Abstract
Background
The comparative efficacy of balloon-based techniques to prepare severely calcified coronary lesions before stenting remains poorly studied.
Aims
We sought to compare stent expansion following preparation of severely calcified coronary lesions with either a super high-pressure balloon or a scoring balloon.
Methods
In this randomised, open-label trial, patients with severely calcified coronary lesions were enrolled at five centres in Germany and Switzerland. After unsuccessful lesion preparation with a standard non-compliant balloon (<30% reduction of baseline diameter stenosis), participants were randomised to predilation with either a super high-pressure balloon or a scoring balloon before drug-eluting stent (DES) implantation. The primary endpoint of the study was stent expansion index as assessed by optical coherence tomography (OCT). The key secondary endpoints included angiographic, strategy and procedural success.
Results
OCT data after DES implantation were available for 70 out of 74 patients (94.6%) enrolled. Lesion preparation with a super high-pressure balloon versus a scoring balloon led to a comparable stent expansion index (0.72±0.12 vs 0.68±0.13; p=0.22). Compared with the scoring balloon, the super high-pressure balloon increased the minimum lumen diameter (2.83±0.34 mm vs 2.65±0.36 mm; p=0.03) and reduced the diameter stenosis (11.6±4.8% vs 14.4±5.6%; p=0.02) without difference in terms of angiographic success (100% vs 97.3%; p>0.99). Strategy success (91.9% vs 83.8%; p=0.48) and procedural success (100% vs 89.2%; p=0.12) were numerically more frequent with the super high-pressure balloon versus the scoring balloon.
Conclusions
In patients with severely calcified coronary artery lesions, preparation with a super high-pressure balloon versus a scoring balloon was associated with comparable stent expansion on intravascular imaging and a trend towards improved angiographic performance.
Visual summary
A ComparIson of Strategies to PrepAre SeveRely CALCified Coronary Lesions: the ISAR-CALC randomised trial.
Introduction
Calcified coronary artery lesions are encountered in a considerable proportion of patients treated with percutaneous coronary intervention (PCI)1. Given the ageing population, and increasing rates of diabetes mellitus and renal failure, the proportion of patients with calcified lesions is expected to increase further in the years to come.
Despite iterative improvements in percutaneous coronary devices and stenting techniques, severely calcified lesions remain a procedural and clinical challenge, even with high-performance drug-eluting stents (DES)2. Extensive calcification of obstructive coronary lesions may impact adversely on successful dilatation prior to stent implantation, thus increasing the likelihood of DES underexpansion, a known correlate of stent failure3.
Optimal lesion preparation is a prerequisite for successful stent implantation and expansion in patients with calcific coronary lesions4. A number of interventional tools are currently available for the preparation of calcified lesions, including debulking, ablation- and balloon-based techniques. The latter comprise standard non-compliant balloons, super high-pressure balloons, cutting or scoring balloons and intravascular lithotripsy5. However, despite the growing interest in this field, the comparative performance of balloon-based techniques to prepare severely calcified coronary lesions before DES implantation remains poorly studied.
Against this background, we conducted a randomised trial in which patients with severely calcified coronary lesions amenable to PCI with DES were allocated to lesion preparation with either a super high-pressure balloon or a scoring balloon before DES implantation after unsuccessful predilation of the target lesion with a standard non-compliant balloon.
Methods
STUDY POPULATION, RANDOMISATION, DEVICES AND INTERVENTION PROTOCOL
The ComparIson of Strategies to PrepAre SeveRely CALCified Coronary Lesions (ISAR-CALC) trial was an investigator-initiated, prospective, randomised, multicentre, assessor-blind, open-label trial (ClinicalTrials.gov Identifier: NCT03487432). Patients were enrolled at five participating centres in Germany and Switzerland between July 2018 and September 2019. A complete list of all inclusion and exclusion criteria is provided in Supplementary Table 1.
Patients who met all inclusion criteria and none of the exclusion criteria were assigned to either a super high-pressure balloon or a scoring balloon in a 1:1 randomisation fashion. The super high-pressure balloon (OPN NC™; SIS Medical AG, Frauenfeld, Switzerland) consists of a rapid-exchange, non-compliant balloon with twin-layer technology developed to deliver inflation pressures ≥35 bar and up to 55 bar6. The scoring balloon (NSE Alpha; B. Braun, Melsungen, Germany) consists of a rapid-exchange, semi-compliant balloon with three triangle-shaped, non-slip, nylon scoring elements attached proximally and distally on the outer surface of the balloon.
The two treatment groups were studied concurrently. Once enrolled in the study, patients received lesion preparation according to randomisation. If satisfactory dilation of the target lesion was not achieved with the allocated study device, additional lesion preparation techniques, such as rotational atherectomy, could be implemented at the discretion of the operator. Crossover to the non-assigned device was not permitted. Following lesion preparation, stenting was performed in the same setting using a latest-generation, thin-strut, biodegradable polymer, everolimus-eluting stent (SYNERGY™️; Boston Scientific, Marlborough, MA, USA). Optical coherence tomography (OCT) imaging for primary endpoint assessment was performed once an optimal angiographic result was achieved after DES implantation, as per the operators’ visual assessment. Stent optimisation after OCT imaging could be performed at the operator’s discretion.
CLINICAL AND IMAGING DATA MANAGEMENT
Relevant data were collected and entered into a dedicated computer database (edc2go; Genae, Antwerp, Belgium). All events were adjudicated and classified by an independent events adjudication committee blinded to the treatment groups. All serious adverse events as well as the primary and secondary endpoints in this trial were monitored on-site. In addition, 25% of all patients with 100% source data verification were monitored in all centres.
Coronary angiograms were digitally recorded and assessed off-line in the quantitative coronary angiographic (QCA) core laboratory (ISAResearch Center, Munich, Germany) using an automated edge detection system (QAngio XA version 7.3; Medis Medical Imaging Systems, Leiden, the Netherlands) by independent personnel unaware of the treatment allocation. Coronary lesion calcification was graded according to the angiographic classification of Mintz et al7. OCT acquisitions were performed with commercially available tools (ILUMIEN™️ OPTIS™️ system and Dragonfly™️ OPTIS™️ imaging catheter; both Abbott Vascular, Santa Clara, CA, USA) according to predefined standard operating procedures of the imaging core laboratory (ISAResearch Center). A detailed description of the protocols for acquisition and analysis of angiographic and OCT data is provided in Supplementary Appendix 2.
ENDPOINTS AND DEFINITIONS
The primary endpoint of the trial was stent expansion index, defined as minimum stent area divided by mean reference lumen area assessed with OCT, as previously described4,8.
Key secondary endpoints were: i) angiographic success, defined as target lesion residual angiographic stenosis <30% in the presence of Thrombolysis In Myocardial Infarction (TIMI) 3 flow; ii) procedural success, defined as angiographic success without the occurrence of major adverse cardiac events (MACE, a composite of cardiac death, target vessel-related myocardial infarction [MI] and repeat revascularisation) up to 30 days; iii) strategy success, defined as procedural success using the assigned study device and stent, without additional devices for lesion preparation; iv) acute lumen gain, defined as minimal lumen diameter (MLD) after balloon angioplasty with the study devices minus baseline MLD; v) need for complementary lesion preparation with rotational atherectomy; vi) incidence of vessel perforation; vii) procedure duration, and viii) contrast volume.
SAMPLE SIZE CALCULATIONS AND STATISTICAL ANALYSIS PLAN
Absence of relevant differences in the primary endpoint among treatment groups was considered the null hypothesis. The alternative hypothesis was that the super high-pressure balloon would be superior to the scoring balloon with regard to achieving improved stent expansion index by OCT. Assuming a stent expansion index of 0.90±0.30 following preparation with a super high-pressure balloon and of 0.70±0.30 following preparation with a scoring balloon, with a two-sided alpha level of 0.05 and a power of 80%, we estimated that a sample size of 37 patients per group (74 patients in total) was required to account also for missing OCT imaging data. Sample size calculation was carried out using nQuery Advisor, Version 7.0 (Statistical Solutions, Cork, Ireland).
All statistical analyses were performed by an independent statistician. Primary and secondary endpoints were analysed on an intention-to-treat basis. Categorical data were expressed as counts and proportions. Differences between groups were checked for significance using the chi-square test (or Fisher’s exact test when the expected cell value was <5). Continuous data were displayed as mean±standard deviation and compared using the Student’s t-test or Mann-Whitney U test, as appropriate. Events were reported as crude incidence. No adjustment was made for the comparisons of primary and secondary endpoints. All tests were two-sided and a p-value <0.05 was considered statistically significant. Statistical analyses were performed in R, Version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
STUDY ORGANISATION
This was an investigator-initiated trial sponsored by Deutsches Herzzentrum München. The study was funded in part by an unrestricted research grant from SIS Medical AG and Boston Scientific. The authors are solely responsible for the design and conduct of the study, analyses, drafting and editing of the report, and its final contents. The ISAR-CALC trial committees are presented in Supplementary Appendix 1.
Results
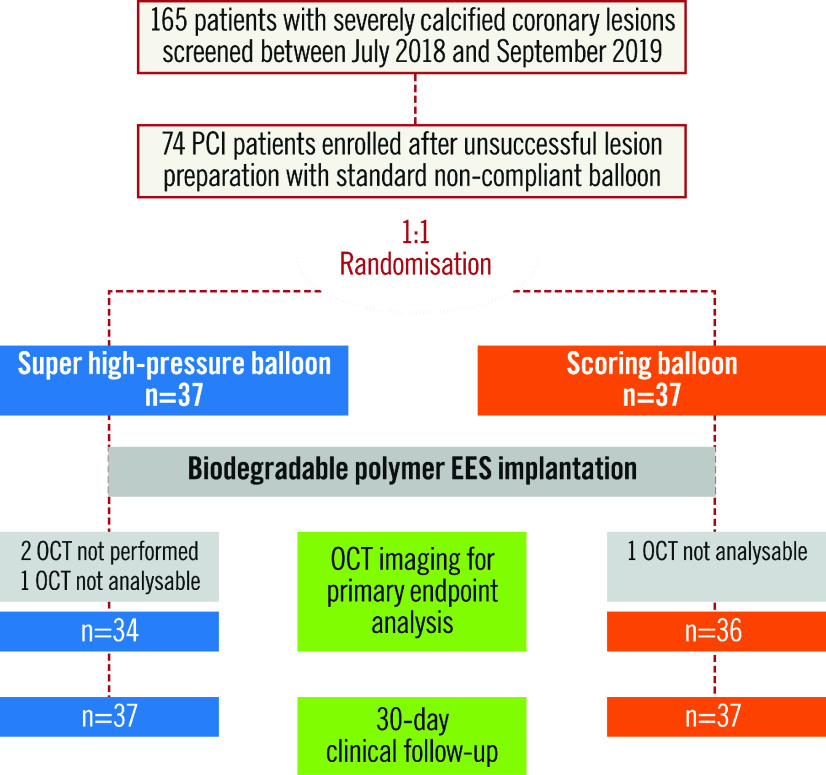
A total of 74 patients with severely calcific coronary lesions were enrolled. The study flow chart is shown in Figure 1. Baseline features of the study patients are shown in Table 1. There were no significant differences between the two groups at baseline. Of interest, the majority of participants were male and suffered from multivessel coronary disease.
Figure 1.
Study flow chart. EES: everolimus-eluting stents; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Table 1. Baseline characteristics.
| Super high-pressure balloon (n=37) | Scoring balloon (n=37) | p-value | |
| Age, years | 73.4±8.5 | 70.9±9.4 | 0.24 |
| Male gender | 86.5% (32/37) | 83.8% (31/37) | >0.99 |
| Height, cm | 175±9 | 172±8 | 0.17 |
| Weight, kg | 81.3±15.0 | 78.1±15.8 | 0.37 |
| Diabetes mellitus | |||
| Non-insulin dependent | 16.2% (6/37) | 24.3% (9/37) | 0.48 |
| Insulin-dependent | 13.5% (5/37) | 13.5% (5/37) | |
| Hypertension | 91.9% (34/37) | 81.1% (30/37) | 0.31 |
| Hyperlipidaemia | 78.4% (29/37) | 75.7% (28/37) | >0.99 |
| Current smoker | 21.6% (8/37) | 13.5% (5/37) | 0.54 |
| Prior myocardial infarction | |||
| >90 days | 10.8% (4/37) | 13.5% (5/37) | >0.99 |
| <90 days | 5.4% (2/37) | 8.1% (3/37) | |
| Prior revascularisation | |||
| PCI | 48.6% (18/37) | 59.5% (22/37) | 0.75 |
| CABG | 16.2% (6/37) | 13.5% (5/37) | |
| Left main disease | 24.3% (9/37) | 18.9% (7/37) | 0.78 |
| Multivessel disease | 91.9% (34/37) | 81.1% (30/37) | 0.26 |
| Ejection fraction, %* | 53.9±10.9 | 55.0±8.1 | 0.71 |
| Creatinine, mg/dl | 1.1±0.3 | 1.0±0.3 | 0.41 |
| Values are n (%) or mean±standard deviation. *Baseline ejection fraction was available in 49 patients. CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention | |||
Angiographic and procedural characteristics are provided in Table 2. There were no significant differences between the two groups at baseline. The angiographic core laboratory confirmed the presence of severely calcified target coronary lesions in all patients treated with the super high-pressure balloon and in all but one patient treated with the scoring balloon.
Table 2. Angiographic* and procedural characteristics.
| Super high-pressure balloon (n=37) | Scoring balloon (n=37) | p-value | |
| Angiographic characteristics | |||
| Target lesion location | |||
| Left anterior descending | 56.8% (21/37) | 51.4% (19/37) | 0.66 |
| Left circumflex | 13.5% (5/37) | 21.6% (8/37) | |
| Right coronary artery | 29.7% (11/37) | 27.0% (10/37) | |
| Bifurcation | 40.5% (15/37) | 37.8% (14/37) | >0.99 |
| Moderate/severe tortuosity | 13.5% (5/37) | 21.6% (8/37) | 0.74 |
| Chronic total occlusion | 2.7% (1/37) | 5.4% (2/37) | >0.99 |
| B2/C lesion | 100% (37/37) | 100% (37/37) | >0.99 |
| Severe calcification | 100% (37/37) | 97.3% (36/37) | >0.99 |
| Vessel diameter, mm | 3.1±0.5 | 2.9±0.4 | 0.11 |
| Lesion length, mm | 23.3±11.5 | 24.8±12.1 | 0.58 |
| Diameter stenosis, pre, % | 69.1±10.2 | 70.6±10.7 | 0.53 |
| Minimal lumen diameter, pre, mm | 0.85±0.36 | 0.82±0.33 | 0.12 |
| Procedural characteristics | |||
| Guiding catheter | |||
| 6 Fr | 43.2% (16/37) | 41.7% (15/37) | >0.99 |
| 7 Fr | 51.4% (19/37) | 55.6% (20/37) | |
| Predilation¶ | |||
| Maximal predilation balloon diameter, mm | 3.1±0.4 | 3.0±0.4 | 0.18 |
| Maximal predilation balloon pressure, bar | 33.8±6.5 | 17.0±4.0 | <0.001 |
| Large residual dissection (>5 mm) | 8.1% (3/37) | 13.5% (5/37) | 0.71 |
| Stent/lesion | 1.5±0.8 | 1.6±0.7 | 0.88 |
| Total stent length, mm | 39.6±18.0 | 41.0±21.5 | 0.75 |
| Minimal stent diameter, mm | 3.2±0.5 | 3.1±0.5 | 0.38 |
| Maximal stent diameter, mm | 3.4±0.5 | 3.2±0.4 | 0.24 |
| Maximal stent implantation pressure, bar | 16.5±3.5 | 16.1±2.9 | 0.62 |
| Post-dilation | 78.4% (29/37) | 83.8% (31/37) | 0.77 |
| Maximal post-dilation balloon diameter, mm | 3.7±0.5 | 3.4±0.6 | 0.11 |
| Maximal post-dilation balloon pressure, bar | 21.3±7.9 | 19.9±3.8 | 0.38 |
| Diameter stenosis, post, % | 11.6±4.8 | 14.4±5.6 | 0.02 |
| Minimal lumen diameter, post, mm | 2.83±0.34 | 2.65±0.36 | 0.03 |
| Acute lumen gain, mm | 1.89±0.42 | 1.83±0.45 | 0.60 |
| Compromised side branch | 13.5% (5/37) | 13.5% (5/37) | >0.99 |
| Values are n (%) or mean±standard deviation. *As adjudicated by the angiographic core laboratory. ¶ Study device. | |||
Target lesions were most frequently located in the left anterior descending artery and involved a bifurcation in approximately 40% of cases. Lesion length and the degree of baseline diameter stenosis did not differ between treatment groups. In the super high-pressure balloon group, the investigational device had a mean diameter of 3.1±0.4 mm and was inflated at a mean pressure of 33.8±6.5 bar. In the scoring balloon group, the control device had a mean diameter of 3.0±0.4 mm and was inflated at a mean pressure of 17.0±4.0 bar. After lesion predilation with the study devices, approximately one third of patients assigned to the scoring balloon received additional dilation with a standard non-compliant balloon (5.4% vs 32.4%, in the super high-pressure balloon and scoring balloon groups, respectively; p=0.012). Of note, three patients per group required rotational atherectomy for complementary lesion preparation due to inability to advance the assigned study device through the target lesions. A total of four lesions (5.4%) could not be treated with the DES selected per protocol due to unavailability; these lesions were treated with a XIENCE everolimus-eluting stent (Abbott Vascular). After DES implantation, the majority of patients received post-dilation with a standard non-compliant balloon without differences in terms of proportions (78.4% vs 83.8%; p=0.77) and maximal dilation pressures (21.3±7.9 bar vs 19.9±3.8 bar; p=0.38) between the super high-pressure balloon and scoring balloon groups.
According to core laboratory analysis, there was no significant difference in terms of acute lumen gain between the super high-pressure balloon and the scoring balloon (1.89±0.42 mm vs 1.83±0.45 mm; p=0.60). Of note, lesion preparation with the super high-pressure balloon significantly increased the final MLD (2.83±0.34 mm vs 2.65±0.36 mm; p=0.03) and reduced the residual diameter stenosis (11.6±4.8% vs 14.4±5.6%; p=0.02) as compared to the scoring balloon.
OCT DATA ANALYSIS
OCT data after DES implantation were available for 70 patients (94.6%) and are reported in Table 3. Three patients had no available OCT in the super high-pressure balloon group due to technical reasons (n=2) or because OCT pullbacks had insufficient quality for analysis (n=1), whereas one patient did not receive OCT in the scoring balloon group due to technical reasons.
Table 3. Optical coherence tomography measurements* after stent implantation.
| Super high-pressure balloon (n=34) | Scoring balloon (n=36) | p-value | |
| Minimal lumen area, mm2 | 6.40±2.15 | 5.77±1.91 | 0.20 |
| Maximal lumen area, mm2 | 11.5±2.97 | 11.1±3.05 | 0.62 |
| Average lumen area, mm2 | 8.59±2.18 | 8.14±1.95 | 0.37 |
| Mean reference area, mm2 | 10.0±2.52 | 9.64±2.39 | 0.50 |
| Minimal stent area, mm2 | 6.33±2.08 | 5.67±1.88 | 0.17 |
| Maximal stent area, mm2 | 10.8±2.87 | 10.6±2.90 | 0.79 |
| Average stent area, mm2 | 8.36±2.13 | 7.98±1.92 | 0.44 |
| *As adjudicated by the imaging core laboratory. | |||
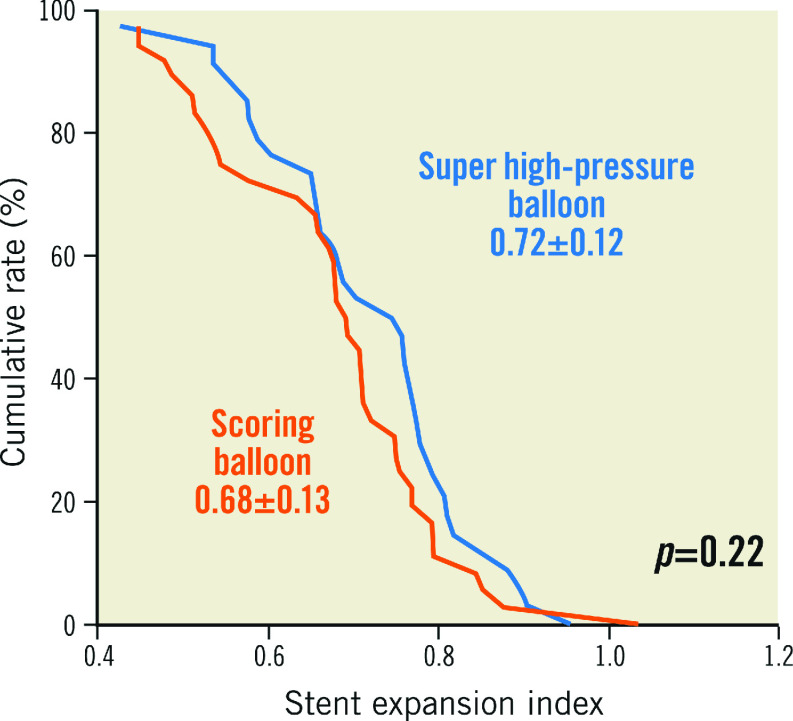
In relation to the primary endpoint of the trial, stent expansion index was comparable in both treatment groups (0.72±0.12 vs 0.68±0.13; p=0.22) (Figure 2). There was no significant difference with respect to other OCT parameters.
Figure 2.
Stent expansion index in patients assigned to either a super high-pressure balloon or a scoring balloon. Cumulative rate distribution for stent expansion index as assessed with optical coherence tomography.
PROCEDURAL AND CLINICAL OUTCOMES
Large residual dissections, coronary perforations with need for covered stents and compromised side branches were observed in a minority of patients among those enrolled, without significant differences between groups. Angiographic success was comparable with either the super high-pressure balloon or the scoring balloon (100% vs 97.3%; p>0.99). Strategy success (91.9% vs 83.8%; p=0.48) and procedural success (100% vs 89.2%; p=0.12) were numerically more frequent with the super high-pressure balloon versus the scoring balloon, without statistical significance. Two patients in the scoring balloon group suffered from an MI due to acute stent thrombosis, which required repeat revascularisation and stenting. No adverse event occurred in the super high-pressure balloon group. The complete list of procedural and in-hospital outcomes is shown in Table 4. The cumulative incidence of 30-day clinical outcomes is reported in Supplementary Table 2. There was no adverse event between discharge and 30-day follow-up.
Table 4. Procedural and in-hospital outcomes.
| Super high- pressure balloon (n=37) | Scoring balloon (n=37) | p-value | |
| Angiographic success | 100% (37/37) | 97.3% (36/37) | >0.99 |
| Final TIMI flow grade <3 | 0 | 2.7% (1/37) | >0.99 |
| Residual diameter stenosis >30%* | 0 | 2.7% (1/37) | >0.99 |
| Strategy success | 91.9% (34/37) | 83.8% (31/37) | 0.48 |
| Stent deployment failure | 0 | 2.7% (1/37) | >0.99 |
| Complementary rotational atherectomy | 8.1% (3/37) | 8.1% (3/37) | >0.99 |
| Procedural success | 100% (37/37) | 89.2% (33/37) | 0.12 |
| In-hospital MACE | 0 | 10.8% (4/37) | 0.11 |
| Cardiac death | 0 | 0 | – |
| Target vessel myocardial infarction | 0 | 5.4% (2/37) | 0.49 |
| Repeat revascularisation | 0 | 8.1% (3/37) | 0.24 |
| Procedure duration, min | 56.2±32.2 | 55.8±38.5 | 0.96 |
| Fluoroscopy time, min | 25.3±22.7 | 24.1±15.5 | 0.79 |
| Contrast volume, ml | 273±108 | 248±107 | 0.32 |
| Coronary perforation | 2.7% (1/37) | 2.7% (1/37) | >0.99 |
| Covered stent implantation | 2.7% (1/37) | 2.7% (1/37) | >0.99 |
| *As adjudicated by the angiographic core laboratory. The cumulative incidence of MACE up to 30 days is reported in Supplementary Table 2. MACE: major adverse cardiac events; TIMI: Thrombolysis In Myocardial Infarction | |||
Discussion
The ISAR-CALC trial represents the first multicentre investigation of the comparative performance of two balloon-based techniques to prepare severely calcified coronary artery lesions before stenting. After unsuccessful preparation with a standard non-compliant balloon at maximal pressure, patients enrolled were randomly assigned to preparation with either a super high-pressure balloon or a scoring balloon before implantation of a latest-generation, thin-strut, everolimus-eluting stent. The principal findings of this trial are that:
– A super high-pressure balloon versus a scoring balloon led to comparable stent expansion index as assessed with OCT imaging.
– There was a trend towards improved angiographic performance with the super high-pressure balloon versus the scoring balloon (namely, increased final MLD and reduced residual stenosis), although angiographic success did not differ between groups.
– The rates of procedural complications and adverse clinical events up to 30 days reflect the anatomical and procedural complexity.
Adequate lesion preparation before stenting is crucial for PCI success: dilatation of fibrocalcific plaque favours stent delivery and allows more homogeneous stent expansion, which, in turn, impacts on acute and long-term outcomes from stenting9. These aspects are of particular importance in the case of PCI for severely calcified coronary lesions, which are associated with higher anatomical complexity, more frequent procedural complications, and stent failure10.
The use of balloon-based techniques to prepare severely calcified coronary lesions offers several potential advantages over ablation- or debulking-based techniques: there is minimal additional training, no extra staff or instrumentation required in the catheterisation laboratory and less risk of downstream embolisation of atheromatous material1. Notably, more than 90% of patients included in the ISAR-CALC trial achieved a strategy success with assigned devices, with rotational atherectomy required only in a minority of patients.
Stent underexpansion is a powerful predictor of stent thrombosis11 and restenosis12. Although not specifically validated for calcific coronary lesions, we chose stent expansion index as the primary endpoint of this trial, deriving the cut-off value for sample size calculations from available evidence4. We found that preparation of severely calcified lesions with a super high-pressure balloon was not superior to a scoring balloon in terms of stent expansion. In relation to this finding, a couple of issues should be mentioned. In contrast to previous investigations13,14, this trial focused on balloon-based techniques for preparation of severely calcified coronary lesions undilatable with standard non-compliant balloons at maximal pressures. The use of a super high-pressure balloon for preparation of these highly complex lesions is probably insufficient to achieve optimal stent expansion as measured by intravascular imaging. However, in contrast with the scoring balloon, a super high-pressure balloon is an established tool for stent optimisation15. Further studies are required to determine whether a super high-pressure balloon is superior to other balloon-based strategies when used for both preparation of calcific lesions and stent optimisation, under the guidance of intravascular imaging.
In this trial, the implementation of a super high-pressure balloon versus a scoring balloon in severely calcified lesions before coronary stenting was associated with larger final MLD and lower residual diameter stenosis according to independent core laboratory analysis. This fact is important; although the overall angiographic success did not differ with either the super high-pressure balloon or the scoring balloon, vessel size and residual diameter stenosis are highly predictive of DES failure16. In addition, although the magnitude of treatment effect for vessel size and diameter stenosis observed with the super high-pressure balloon was modest, the marginal gain may be of clinical relevance in this particular population that is prone to stent failure. However, the current findings are hypothesis-generating and require validation in studies powered for angiographic measures of efficacy.
There is concern regarding the potential risk of coronary perforation with super high-pressure balloons due to excessive mechanical vascular trauma associated with high dilation pressures in calcified lesions1. Although this trial was not powered to address such rarely occurring clinical endpoints, the overall incidence of adverse events was low in both treatment groups. However, in line with previous data14, we found that adverse events were numerically more frequent among patients treated with a scoring balloon. Approximately one third of patients assigned to a scoring balloon received complementary lesion preparation with repeat, additional non-compliant balloons before stenting. In contrast, the super high-pressure balloon was the sole therapy used for lesion preparation in the majority of cases. The variance in complementary lesion preparation is probably due to the unsatisfactory angiographic appearance after scoring balloon dilation as per the visual estimation of operators. However, the angiographic core laboratory analysis found no significant difference in terms of acute gain between treatment groups.
Study limitations
This trial has several limitations, which deserve discussion.
– The hypothesised superiority of a super high-pressure balloon versus a scoring balloon in terms of stent expansion index could not be demonstrated. In this regard, any consideration regarding other endpoints should be interpreted with caution and remains exploratory in nature.
– Since randomisation occurred after unsuccessful lesion preparation with a standard non-compliant balloon, baseline OCT pullbacks were not routinely performed, which represents a major limitation of the present study. However, the value of baseline imaging such as OCT17 to guide the selection of interventional techniques and predict the relative performance in calcific coronary lesions remains undisputed, and the threshold for imaging-guided interventions in this setting should be as low as possible.
– The present results cannot be extrapolated to balloon-based technologies or DES platforms different from those selected for this trial. Amongst others, the novel option of intravascular lithotripsy, which uses acoustic shock waves in a balloon-based system to fracture severe calcifications in the vessel wall, is promising18. Whether this latter technology is alternative or complementary to a super high-pressure balloon in the contemporary algorithm for the imaging-guided management of severe coronary calcifications undilatable with conventional non-compliant or scoring balloons (as suggested from the study of Kassimis et al19) warrants further investigation in properly designed head-to-head comparisons.
– This trial enrolled selected patients, excluding, amongst others, those with a history of MI within seven days. This aspect limits the external validity of the current findings.
Conclusions
The preparation of severely calcified coronary lesions with either a super high-pressure balloon or a scoring balloon was associated with comparable stent expansion on intravascular imaging and a trend towards improved angiographic performance. The role of the super high-pressure balloon for lesion preparation and imaging-guided optimisation of drug-eluting stents in severely calcified coronary lesions warrants further investigation.
Impact on daily practice
Percutaneous treatment of severely calcified coronary lesions is associated with procedural complications and adverse clinical events. So far, there has been no randomised comparison of balloon-based techniques to prepare severely calcified coronary lesions. In this randomised trial, the preparation of severely calcified coronary lesions with either a super high-pressure balloon or a scoring balloon was associated with comparable stent expansion on intravascular imaging and a trend towards improved angiographic performance.
Supplementary data
ISAR-CALC trial committees.
Detailed description of the protocols for acquisition and analysis of angiographic and OCT data.
Inclusion/exclusion criteria of the ISAR-CALC trial.
Cumulative 30-day clinical outcomes.
Visual summary.
A ComparIson of Strategies to PrepAre SeveRely CALCified Coronary Lesions: the ISAR-CALC randomised trial.
Acknowledgments
Acknowledgements
We are grateful to the following collaborators: Miroslaw Ferenc, MD; Jens Wiebe, MD; Felix Voll, MD; Nader Mankerious, MD; Phillip Münch, MD; Stefanie Brunner; Susanne Pinieck; Nonglag Rifatov; Brigitta Mehmann.
Funding
The ISAR-CALC trial is an investigator-initiated trial which was supported by an unrestricted research grant from SIS Medical AG and Boston Scientific.
Conflict of interest statement
A. Allali reports personal fees from Boston Scientific, outside the submitted work. M. Ayoub reports personal fees from Boston Scientific, outside the submitted work. F. Cuculi reports personal fees from SIS Medical AG, outside the submitted work. M. Bossard reports grants from SIS Medical Switzerland, and personal fees from Abbott Vascular, during the conduct of the study. S. Kufner reports personal fees from AstraZeneca and personal fees from Bristol-Myers Squibb, outside the submitted work. M. Fusaro reports personal fees from SIS Medical AG, outside the submitted work. M. Joner reports personal fees from Biotronik, personal fees from OrbusNeich, grants and personal fees from Boston Scientific, grants and personal fees from Edwards, personal fees from ReCor, personal fees from AstraZeneca, and grants from Amgen, outside the submitted work. R.A. Byrne reports grants to the institution of prior employment from SIS Medical and from Boston Scientific related to the conduct of the study, and grants from CeloNova Biosciences to the institution of prior employment, outside the submitted work. S. Cassese reports personal fees and grants to the institution from SIS Medical AG, outside the submitted work. The other authors have no conflicts of interest to declare.
Abbreviations
- DES
drug-eluting stent(s)
- EES
everolimus-eluting stent(s)
- MACE
major adverse cardiac events
- MLD
minimal lumen diameter
- OCT
optical coherence tomography
- PCI
percutaneous coronary intervention
Contributor Information
Tobias Rheude, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany.
Himanshu Rai, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany.
Gert Richardt, Heart Center, Segeberger Kliniken, Bad Segeberg, Germany.
Abdelhakim Allali, Heart Center, Segeberger Kliniken, Bad Segeberg, Germany.
Mohamed Abdel-Wahab, Department of Cardiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany.
Dmitriy S. Sulimov, Department of Cardiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany.
Kambis Mashayekhi, Division of Cardiology and Angiology II, University Heart Center Freiburg-Bad Krozingen, Bad Krozingen, Germany.
Mohamed Ayoub, Division of Cardiology and Angiology II, University Heart Center Freiburg-Bad Krozingen, Bad Krozingen, Germany.
Florim Cuculi, Department of Cardiology, Kantonspital Luzern, Lucerne, Switzerland.
Matthias Bossard, Department of Cardiology, Kantonspital Luzern, Lucerne, Switzerland.
Sebastian Kufner, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany.
Erion Xhepa, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany.
Adnan Kastrati, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Massimiliano Fusaro, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany.
Michael Joner, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Robert A. Byrne, Cardiology Department, Mater Private Hospital, Dublin, Ireland; Cardiovascular Research, Royal College of Surgeons in Ireland, Dublin, Ireland.
Salvatore Cassese, Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Munich, Germany.
References
- De Maria GL, Scarsini R, Banning AP. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc Interv. 2019;12:1465–78. doi: 10.1016/j.jcin.2019.03.038. [DOI] [PubMed] [Google Scholar]
- Buiten RA, Ploumen EH, Zocca P, Doggen CJM, van Houwelingen KG, Danse PW, Schotborgh CE, Stoel MG, Scholte M, Linssen GCM, de Man FHAF, von Birgelen C. Three contemporary thin-strut drug-eluting stents implanted in severely calcified coronary lesions of participants in a randomized all-comers trial. Catheter Cardiovasc Interv. 2020;96:E508–15. doi: 10.1002/ccd.28886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Généreux P, Redfors B, Witzenbichler B, Arsenault MP, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Francese DP, Marquis-Gravel G, Mintz GS, Kirtane AJ, Maehara A, Mehran R, Stone GW. Two-year outcomes after percutaneous coronary intervention of calcified lesions with drug-eluting stents. Int J Cardiol. 2017;231:61–7. doi: 10.1016/j.ijcard.2016.12.150. [DOI] [PubMed] [Google Scholar]
- Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de la Torre Hernandez JM, Escaned J, Hill J, Prati F, Colombo A, Di Mario C, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G ESC Scientific Document Group. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- Sorini Dini C, Nardi G, Ristalli F, Mattesini A, Hamiti B, Di Mario C. Contemporary Approach to Heavily Calcified Coronary Lesions. Interv Cardiol. 2019;14:154–63. doi: 10.15420/icr.2019.19.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco GG, Ghione M, Mattesini A, Dall’Ara G, Ghilencea L, Kilickesmez K, De Luca G, Fattori R, Parisi R, Marino PN, Lupi A, Foin N, Di Mario C. Very high-pressure dilatation for undilatable coronary lesions: indications and results with a new dedicated balloon. EuroIntervention. 2016;12:359–65. doi: 10.4244/EIJY15M06_04. [DOI] [PubMed] [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, Ditrano CJ, Leon MB. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91:1959–65. doi: 10.1161/01.CIR.91.7.1959. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Otsuka T, Hibi K, Räber L. The Value of Intracoronary Imaging and Coronary Physiology When Treating Calcified Lesions. Interv Cardiol. 2019;14:164–8. doi: 10.15420/icr.2019.16.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13:696–705. doi: 10.4244/EIJ-D-17-00473. [DOI] [PubMed] [Google Scholar]
- Redfors B, Maehara A, Witzenbichler B, Weisz G, Stuckey TD, Henry TD, McAndrew T, Mehran R, Kirtane AJ, Stone GW, Généreux P. Outcomes After Successful Percutaneous Coronary Intervention of Calcified Lesions Using Rotational Atherectomy, Cutting-Balloon Angioplasty, or Balloon-Only Angioplasty Before Drug-Eluting Stent Implantation. J Invasive Cardiol. 2017;29:378–86. [PubMed] [Google Scholar]
- Adriaenssens T, Joner M, Godschalk TC, Malik N, Alfonso F, Xhepa E, De Cock D, Komukai K, Tada T, Cuesta J, Sirbu V, Feldman LJ, Neumann FJ, Goodall AH, Heestermans T, Buysschaert I, Hlinomaz O, Belmans A, Desmet W, ten Berg JM, Gershlick AH, Massberg S, Kastrati A, Guagliumi G, Byrne RA Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Investigators. Optical Coherence Tomography Findings in Patients With Coronary Stent Thrombosis: A Report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation. 2017;136:1007–21. doi: 10.1161/CIRCULATIONAHA.117.026788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joner M, Koppara T, Byrne RA, Castellanos MI, Lewerich J, Novotny J, Guagliumi G, Xhepa E, Adriaenssens T, Godschalk TC, Malik N, Alfonso F, Tada T, Neumann FJ, Desmet W, ten Berg JM, Gershlick AH, Feldman LJ, Massberg S, Kastrati A Prevention of PRESTIGE Investigators. Neoatherosclerosis in Patients With Coronary Stent Thrombosis: Findings From Optical Coherence Tomography Imaging (A Report of the PRESTIGE Consortium). JACC Cardiovasc Interv. 2018;11:1340–50. doi: 10.1016/j.jcin.2018.02.029. [DOI] [PubMed] [Google Scholar]
- Ali ZA, Nef H, Escaned J, Werner N, Banning AP, Hill JM, De Bruyne B, Montorfano M, Lefevre T, Stone GW, Crowley A, Matsumura M, Maehara A, Lansky AJ, Fajadet J, Di Mario C. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses: The Disrupt CAD II Study. Circ Cardiovasc Interv. 2019;12:e008434. doi: 10.1161/CIRCINTERVENTIONS.119.008434. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab M, Toelg R, Byrne RA, Geist V, El-Mawardy M, Allali A, Rheude T, Robinson DR, Abdelghani M, Sulimov D, Kastrati A, Richardt G. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ Cardiovasc Interv. 2018;11:e007415. doi: 10.1161/CIRCINTERVENTIONS.118.007415. [DOI] [PubMed] [Google Scholar]
- Secco GG, Buettner A, Parisi R, Pistis G, Vercellino M, Audo A, Kambis M, Garbo R, Porto I, Tarantini G, Di Mario C. Clinical Experience with Very High-Pressure Dilatation for Resistant Coronary Lesions. Cardiovasc Revasc Med. 2019;20:1083–7. doi: 10.1016/j.carrev.2019.02.026. [DOI] [PubMed] [Google Scholar]
- Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100:153–9. doi: 10.1136/heartjnl-2013-304933. [DOI] [PubMed] [Google Scholar]
- Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, Usui E, Yonetsu T, Haag E, Shlofmitz RA, Kakuta T, Maehara A. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182–9. doi: 10.4244/EIJ-D-17-00962. [DOI] [PubMed] [Google Scholar]
- Hill JM, Kereiakes DJ, Shlofmitz RA, Klein AJ, Riley RF, Price MJ, Herrmann HC, Bachinsky W, Waksman R, Stone GW Disrupt CAD III Investigators. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J Am Coll Cardiol. 2020;76:2635–46. doi: 10.1016/j.jacc.2020.09.603. [DOI] [PubMed] [Google Scholar]
- Kassimis G, Didagelos M, De Maria GL, Kontogiannis N, Karamasis GV, Katsikis A, Sularz A, Karvounis H, Kanonidis I, Krokidis M, Ziakas A, Banning AP. Shockwave Intravascular Lithotripsy for the Treatment of Severe Vascular Calcification. Angiology. 2020;71:677–88. doi: 10.1177/0003319720932455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ISAR-CALC trial committees.
Detailed description of the protocols for acquisition and analysis of angiographic and OCT data.
Inclusion/exclusion criteria of the ISAR-CALC trial.
Cumulative 30-day clinical outcomes.