Abstract
Aims
Failure of transcatheter mitral valve repair (fTMVR) therapy has a decisive prognostic influence, and complex retreatment is of higher risk. The aim of this analysis was to evaluate the survival outcome following percutaneous procedures and surgery after unsuccessful TMVR interventions for different aetiologies.
Methods and results
Of 824 consecutive patients who had been treated with the MitraClip device at our institution, between September 2009 and May 2019, 63 (7.6%) symptomatic patients with therapy failure and persistent or recurrent mitral regurgitation (MR) underwent reinterventions. An outcome analysis for primary (PMR) and secondary mitral regurgitation (SMR) and subsequent percutaneous versus surgical treatment was carried out. MitraClip reinterventions were performed in 36 patients (57.1%; n=26 SMR, n=10 PMR), while 27 (42.9%; n=13 SMR, n=14 PMR) underwent open heart surgery. Surgical patients with PMR showed lower mortality than patients with SMR (p<0.0001) and ReClip patients with PMR (p=0.073). Atrial fibrillation (HR 2.915, 95% CI: [1.311, 6.480]), prior open heart surgery (2.820 [1.215, 6.544]) and chronic obstructive pulmonary disease (2.506 [1.099, 5.714]) increased the risk of death. The level of post-interventional MR had no relevant impact on survival.
Conclusions
We conclude that, after SMR and failed TMVR, reclipping is an appropriate treatment option for symptomatic patients. For PMR patients, surgery must be favoured over a reclipping procedure. However, patients with atrial fibrillation, prior open heart surgery and chronic obstructive pulmonary disease are at risk of reduced survival after reinterventions.
Introduction
Since 2008, the percutaneous variation of the Alfieri double-orifice technique using the MitraClip™ device (Abbott Vascular, Santa Clara, CA, USA) has increasingly been used to treat patients with primary and secondary mitral regurgitation (MR)1,2. With experience, the periprocedural success rate has increased over recent years, to almost 100% in the prospective COAPT trial3. However, procedural failures of transcatheter mitral valve repair (fTMVR) therapy have had a profound influence on prognosis, and retreatment is complex due to the high risk4. At present, data on larger patient cohorts undergoing different reintervention strategies following unsuccessful TMVR, including survival analyses, are not available. In this analysis we evaluated the midterm outcome of surgical and percutaneous interventional treatments (ReClip) for different aetiologies.
Materials and methods
PATIENTS
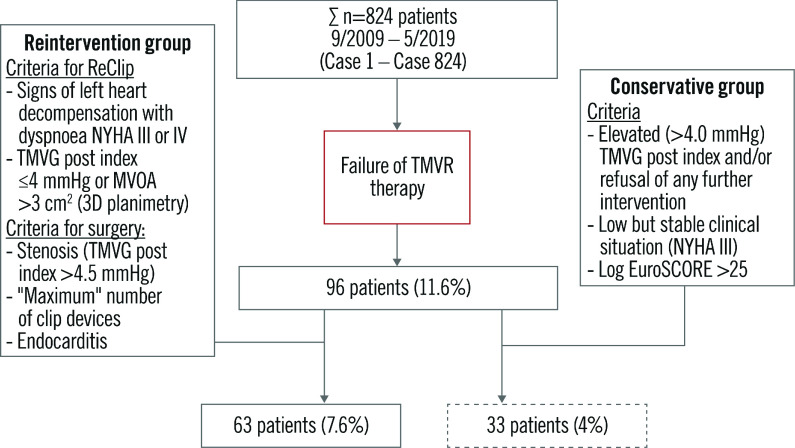
All consecutive patients from September 2009 (case 1) to May 2019 (case 824) who had MitraClip interventions from cardiologists of the Department of Cardiology, Asklepios Klinik St. Georg in Hamburg were included as having had an index procedure. All patients had been considered inoperable or at high surgical risk at the time of index clipping. Of these, 96 patients were included after the diagnosis of fTMVR therapy. Following detailed discussions, the Heart Team decided to schedule 63 patients for reinterventions. On the basis of criteria for reintervention and a conservative approach (Figure 1), the patients were stratified into intervention groups, and an outcome analysis for primary mitral regurgitation (PMR) and secondary mitral regurgitaton (SMR) with subsequent percutaneous versus surgical treatment was carried out. All patients in the reintervention groups suffered from persistent or recurrent, at least moderate to severe, MR (≥3+). All were highly symptomatic despite adequate medical therapy and were either presented for re-evaluation by their general practitioner/authorised cardiologist or treated immediately after the index intervention. All patients, including those in the conservative group, received optimal medical and device treatment before and after reintervention, as judged by the Heart Team.
Figure 1.
Flow chart of reinterventions after failure of TMVR therapy in 824 patients.
ECHOCARDIOGRAPHY
All patients underwent two-dimensional transthoracic echocardiography on commercially available echocardiographic systems (Vivid E9 and E95; GE Vingmed Ultrasound AS, Horten, Norway, or iE33 and Epiq7; Philips Medical Systems, Andover, MA, USA) before and after reintervention. Standard parameters of left ventricular dimension as well as left and right ventricular function were assessed according to recent guidelines5,6. Preprocedural, intraprocedural and post-procedural valve analysis was carried out by transoesophageal echocardiography. MR grading was based on a previously reported technique7.
REPEAT INTERVENTIONS
The technical details of reinterventions have already been described in detail elsewhere8,9. MR reduction to moderate or less was defined as a technical success in the ReClip group according to the Mitral Valve Academic Research Consortium (MVARC) criteria. The transmitral valve gradient (TMVG) should remain ≤5 mmHg10. Our general exclusion criteria for reinterventions in the event of unsuccessful MitraClip insertion (ongoing resuscitation and/or serious sepsis) did not apply.
REPEAT INTERVENTION SURGERY GROUP
As reported previously8, concomitant tricuspid valve repairs were performed in surgical patients with insufficiency grade 2 or more and/or annulus dilation (>40 mm), and standardised ablation procedures, including occlusion of the left atrial appendage, were carried out in patients with atrial fibrillation. All patients required closure of an iatrogenic atrial septal defect during open heart surgery.
FOLLOW-UP INVESTIGATIONS
After hospital discharge, follow-up visits were scheduled at 30 days and 12 months, and an annual telephone follow-up was conducted thereafter (up to 10 years).
STATISTICAL ANALYSIS
Continuous data were summarised as means±standard deviations or as medians (25/75th percentiles) as appropriate. Categorical data were presented as number (%). Poisson, chi-square, Fisher’s exact and Wilcoxon tests were used to examine differences between groups. Baseline, echo and procedural data were individually associated with overall survival and adjusted with the interaction of mitral insufficiency and intervention type. Hazard ratios and 95% confidence intervals for each model were calculated. All p-values were two-sided and a p-value <0.05 was considered significant. All calculations were performed using statistical analysis software R (R Core Team, 2020; R Foundation for Statistical Computing, Vienna, Austria).
ETHICS
The Hamburg General Medical Council Ethics Committee approved the protocol for this study.
Results
Procedural failures or clip device dysfunctions with persistent or recurrent MR of grades >2+ were found post-interventionally in 96 patients (11.6%); of these, 63 patients (7.6%) were treated interventionally and surgically (ReClip/Surgery group) and 33 (4%) were without interventional/surgical treatment (conservative group). Baseline characteristics of all groups are given in Table 1. There were highly significant differences between the groups: these were related to age, implantable cardioverter-defibrillator (ICD)/cardiac resychronisation therapy (CRT), pulmonary artery hypertension and hypertension (p<0.001).
Table 1. Patient baseline characteristics.
| Patients | Surgery n=27 | ReClip n=36 | Conservative n=33 | |
| Female, n (%) | 10 (37) | 10 (28) | 12 (36) | |
| Age, years | 72±9 | 75±9 | 75±10 | |
| Renal impairment, n (%) | 15 (56) | 17 (47) | 21 (64) | |
| NYHA | Class III, n (%) | 19 (70) | 25 (69) | 18 (55) |
| Class IV, n (%) | 8 (30) | 11 (31) | 15 (45) | |
| NT-pro BNP, pg/ml* | 2,552 [1,302-5,325] | 2,932 [1,453-6,517] | 3,269 [709-5,965] | |
| ICD, n (%) | 6 (22) | 6 (17) | 4 (12) | |
| CRT-D, n (%) | 2 (7) | 11 (31) | 4 (12) | |
| Log EuroSCORE pre index, % | 19.1±14.3 | 21.3±14.9 | 30.8±20.2 | |
| STS score pre index, % | 4.3±3.7 | 4.5±3.4 | 7.1±7.0 | |
| PAH, n (%) | 24 (89) | 10 (28) | 29 (88) | |
| PAD, n (%) | 7 (26) | 5 (14) | 7 (21) | |
| Hypertension, n (%) | 18 (67) | 25 (69) | 30 (91) | |
| CAD, n (%) | 14 (52) | 26 (72) | 23 (70) | |
| Previous cardiac surgery, n (%) | 7 (26) | 6 (17) | 14 (42) | |
| Diabetes, n (%) | 10 (37) | 10 (28) | 11 (33) | |
| Hyperlipidaemia | 11 (41) | 18 (50) | 11 (34) | |
| Atrial fibrillation, n (%) | 20 (74) | 26 (72) | 25 (76) | |
| COPD, n (%) | 4 (15) | 5 (14) | 7 (21) | |
| Body mass index, kg/m² | 26.0±5.0 | 24.4±4.3 | 26.5±5.5 | |
| x±s represents mean±1 standard deviation. * Median [IQR]. Numbers after frequencies are percentages. CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CRT-D: cardiac resynchronisation therapy - defibrillator; EuroSCORE: European System for Cardiac Operative Risk Evaluation; ICD: implantable cardioverter-defibrillator; NYHA: New York Heart Association; PAD: peripheral arterial disease; PAH: pulmonary artery hypertension; STS: Society of Thoracic Surgeons | ||||
PATIENT CHARACTERISTICS OF THE INTERVENTIONAL GROUPS
In patients with recurrent or persistent MR and severe symptoms, the pathomechanisms of MR were leaflet tear/single leaflet device attachment (SLDA) (Figure 2), a restrictive or torn visual thread caused by the clip material, and/or acquired device endocarditis. In the surgical group, pathomechanisms were supported by intraoperative findings that showed combinations of leaflet tear and SLDA. In four cases, devices suffered technical dysfunctions as part of the index intervention (three cases of “loss” of clip that was still connected to the clip delivery system with a nitinol wire and one case after an atrial septal device embolisation).
Figure 2.
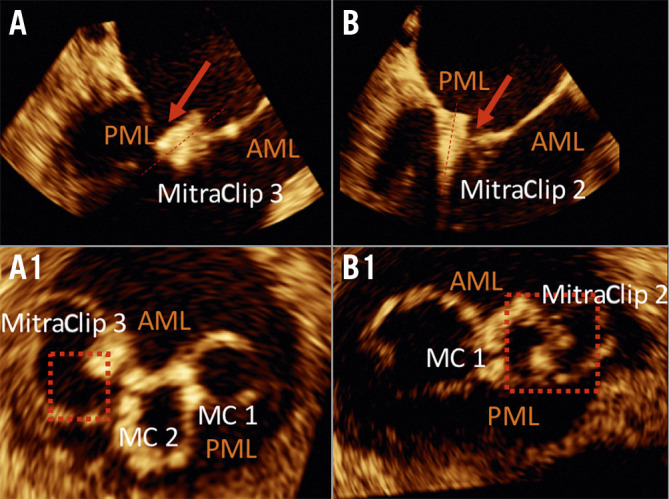
Images of leaflet/MitraClip connections. Top panels show diastolic transoesophageal echocardiography long-axis views. The corresponding 3D planimetry views are shown in the bottom panels. A) & A1) A single leaflet device attachment (SLDA). The clip position was directed completely towards the atrium and there was no longer any leaflet insertion. B) & B1) Isolated leaflet injuries or a tear with minimal mobility as well as a minimal leaflet insertion of the MitraClip device with a slight tilt of the ventricular device tip towards the atrium. AML: anterior mitral leaflet; MC: MitraClip; PML: posterior mitral leaflet
The ReClip and surgery groups and the conservative collective had calculated risks at the time of index clipping measured by the logistic EuroSCORE (LogES) of 19.1±11.3%, 21.3±14.9% and 30.8±20.2%, respectively; in the early mortality group between intervention groups, risks of 34.8±15.7% (ReClip) and 42.8±20.1% (surgery) were calculated, respectively. On average, 1.4±0.7 (ReClip) versus 2.0±1.0 (surgery) versus 2.1±0.6 (conservative group) clips per patient were implanted prior to reintervention. There were highly significant differences in TMVG post index procedure between the ReClip and surgical groups and the ReClip and conservative groups (p<0.001).
All relevant echocardiography data, aetiology of the mitral valve at baseline, mechanisms of residual MR, mean gradient post index procedure and the number of implanted clips in the intervention and conservative groups are summarised in Table 2.
Table 2. Echocardiographic measurements prior to and after the index procedure according to groups.
| Patients | Surgery PMR n=14 | ReClip PMR n=10 | Surgery SMR n=13 | ReClip SMR n=26 | Conservative PMR n=13 | Conservative SMR n=16 | Conservative Mixed n=4 | |||||||
| LVEDD, mm | 52±8 | 59±13 | 66±10 | 66±10 | 55±7 | 63±11 | 56±10 | |||||||
| LVESD, mm | 32±7 | 42±17 | 56±13 | 55±12 | 38±9 | 50±12 | 38±7 | |||||||
| LVEF, % | 56±9 | 52±12 | 33±12 | 30±10 | 52±10 | 39±13 | 48±14 | |||||||
| Morpholgy MR†, n | Flail | 4 | Flail | 3 | 1 | 3 | 1 | 6 | Flail | 5 | 1 | 3 | 1+Prolapse | 1 |
| Prolapse | 8 | Prolapse | 6 | 3b | 8 | 3b | 16 | Prolapse | 4 | 3b | 8 | 1+Flail | 1 | |
| Bileaflet prolapse | 2 | Bileaflet prolapse | 1 | 1/3b | 2 | 1/3b | 4 | Other‡ | 4 | 1/3b | 5 | Degenerative+3b | 2 | |
| Residual MR¶ | 2 | 8 | 4 | 13 | 6 | 7 | 1 | |||||||
| Leaflet tear/SLDA | 9 | 2 | 3 | 13 | 5 | 9 | 3 | |||||||
| Device endocarditis | 1 | – | 2 | – | – | – | – | |||||||
| Device failure | 1 | 0 | 3 | 0 | 2 | 0 | 0 | |||||||
| Restricted chords | 1 | – | 1 | – | – | – | – | |||||||
| TMVG post index§ | 5.0 [3.0-7.5]* | 3.0 [2.5-3.9]* | 4.7 [3.2-5.8]* | 3.0 [2.1-3.0]* | 5.0 [5.0-5.9]* | 4.0 [3.1-5.0]* | 4.6 [4.5-5.3]* | |||||||
| Number of MitraClips post index¥ | 2.1±0.8 | 1.4±0.5 | 2.0±1.2 | 1.5±0.7 | 2.1±0.6 | 2.2±0.7 | 2.0±0.8 | |||||||
PROCEDURAL AND IN-HOSPITAL OUTCOME
The mean time from the index procedure to the reintervention was highly significantly shorter in the surgical group (p<0.001). Of note, one patient in the surgical group was accepted in an emergency situation. MitraClip reinterventions were performed on 36 patients (57.1%; n=26 SMR, n=10 PMR), while 27 (42.9%; n=13 SMR, n=14 PMR) underwent open heart surgery. The mean grade of MR after clip intervention was mild to moderate (grade 1-2) in the PMR group versus moderate (grade 2) in the SMR group, whereas the transmitral gradient increased in the PMR group to 4.0 (2.2-4.0) mmHg, and in the SMR group to 3.8 (3.0-5.0) mmHg (p=0.71). There was no residual MR in the surgical group. Relevant echocardiographic data after repeat interventions are given in Table 2 and Table 3.
Table 3. Procedural outcome and procedure-related complications according to intervention groups.
| Patients | Surgery PMR n=14 | ReClip PMR n=10 | Surgery SMR n=13 | ReClip SMR n=26 | p-value | |
| MR pre ReClip/surgery >2+, n (%) | 14 (100) | 10 (100) | 13 (100) | 26 (100) | >0.999 | |
| MR post ReClip/surgery | 0 | 1.5 [1-2]* | 0 | 2 [1.5-2]* | <0.001 | |
| TMVG post ReClip, mmHg | / | 4.0 [2.2-4.0]* | / | 3.8 [3.0-5.0]* | 0.71 | |
| TAPSE post ReClip/surgery, mm | 14±3 | 20±5 | 15±2 | 16±4 | 0.005 | |
| Duration until reintervention, months | 1.3 [0.9-12]* | 12 [2-19]* | 0.4 [0.2-1.4]* | 4 [0.8-13]* | <0.001 | |
| Total LOS, days | 17.0 [15.0-21.0]* | 6.5 [4.5-14.5]* | 11.0 [8.0-25.0]* | 5.5 [4.0-7.8]* | <0.001 | |
| Ventilation >48 hours, n (%) | 4 (31) | 1 (10) | 6 (46) | 1 (4) | 0.005 | |
| Stroke | 0 | 0 | 0 | 0 | >0.999 | |
| Post acute kidney injury‡ (yes), n (%) | 2 (14) | 1 (10) | 3 (23) | 2 (8) | 0.597 | |
| Post-intervention blood transfusion (yes), n (%) | 13 (93) | 1 (10) | 12 (92) | 2 (8) | <0.001 | |
| Intrahospital death, n (%) | 0 | 1 (10) | 5 (39) | 3 (11) | 0.036 | |
| Discharge status | Home | 4 (29) | 6 (60) | 2 (15) | 21 (81) | <0.001 |
| Rehabilitation clinic | 10 (71) | 3 (30) | 6 (46) | 2 (8) | <0.001 | |
| Numbers after frequencies are percentages. ‡ Stage II or III modified RIFLE criteria. *Median [IQR]. LOS: in-hospital length of stay after reintervention; TMVG: transmitral valve gradient | ||||||
According to the MVARC procedural success criteria, 22 (61.1%) of the 36 ReClip patients met the criteria, while 14 (38.9%) did not (n=11 SMR, n=3 PMR). As part of the surgical intervention in a total of 27 patients, 21 had a biological mitral valve prosthesis (n=11 PMR, n=10 SMR), while a reconstruction was carried out in 6 patients (n=3 PMR, n=3 SMR).
The median length of hospital stay (LOS) was 6.0 (4.0-8.2) days in the ReClip group and 16.5 (11.0-24.0) days in the surgical group (p<0.001). Further, the vast majority of patients in the surgical group needed prolonged mechanical ventilation (>48 hours) and a post-procedural blood transfusion (p=0.005 and p<0.001, respectively). Procedural outcomes and procedure-related complications are shown in Table 3.
IN-HOSPITAL DEATH AND 30-DAY OUTCOMES
Within 30 days there were six deaths (16.7%, n=5 SMR, n=1 PMR) in the ReClip group and five deaths (18.5%, all SMR) in the surgical group. All five patients in the surgical group died in hospital, while four in-hospital deaths were from the ReClip group. The cause of death in the ReClip group was cardiac in one patient with low output syndrome, and multiorgan failure in two patients in the course of sepsis (one after left ventricular assist device [LVAD] implantation despite successful ReClip); in three patients the cause remains unknown. In the surgically treated group, the cause of death was cardiac in one patient with low output syndrome, uncontrolled sepsis in three patients and uncontrollable bleeding in one patient after surgical intervention.
FOLLOW-UP AND SURVIVAL OUTCOME
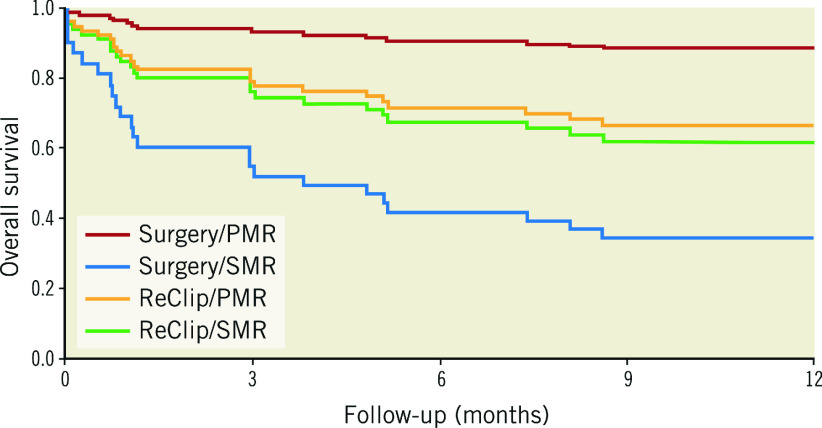
The follow-up durations were 13 (3.9-58.2) months in the surgical group and 19 (3.0-45.6) months in the ReClip group. During follow-up, a total of 21 deaths in the ReClip group versus 15 deaths in the surgical group were recorded. The survival follow-ups after failed MitraClip procedures in the four intervention groups are shown in Figure 3. Surgically treated patients with PMR showed significantly lower mortality than patients with functional genesis (p<0.0001) and there was a trend towards higher survival compared with ReClip treatment in PMR patients (p=0.073).
Figure 3.
Cox PH model-based survival of the intervention groups. Survival curves are drawn from model-based predictions of overall survival. A Cox proportional hazards model is used including aetiology, the type of intervention and an interaction term of intervention and aetiology.
PROGNOSTIC OUTCOME OF BASELINE AND POST-INTERVENTIONAL PARAMETERS
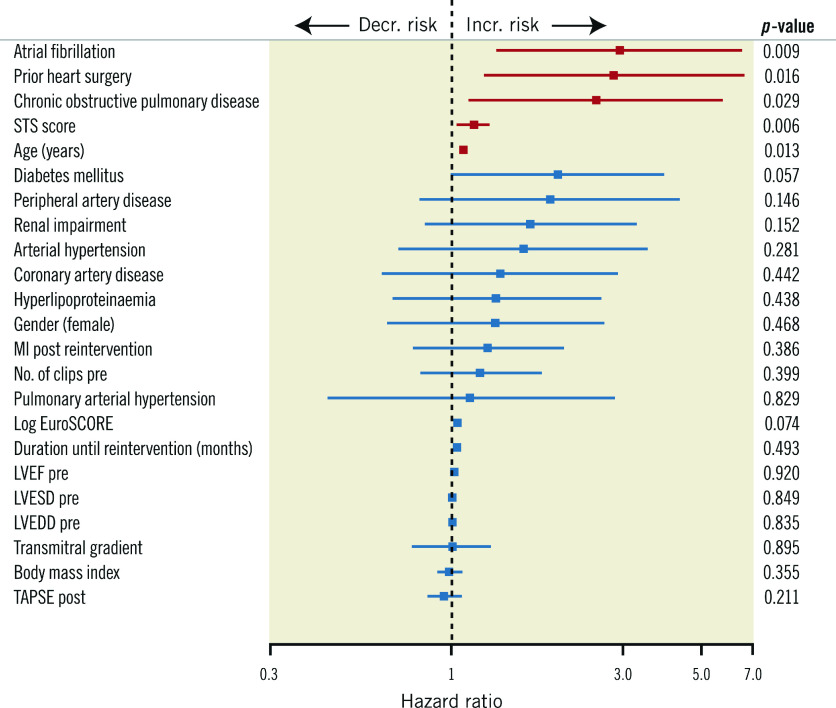
Analyses of hazard ratios demonstrated a significant (2.9-, 2.8-, 2.5-fold) lower survival for patients with atrial fibrillation, prior open heart surgery and chronic obstructive pulmonary disease, respectively. In addition, the Society of Thoracic Surgeons (STS) scores and age had a significant impact (p=0.006, p=0.013, respectively). The corresponding hazard ratios for the baseline parameters examined are shown in Figure 4. The post-intervention MR level and the tricuspid annular plane systolic excursion (TAPSE) had no impact on survival (p=0.386, p=0.211, respectively).
Figure 4.
Plot of hazard ratios and 95% confidence intervals representing the individual effects of baseline, echo and procedural data on survival adjusted for MI and intervention type. LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter
Discussion
Failure of TMVR therapy is a rare event. In the German TRAMI registry, MitraClip failure occurred in 3% of patients11 and, in other registries such as COAPT, it occurred in <1.5% of patients3. Unique to this single-centre study of 63 patients treated with TMVR therapy is that a repeat intervention using the MitraClip device was able to reduce MR despite unfavourable conditions of SLDA and leaflet tear regardless of the valve aetiology. Surgically intervened SMR patients showed a poorer outcome compared to ReClip patients and had a significantly higher mortality compared to surgically treated PMR patients. Surgically treated PMR patients had a very good midterm outcome.
INTERVENTIONAL CONSIDERATIONS AND IMPACT OF DIFFERENT INTERVENTION STRATEGIES
In our study, the main indications for reintervention in both groups were a high-grade residual MR, an SLDA and a leaflet tear caused by the MitraClip device. This pattern was also observed in various smaller case series12,13. Important considerations for decision making between reintervention strategies are the number of implanted clip devices, the remaining TMVG and the orifice area after fTMVR. In particular, in our surgery group and the conservative collective, patients showed a significantly higher TMVG in comparison to the ReClip group and a higher number of implanted clips between the ReClip and no intervention groups. The number of implanted clips impacted negatively on the repair procedure8 and, in combination with a prior multi-clip strategy, induced leaflet damage that dramatically reduced the option of ReClip treatment; a prosthesis had to be implanted. In our surgical SMR population, only 3 (23%) of 13 patients could be reconstructed (repeat repair). Goldstein et al14 did not report any outcome advantage between SMR patients with a reconstructed mitral valve compared to patients with a prosthetic replacement. However, one limitation is that, compared to our data, the patients did not have previous surgery. Possible deterioration of the patient’s condition before renewed surgical intervention also increases the perioperative risk considerably8.
Another consideration is the pathomechanism of MR after fTMVR. In the two ReClip groups, a higher proportion of patients with PMR were without leaflet injury, and treatment of these was more likely to succeed9. Analysis of our data showed that approximately two out of three of the interventionally treated patients met the procedural criteria according to the MVARC definition. It must be emphasised that, despite unfavourable leaflet-specific requirements, an acceptable ratio of transmitral gradient (≤5 mmHg) to MR reduction (≤moderate) was achieved in the majority of patients.
PROGNOSTIC PREDICTORS
It has recently been described that residual MR and the TMVG after MitraClip therapy have a decisive prognostic influence on the outcome4,11,15. However, in contrast to other studies, we showed no significant prognostic impact of residual MR in both intervention groups or in the ReClip group for TMVG. This may be attributed to the positive effect of ReClip intervention on reduction of MR without creating stenosis. However, there are no comparative studies between ReClip patients and primarily successfully intervened patients, so that the prognostic effect of ReClip intervention remains unclear.
In previous studies of patients who had undergone surgical intervention, prognostic predictors of a poor outcome were summarised as restricted left ventricular (LV) function, the level of the LogES and EuroSCORE II, and the preoperative presence of cardiogenic shock8,16,17. The level of pre-interventional LogES is described as a general surgical risk indicator and ≥20 at baseline is associated with a restricted prognosis in MitraClip patients18,19. The analysis of LogES for our conservative group supports the conservative approach of not considering reintervention.
A strong negative outcome predictor is the pre-interventional presence of atrial fibrillation. Herrmann et al20 investigated the influence of pre-interventional atrial fibrillation on outcomes in the EVEREST II patient cohort: 27% of patients had atrial fibrillation and, of those, there was no negative influence on MitraClip or in the surgical patient cohort compared to patients without atrial fibrillation. In contrast to this finding, our data for over 70% of patients in each group show a significantly higher number of atrial fibrillation patients, which may indicate an older cohort with advanced cardiac remodelling.
Furthermore, the influence of established surgical and interventional risk factors, such as age, prior heart surgery, chronic obstructive pulmonary disease21 and an STS score reflecting a poor outcome after surgical intervention, was confirmed, although their influence on outcomes varied.
Limitations
This study was not undertaken to evaluate the results of MitraClip implantations in general. Instead, we focused on symptomatic patients with failure of MitraClip therapy, persistence or recurrence of MR, and therefore indications for reinterventions. We present a retrospective single-centre analysis. The total number of a heterogeneous group of patients we dealt with was only 63. The patients were not randomised to either reclipping or surgery. However, decision making remained difficult after fTMVR procedures, because those patients had previously been categorised as inoperable. Due to the follow-up over 10 years, the cause of death in some cases could not be evaluated. Because of the retrospective nature of this study, it was not possible to collect detailed data on re-hospitalisation, clinical deterioration or serial echocardiographic parameters in every patient. There was no comparable control group treated conservatively. Therefore, prospective randomised studies of larger cohorts with systematic follow-up are required to confirm these preliminary data.
Conclusions
We conclude that, whenever possible, in SMR and MitraClip failure, reclipping should be considered as the primary treatment option in symptomatic patients. The ReClip strategy can deliver acceptable results even with unfavourable leaflet morphology such as leaflet tear or SLDA. In PMR patients, surgery, if possible, should be favoured over a ReClip procedure. Surgically intervened patients and those with SMR after unsuccessful clipping showed limited survival and a high postoperative mortality, such that an indication for surgical reintervention in SMR patients should always be made carefully by a Heart Team.
Impact on daily practice
Open heart mitral valve surgery in patients with PMR demonstrates significantly lower mortality than in patients with SMR and exhibits a trend towards higher survival compared to ReClip treatment. A ReClip intervention can deliver acceptable results even with an unfavourable leaflet morphology such as leaflet tear and SLDA, and can be recommended in patients with SMR. However, survival is significantly lower in patients with atrial fibrillation, prior open heart surgery or chronic obstructive pulmonary disease.
Acknowledgments
Conflict of interest statement
K-H. Kuck has received research grants from Abbott Vascular. The other authors have no conflicts of interest to declare.
Abbreviations
- fTMVR
failure of transcatheter mitral valve repair
- LogES
logistic regression version of the European System for Cardiac Operative Risk Evaluation
- MR
mitral regurgitation
- NYHA
New York Heart Association
- PMR
primary mitral regurgitation
- SLDA
single leaflet device attachment
- SMR
secondary mitral regurgitation
- STS
Society of Thoracic Surgeons
- TAPSE
tricuspid annular plane systolic excursion
- TMVG
transmitral valve gradient
- TMVR
transcatheter mitral valve repair
Contributor Information
Hannes Alessandrini, Department of Cardiology, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Ansgar Dreher, Department of Cardiology, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Claudia Harr, Department of Cardiology, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Peter Wohlmuth, Proresearch Institute, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Felix Meincke, Department of Cardiology, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Samer Hakmi, Department of Cardiology, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Timm Ubben, Department of Cardiology, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Karl-Heinz Kuck, LANS Medicum, Hamburg, Germany.
Kambiz Hassan, Department of Cardiac Surgery, Asklepios Klinik St. Georg, Hamburg, Germany.
Stephan Willems, Department of Cardiology, Asklepios Klinik Sankt. Georg, Hamburg, Germany.
Michael Schmoeckel, Department of Cardiac Surgery, Asklepios Klinik St. Georg, Hamburg, Germany.
Stephan Geidel, Department of Cardiac Surgery, Asklepios Klinik St. Georg, Hamburg, Germany.
References
- Alfieri O, Maisano F, De Bonis M, Stefano PL, Torracca L, Oppizzi M, La Canna G. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122:674–81. doi: 10.1067/mtc.2001.117277. [DOI] [PubMed] [Google Scholar]
- Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D EVEREST Investigators. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54:686–94. doi: 10.1016/j.jacc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ COAPT Investigators. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307–18. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- Puls M, Tichelbäcker T, Bleckmann A, Hünlich M, von der Ehe K, Beuthner E, Rüter K, Beißbarth T, Seipelt R, Schöndube F, Hasenfuss G, Schillinger W. Failure of acute procedural success predicts adverse outcome after percutaneous edge-to-edge mitral valve repair with MitraClip. EuroIntervention. 2014;9:1407–17. doi: 10.4244/EIJV9I12A238. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano L, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Foster E, Wasserman HS, Gray W, Homma S, Di Tullio MR, Rodriguez L, Stewart WJ, Whitlow P, Block P, Martin R, Merlino J, Herrmann HC, Wiegers SE, Silvestry FE, Hamilton A, Zunamon A, Kraybill K, Gerber IL, Weeks SG, Zhang Y, Feldman T. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am J Cardiol. 2007;100:1577–83. doi: 10.1016/j.amjcard.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Geidel S, Wohlmuth P, Schmoeckel M. Survival Prediction in Patients Undergoing Open-Heart Mitral Valve Operation After Previous Failed MitraClip Procedures. Ann Thorac Surg. 2016;101:952–8. doi: 10.1016/j.athoracsur.2015.08.086. [DOI] [PubMed] [Google Scholar]
- Kreidel F, Frerker C, Schlüter M, Alessandrini H, Thielsen T, Geidel S, Schäfer U, Kuck KH. Repeat MitraClip Therapy for Significant Recurrent Mitral Regurgitation in High Surgical Risk Patients: Impact of Loss of Leaflet Insertion. JACC Cardivasc Interv. 2015;8:1480–9. doi: 10.1016/j.jcin.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Stone GW, Adams DH, Abraham WT, Kappetein AP, Généreux P, Vranckx P, Mehran R, Kuck KH, Leon MB, Piazza N, Head SJ, Filippatos G, Vahanian AS Mitral Valve Academic Research Consortium (MVARC) Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 2: Endpoint Definitions: A Consensus Document From the Mitral Valve Academic Research Consortium. J Am Coll Cardiol. 2015;66:308–21. doi: 10.1016/j.jacc.2015.05.049. [DOI] [PubMed] [Google Scholar]
- Puls M, Lubos E, Boekstegers P, von Bardeleben RS, Ouarrak T, Butter C, Zuern CS, Bekeredjian R, Sievert H, Nickenig G, Eggebrecht H, Senges J, Schillinger W. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J. 2016;37:703–12. doi: 10.1093/eurheartj/ehv627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenziano M, Skipper E, Heimansohn D, Letsou GV, Woo J, Kron I, Alexander J, Cleveland J, Kong B, Davidson M, Vassiliades T, Krieger K, Sako E, Tibi P, Galloway A, Foster E, Feldman T, Glower D EVEREST Investigators. Surgical revision after percutaneous mitral repair with the MitraClip device. Ann Thorac Surg. 2010;89:72–80. doi: 10.1016/j.athoracsur.2009.08.063. [DOI] [PubMed] [Google Scholar]
- Alozie A, Westphal B, Kische S, Kaminski A, Paranskaya L, Bozdag-Turan I, Ortak J, Schubert J, Steinhoff G, Ince H. Surgical revision after percutaneous mitral valve repair by edge-to-edge device. When the strategy fails in the highest risk surgical population. Eur J Cardiothorac Surg. 2014;46:55–60. doi: 10.1093/ejcts/ezt535. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, Thourani V, Argenziano M, Gammie JS, Mack M, Demers P, Atluri P, Rose EA, O’Sullivan K, Williams DL, Bagiella E, Michler RE, Weisel RD, Miller MA, Geller NL, Taddei-Peters WC, Smith PK, Moquete E, Overbey JR, Kron IL, O’Gara PT, Acker MA CTSN. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med. 2016;374:344–53. doi: 10.1056/NEJMoa1512913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuß M, Schau T, Isotani A, Pilz M, Schöpp M, Butter C. Elevated Mitral Valve Pressure Gradient After MitraClip Implantation Deteriorates Long-Term Outcome in Patients With Severe Mitral Regurgitation and Severe Heart Failure. JACC Cardiovasc Interv. 2017;10:931‑9. doi: 10.1016/j.jcin.2016.12.280. [DOI] [PubMed] [Google Scholar]
- Kreidel F, Alessandrini H, Wohlmuth P, Schmoeckel M, Geidel S. Is Surgical or Catheter-based Interventions an Option After an Unsuccessful Mitral Clip? Semin Thorac Cardiovasc Surg. 2018;30:152–7. doi: 10.1053/j.semtcvs.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Ehlmidi Y, Voss B, Lange R. Surgical mitral valve intervention following a failed MitraClip procedure. EuroIntervention. 2016;12:Y102–6. doi: 10.4244/EIJV12SYA27. [DOI] [PubMed] [Google Scholar]
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- Paranskaya L, D´Ancona G, Bozdag-Turan I, Akin I, Kische S, Turan GR, Divchev D, Rehders T, Schneider H, Ortak J, Nienaber CA, Ince H. Early and mid-term outcomes of percutaneous mitral valve repair with the MitraClip®: comparative analysis of different EuroSCORE strata. EuroIntervention. 2012;8:571–8. doi: 10.4244/EIJV8I5A88. [DOI] [PubMed] [Google Scholar]
- Herrmann HC, Gertz ZM, Silvestry FE, Wiegers SE, Woo JY, Hermiller J, Segar D, Heimansohn D, Gray W, Homma S, Argenziano M, Wang A, Jollis J, Lampert MB, Alexander J, Mauri L, Foster E, Glower D, Feldmann T. Effects of atrial fibrillation on treatment of mitral regurgitation in the EVEREST II (Endovascular Valve Edge-to-Edge Repair Study) randomized trial. J Am Coll Cardiol. 2012;59:1312–9. doi: 10.1016/j.jacc.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012;42:S1–44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]