227) versus 221 (161–291), p<0.001 |
AUC: area under the curve; CI: confidence interval; ED: emergency department; ICU: intensive care unit; OR: odds ratio; NR: not reported; P: prospective; PH: pulmonary hypertension; R: retrospective. #: from multivariate analysis. ¶: median and interquartile range.
NLR
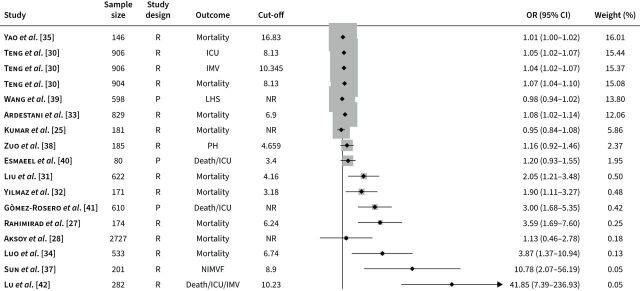
Studies describing the association between the NLR and clinical outcomes in AECOPD have been published between 2017 and 2022. Outcomes investigated included mortality (12 studies) [25–36], ICU transfer (one study) [30], need for invasive ventilation (one study) [30], noninvasive ventilation failure (one study) [37], pulmonary hypertension (one study) [38], length of hospitalisation (one study) [39] and composite end-points (three studies) [40–42]. One study investigated three outcomes separately [30]. Three studies were prospective [39–41]. 15 studies reported independent associations between the NLR and clinical outcomes using multivariate logistic regression [25, 27, 28, 30–35, 38–42], and in 12 receiver operating characteristic (ROC) analysis was conducted to evaluate the prediction performance of specific cut-off values (table 1) [26, 27, 30, 31, 33–38, 40, 42]. All reported cut-off values were calculated by ROC analysis in each study. All studies described the use of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, barring two [27, 33]. A plot of the associations (ORs) between the NLR and adverse outcomes is described in figure 1.
FIGURE 1.
Odds ratios (ORs) obtained from forest plots, squares (proportional to weights) and associated confidence intervals (CIs) describing the association between the neutrophil-to-lymphocyte ratio and adverse outcomes. The vertical line represents the value of no effect. All ORs were from multivariate analysis. ICU: intensive care unit; IMV: invasive mechanical ventilation; LHS: length of hospital stay; NIMVF: noninvasive mechanical ventilation failure; NR: not reported; P: prospective; PH: pulmonary hypertension; R: retrospective.
Mortality
Kumar et al. [25] reported that the NLR was not independently associated with mortality after adjusting for age, haemoglobin, neutrophil count, urea and PLR. Yao et al. [26] reported that the NLR was significantly higher in nonsurvivors than survivors, and a significant positive correlation was observed between the NLR and CRP concentrations. Notably, combining the NLR with other parameters did not significantly improve the predictive capacity, suggesting that the NLR per se may be a useful prognostic marker. Rahimirad et al. [27] reported that the NLR was independently associated with in-hospital mortality after adjusting for age, sex, anaemia and thrombocytopenia. Aksoy et al. [28] reported that in multivariate analysis the NLR was not significantly associated with mortality after adjusting for age, CRP, albumin, platelet count/mean platelet volume ratio, use of steroids, hospital stay >7 days and eosinophilia. Similarly, Ergun et al. [29] failed to observe significant differences in NLR values between survivors and nonsurvivors. In this study, significant associations were reported between the NLR and white blood cell count (r=0.397, p<0.001) and CRP concentrations (r=0.190, p=0.028). Teng et al. [30] reported that, in multivariate logistic regression, the NLR was independently associated with mortality, together with renal failure, heart failure and upper gastrointestinal bleeding. Liu et al. [31] reported that the NLR was independently associated with mortality at 90 days after adjusting for smoking, blood pressure, haemoglobin, CRP and pH. Similarly, Yilmaz et al. [32] and Ardestani et al. [33] reported independent associations between the NLR and in-hospital mortality, and Luo et al. [34] reported independent associations between the NLR and 28-day mortality. ROC analysis showed that, of all the variables considered, the NLR had the highest area under the curve. Notably, combining NLR values with the PLR and CRP concentrations further increased the predictive performance [31]. Yao et al. [35] reported that, in AECOPD patients with heart failure, 28-day mortality was independently associated with brain natriuretic peptide (NT-proBNP) and the NLR. In line with the study by Luo et al. [34], combining NLR values with CRP/albumin and NT-proBNP increased the predictive performance. Finally, Karauda et al. [36] reported a relatively high area under the curve (AUC), 0.96, for the NLR in regard to in-hospital mortality.
Other adverse outcomes
Teng et al. [30] reported that the NLR was significantly associated with the risk of transfer to ICU and the need for invasive mechanical ventilation after adjusting for diabetes mellitus, osteoarthritis, reflux esophagitis, renal failure, hyperlipidaemia and upper gastrointestinal bleeding. Sun et al. [37] reported that the capacity of the NLR to predict noninvasive mechanical ventilation failure was superior to that of leukocyte count, CRP and procalcitonin. The NLR was also independently associated with noninvasive mechanical ventilation failure in multivariate logistic regression after adjusting for leukocyte count, procalcitonin, and CRP. Zuo et al. [38] observed that AECOPD patients with pulmonary hypertension had significantly higher median NLR values than patients without it. However, in multivariate analysis, the association between the NLR and pulmonary hypertension was no longer significant. Wang et al. [39] reported significant differences in the NLR between AECOPD patients with normal (<7 days), mildly prolonged (between 7 and 10 days) and significantly prolonged (≥11 days) hospitalisation. In particular, the NLR and erythrocyte sedimentation rate (ESR) were significantly higher in those with mild and prolonged hospitalisation compared to the reference group. However, no significant differences in NLR and ESR were observed between the mild and the significantly prolonged hospitalisation group. In logistic regression, NLR, ESR, hypertension and chronic cor pulmonale remained independently associated with length of stay. Esmaeel et al. [40] reported that thrombocytopenia, renal function and GOLD group, but not the NLR, were independently associated with a composite outcome of ICU transfer and/or death in multivariate logistic regression. Gòmez-Rosero et al. [41] reported that the NLR was independently associated with a composite outcome of ICU transfer and death in multivariate analysis. In this study, NLR values >5 decreased the probability of being discharged alive by 27% and increased the length of stay by 37% compared to values ≤5. Similarly, Lu et al. [42] reported that NLR values were significantly associated with a composite outcome of death, ICU transfer or need for invasive mechanical ventilation after adjusting for age, sex, body mass index, smoking status, comorbidities, GOLD grade and therapy.
PLR
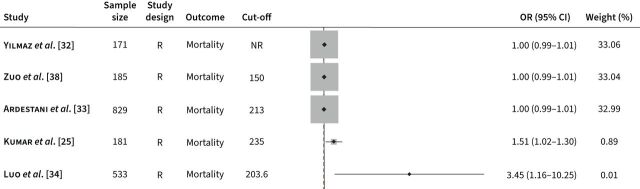
10 studies, published between 2017 and 2021, have investigated the association between the PLR and clinical outcomes in AECOPD. Outcomes investigated were limited to mortality (nine studies) [25–29, 32–35] and pulmonary hypertension (one study) [38]. All studies were retrospective [25–29, 32–35, 38]. Four studies reported independent associations between the NLR and clinical outcomes in multivariate logistic regression [25, 33, 34, 38], and in six ROC analysis was conducted to evaluate the prediction performance of specific cut-off values (table 2) [25–27, 33, 34, 38]. All reported cut-off values were calculated by ROC analysis in each study. All studies described the use of the GOLD guidelines, barring two [27, 33]. A plot of the associations (ORs) between the PLR and adverse outcomes is described in figure 2.
FIGURE 2.
Odds ratios (ORs) obtained from forest plots, squares (proportional to weights) and associated confidence intervals (CIs) describing the association between the platelet-to-lymphocyte ratio and adverse outcomes. The vertical line represents the value of no effect. All ORs were from multivariate analysis. R: retrospective; NR: not reported.
Mortality and pulmonary hypertension
Kumar et al. [25] reported, in multivariate logistic regression, that the PLR was independently associated with mortality after adjusting for age, haemoglobin, neutrophil count, urea concentration and NLR. In the study by Yao et al. [26], in-hospital nonsurvivors had higher PLR values than survivors. ROC analysis revealed that, using a cut-off value of 182.68, PLR predicted in-hospital mortality with an AUC of 0.639. Combining the PLR with other markers, particularly the NLR, increased the AUC to 0.800. Rahimirad et al. [27] reported nonsignificant differences in PLR values between nonsurvivors and survivors. The poor predictive performance in this study was further confirmed by ROC curve analysis (AUC=0.576). These results are similar to those by Aksoy et al. [28], Ergun et al. [29] and Yao et al. [35] reporting nonsignificant differences in PLR between AECOPD survivors and nonsurvivors. Ardestani et al. [33] also reported a nonsignificant difference in PLR values between AECOPD patients who died during hospitalisation and those who were alive on discharge. Accordingly, the PLR was not significantly associated with mortality in multivariate regression. Yilmaz et al. [32] reported significant differences in the PLR between survivors and nonsurvivors; however, no independent associations were observed in multivariate analysis. Luo et al. [34] reported an independent association between PLR values and mortality in multivariate logistic regression. Finally, Zuo et al. [38] reported that the PLR values in AECOPD patients with pulmonary hypertension were significantly higher than those without. However, there was no independent association between the PLR and pulmonary hypertension in multivariate analysis.
Discussion
Despite the relatively high prevalence of AECOPD in patients with COPD and the significant burden of disease exacerbations to patients, relatives, caregivers and healthcare systems, the identification of the exact mechanisms triggering this state as well as objective markers of severity and response to treatment remain an unaddressed issue. There is very good evidence that a heterogeneous group of factors ultimately favours the development of acute bursts of inflammation in the airways. This phenomenon appears to be part of the natural progression of COPD in most patients, which is typically characterised by a state of chronic local and systemic inflammation [1, 43]. It is widely reported that patients with COPD show increased concentrations of serum inflammatory biomarkers. In particular, about 70% of subjects with COPD have been reported to have elevated serum concentrations of at least one inflammatory parameter [44]. Several lines of evidence suggest that clinical and functional parameters, disease progression and development of comorbidities in COPD are strictly related to the persistent elevation of such inflammatory markers [45–47]. Serum CRP, interleukin-6 and tumour necrosis factor-α have been extensively studied as inflammation biomarkers both in COPD and in AECOPD [46]. However, apart from CRP, several limitations related to costs and turnaround time prevent the routine use of these biomarkers in clinical practice. In contrast, inflammatory indexes that are easily derived from routine haematological parameters, particularly the NLR and the PLR, are increasingly studied in inflammatory states, including COPD, in view of their simple and rapid determination and easy interpretation. The potential role of both the NLR and the PLR in clinical practice is supported by their capacity to predict adverse outcomes in a wide range of disease states [11–22]. In the context of COPD, it is well known that a state of chronic inflammation results not only in alterations of neutrophils and platelets but also in the increased production and release of specific inflammatory mediators by these cell types, which ultimately lead to irreversible airway damage. For example, neutrophil activation favours the release of several enzymes such as neutrophil elastase, cathepsin G, proteinase-3, matrix metalloproteinase (MMP)-8, MMP-9 and myeloperoxidase (MPO), which actively contribute to the pathophysiological mechanisms of emphysema and COPD [48]. It has been reported that MPO mediates the bactericidal effects of neutrophils [49]. However, at the same time, there is evidence that MPO can favour the initiation of oxidative tissue damage and alterations in cellular homeostasis and increase the response of lung epithelial cells to pro-inflammatory stimuli [50, 51]. Neutrophil elastase can stimulate the production and secretion of mucin, leading to excessive mucus secretion and airway obstruction [52]. Furthermore, MMP can degrade extracellular matrices, leading to structural damage and airway remodelling in COPD patients [52]. Platelets also play a key role in the modulation of inflammation and immune responses. Platelet p-selectin expression and the subsequent formation of platelet-leukocyte aggregates upregulate leukocyte pro-inflammatory functions. In addition, platelet α-granules contain several types of cytokines with predominant pro-inflammatory effects [53, 54]. For these reasons, it has been suggested that indices that reflect an increase in the number of circulating neutrophils and platelets in relation to lymphocytes may be particularly useful as biomarkers of disease severity and outcome in AECOPD.
Our narrative review has shown that the current evidence supporting the presence of significant associations with several pre-defined adverse clinical outcomes in patients with AECOPD is stronger for the NLR than the PLR. Furthermore, among the 18 studies evaluating the NLR, 15 used multivariate logistic regression to assess the presence of independent associations with adverse outcomes [25, 27, 28, 30–35, 37–42], with significant relationships reported in 10 (OR 1.042–41.85) [27, 30–34, 37, 39, 41, 42]. It is important to emphasise that, in two studies [37, 42], the particularly high OR (10.783 and 41.85, respectively) likely reflects the use of relatively high NLR cut-offs (8.9 and 10.23, respectively), and consequently a greater magnitude of risk, between 10 and 40 times. Although NLR cut-off values were reported for various adverse outcomes, the number of studies investigating outcomes other than mortality was limited [30, 37–42].
In contrast to the NLR, the association between the PLR and adverse outcomes in AECOPD patients using multivariate logistic regression analysis was investigated in only five studies [25, 32–34, 38], with two reporting the presence of independent associations with mortality [25, 34]. Therefore, more research is warranted to investigate the predictive capacity of the PLR in AECOPD.
Limitations of our narrative review include the lack of a systematic assessment of publication bias as well as inherent limitations of the studies identified, particularly their predominantly retrospective nature, the relatively short follow-up (<90 days) and the lack of serial assessments of the NLR and the PLR and their association with clinical progress. Furthermore, information regarding the use of corticosteroids, antibiotics and bronchodilators was inconsistent. Therefore, well-designed prospective clinical studies with longer-term follow-ups are warranted to validate the use of these biomarkers in routine clinical practice. Such studies should ideally include AECOPD patients with a wide range of age, comorbidities, frailty and ethnic background, and assess several clinical end-points. The issue of whether the prognostic capacity of the NLR and/or the PLR is superior to that provided by established inflammatory biomarkers, e.g. CRP, is particularly important in order to justify their introduction in clinical practice. In this context, some of the identified studies reported the presence of significant positive associations between the NLR and CRP concentrations, raising the possibility that the NLR provides redundant clinical information [26, 29, 37]. However, in other studies, the prognostic capacity of the NLR was either independent [31, 33] or superior to that of the CRP [31, 37]. Furthermore, in one study, the combination of NLR, PLR and CRP concentrations further increased the predictive ability compared to the NLR alone [31]. Therefore, appropriate statistical analyses are also warranted in the proposed prospective studies to identify the best biomarker, or combination of biomarkers with or without other clinical characteristics, for prognosis in AECOPD.
Questions for future research
Do the NLR and the PLR on admission significantly predict short- and long-term outcomes in patients admitted for AECOPD in appropriately designed prospective studies?
Do the temporal changes in the NLR and the PLR during hospitalisation correlate with changes in clinical status and response to treatment?
Can the NLR and PLR guide the selection of specific therapies and/or care pathways in patients with AECOPD?
Does the combination of the NLR and/or the PLR with other inflammatory biomarkers and patient characteristics further improve the prognostic capacity in AECOPD?
Conclusions
This narrative review has shown that the NLR, a simple and inexpensive inflammatory index that has been increasingly investigated in a wide range of disease states, including COPD, appears to be a promising predictor of mortality in AECOPD patients. Cut-off values for mortality and other adverse outcomes have been identified, providing the basis for the adequate design of larger prospective studies that investigate several clinical end-points. By contrast, the current evidence regarding the prognostic value of the PLR is less clear and requires further studies. Pending the results of well-designed prospective studies, the routine use of these inflammatory markers may potentially influence the management of AECOPD.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: The authors declare no conflict of interest.
Support statement: This research was supported by grants from the Sardinian Fondo di Sviluppo e Coesione (FSC) 2014–2020, Patto per lo Sviluppo della Regione Sardegna, LR7-2017 (RASSR82005) and Fondo di Ateneo per la Ricerca, annualità 2020. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Ko FW, Chan KP, Hui DS, et al. Acute exacerbation of COPD. Respirology 2016; 21: 1152–1165. doi: 10.1111/resp.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One 2016; 11: e0152618. doi: 10.1371/journal.pone.0152618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gayle A, Dickinson S, Morris K, et al. What is the impact of GOLD 2017 recommendations in primary care? – A descriptive study of patient classifications, treatment burden and costs. Int J Chron Obstruct Pulmon Dis 2018; 13: 3485–3492. doi: 10.2147/COPD.S173664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J 2017; 49: 1700214. doi: 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 5.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 6.Peng Z, Zhang W, Qiao J, et al. Melatonin attenuates airway inflammation via SIRT1 dependent inhibition of NLRP3 inflammasome and IL-1beta in rats with COPD. Int Immunopharmacol 2018; 62: 23–28. doi: 10.1016/j.intimp.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 7.Cheng Q, Fang L, Feng D, et al. Memantine ameliorates pulmonary inflammation in a mice model of COPD induced by cigarette smoke combined with LPS. Biomed Pharmacother 2019; 109: 2005–2013. doi: 10.1016/j.biopha.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 9.Keene JD, Jacobson S, Kechris K, et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med 2017; 195: 473–481. doi: 10.1164/rccm.201607-1330OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noell G, Cosio BG, Faner R, et al. Multi-level differential network analysis of COPD exacerbations. Eur Respir J 2017; 50: 1700075. doi: 10.1183/13993003.00075-2017 [DOI] [PubMed] [Google Scholar]
- 11.Xie X, Li X, Tang W, et al. Prognostic value of the neutrophil-to-lymphocyte ratio and primary tumor location in epidermal growth factor receptor-mutated metastatic non-small cell lung cancer. J Cancer Res Ther 2021; 17: 1618–1625. doi: 10.4103/jcrt.jcrt_1442_21 [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Sun J, Jiang Z, et al. Risk factors and prognostic index model for pancreatic cancer. Gland Surg 2022; 11: 186–195. doi: 10.21037/gs-21-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B, Xiong F, Yi S, et al. Prognostic and clinicopathologic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: an update meta-analysis. Technol Cancer Res Treat 2022; 21: 15330338211070140. doi: 10.1177/15330338211070140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Xin S, Xu B. Value research of NLR, PLR, and RDW in prognostic assessment of patients with colorectal cancer. J Healthc Eng 2022; 2022: 7971415. doi: 10.1155/2022/7971415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi K, Westhuyzen J, Gortman A, et al. Prognostic value of the neutrophil-lymphocyte ratio in triple negative breast cancer patients. Ann Clin Lab Sci 2022; 52: 33–39. [PubMed] [Google Scholar]
- 16.Al Jarroudi O, El Bairi K, Abda N, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of outcomes in inflammatory breast cancer. Biomark Med 2021; 15: 1289–1298. doi: 10.2217/bmm-2020-0717 [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Song S, Zhang L, et al. Preoperative platelet-lymphocyte ratio predicts recurrence of laryngeal squamous cell carcinoma. Future Oncol 2020; 16: 209–217. doi: 10.2217/fon-2019-0527 [DOI] [PubMed] [Google Scholar]
- 18.Liu N, Mao J, Tao P, et al. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Medicine 2022; 101: e28617. doi: 10.1097/MD.0000000000028617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho J, Liang S, Lim SHH, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio reflect disease activity and flares in patients with systemic lupus erythematosus - A prospective study. Joint Bone Spine 2022; 89: 105342. doi: 10.1016/j.jbspin.2022.105342 [DOI] [PubMed] [Google Scholar]
- 20.Pan Q, Zhang W, Li X, et al. Sex difference in the association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Angiology 2022; 73: 470–477. doi: 10.1177/00033197211070884 [DOI] [PubMed] [Google Scholar]
- 21.Pinna A, Porcu T, Paliogiannis P, et al. Complete blood cell count measures in retinal artey occlusions. Acta Ophthalmol 2021; 99: 637–643. doi: 10.1111/aos.14699 [DOI] [PubMed] [Google Scholar]
- 22.Zhao WM, Tao SM, Liu GL. Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail 2020; 42: 1059–1066. doi: 10.1080/0886022X.2020.1832521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paliogiannis P, Fois AG, Sotgia S, et al. The neutrophil-to-lymphocyte ratio as a marker of chronic obstructive pulmonary disease and its exacerbations: a systematic review and meta-analysis. Eur J Clin Invest 2018; 48: e12984. doi: 10.1111/eci.12984 [DOI] [PubMed] [Google Scholar]
- 24.Paliogiannis P, Fois AG, Sotgia S, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev 2018; 27: 170113. doi: 10.1183/16000617.0113-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar P, Law S, Sriram KB. Evaluation of platelet lymphocyte ratio and 90-day mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis 2017; 9: 1509–1516. doi: 10.21037/jtd.2017.05.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 2285–2290. doi: 10.2147/COPD.S141760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimirad S, Ghaffary MR, Rahimirad MH, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. Tuberk Toraks 2017; 65: 25–31. doi: 10.5578/tt.27626 [DOI] [PubMed] [Google Scholar]
- 28.Aksoy E, Gungor S, Agca MC, et al. A revised treatment approach for hospitalized patients with eosinophilic and neutrophilic exacerbations of chronic obstructive pulmonary disease. Turk Thorac J 2018; 19: 193–200. doi: 10.5152/TurkThoracJ.2018.18004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ergun R, Ergan B. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as mortality predictors in critically ill COPD patients. J Ponte 2018; 74: 256–269. doi: 10.21506/j.ponte.2018.4.20 [DOI] [Google Scholar]
- 30.Teng F, Ye H, Xue T. Predictive value of neutrophil to lymphocyte ratio in patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One 2018; 13: e0204377. doi: 10.1371/journal.pone.0204377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Liu J, Zou Y. Relationship between neutrophil-lymphocyte ratio and short-term prognosis in the chronic obstructive pulmonary patients with acute exacerbation. Biosci Rep 2019; 39: BSR20190675. doi: 10.1042/BSR20190675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz G, Salihoglu Z. Does mean platelet volume/platelet count ratio and red blood cell distribution width predict in-hospital mortality in patients admitted for acute exacerbation of chronic obstructive pulmonary disease? J Immunol Clin Microbiol 2019; 4: 18–25. [Google Scholar]
- 33.Ardestani ME, Alavi-Naeini N. Evaluation of the relationship of neutrophil-to lymphocyte ratio and platelet-to-lymphocyte ratio with in-hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J 2021; 15: 382–388. doi: 10.1111/crj.13312 [DOI] [PubMed] [Google Scholar]
- 34.Luo Z, Zhang W, Chen L, et al. Prognostic value of neutrophil:lymphocyte and platelet:lymphocyte ratios for 28-day mortality of patients with AECOPD. Int J Gen Med 2021; 14: 2839–2848. doi: 10.2147/IJGM.S312045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao C, Wang L, Shi F, et al. Optimized combination of circulating biomarkers as predictors of prognosis in AECOPD patients complicated with Heart Failure. Int J Med Sci 2021; 18: 1592–1599. doi: 10.7150/ijms.52405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karauda T, Kornicki K, Jarri A, et al. Eosinopenia and neutrophil-to-lymphocyte count ratio as prognostic factors in exacerbation of COPD. Sci Rep 2021; 11: 4804. doi: 10.1038/s41598-021-84439-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun W, Luo Z, Jin J, et al. The neutrophil/lymphocyte ratio could predict noninvasive mechanical ventilation failure in patients with acute exacerbation of chronic obstructive pulmonary disease: a retrospective observational study. Int J Chron Obstruct Pulmon Dis 2021; 16: 2267–2277. doi: 10.2147/COPD.S320529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuo H, Xie X, Peng J, et al. Predictive value of novel inflammation-based biomarkers for pulmonary hypertension in the acute exacerbation of chronic obstructive pulmonary disease. Anal Cell Pathol 2019; 2019: 5189165. doi: 10.1155/2019/5189165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Yang T, Yu X, et al. Risk factors for length of hospital stay in acute exacerbation chronic obstructive pulmonary disease: a multicenter cross-sectional study. Int J Gen Med 2022; 15: 3447–3458. doi: 10.2147/IJGM.S354748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esmaeel HM, Ahmed HA. The refined ABCD assessment and non-costly laboratory parameters are outcome predictors in acute exacerbation of COPD. Egypt J Chest Dis Tubercul 2017; 66: 599–603. doi: 10.1016/j.ejcdt.2017.06.004 [DOI] [Google Scholar]
- 41.Gòmez-Rosero JA, Caceres-Galvis C, Ascuntar J, et al. Biomarkers as a prognostic factor in COPD exacerbation: a cohort study. COPD 2021; 18: 325–332. doi: 10.1080/15412555.2021.1922370 [DOI] [PubMed] [Google Scholar]
- 42.Lu FY, Chen R, Li N, et al. Neutrophil-to-lymphocyte ratio predicts clinical outcome of severe acute exacerbation of COPD in frequent exacerbators. Int J Chron Obstruct Pulmon Dis 2021; 16: 341–349. doi: 10.2147/COPD.S290422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wouters EF, Reynaert NL, Dentener MA, et al. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: is there a connection? Proc Am Thorac Soc 2009; 6: 638–647. doi: 10.1513/pats.200907-073DP [DOI] [PubMed] [Google Scholar]
- 44.Oh JY, Sin DD. Lung inflammation in COPD: why does it matter? F1000 Med Rep 2012; 4: 23. doi: 10.3410/M4-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritchie AI, Wedzicha JA. Definition, causes, pathogenesis, and consequences of chronic obstructive pulmonary disease exacerbations. Clin Chest Med 2020; 41: 421–438. doi: 10.1016/j.ccm.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su B, Liu T, Fan H, et al. Inflammatory markers and the risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One 2016; 11: e0150586. doi: 10.1371/journal.pone.0150586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med 2015; 4: 68. doi: 10.1186/s40169-015-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol 2006; 2: 98–108. doi: 10.1186/1710-1492-2-3-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012; 225: 456–460. doi: 10.1016/j.atherosclerosis.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 50.Davies MJ, Hawkins CL. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid Redox Signal 2020; 32: 957–981. doi: 10.1089/ars.2020.8030 [DOI] [PubMed] [Google Scholar]
- 51.Haegens A, Vernooy JH, Heeringa P, et al. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur Respir J 2008; 31: 252–260. doi: 10.1183/09031936.00029307 [DOI] [PubMed] [Google Scholar]
- 52.Hao W, Li M, Zhang Y, et al. Severity of chronic obstructive pulmonary disease with ‘exacerbator with emphysema phenotype' is associated with potential biomarkers. Postgrad Med J 2020; 96: 28–32. doi: 10.1136/postgradmedj-2019-136599 [DOI] [PubMed] [Google Scholar]
- 53.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost 2015; 114: 449–458. doi: 10.1160/TH14-12-1067 [DOI] [PubMed] [Google Scholar]
- 54.Mallah H, Ball S, Sekhon J, et al. Platelets in chronic obstructive pulmonary disease: an update on pathophysiology and implications for antiplatelet therapy. Respir Med 2020; 171: 106098. doi: 10.1016/j.rmed.2020.106098 [DOI] [PubMed] [Google Scholar]